The Relationship between Microbial Communities in Coffee Fermentation and Aroma with Metabolite Attributes of Finished Products
Abstract
:1. Introduction
2. Materials and Methods
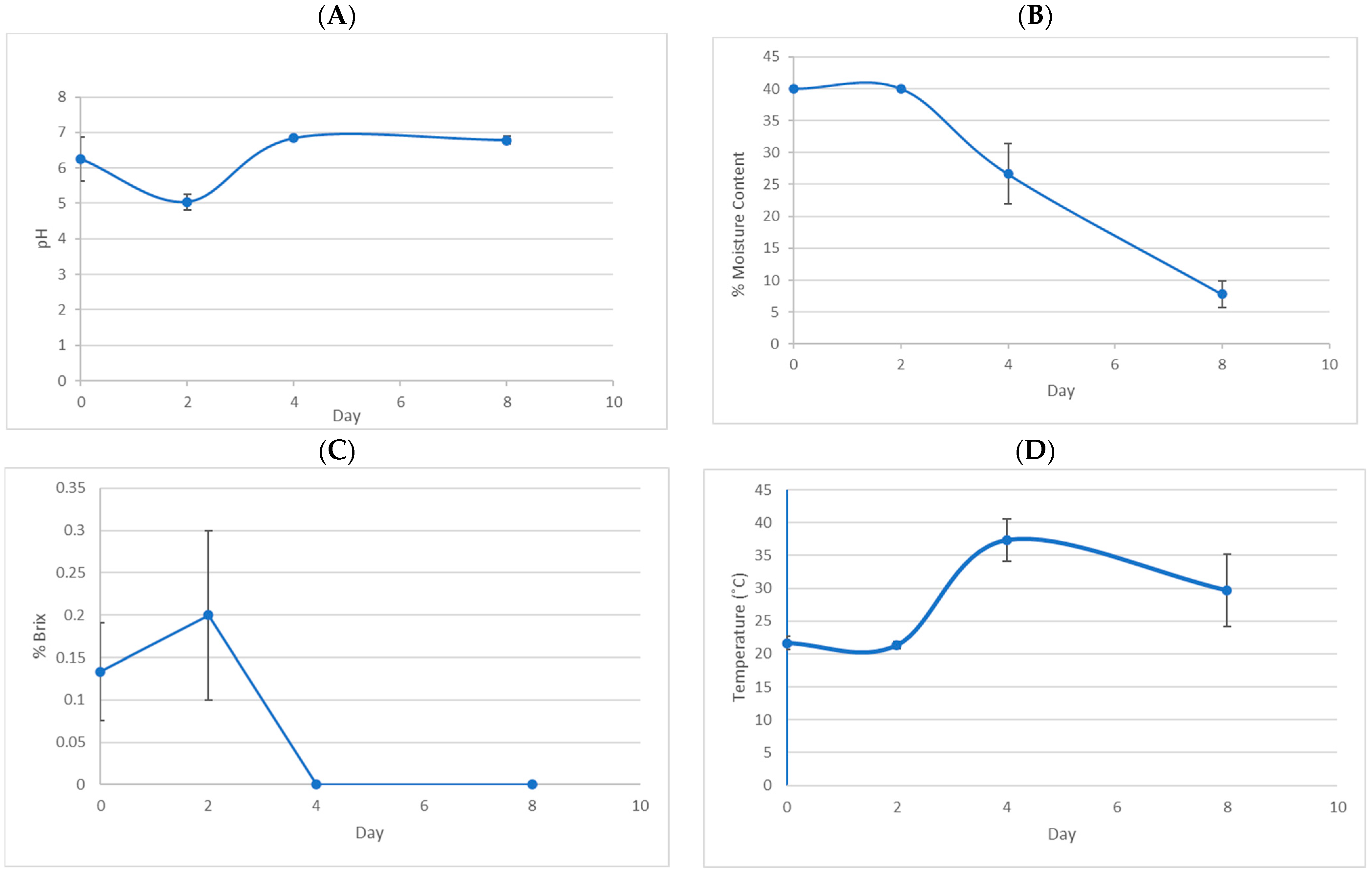
2.1. On-Site Wet Process Coffee Fermentation
2.2. Microbiome Sequencing and Bioinformatic Analysis of Bacterial and Fungal Communities
× Average Molecular Weight of a DNA bp (660 g/mole/bp) ÷ Avogadros Number (6.022 × 1023/mole)
2.3. Extraction and Analysis of Volatile Compounds Using Headspace Stir Bar Sorptive Extraction (HSSE) and Gas Chromatograph–Mass Spectrometer Triple Quadrupole (GCTQ)
2.4. Biochemical Compound Analysis Using UHPLC
2.5. Data Analysis
3. Result and Discussion
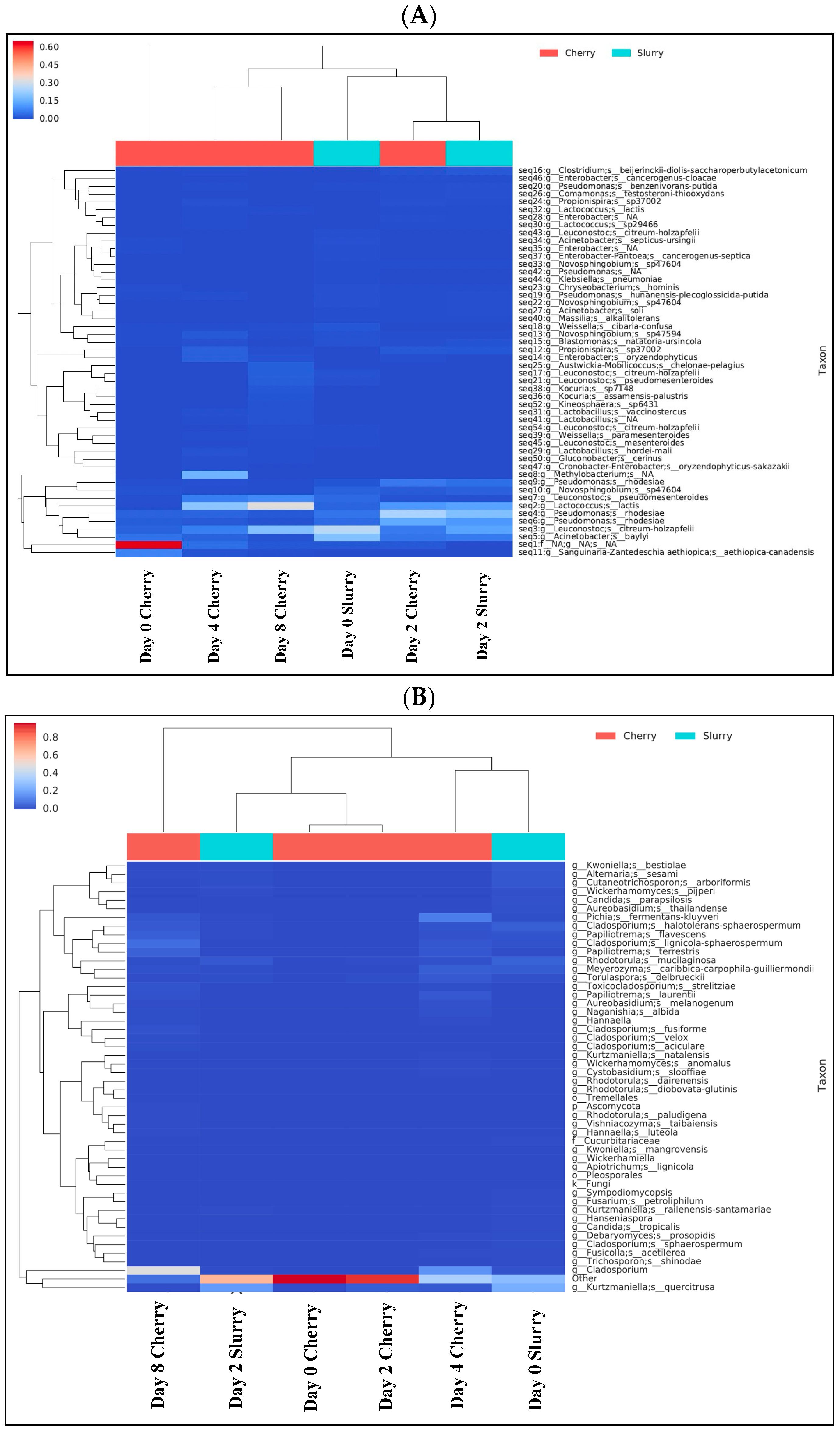
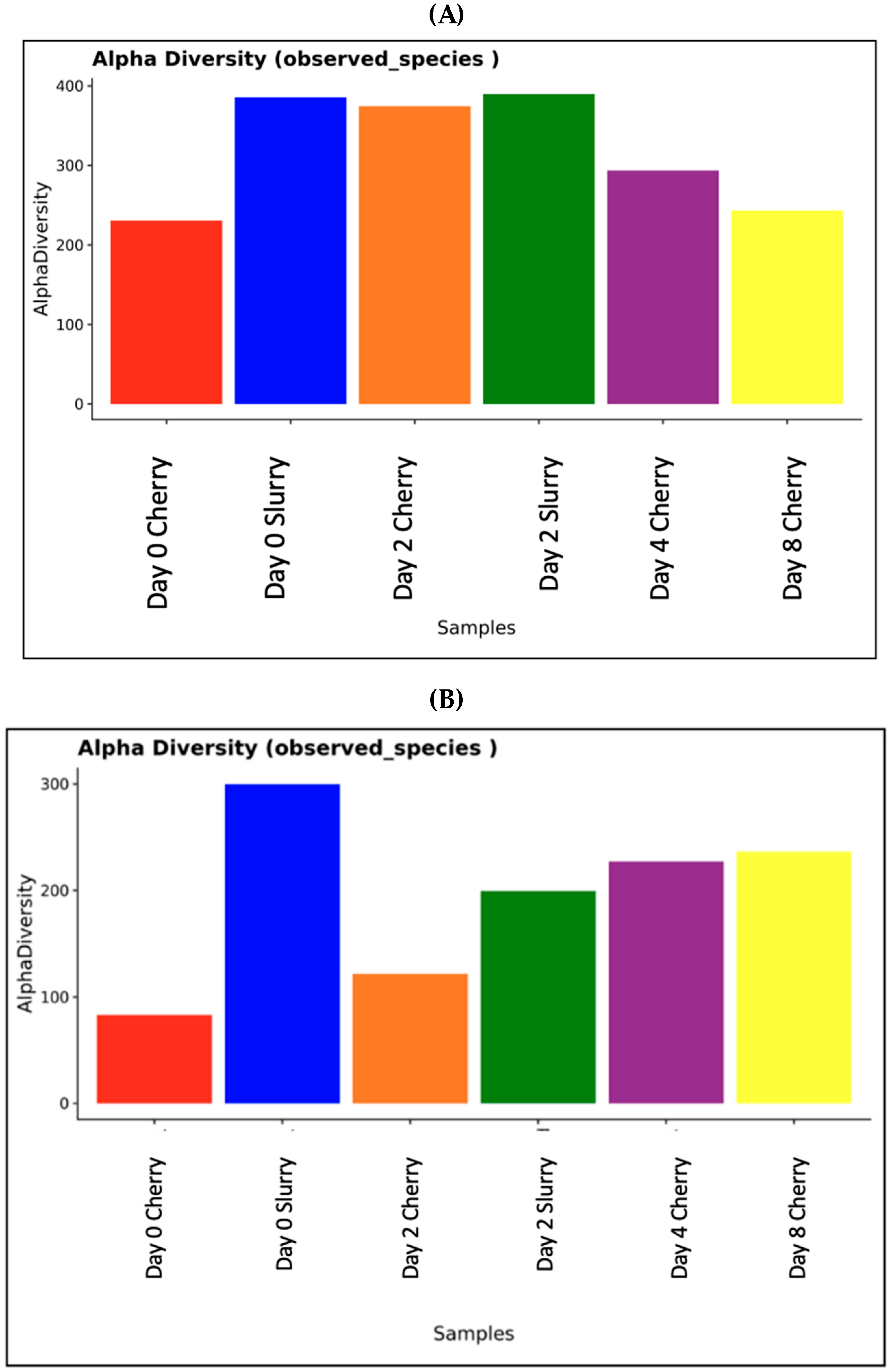
3.1. Metagenomic Analysis of Microbial Community during Coffee Fermentation
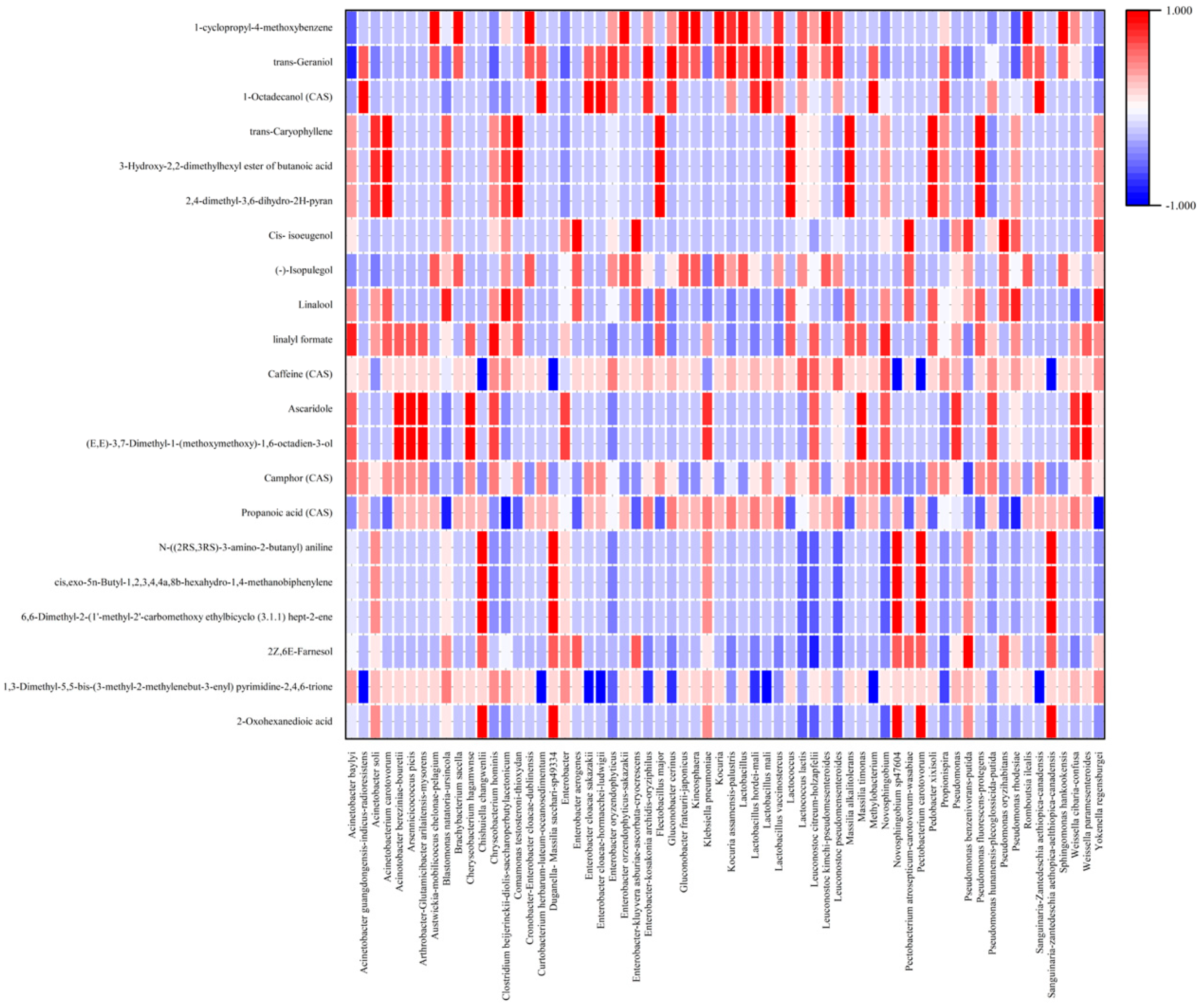
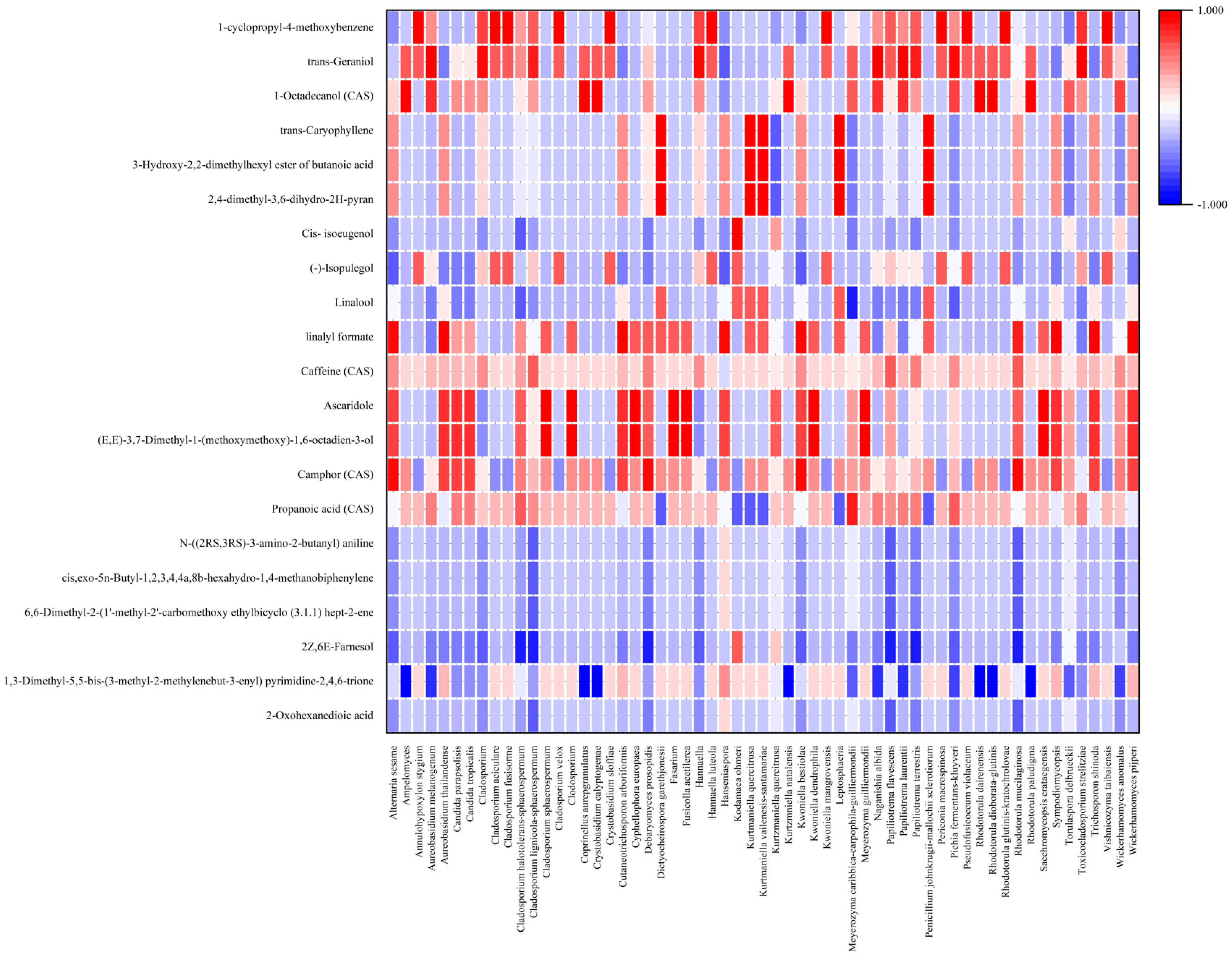
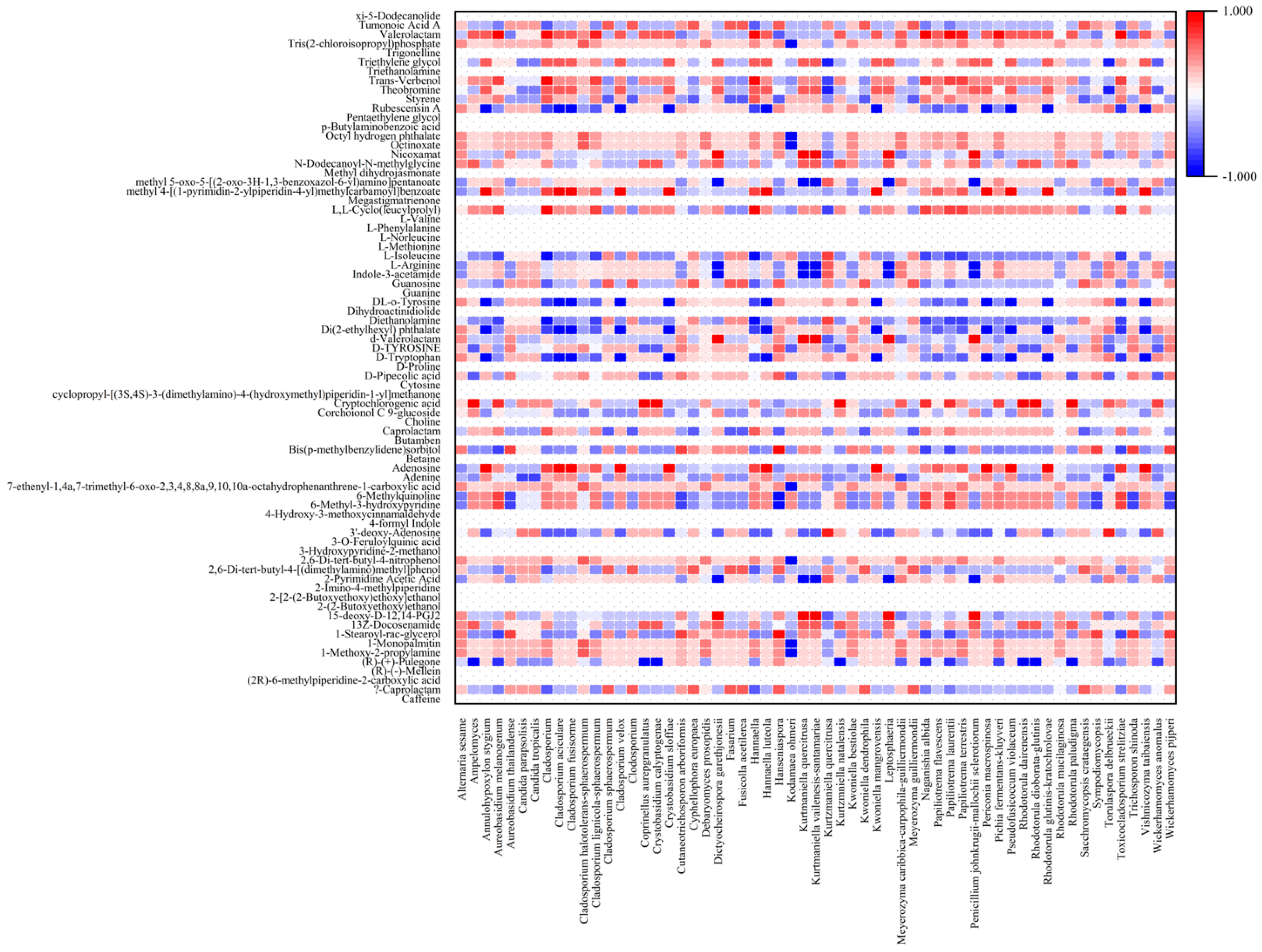
3.2. The Correlation between the Microbial Community and the Developed Flavor/Aroma
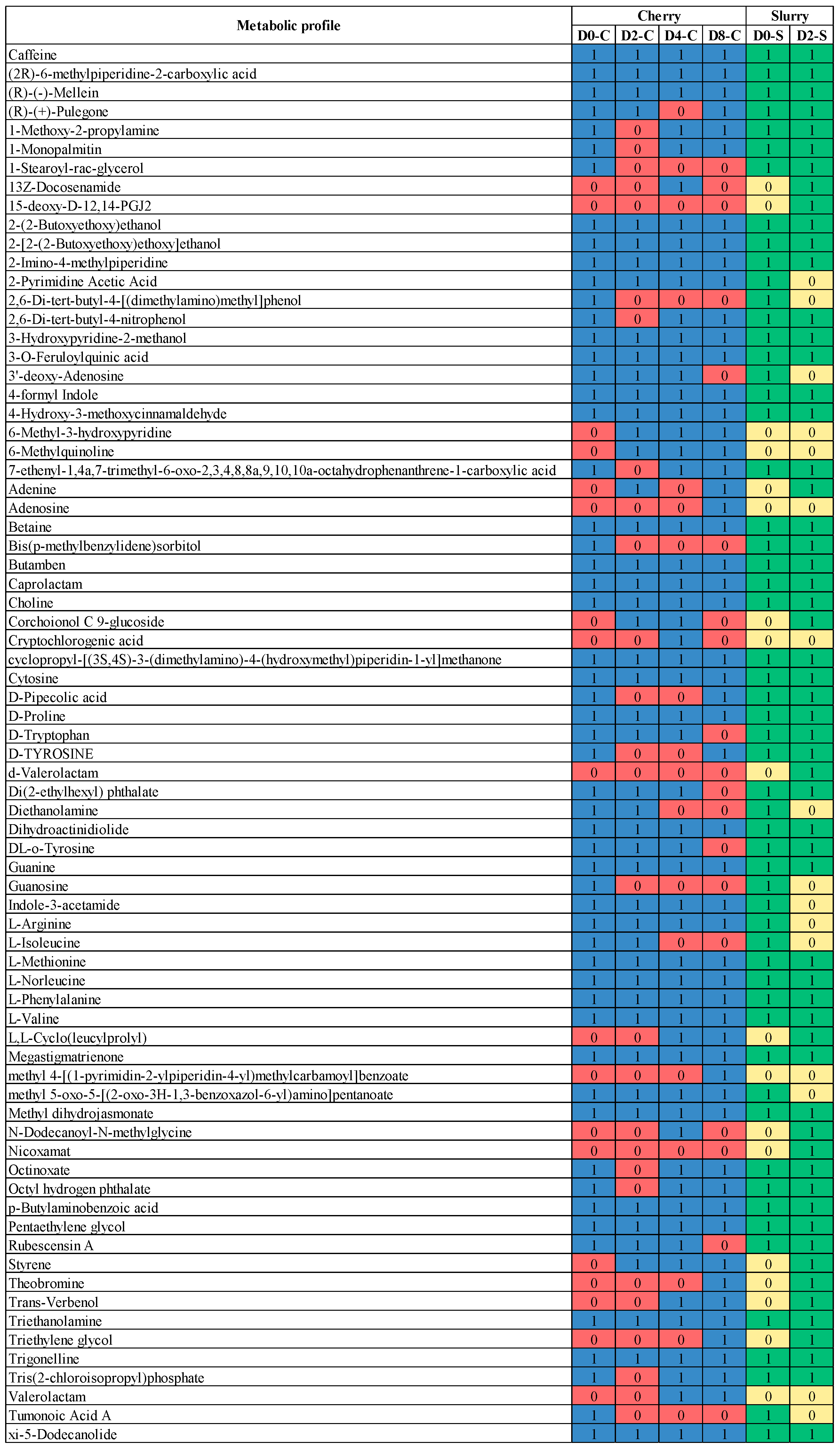
3.3. Metabolic Profiles Produced by the Microbial Community in Coffee Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Office of Agricultural Economics (Thailand). 2022. Available online: https://www.oae.go.th/view/1/Home/EN-US (accessed on 7 July 2024).
- Pimenta, C.J.; Angélico, C.L.; Chalfoun, S.M. Challengs in coffee quality: Cultural, chemical and microbiological aspects. Ciência Agrotecnol. 2018, 42, 337–349. [Google Scholar] [CrossRef]
- Avallone, S.; Brillouet, J.M.; Guyot, B.; Olguin, E.; Guiraud, J.P. Involvement of pectolytic micro-organisms in coffee fermentation. Int. J. Food Sci. Technol. 2002, 37, 191–198. [Google Scholar] [CrossRef]
- Masoud, W.; Bjørg, C.L.; Jespersen, L.; Jakobsen, M. Yeast involved in fermentation of Coffea arabica in East Africa determined by genotyping and by direct denaturating gradient gel electrophoresis. Yeast 2004, 21, 549–556. [Google Scholar] [CrossRef]
- HaileMKang, W.H. Isolation, identification, and characterization of pectinolytic yeasts for starter culture in coffee fermentation. Microorganisms 2019, 7, 401. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. The role of wet fermentation in enhancing coffee flavor, aroma and sensory quality. Eur. Food Res. Technol. 2021, 247, 485–498. [Google Scholar] [CrossRef]
- Silva, C.F.; Batista, L.R.; Abreu, L.M.; Dias, E.S.; Schwan, R.F. Succession of bacterial and fungal communities during natural coffee (Coffea arabica) fermentation. Food Microbiol. 2008, 25, 951–957. [Google Scholar] [CrossRef]
- Jakobsen, M.; Narvhus, J. Yeasts and their possible beneficial and negative effects on the quality of dairy products. Int. Dairy J. 1996, 6, 755–768. [Google Scholar] [CrossRef]
- Viljoen, B.C. Yeast ecological interactions. Yeast’Yeast, Yeast’Bacteria, Yeast’Fungi interactions and yeasts as biocontrol agents. In Yeasts in Food and Beverages; Springer: Berlin/Heidelberg, Germany, 2006; pp. 83–110. [Google Scholar]
- Aculey, P.C.; Snitkjaer, P.; Owusu, M.; Bassompiere, M.; Takrama, J.; Nørgaard, L.; Petersen, M.A.; Nielsen, D.S. Ghanaian cocoa bean fermentation characterized by spectroscopic and chromatographic methods and chemometrics. J. Food Sci. 2010, 75, S300–S307. [Google Scholar] [CrossRef]
- Pregolini, V.B.; de Melo Pereira, G.V.; da Silva Vale, A.; de Carvalho Neto, D.P.; Soccol, C.R. Influence of environmental microbiota on the activity and metabolism of starter cultures used in coffee beans fermentation. Fermentation 2021, 7, 278. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Coffee, tea, cocoa. In Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; pp. 938–970. [Google Scholar]
