A Preliminary Study on the Effect of Adding Sugarcane Syrup on the Flavor of Barley Lager Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Physicochemical Parameters
2.3. Determination of Bittering Value of Beer
2.4. Beer Sensory Appetite Experiment
2.5. Determination of Fermentable Sugars
2.6. Determination of Free Amino Acids
2.7. Determination of Volatile Aroma Substances in Lager Beer
2.8. Determination of Antioxidant Power of Beer
2.9. Determination of Volatile Aging Substances
2.10. Methods of Data Analysis
3. Results and Discussion
3.1. Influence of Process Parameters on the Finished Beer
3.2. Determination of the Optimal Fermentation Process
3.3. Changes in Substances during Fermentation
3.3.1. Fermentable Sugars
3.3.2. Free Amino Nitrogen
3.4. Detection of Aroma Substances in Beer
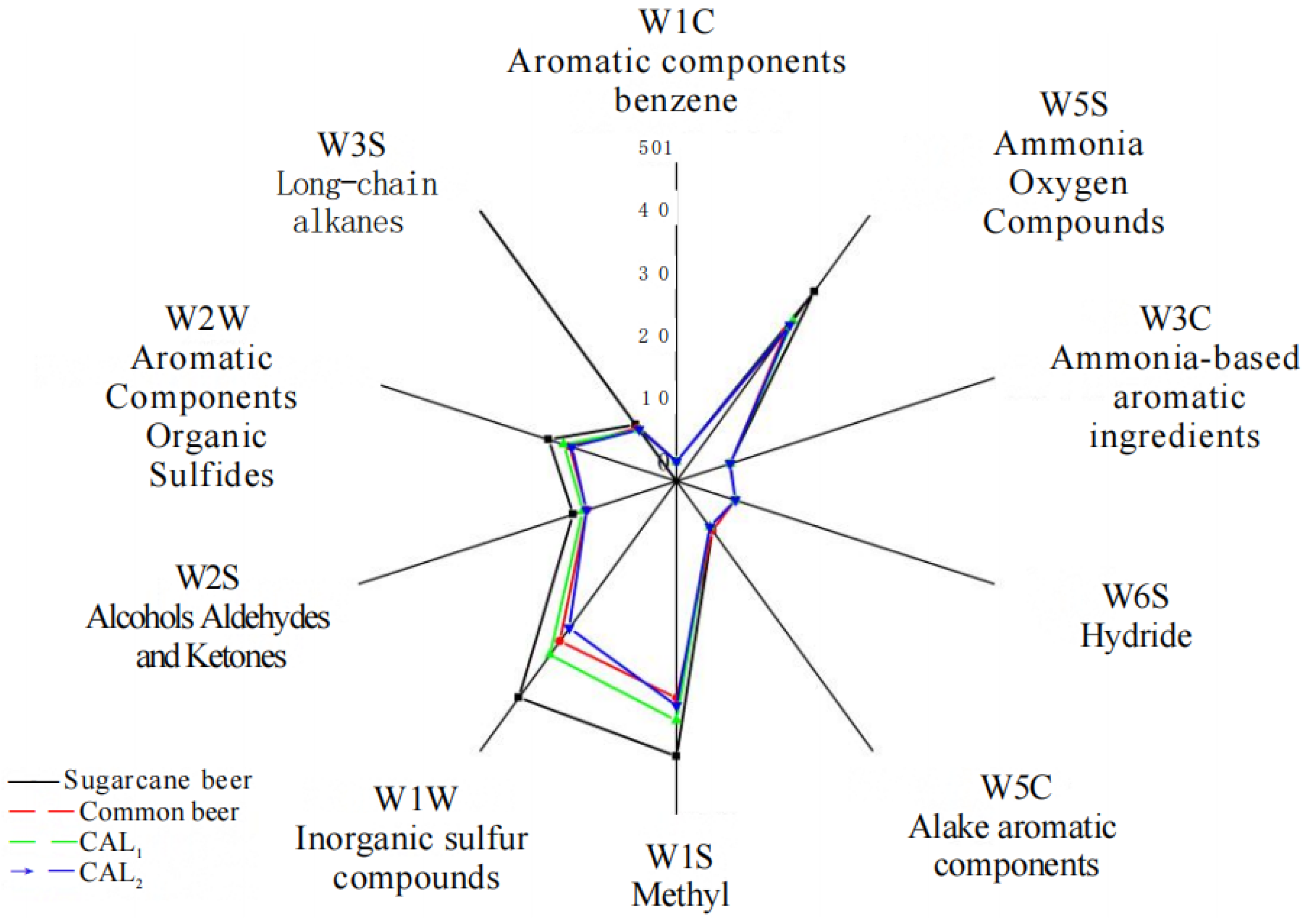
3.4.1. The Electronic Nose
3.4.2. GC-MS
3.5. Determination of Lager Aging Characteristic Aroma
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.L.; García-Caro, R.D.L.C.; Zhang, P.; Carlin, S.; Gottlieb, A.; Petersen, M.A.; Vrhovsek, U.; Bond, U. Packing a Punch: Understanding How Flavours Are Produced in Lager Fermentations. FEMS Yeast Res. 2021, 21, foab040. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The Soul of Beer’s Aroma—A Review of Flavour-Active Esters and higher alcohols Produced by the Brewing Yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gąsior, J.; Pietrzak, W. Volatile Compounds Content, Physicochemical Parameters, and Antioxidant Activity of Beers with Addition of Mango Fruit (Mangifera indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.; Tsuchiya, F.; Ishimaru, M.; Oka, K.; Komura, H. Different Beers with Different Hops. Relevant Compounds for Their Aroma Characteristics. J. Agric. Food Chem. 2013, 61, 4758–4764. [Google Scholar] [CrossRef]
- Lehnhardt, F.; Becker, T.; Gastl, M. Flavor Stability Assessment of Lager Beer: What We Can Learn by Comparing Established Methods. Eur. Food Res. Technol. 2020, 246, 1105–1118. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Deng, W.; Cai, J.; Chen, J. Evaluation of Aroma Characteristics of Sugarcane (Saccharum officinarum L.) Juice Using Gas Chromatography-Mass Spectrometry and Electronic Nose. LWT 2019, 108, 400–406. [Google Scholar] [CrossRef]
- Wang, H.M.; Ding, C.H. Application of Syrup in Beer Brewing. Liquor. Mak. Sci. Technol. 2004, 03, 58–60. [Google Scholar] [CrossRef]
- Dabija, A.; Ciocan, M.E.; Chetrariu, A.; Codină, G.G. Maize and Sorghum as Raw Materials for Brewing: A Review. Appl. Sci. 2021, 11, 3139. [Google Scholar] [CrossRef]
- Bogdan, P.; Kordialik-Bogacka, E. Alternatives to Malt in Brewing. Trends Food Sci. Technol. 2017, 65, 1–9. [Google Scholar] [CrossRef]
- Piddocke, M.P.; Kreisz, S.; Heldt-Hansen, H.P.; Nielsen, K.F.; Olsson, L. Physiological Characterization of Brewer’s Yeast in High-Gravity Beer Fermentations with Glucose or Maltose Syrups as Adjuncts. Appl. Microbiol. Biotechnol. 2009, 84, 453–464. [Google Scholar] [CrossRef]