- Arrey, G.; Li, G.; Murphy, R.; Guimaraes, L.; Alizadeh, S.; Poulsen, M.; Regenberg, B. Isolation, characterization, and genome assembly of Barnettozyma botsteinii sp. nov. and novel strains of Kurtzmaniella quercitrusa isolated from the intestinal tract of the termite Macrotermes bellicosus. G3 2021, 11, 342. [Google Scholar]
- Vilela, D.M.; Pereira, G.V.; Silva, C.F.; Batista, L.R.; Schwan, R.F. Molecular ecology and polyphasic characterization of the microbiota associated with semi-dry processed coffee (Coffea Arab. L.). Food Microbiol. 2010, 27, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.V.; Amore, T.D.; Teixeira, E.C.; de Medeiros Burkert, J.F. Carotenoid production by Rhodotorula mucilaginosa in batch and fed-batch fermentation using agroindustrial byproducts. Food Technol. Biotechnol. 2019, 57, 388. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.L.; Ribeiro, L.S.; Miguel, M.G.; Batista, L.R.; Schwan, R.F.; Medeiros, F.H.; Silva, C.F. Yeasts prevent ochratoxin A contamination in coffee by displacing Aspergillus Carbonarius. Biol. Control. 2021, 155, 104512. [Google Scholar] [CrossRef]
- Muleta, D.; Assefa, F.; Granhall, U. In vitro antagonism of rhizobacteria isolated from Coffea arabica L. against emerging fungal coffee pathogens. Eng. Life Sci. 2007, 7, 577–586. [Google Scholar] [CrossRef]
- Mathews, S.L.; Pawlak, J.; Grunden, A.M. Bacterial biodegradation and bioconversion of industrial lignocellulosic streams. Appl. Microbiol. Biotechnol. 2015, 99, 2939–2954. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shao, T.; Li, J.; Zhao, J.; Dong, Z. Fermentative products and bacterial community structure of C4 forage silage in response to epiphytic microbiota from C3 forages. Anim. Biosci. 2022, 35, 1860. [Google Scholar] [CrossRef] [PubMed]
- Maisuria, V.; Nerurkar, A. Biochemical properties and thermal behavior of pectate lyase produced by Pectobacterium carotovorum subsp. carotovorum BR1 with industrial potentials. Biochem. Eng. J. 2012, 63, 22–30. [Google Scholar] [CrossRef]
- Björkroth, K.; Holzapfel, W. Genera Leuconostoc, Oenococcus and Weissella. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 267–319. [Google Scholar]
- Robert, H.; Gabriel, V.; Fontagné-Faucher, C. Biodiversity of lactic acid bacteria in French wheat sourdough as determined by molecular characterization using species-specific PCR. Int. J. Food Microbiol. 2009, 135, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Heo, G.Y.; Lee, J.W.; Oh, Y.J.; Park, J.A.; Park, Y.H.; Pyun, Y.R.; Ahn, J.S. Analysis of kimchi microflora using denaturing gradient gel electrophoresis. Int. J. Food Microbiol. 2005, 102, 143–150. [Google Scholar] [CrossRef]
- Malik, A.; Radji, M.; Kralj, S.; Dijkhuizen, L. Screening of lactic acid bacteria from Indonesia reveals glucansucrase and fructansucrase genes in two different Weissella confusa strains from soya. FEMS Microbiol. Lett. 2009, 300, 131–138. [Google Scholar] [CrossRef]
- Martín, R.; Heilig, H.G.; Zoetendal, E.G.; Jiménez, E.; Fernández, L.; Smidt, H.; Rodríguez, J.M. Cultivation-independent assessment of the bacterial diversity of breast milk among healthy women. Res. Microbiol. 2007, 158, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Park, J.Y.; Jeong, H.R.; Heo, H.J.; Han, N.S.; Kim, J.H. Probiotic properties of Weissella strains isolated from human faeces. Anaerobe 2012, 18, 96–102. [Google Scholar] [CrossRef]
- Leong, K.H.; Chen, Y.S.; Pan, S.F.; Chen, J.J.; Wu, H.C.; Chang, Y.C.; Yanagida, F. Diversity of lactic acid bacteria associated with fresh coffee cherries in Taiwan. Curr. Microbiol. 2014, 68, 440–447. [Google Scholar] [CrossRef]
- Picon, A.; López-Pérez, O.; Torres, E.; Garde, S.; Nuñez, M. Contribution of autochthonous lactic acid bacteria to the typical flavour of raw goat milk cheeses. Int. J. Food Microbiol. 2019, 299, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Zhu, H.; Tong, L.; Zhou, S.; Yang, R.; Niu, M. Volatile profiles of fresh rice noodles fermented with pure and mixed cultures. Food Res. Int. 2019, 119, 152–160. [Google Scholar] [CrossRef]
- de Carvalho Neto, D.P.; de Melo Pereira, G.V.; Finco, A.M.; Letti, L.A.; da Silva, B.J.; Vandenberghe, L.P.; Soccol, C.R. Efficient coffee beans mucilage layer removal using lactic acid fermentation in a stirred-tank bioreactor: Kinetic, metabolic and sensorial studies. Food Biosci. 2018, 26, 80–87. [Google Scholar] [CrossRef]
- Huch, M.; Franz, C. Coffee: Fermentation and microbiota. In Advances in Fermented Foods and Beverages; Elsevier: Amsterdam, The Netherlands, 2015; pp. 501–513. [Google Scholar]
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Zhao, F.; Zhao, F.; Chen, Y.; Liu, S.Q. Potential of lactic acid bacteria to modulate coffee volatiles and effect of glucose supplementation: Fermentation of green coffee beans and impact of coffee roasting. J. Sci. Food Agric. 2019, 99, 409–420. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; da Silva Vale, A.; de Carvalho Neto, D.P.; Muynarsk, E.S.; Soccol, V.T.; Soccol, C.R. Lactic acid bacteria: What coffee industry should know? Curr. Opin. Food Sci. 2020, 31, 1–8. [Google Scholar] [CrossRef]
- Klimankova, E.; Holadová, K.; Hajšlová, J.; Čajka, T.; Poustka, J.; Koudela, M. Aroma profiles of five basil (Ocimum basilicum L.) cultivars grown under conventional and organic conditions. Food Chem. 2008, 107, 464–472. [Google Scholar] [CrossRef]
- Jennings, W. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA; Wiley Online Library: Hoboken, NJ, USA, 1980. [Google Scholar]
- Lisko, J.G.; Lee, G.E.; Kimbrell, J.B.; Rybak, M.E.; Valentin-Blasini, L.; Watson, C.H. Caffeine concentrations in coffee, tea, chocolate, and energy drink flavored e-liquids. Nicotine Tob. Res. 2017, 19, 484–492. [Google Scholar] [CrossRef]
- Chisholm, M.G.; Wilson, M.A.; Gaskey, G.M. Characterization of aroma volatiles in key lime essential oils (Citrus aurantifolia Swingle). Flavour Fragr. J. 2003, 18, 106–115. [Google Scholar] [CrossRef]
- Duke, J.D. Duke’s Phytochemical and Ethnobotanical Databases; United States Department of Agriculture: Washington, DC, USA; Agricultural Research Service: Washington, DC, USA, 2004. [Google Scholar]
- Jiang, K.; Xu, K.; Wang, J.; Meng, F.; Wang, B. Based on HS-SPME-GC-MS combined with GC-O-MS to analyze the changes of aroma compounds in the aging process of Citri Reticulatae Pericarpium. Food Biosci. 2023, 54, 102798. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Guchu, E.; Castro-Vázquez, L.; de Torres, C.; Pérez-Coello, M.S. Aroma-active compounds of American, French, Hungarian and Russian oak woods, studied by GC–MS and GC–O. Flav. Frag. J. 2008, 23, 93–98. [Google Scholar] [CrossRef]
- Chung, T.Y.; Eiserich, J.P.; Shibamoto, T. Volatile compounds isolated from edible Korean chamchwi (Aster Scaber Thunb). J. Agric. Food Chem. 1993, 41, 1693–1697. [Google Scholar] [CrossRef]