- Asikin, Y.; Wada, K.; Imai, Y.; Kawamoto, Y.; Mizu, M.; Mutsuura, M.; Takahashi, M. Compositions, Taste Characteristics, Volatile Profiles, and Antioxidant Activities of Sweet Sorghum (Sorghum bicolor L.) and Sugarcane (Saccharum officinarum L.) Syrups. Food Meas. 2018, 12, 884–891. [Google Scholar] [CrossRef]
- Feng, S.; Luo, Z.; Zhang, Y.; Zhong, Z.; Lu, B. Phytochemical Contents and Antioxidant Capacities of Different Parts of Two Sugarcane (Saccharum officinarum L.) Cultivars. Food Chem. 2014, 151, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Guo, M.; Du, J.; Zhang, Z. Cloudy barley Beer Enriched with Okra [Abelmoschus esculentus (L.) Moench]: Effects on Volatile Compound and Sensorial Attributes. Int. J. Food Prop. 2018, 21, 289–300. [Google Scholar] [CrossRef]
- Vesely, P.; Lusk, L.; Basarova, G.; Seabrooks, J.; Ryder, D. Analysis of Aldehydes in Beer Using Solid-Phase Microextraction with On-Fiber Derivatization and Gas Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 6941–6944. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Guo, W.; Shen, Y.; Dai, J.; Zhang, H.; Fu, M.; Wang, X. In Situ Assay of the Reducing Sugars in Hydrophilic Natural Deep Eutectic Solvents by a Modified DNS Method. J. Mol. Liq. 2023, 385, 122286. [Google Scholar] [CrossRef]
- Hahn, C.D.; Lafontaine, S.R.; Pereira, C.B.; Shellhammer, T.H. Evaluation of Nonvolatile Chemistry Affecting Sensory Bitterness Intensity of Highly Hopped Beers. J. Agric. Food Chem. 2018, 66, 3505–3513. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.F.; Affonso, A.D.; Lehman, R.M. Impact of Specialty Malts on Wort and Beer Characteristics. Fermentation 2021, 7, 137. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, L.; Lu, Y.; Yang, L. Analysis of Amino Acids in Foodstuff by Nuclear Magnetic Resonance. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics, Yantai, China, 16–18 October 2010; IEEE: Yantai, China, 2010; pp. 750–754. [Google Scholar]
- Men, H.; Shi, Y.; Fu, S.; Jiao, Y.; Qiao, Y.; Liu, J. Mining Feature of Data Fusion in the Classification of Beer Flavor Information Using E-Tongue and E-Nose. Sensors 2017, 17, 1656. [Google Scholar] [CrossRef] [PubMed]
- Molina-Calle, M.; Priego-Capote, F.; Luque De Castro, M.D. Headspace−GC–MS Volatile Profile of Black Garlic vs Fresh Garlic: Evolution along Fermentation and Behavior under Heating. LWT 2017, 80, 98–105. [Google Scholar] [CrossRef]
- Gaulejac, N.S.-C.; Provost, C.; Vivas, N. Comparative study of polyphenol scavenging activities assessed by different methods. J. Agric. Food Chem. 1998, 47, 425–431. [Google Scholar] [CrossRef]
- Aguiar, D.; Pereira, A.C.; Marques, J.C. Assessment of the Prediction Power of Forced Ageing Methodology on Lager Beer Aldehyde Evolution during Maritime Transportation. Molecules 2023, 28, 4201. [Google Scholar] [CrossRef]