- Berger, R.G.; Drawert, F.; Kollmannsberger, H. The flavour of cape gooseberry (Physalis peruviana L.). Z Leb. Unters Forsch 1989, 188, 122–126. [Google Scholar] [CrossRef]
- Vaneechoutte, M.; Dijkshoorn, L.; Nemec, A.; Kämpfer, P.; Wauters, G. Acinetobacter, Chryseobacterium, Moraxella, and other nonfermentative Gram-negative rods. In Manual of Clinical Microbiology; Wiley Online Library: Hoboken, NJ, USA, 2011; pp. 714–738. [Google Scholar]
- Zhu, Y.; Zhang, F.; Zhang, C.; Yang, L.; Fan, G.; Xu, Y.; Sun, B.; Li, X. Dynamic microbial succession of Shanxi aged vinegar and its correlation with flavor metabolites during different stages of acetic acid fermentation. Sci. Rep. 2018, 8, 8612. [Google Scholar] [CrossRef]
- Martinez, S.J.; Simão, J.B.; Pylro, V.S.; Schwan, R.F. The altitude of coffee cultivation causes shifts in the microbial community assembly and biochemical compounds in natural induced anaerobic fermentations. Front. Microbiol. 2021, 12, 671395. [Google Scholar] [CrossRef]
- Philippe, C.; Krupovic, M.; Jaomanjaka, F.; Claisse, O.; Petrel, M.; Le Marrec, C. Bacteriophage GC1, a novel tectivirus infecting Gluconobacter cerinus, an acetic acid bacterium associated with wine-making. Viruses 2018, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Schink, B. Propionispira. Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; pp. 1–4. [Google Scholar]
- O’mahony, T.; Rekhif, N.; Cavadini, C.; Fitzgerald, G.F. The application of a fermented food ingredient containing ‘variacin’, a novel antimicrobial produced by Kocuria varians, to control the growth of Bacillus cereus in chilled dairy products. J. Appl. Microbiol. 2001, 90, 106–114. [Google Scholar] [CrossRef]
- Cárdenas, E.L.; Zapata-Zapata, A.D.; Kim, D. Hydrogen production from coffee mucilage in dark fermentation with organic wastes. Energies 2018, 12, 71. [Google Scholar] [CrossRef]
- Vega, F.E.; Pava-Ripoll, M.; Posada, F.; Buyer, J.S. Endophytic bacteria in Coffea arabica L. An International Journal on Biochemistry, Physiology, Genetics, Morphology, and Ecology of Microorganisms. J. Basic Microbiol. 2005, 45, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kim, M.S.; Jung, M.J.; Nam, Y.D.; Park, E.J.; Roh, S.W.; Bae, J.W. Brachybacterium squillarum sp. nov., isolated from salt-fermented seafood. Int. J. Syst. Evol. Microbiol. 2011, 61, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yin, Y.; Wang, J. Microbial community diversity during fermentative hydrogen production inoculating various pretreated cultures. Int. J. Hydrogen Energy 2019, 44, 13147–13156. [Google Scholar] [CrossRef]
- Bhandarkar, N.S.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Brown, L.; Panchal, S.K. Coffee pulp, a by-product of coffee production, modulates gut microbiota and improves metabolic syndrome in high-carbohydrate, high-fat diet-fed rats. Pathogens 2021, 10, 1369. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.M.; Batista, N.N.; Miguel, M.G.; Simão, J.B.; Soares, J.R.; Schwan, R.F. Coffee growing altitude influences the microbiota, chemical compounds and the quality of fermented coffees. Food Res. Int. 2020, 129, 108872. [Google Scholar] [CrossRef] [PubMed]
- Vinícius de Melo Pereira, G.; Soccol, V.T.; Brar, S.K.; Neto, E.; Soccol, C.R. Microbial ecology and starter culture technology in coffee processing. Crit. Rev. Food Sci. Nutr. 2017, 57, 2775–2788. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.F.; Narciso, J.A.; Collins, R.P. Odorous constituents of Penicillium decumbens. Mycologia 1975, 67, 1158–1165. [Google Scholar] [CrossRef]