- Ortiz, R.M. Analysis of Selected Aldehydes in Packaged Beer by Solid-Phase Microextraction (SPME)–Gas Chromatography (GC)–Negative Chemical Ionization Mass Spectrometry (NCIMS). J. Am. Soc. Brew. Chem. 2015, 73, 266–274. [Google Scholar] [CrossRef]
- Ojala, M.; Kotiaho, T.; Siirilä, J.; Sihvonen, M.L. Analysis of aldehydes and ketones from beer as O-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine derivatives. Talanta 1994, 41, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Silva, R.D.; Chaves, S.R.; Sousa, M.J.; Côrte-Real, M. The Impact of Acetate Metabolism on Yeast Fermentative Performance and Wine Quality: Reduction of Volatile Acidity of Grape Musts and Wines. Appl. Microbiol. Biotechnol. 2011, 89, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M. Hop Analysis, Cohumulone Factor, and the Bitterness of Beer: Review and Critical Evaluation. J. Inst. Brew. 1960, 66, 35–50. [Google Scholar] [CrossRef]
- Černá, S.; Benešová, K. Stanovení Organických Kyselin ve Speciálních Pivech A Nápojích Na Bázi Piva Pomocí Kapilární Izotachoforézy. Chem. Listy 2023, 117, 516–521. [Google Scholar] [CrossRef]
- Dušek, M.; Olšovsk, J.; Krofta, K.; Jurková, M.; Mikyska, A. Qualitative Determination of β-Acids and Their Transformation Products in Beer and Hop Using HR/AM-LC-MS/MS. J. Agric. Food Chem. 2014, 62, 7690–7697. [Google Scholar] [CrossRef] [PubMed]
- Meneses, F.J.; Henschke, P.A.; Jiranek, V. A Survey of Industrial Strains of Saccharomyces cerevisiae Reveals Numerous Altered Patterns of Maltose and Sucrose Utilisation. J. Inst. Brew. 2002, 108, 310–321. [Google Scholar] [CrossRef]
- Hambraeus, G.; Nyberg, N. Enzymatic Hydrogenation of Trans-2-Nonenal in Barley. J. Agric. Food Chem. 2005, 53, 8714–8721. [Google Scholar] [CrossRef]
- Bushnell, S.E.; Guinard, J.-X.; Bamforth, C.W. Effects of Sulfur Dioxide and Polyvinylpolypyrrolidone on the Flavor Stability of Beer as Measured by Sensory and Chemical Analysis. J. Am. Soc. Brew. Chem. 2003, 61, 133–141. [Google Scholar] [CrossRef]
- Andersen, M.L.; Skibsted, L.H. Electron Spin Resonance Spin Trapping Identification of Radicals Formed during Aerobic Forced Aging of Beer. J. Agric. Food Chem. 1998, 46, 1272–1275. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of Staling Compounds to the Aged Flavour of Lager Beer by Studying Their Flavour Thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The Chemistry of Beer Aging—A Critical Review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]

| Addition of Sugar Cane Syrup | Physical and Chemical Indicators Related to Sugar Cane Beer | ||||||
|---|---|---|---|---|---|---|---|
| Alcoholic Strength (%) | Total Acidity (mL/100 mL) | pH | Residual Sugar (g/L) | Color (EBC) | Bitterness Value (BU) | Senior Alcohol (mg/L) | |
| OWBL (10°P) | 5.6 d ± 0.3 | 1.96 c ± 0.10 | 4.77 c ± 0.02 | 9.41 b ± 0.02 | 13.35 bc ± 0.32 | 19.8 a ± 1.0 | 116.67 a ± 22.53 |
| 5% | 6.0 c ± 0.1 | 1.53 f ± 0.11 | 4.78 bc ± 0.01 | 9.55 b ± 0.04 | 12.88 c ± 0.36 | 18.0 b ± 0.5 | 94.39 b ± 18.64 |
| 10% | 6.5 b ± 0.1 | 1.53 f ± 0.11 | 4.77 bc ± 0.00 | 9.19 b ± 0.13 | 13.61 b ± 0.16 | 17.4 b ± 1.4 | 97.00 b ± 20.01 |
| 15% | 6.1 c ± 0.2 | 1.57 ef ± 0.03 | 4.78 bc ± 0.01 | 9.40 b ± 0.21 | 12.78 c ± 0.20 | 16.6 bc ± 0.9 | 100.94 bc ± 19.05 |
| 20% | 6.2 c ± 0.1 | 1.63 de ± 0.01 | 4.80 b ± 0.01 | 7.65 c ± 1.23 | 13.05 bc ± 0.67 | 16.3 bc ± 0.5 | 100.83 bc ± 24.56 |
| 25% | 7.2 a ± 0.1 | 1.67 d ± 0.03 | 4.61 d ± 0.00 | 8.72 b ± 0.04 | 13.24 bc ± 0.24 | 16.6 bc ± 1.2 | 119.72 a ± 16.88 |
| 30% | 6.6 b ± 0.1 | 2.30 a ± 0.01 | 4.63 d ± 0.01 | 9.25 b ± 0.51 | 13.04 bc ± 0.21 | 15.4 c ± 0.6 | 108.45 c ± 24.23 |
| 50% | 6.7 b ± 0.1 | 2.20 b ± 0.00 | 4.83 a ± 0.01 | 12.50 a ± 0.51 | 17.6 a ± 0.20 | 13.6 d ± 0.9 | 105.88 c ± 19.65 |
| Wort Consistency | Physical and Chemical Indicators Related to Sugar Cane Beer | ||||||
|---|---|---|---|---|---|---|---|
| Alcoholic Strength (%) | Total Acidity (mL/100 mL) | pH | Residual Sugar (g/L) | Color (EBC) | Bitterness Value (BU) | Senior Alcohol (mg/L) | |
| 6°P | 3.6 e ± 0.1 | 1.21 c ± 0.10 | 4.48 b ± 0.13 | 5.60 e ± 0.41 | 7.54 c ± 0.59 | 17.1 a ± 0.1 | 92.32 a ± 17.75 |
| 8°P | 4.4 d ± 0.0 | 1.23 c ± 0.03 | 4.54 b ± 0.04 | 7.52 d ± 0.23 | 10.40 bc ± 1.13 | 15.6 b ± 0.0 | 97.67 a ± 20.54 |
| 10°P | 5.2 c ± 0.0 | 1.49 bc ± 0.41 | 4.62 ab ± 0.10 | 10.13 c ± 0.43 | 12.42 b ± 0.66 | 14.2 c ± 0.1 | 103.48 ab ± 25.12 |
| 12°P | 6.2 b ± 0.0 | 1.88 ab ± 0.12 | 4.73 a ± 0.01 | 12.54 b ± 0.30 | 13.65 b ± 0.42 | 11.9 d ± 1.1 | 106.35 ab ± 18.04 |
| 14°P | 7.2 a ± 0.0 | 2.19 a ± 0.24 | 4.63 ab ± 0.10 | 15.41 a ± 0.69 | 18.58 a ± 4.48 | 9.2 e ± 0.5 | 119.2 b ± 18.91 |
| OWBL (10°P) | 5.6 d ± 0.3 | 1.96 c ± 0.10 | 4.77 c ± 0.02 | 9.41 b ± 0.02 | 13.35 bc ± 0.32 | 19.8 a ± 1.0 | 114.25 b ± 22.06 |
| Temp | Physical and Chemical Indicators Related to Sugar Cane Beer | ||||||
|---|---|---|---|---|---|---|---|
| Alcoholic Strength (%) | Total Acidity (mL/100 mL) | pH | Residual Sugar (g/L) | Color (EBC) | Bitterness Value (BU) | Higher Alcohol (mg/L) | |
| 10 °C | 5.3 b ± 0.0 | 1.60 b ± 0.01 | 4.82 a ± 0.01 | 11.28 a ± 0.51 | 14.3 b ± 0.30 | 20.5 a ± 1.0 | 90.24 a ± 19.23 |
| 12 °C | 5.3 b ± 0.1 | 1.60 b ± 0.01 | 4.68 a ± 0.01 | 10.13 b ± 0.25 | 14.7 ab ± 0.30 | 17.3 b ± 1.5 | 109.15 b ± 18.69 |
| 14 °C | 5.7 a ± 0.0 | 1.80 a ± 0.10 | 4.86 a ± 0.01 | 8.06 c ± 0.31 | 15.20 a ± 0.10 | 19.05 ab ± 1.1 | 124.49 c ± 22.72 |
| Level | Factors | |||
|---|---|---|---|---|
| Amount of Syrup Added (%) | Wort Consistency (°P) | Fermentation Temperature (°C) | Hops Added (%) | |
| 1 | 10 | 8 | 8 | 0.02 |
| 2 | 20 | 10 | 10 | 0.04 |
| 3 | 30 | 12 | 12 | 0.06 |
| Experiment Number | A | B | C | D | Sensory Evaluation |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 76 |
| 2 | 1 | 2 | 2 | 2 | 91 |
| 3 | 1 | 3 | 3 | 3 | 69 |