- Moayedi, Y.; Greenberg, S.A.; Jenkins, B.A.; Marshall, K.L.; Dimitrov, L.V.; Nelson, A.M.; Owens, D.M.; Lumpkin, E.A. Camphor white oil induces tumor regression through cytotoxic T cell-dependent mechanisms. Mol. Carcinog. 2019, 58, 722–734. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; Neto, E.; Soccol, V.T.; Medeiros, A.B.; Woiciechowski, A.L.; Soccol, C.R. Conducting starter culture-controlled fermentations of coffee beans during on-farm wet processing: Growth, metabolic analyses and sensorial effects. Food Res. Int. 2015, 75, 348–356. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Soccol, V.T.; Pandey, A.; Medeiros, A.B.; Lara, J.M.; Gollo, A.L.; Soccol, C.R. Isolation, selection and evaluation of yeasts for use in fermentation of coffee beans by the wet process. Int. J. Food Microbiol. 2014, 188, 60–66. [Google Scholar] [CrossRef]
- Evangelista, S.R.; Miguel, M.G.; de Souza Cordeiro, C.; Silva, C.F.; Pinheiro, A.C.; Schwan, R.F. Inoculation of starter cultures in a semi-dry coffee (Coffea arabica) fermentation process. Food Microbiol. 2014, 44, 87–95. [Google Scholar] [CrossRef]
- Albertin, W.; Chasseriaud, L.; Comte, G.; Panfili, A.; Delcamp, A.; Salin, F.; Marullo, P.; Bely, M. Winemaking and bioprocesses strongly shaped the genetic diversity of the ubiquitous yeast Torulaspora Delbrueckii. PLoS ONE 2014, 9, e94246. [Google Scholar] [CrossRef]
- Lambrechts, M.; Pretorius, I. Yeast and its importance to wine aroma. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A. Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef]
- Fernandes, T.; Silva-Sousa, F.; Pereira, F.; Rito, T.; Soares, P.; Franco-Duarte, R.; Sousa, M.J. Biotechnological importance of Torulaspora delbrueckii: From the obscurity to the spotlight. J. Fungi 2021, 7, 712. [Google Scholar] [CrossRef]
- Mariyam, S.; Widiyastuti, R.J.; Karyadi, J.N.; Amanah, H.Z.; Kistanti, A. Physiochemical Characteristic of Fermented Coffee with yeast addition (Hanseniaspora uvarum and Candida parapsilosis). In Proceedings of the International Conference on Sustainable Environment, Agriculture and Tourism (ICOSEAT 2022), Bangka Island, Indonesia, 21–22 July 2022; Atlantis Press: Amsterdam, The Netherlands, 2022. [Google Scholar]
- de Carvalho Neto, D.P.; de Melo Pereira, G.V.; Tanobe, V.O.; Thomaz Soccol, V.G.; da Silva, B.J.; Rodrigues, C.; Soccol, C.R. Yeast diversity and physicochemical characteristics associated with coffee bean fermentation from the Brazilian Cerrado Mineiro region. Fermentation 2017, 3, 11. [Google Scholar] [CrossRef]
- Pereira, T.S.; Batista, N.N.; Pimenta, L.P.; Martinez, S.J.; Ribeiro, L.S.; Naves, J.A.; Schwan, R.F. Self-induced anaerobiosis coffee fermentation: Impact on microbial communities, chemical composition and sensory quality of coffee. Food Microbiol. 2022, 103, 103962. [Google Scholar] [CrossRef]
- Pinkas, J.M.; Battista, K.; Morille-Hinds, T. Microbiological spoilage of spices, nuts, cocoa, and coffee. In Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: New York, NY, USA, 2009; pp. 325–350. [Google Scholar]
- Silva, C.F.; Vilela, D.M.; de Souza Cordeiro, C.; Duarte, W.F.; Dias, D.R.; Schwan, R.F. Evaluation of a potential starter culture for enhance quality of coffee fermentation. World J. Microbiol. Biotechnol. 2013, 29, 235–247. [Google Scholar] [CrossRef]
- Guneser, O.; Demirkol, A.; Yuceer, Y.K.; Togay, S.O.; Hosoglu, M.I.; Elibol, M. Production of flavor compounds from olive mill waste by Rhizopus oryzae and Candida Tropicalis. Braz. J. Microbiol. 2017, 48, 275–285. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Antioxidant properties of fermented green coffee beans with Wickerhamomyces anomalus (Strain KNU18Y3). Fermentation 2020, 6, 18. [Google Scholar] [CrossRef]