| 4 | 2 | 1 | 2 | 3 | 72 |
| 5 | 2 | 2 | 3 | 1 | 87 |
| 6 | 2 | 3 | 1 | 2 | 86 |
| 7 | 3 | 1 | 3 | 2 | 78 |
| 8 | 3 | 2 | 1 | 3 | 62 |
| 9 | 3 | 3 | 2 | 1 | 73 |
| K1 | 236 | 226 | 224 | 236 | |
| K2 | 245 | 240 | 236 | 255 | |
| K3 | 213 | 228 | 234 | 203 | |
| k1 | 78.667 | 75.333 | 74.667 | 78.667 | |
| k2 | 81.667 | 80.000 | 78.667 | 85.000 | |
| k3 | 71.000 | 76.000 | 78.000 | 67.667 | |
| R | 10.667 | 4.667 | 4.000 | 17.333 |
| Project | Standards | LS | OWBL (10°P) |
|---|---|---|---|
| Alcoholic strength (%) | 10.1°P–11.0°P ≥ 3.7 | 4.9–5.3 | 4.7–4.9 |
| Total acidity (mg/100 mL) | 10.1°P–14.0°P ≤ 2.6 | 1.0–2.0 | 1.6–2.4 |
| Diacetyl (mg/L) | ≤0.10 | 0.07 | 0.09 |
| CO2 (mass fraction) | 0.35–0.65 | 0.40 | 0.45 |
| Higher alcohol (mg/L) | 50–120 | 109.15 | 105.42 |
| Amount of hops added (%) | Bitterness value 13 ≤ x ≤ 15 | 14.01 | 14.9 |
| Glucose (g/L) | Fructose (g/L) | Maltose (g/L) | Sucrose (g/L) | Maltotriose (g/L) | |
|---|---|---|---|---|---|
| 10°P Wort | 8.39 ± 0.26 | 2.43 ± 0.11 | 80.6 ± 6.8 | 1.42 ± 0.02 | 5.28 ± 0.42 |
| 10°P Cane Juice | 0.63 ± 0.03 | 0.79 ± 0.05 | 0 | 11.58 ± 1.35 | 0 |
| 10°P OWBL (mg/100 mL) | 0.68 ± 0.02 | 0.12 ± 0.01 | 3.07 ± 0.22 | 0.87 ± 0.03 | 0.29 ± 0.01 |
| LS (10°P substrate) (mg/100 mL) | 1.22 ± 0.03 | 0.36 ± 0.01 | 2.62 ± 0.12 | 0.92 ± 0.12 | 0.38 ± 0.01 |
| Amino Acid Type | LS (10°P Substrate) (mg/100 mL) | 10°P OWBL (mg/100 mL) | Control Wort (mg/100 mL) | Sugar Cane Juice (mg/100 mL) |
|---|---|---|---|---|
| Asp | 5.85 ± 0.47 | 7.22 ± 0.64 | 9.17 ± 0.78 | 13.45 ± 1.04 |
| Thr | 0.93 ± 0.04 | 2.13 ± 0.18 | 6.79 ± 0.47 | 2.92 ± 0.34 |
| Ser | 1.91 ± 0.16 | 2.35 ± 0.28 | 6.88 ± 5.35 | 8.41 ± 0.96 |
| Glu | 14.29 ± 1.54 | 8.03 ± 0.78 | 13.24 ± 1.64 | 81.56 ± 8.13 |
| Gly | 2.96 ± 0.13 | 4.16 ± 0.31 | 3.88 ± 0.28 | 0.79 ± 0.04 |
| Ala | 8.99 ± 0.68 | 12.2 ± 1.51 | 11.2 ± 1.67 | 7.35 ± 0.76 |
| Cys | 0.24 ± 0.03 | 0.37 ± 0.02 | 0.54 ± 0.06 | 0.11 ± 0.01 |
| Val | 2.54 ± 0.03 | 8.72 ± 0.79 | 12.32 ± 1.36 | 5.25 ± 0.04 |
| Met | 0.47 ± 0.04 | 2.12 ± 0.31 | 4.33 ± 0.42 | 0.64 ± 0.05 |
| Ile | 0.76 ± 0.06 | 4.02 ± 0.36 | 7.54 ± 0.69 | 2.83 ± 0.29 |
| Leu | 0.78 ± 0.08 | 7.12 ± 0.82 | 16.60 ± 1.72 | 2.16 ± 0.23 |
| Tyr | 6.06 ± 0.53 | 10.78 ± 1.32 | 12.96 ± 1.32 | 2.26 ± 0.19 |
| Phe | 2.77 ± 0.31 | 11.26 ± 1.41 | 16.33 ± 1.75 | 2.12 ± 0.18 |
| Lys | 0 ± 0 | 5.56 ± 0.53 | 10.07 ± 0.99 | 0.79 ± 0.09 |
| NH3 | 1.46 ± 0.14 | 2.56 ± 0.28 | 3.12 ± 0.35 | 0.68 ± 0.07 |
| His | 1.49 ± 0.11 | 4.15 ± 0.40 | 5.61 ± 0.61 | 2.67 ± 0.19 |
| Arg | 1.19 ± 0.09 | 8.49 ± 0.72 | 11.7 ± 1.25 | 1.34 ± 0.13 |
| Hyp | 1.53 ± 0.08 | 2.81 ± 0.19 | 7.94 ± 0.81 | 3.19 ± 0.27 |
| Pro | 35.81 ± 2.67 | 41.71 ± 5.96 | 41.98 ± 3.78 | 3.16 ± 0.21 |
| NO | Volatile Compounds | RI | CAS | Relative Content (%) | Aroma Characteristic | |||
|---|---|---|---|---|---|---|---|---|
| OWBL | LS | CAL1 | CAL2 | |||||
| 1 | Acetaldehyde | 710 | 75-07-0 | 0.082 a ± 0.014 | NQ | 0.031 b ± 0.006 | NQ | Pungent, ethereal, aldehydic, fruity |