- Serra, J.L.; Moura, F.G.; de Melo Pereira, G.V.; Soccol, C.R.; Rogez, H.; Darnet, S. Determination of the microbial community in Amazonian cocoa bean fermentation by Illumina-based metagenomic sequencing. LWT 2019, 106, 229–239. [Google Scholar] [CrossRef]
- Kayanna, N.; Suppavorasatit, I.; Bankeeree, W.; Lotrakul, P.; Punnapayak, H.; Prasongsuk, S. Production of prebiotic aubasidan-like β-glucan from Aureobasidium thailandense NRRL 58543 and its potential as a functional food additive in gummy jelly. LWT 2022, 163, 113617. [Google Scholar] [CrossRef]
- Pacheco-Montealegre, M.E.; Dávila-Mora, L.L.; Botero-Rute, L.M.; Reyes, A.; Caro-Quintero, A. Fine resolution analysis of microbial communities provides insights into the variability of cocoa bean fermentation. Front. Microbiol. 2020, 11, 650. [Google Scholar] [CrossRef]
- Bezus, B.; Cavello, I.; Contreras-Esquivel, J.C.; Cavalitto, S. Pectinases produced by extremophilic yeasts: From cold environments to the food industry. In Value-Addition in Food Products and Processing Through Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 437–452. [Google Scholar]
- Wingfield, B.D.; Bills, G.F.; Dong, Y.; Huang, W.; Nel, W.J.; Swalarsk-Parry, B.S.; Vaghefi, N.; Wilken, P.M.; An, Z.; De Beer, Z.W.; et al. Draft genome sequence of Annulohypoxylon stygium, Aspergillus mulundensis, Berkeleyomyces basicola (syn. Thielaviopsis basicola), Ceratocystis smalleyi, two Cercospora beticola strains, Coleophoma Cylind. Fusarium Fracticaudum Phialophora Cf. Hyalina Morchella Septimelata. IMA Fungus 2018, 9, 199–223. [Google Scholar]
- Liu, L.; Guo, J.; Zhou, X.F.; Li, Z.; Zhou, H.X.; Song, W.Q. Characterization and Secretory Expression of a Thermostable Tannase from Aureobasidium melanogenum T9: Potential Candidate for Food and Agricultural Industries. Front. Bioengineer. Biotechnol. 2022, 9, 769816. [Google Scholar] [CrossRef]
- Rout, S.; Banerjee, R. Production of Tannase under mSSF and Its Application in Fruit Juice Debittering. Indian J. Biotechnol. 2006, 5. [Google Scholar]
- Mussatto, S.I.; Machado, E.M.; Martins, S.; Teixeira, J.A. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Bytof, G.; Knopp, S.E.; Schieberle, P.; Teutsch, I.; Selmar, D. Influence of processing on the generation of γ-aminobutyric acid in green coffee beans. Eur. Food Res. Technol. 2005, 220, 245–250. [Google Scholar] [CrossRef]
- Seninde, D.R.; Chambers, I.V.E. Coffee flavor: A review. Beverages 2020, 6, 44. [Google Scholar] [CrossRef]
- Winkelman, D.L.; Beck, C.L.; Ypey, D.L.; O’Leary, M.E. Inhibition of the A-type K+ channels of dorsal root ganglion neurons by the long-duration anesthetic butamben. J. Pharmacol. Exp. Therapeu. 2005, 314, 1177–1186. [Google Scholar]
- Shulman, M.; Lubenow, T.R.; Nath, H.A.; Blazek, W.; McCarthy, R.J.; Ivankovich, A.D. Nerve blocks with 5% butamben suspension for the treatment of chronic pain syndromes. Reg. Anesth. Pain Med. 1998, 23, 395–401. [Google Scholar]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in inflammation: Mechanistic aspects and applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]
- Petrović, Z.D.; Čomić, L.; Stefanović, O.; Simijonović, D.; Petrović, V.P. Antimicrobial activity of the ionic liquids triethanolamine acetate and diethanolamine chloride, and their corresponding Pd (II) complexes. J. Mol. Liq. 2012, 170, 61–65. [Google Scholar] [CrossRef]