| 2 | Isobutyl acetate | 997 | 110-19-0 | 0.038 a ± 0.007 | NQ | 0.068 b ± 0.014 | 0.141 c ± 0.020 | Sweet, fruity, ethereal, banana, tropical |
| 3 | Ethyl valerate | 1118 | 539-82-2 | 0.028 a ± 0.004 | NQ | NQ | NQ | Sweet, fruity, apple, pineapple, green, tropical |
| 4 | 2-Heptanone | 1126 | 110-43-0 | 0.011 a ± 0.001 | NQ | NQ | NQ | Fruity, spicy, sweet, herbal, coconut, woody |
| 5 | Dipentene | 1140 | 7705-14-8 | 0.046 a ± 0.007 | NQ | 0.033 b ± 0.009 | NQ | Citrus, herbal, terpene, camphor |
| 6 | Methylheptenone | 1295 | 110-93-0 | 0.024 a ± 0.004 | NQ | NQ | 0.024 a ± 0.005 | Citrus, green, musty Lemongrass, apple |
| 7 | 2-Nonanone | 1385 | 821-55-6 | 0.045 a ± 0.007 | 0.021 b ± 0.004 | 0.026 b ± 0.003 | 0.026 b ± 0.003 | Fresh, sweet, green, weedy, earthy, herbal |
| 8 | Decanal | 1545 | 112-31-2 | 1.152 a ± 0.326 | 0.141 b ± 0.003 | 0.187 b ± 0.002 | 0.511 c ± 0.011 | Sweet, aldehydic, waxy, orange peel, citrus, floral |
| 9 | Octanol | 1597 | 111-87-5 | 0.895 a ± 0.178 | NQ | 0.791 a ± 0.136 | 0.867 a ± 0.147 | Waxy, green, orange, aldehydic, rose, mushroom |
| 10 | Undecanal | 1639 | 112-44-7 | 0.062 a ± 0.014 | NQ | NQ | NQ | Waxy, soapy, floral, aldehydic, citrus, green, fatty, fresh laundry |
| 11 | Ethyl 4-trans-decenoate | 1729 | 76649-16-6 | 0.372 a ± 0.046 | NQ | NQ | NQ | Green, fruity Waxy, cognac |
| 12 | Acetic acid, decyl ester | 1744 | 112-17-4 | 0.036 a ± 0.005 | NQ | NQ | 0.057 b ± 0.009 | Waxy, clean, fresh laundered cloths, citrus, soapy |
| 13 | (E)-methyl geranate | 1759 | 1189-09-9 | 0.617 a ± 0.092 | 0.386 b ± 0.063 | 0.127 c ± 0.021 | 0.325 b ± 0.057 | Waxy, green Fruity, flower |
| 14 | Alpha-terpineol | 1762 | 10482-56-1 | 0.043 a ± 0.006 | 0.02 b ± 0.003 | 0.038 a ± 0.004 | 0.095 c ± 0.013 | Lilac, floral, terpenic |
| 15 | Cyclooctane | 1799 | 292-64-8 | 0.987 a ± 0.167 | NQ | 0.377 b ± 0.073 | NQ | Camphor odor |
| 16 | Phenethyl isobutyrate | 1956 | 103-48-0 | 0.057 a ± 0.011 | NQ | NQ | NQ | Floral, fruity, rose, tea, peach, pastry |
| 17 | 2-Acetylpyrrole | 1978 | 1072-83-9 | 0.046 a ± 0.008 | NQ | NQ | NQ | Musty, nut, skin, maraschino cherry, coumarinic, licorice, walnut, bready |
| 18 | Gamma-nonanolactone | 2025 | 104-61-0 | 0.053 a ± 0.012 | NQ | NQ | NQ | Coconut, creamy, waxy, sweet, buttery, oily |
| 19 | Ethyl acetate | 891 | 141-78-6 | 1.638 a ± 0.347 | 1.761 a ± 0.331 | 2.472 b ± 0.421 | 2.375 b ± 0.397 | Ethereal, fruity, sweet, weedy, green |
| 20 | Ethanol | 895 | 64-17-5 | 14.774 b ± 2.358 | 12.073 a ± 1.872 | 18.836 d ± 2.468 | 13.821 ab ± 2.181 | Strong alcoholic, ethereal, medical |
| 21 | Ethyl butyrate | 1045 | 105-54-4 | 0.248 a ± 0.026 | 0.214 b ± 0.031 | 0.268 c ± 0.038 | 0.217 b ± 0.0025 | Fruity, juicy, fruit, pineapple, cognac |
| 22 | Propan-1-ol | 1059 | 71-23-8 | 0.080 a ± 0.012 | 0.065 b ± 0.011 | 0.083 a ± 0.012 | 0.090 c ± 0.021 | Alcoholic, fermented fusel, musty |
| 23 | Ethyl isovalerate | 1062 | 108-64-5 | NQ | NQ | NQ | 0.011 a ± 0.001 | Fruity, sweet, apple, pineapple, tutti frutti |
| 24 | Isobutyl isobutyrate | 1068 | 97-85-8 | NQ | NQ | NQ | 0.02 a ± 0.002 | Ethereal, fruity, tropical fruit, pineapple, grape skin, banana |
| 25 | Isobutyl alcohol | 1078 | 78-83-1 | 0.506 a ± 0.083 | 0.620 b ± 0.096 | 0.504 a ± 0.092 | 0.632 b ± 0.101 | Ethereal, winey, cortex |
| 26 | Isoamyl acetate | 1110 | 123-92-2 | 4.213 a ± 0.7235 | 6.493 b ± 0.925 | 10.603 c ± 1.532 | 12.447 cd ± 1.826 | Sweet, fruity, banana, solvent |
| 27 | Myrcene | 1122 | 123-35-3 | 0.025 a ± 0.003 | 0.019 b ± 0.002 | 0.029 c ± 0.003 | 0.048 d ± 0.005 | Peppery, terpene, spicy, balsam, plastic |
| 28 | Isopentyl isobutyrate | 1176 | 2050-01-3 | 0.018 a ± 0.002 | NQ | 0.123 b ± 0.025 | 0.166 c ± 0.037 | Fruity, ethereal, tropical, green grape, cherry, unripe banana, apple, cocoa |
| 29 | 3-Methyl-1-butanol | 1200 | 123-51-3 | 13.623 a ± 1.793 | 11.607 b ± 1.467 | 13.146 a ± 1.983 | 11.17 b ± 1.249 | Fusel, oil, alcoholic, whiskey, fruity, banana |
| 30 | 2-Pentylfuran | 1213 | 3777-69-3 | NQ | NQ | NQ | 0.01 a ± 0.001 | Fruity, green, earthy, beany, vegetable, metallic |
| 31 | Ethyl caproate | 1219 | 123-66-0 | 4.851 a ± 0.865 | 3.433 b ± 0.547 | 6.19 c ± 1.223 | 4.319 ab ± 0.792 | Sweet, fruity, pineapple, waxy, green, banana |
| 32 | 3,7-Dimethyl-1 | 1221 | 13877-91-3 | NQ | NQ | NQ | 0.018 a ± 0.003 | Citrus, tropical, green, terpene, woody, green |
| 33 | Hexyl acetate | 1228 | 142-92-7 | 0.079 a ± 0.013 | 0.135 b ± 0.028 | 0.149 bc ± 0.033 | 0.02 d ± 0.003 | Fruity, green, apple, banana, sweet |
| 34 | 2-Methylbutyl 2-methylbutyrate | 1230 | 2445-78-5 | NQ | NQ | NQ | 0.024 a ± 0.003 | Sweet, fruity, ester, berry, green, waxy, apple |
| 35 | Hexyl methyl ketone | 1241 | 111-13-7 | 0.075 a ± 0.013 | 0.018 b ± 0.003 | 0.066 c ± 0.012 | 0.066 c ± 0.013 | Earthy, weedy, natural, woody, herbal |
| 36 | Isopentyl isopentanoate | 1273 | 659-70-1 | NQ | NQ | NQ | 0.035 a ± 0.005 | Sweet, fruity, green, ripe, apple, jammy, tropical |
| Ethyl heptanoate | 1281 | 106-30-9 | 0.151 a ± 0.024 | 0.100 b ± 0.013 | 0.116 ba ± 0.015 | 0.274 c ± 0.044 | Fruity, pineapple, cognac, rum wine | |
| 38 | Hexanol | 1299 | 111-27-3 | 0.059 a ± 0.009 | 0.039 b ± 0.006 | 0.037 b ± 0.005 | NQ | Ethereal, fusel, oil, fruity, alcoholic, sweet, green |
| 39 | (Z)-4-Heptenal | 1331 | 6728-31-0 | NQ | NQ | 0.027 a ± 0.004 | 0.035 b ± 0.006 | Oily, fatty, green, dairy, milky, creamy |
| 40 | 2-Hthylhexyl acetate | 1369 | 103-09-3 | 0.021 a ± 0.003 | 0.020 a ± 0.003 | NQ | NQ | Earthy, herbal, humus, undergrowth |
| 41 | Nonyl aldehyde | 1391 | 124-19-6 | NQ | NQ | 0.073 a ± 0.012 | 0.197 b ± 0.037 | Waxy, aldehydic, rose, fresh, orris, orange peel, fatty, peely |
| 42 | Ethyl caprylate | 1430 | 106-32-1 | 19.674 a ± 3.85 | 19.791 a ± 4.03 | 17.016 ab ± 3.42 | 15.357 b ± 2.86 | Fruity, wine, waxy, sweet, apricot, banana, brandy, pear |