- Lanznaster, D.; Dal-Cim, T.; Piermartiri, T.C.; Tasca, C.I. Guanosine: A neuromodulator with therapeutic potential in brain disorders. Aging Dis. 2016, 7, 657. [Google Scholar] [CrossRef]
- Nakamura, K.; Yoshikawa, N.; Yamaguchi, Y.; Kagota, S.; Shinozuka, K.; Kunitomo, M. Anti-Tumor Effect of Cordycepin (3’-Deoxy Adenosine) through Adenosine A (3) Receptor. In Anticancer Research; International Anticancer Research Editorial Office: Athens, Greece, 2004. [Google Scholar]
- Yehia, R.; Hathout, R.M.; Attia, D.A.; Elmazar, M.M.; Mortada, N.D. Anti-tumor efficacy of an integrated methyl dihydrojasmonate transdermal microemulsion system targeting breast cancer cells: In vitro and in vivo studies. Colloids Surf. B 2017, 155, 512–521. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, M.H.; Lee, H.; Jo, S.Y.; Yang, W.M. (R)-(+)-pulegone suppresses allergic and inflammation responses on 2, 4-dinitrochlorobenzene-induced atopic dermatitis in mice model. J. Dermatol. Sci. 2018, 91, 292–300. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Da Costa, K.A. Choline: An essential nutrient for public health. Nutri. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef]
- Ma, X.; Okyere, S.K.; Hu, L.; Wen, J.; Ren, Z.; Deng, J.; Hu, Y. Anti-inflammatory activity and mechanism of cryptochlorogenic acid from ageratina adenophora. Nutrients 2022, 14, 439. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Microbiome and One Health: Potential of Novel Metabolites from the Gut Microbiome of Unique Species for Human Health. Microorganisms 2023, 11, 481. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Oñatibia-Astibia, A.; Franco, R. The relevance of theobromine for the beneficial effects of cocoa consumption. Front. Pharmacol. 2015, 6, 30. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Tan, W.; Lv, B.; Dai, Y.; Wang, Y.; Zhang, Z.; Wang, X. Dual-screening of anti-inflammatory and antioxidant active ingredients of shenxiang suhe pill and its potential multi-target therapy for coronary heart disease. Biomed. Pharmacother. 2020, 129, 110283. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Isolation and quantification of major chlorogenic acids in three major instant coffee brands and their potential effects on H2O2-induced mitochondrial membrane depolarization and apoptosis in PC-12 cells. Food Funct. 2013, 4, 1632–1638. [Google Scholar] [CrossRef]
- Padilla, J.; Kaur, K.; Cao, H.J.; Smith, T.J.; Phipps, R.P. Peroxisome proliferator activator receptor-γ agonists and 15-deoxy-Δ12, 1412, 14-PGJ2 induce apoptosis in normal and malignant B-lineage cells. J. Immunol. 2000, 165, 6941–6948. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, L.; Linden, J.; Berne, R.M. The cardiac effects of adenosine. Prog. Cardiovasc. Dis. 1989, 32, 73–97. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Chen, J.F.; Cunha, R.A.; Svenningsson, P.; Vaugeois, J.M. Adenosine and brain function. Int. Rev. Neurobiol. 2005, 63, 191–270. [Google Scholar]


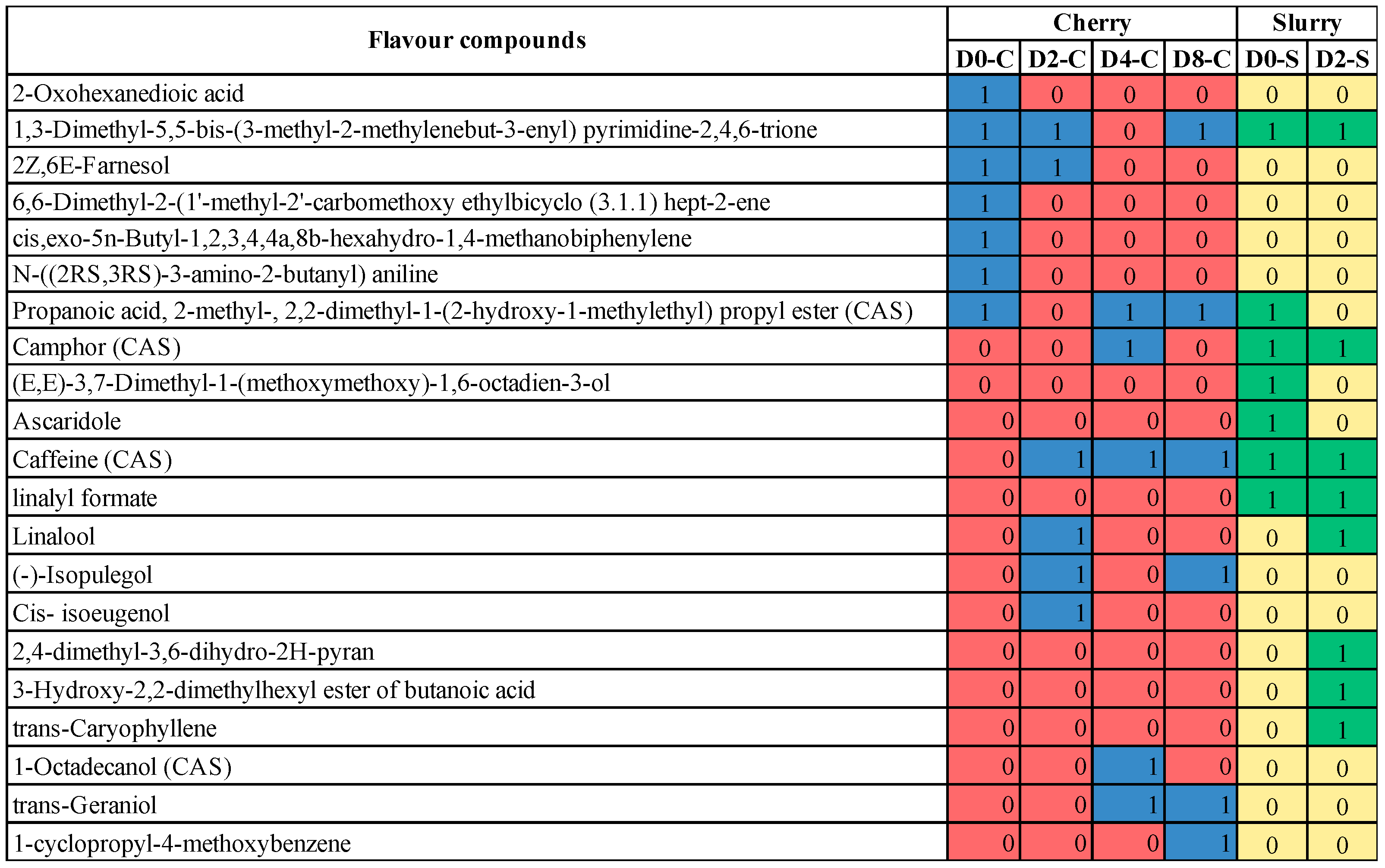

| Day | Compound | Chemical Formula | Flavor/Aroma | References |
|---|---|---|---|---|
| Day 0 (Cherry) | 2-Oxohexanedioic acid | C6H8O5 | ||
| 2Z,6E-Farnesol | C15H26O | |||
| Propanoic acid, 2-methyl-, 2,2-dimethyl-1-(2-hydroxy-1-methylethyl) propyl ester (CAS) | C12H24O3 | |||
| N-((2RS,3RS)-3-amino-2-butanyl) aniline | C10H16N2 | |||
| 1,3-Dimethyl-5,5-bis-(3-methyl-2-methylenebut-3-enyl) pyrimidine-2,4,6-trione | C18H24N2O3 | |||
| 6,6-Dimethyl-2-(1′-methyl-2′-carbomethoxy ethylbicyclo (3.1.1) hept-2-ene | C14H22O2 | |||
| cis,exo-5n-Butyl-1,2,3,4,4a,8b-hexahydro-1,4-methanobiphenylene | C17H22 | |||
| cis,exo-5n-Butyl-1,2,3,4,4a,8b-hexahydro-1,4-methanobiphenylene | C17H22 | |||
| Day 0 (Slurry) | Camphor (CAS) | C10H16O | Camphoreous | [34] |
| linalyl formate | C11H18O2 | Citrus, herbal, bergamot Lavender Soapy Fatty, green, woody | [35] | |
| (E,E)-3,7-Dimethyl-1-(methoxymethoxy)-1,6-octadien-3-ol | C12H22O3 | |||
| Ascaridole | C10H16O2 | |||
| Propanoic acid, 2-methyl-, 2,2-dimethyl-1-(2-hydroxy-1-methylethyl) propyl ester (CAS) | C12H24O3 | |||
| 1,3-Dimethyl-5,5-bis-(3-methyl-2-methylenebut-3-enyl) pyrimidine-2,4,6-trione | C18H24N2O3 | |||
| Caffeine (CAS) | C8H10N4O2 | Odorless | [36] | |
| Day 2 (Cherry) | Linalool | C10H18O | Citrus, floral, sweet bois de rose Woody Green Blueberry | [37] |
| (-)-Isopulegol | C10H18O | Minty cooling, medicinal Woody | [38] [39] | |
| 2Z,6E-Farnesol | C15H26O | |||
| Cis-isoeugenol | C10H12O2 | Sweet spicy, carnation Phenolic Floral | [40] | |
| 1,3-Dimethyl-5,5-bis-(3-methyl-2-methylenebut-3-enyl) pyrimidine-2,4,6-trione | C18H24N2O3 | |||
| Caffeine (CAS) | C8H10N4O2 | Odorless | [36] | |
| Day 2 (Slurry) | Linalool | C10H18O | Citrus Floral Sweet bois de rose Woody Green, blueberry | [37] |
| Camphor (CAS) | C10H16O | Camphoreous | [34] | |
| Linalyl formate | C11H18O2 | Citrus, herbal, bergamot Lavender, soapy, fatty, green Woody | [35] | |
| 2,4-dimethyl-3,6-dihydro-2H-pyran | C7H12O | |||
| 3-Hydroxy-2,2-dimethylhexyl ester of butanoic acid | C12H24O3 | |||
| trans-Caryophyllene | C15H24 | |||
| 1,3-Dimethyl-5,5-bis-(3-methyl-2-methylenebut-3-enyl) pyrimidine-2,4,6-trione | C18H24N2O3 | |||
| Caffeine (CAS) | C8H10N4O2 | Odorless | [36] | |
| Day 4 (Cherry) | Camphor (CAS) | C10H16O | Camphoreous | [34] |
| trans-Geraniol | C10H18O | Sweet floral, fruity Rose Waxy Citrus | [41] | |
| Propanoic acid, 2-methyl-, 2,2-dimethyl-1-(2-hydroxy-1-methylethyl) propyl ester (CAS) | C12H24O3 | |||
| 1-Octadecanol (CAS) | C18H38O | Bland | [42] | |
| Caffeine (CAS) | C8H10N4O2 | Odorless | [36] | |
| Day 8 (Cherry) | (-)-Isopulegol | C10H18O | Minty cooling, medicinal Woody | [38] [39] |
| 1-cyclopropyl-4-methoxybenzene | C10H12O | |||
| trans-Geraniol | C10H18O | Sweet floral, fruity Rose Waxy Citrus | [41] | |
| Propanoic acid, 2-methyl-, 2,2-dimethyl-1-(2-hydroxy-1-methylethyl) propyl ester (CAS) | C12H24O3 | |||
| 1,3-Dimethyl-5,5-bis-(3-methyl-2-methylenebut-3-enyl) pyrimidine-2,4,6-trione | C18H24N2O3 | |||
| Caffeine (CAS) | C8H10N4O2 | Odorless | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todhanakasem, T.; Van Tai, N.; Pornpukdeewattana, S.; Charoenrat, T.; Young, B.M.; Wattanachaisaereekul, S. The Relationship between Microbial Communities in Coffee Fermentation and Aroma with Metabolite Attributes of Finished Products. Foods 2024, 13, 2332. https://doi.org/10.3390/foods13152332
Todhanakasem T, Van Tai N, Pornpukdeewattana S, Charoenrat T, Young BM, Wattanachaisaereekul S. The Relationship between Microbial Communities in Coffee Fermentation and Aroma with Metabolite Attributes of Finished Products. Foods. 2024; 13(15):2332. https://doi.org/10.3390/foods13152332
Chicago/Turabian StyleTodhanakasem, Tatsaporn, Ngo Van Tai, Soisuda Pornpukdeewattana, Theppanya Charoenrat, Briana M. Young, and Songsak Wattanachaisaereekul. 2024. "The Relationship between Microbial Communities in Coffee Fermentation and Aroma with Metabolite Attributes of Finished Products" Foods 13, no. 15: 2332. https://doi.org/10.3390/foods13152332