| 43 | Furfural | 1482 | 98-01-1 | NQ | NQ | 0.321 a ± 0.064 | 0.144 b ± 0.022 | Sweet, woody, almond, fragrant, baked bread |
| 44 | Octyl acetate | 1503 | 112-14-1 | 0.197 a ± 0.033 | 0.217 a ± 0.029 | 0.414 b ± 0.061 | 0.687 c ± 0.106 | Green, earthy, mushroom, herbal, waxy |
| 45 | Camphor | 1558 | 76-22-2 | NQ | 0.019 a ± 0.003 | NQ | NQ | Camphoreous |
| 46 | 2-Nonanol | 1567 | 628-99-9 | 0.140 a ± 0.028 | 0.056 b ± 0.009 | 0.034 c ± 0.005 | NQ | Waxy, green, creamy, citrus, orange, cheese, fruity |
| 47 | Ethyl nonanoate | 1578 | 123-29-5 | 0.269 a ± 0.051 | 0.321 a ± 0.063 | 0.098 b ± 0.0156 | 0.054 c ± 0.008 | Fruity, rose, waxy, rum, wine, natural, tropical |
| 48 | Linalool | 1588 | 78-70-6 | 0.539 a ± 0.088 | 0.453 b ± 0.072 | 0.474 b ± 0.078 | 0.680 c ± 0.117 | Citrus, floral, sweet, bois de rose, woody, green, blueberry |
| 49 | Octanoicacid | 1592 | 5461-6-3 | 0.017 a ± 0.003 | 0.035 b ± 0.006 | NQ | NQ | Fruity, green, oily, floral |
| 50 | Carbonochl | 1633 | 7452-59-7 | NQ | 0.586 a ± 0.089 | NQ | NQ | Sugar cane aroma, fruity |
| 51 | 2-Decanol | 1656 | 1120-06-5 | 0.030 a ± 0.005 | 0.017 b ± 0.002 | NQ | 0.051 c ± 0.009 | NF |
| 52 | Ethyl caprate | 1690 | 110-38-3 | 6.257 a ± 1.331 | 4.795 b ± 0.874 | 1.401 c ± 0.258 | 2.275 c ± 0.367 | Sweet, waxy, fruity, apple, grape, oily, brandy |
| 53 | Acetophenone | 1705 | 98-86-2 | NQ | 0.032 a ± 0.005 | NQ | NQ | Sweet, pungent, hawthorn, mimosa, almond, acacia, chemical |
| 54 | Myrcene | 1721 | 123-35-3 | NQ | NQ | 0.357 a ± 0.053 | 0.191 b ± 0.031 | Peppery, terpene, spicy, balsam, plastic, |
| 55 | Ethyl benzoate | 1739 | 93-89-0 | 0.082 a ± 0.012 | 0.091 b ± 0.014 | 0.056 c ± 0.008 | 0.064 d ± 0.009 | Fruity, dry, musty, sweet, wintergreen |
| 56 | ethyl 9-decenoate | 1748 | 67233-91-4 | 4.062 a ± 0.879 | 3.96 a ± 0.697 | 0.369 b ± 0.652 | 0.725 b ± 0.117 | Fruity, fatty |
| 57 | 2-Dodecanol | 1771 | 10203-28-8 | 0.205 a ± 0.031 | 0.139 b ± 0.024 | NQ | NQ | NF |
| 58 | 4-Ethylbenzaldehyde | 1782 | 4748-78-1 | NQ | 0.038 a ± 0.005 | NQ | NQ | Bitter, almond, sweet, anise |
| 59 | Geranyl acetate | 1790 | 105-87-3 | 0.031 a ± 0.005 | 0.029 a ± 0.004 | 0.034 a ± 0.005 | 0.030 a ± 0.006 | Floral, rose, lavender, green, waxy |
| 60 | Decyl alcohol | 1800 | 112-30-1 | NQ | 0.678 a ± 0.098 | NQ | 0.488 b ± 0.078 | Fatty, waxy, floral, orange, sweet, clean, watery |
| 61 | Citronellol | 1804 | 106-22-9 | 0.508 a ± 0.089 | 0.346 b ± 0.064 | 0.352 b ± 0.059 | 0.174 c ± 0.031 | Floral, leather, waxy, rose bud, citrus |
| 62 | Ethyl phenylacetate | 1807 | 101-97-3 | 0.027 a ± 0.004 | 0.049 b ± 0.001 | 0.029 a ± 0.005 | 0.036 c ± 0.004 | Sweet, floral, honey, rose, balsam, cocoa |
| 63 | Nerol | 1810 | 106-25-2 | 0.028 a ± 0.004 | 0.032 ab ± 0.006 | NQ | 0.009 c ± 0.001 | Sweet, natural, neroli, citrus, magnolia |
| 64 | Beta-damascenone | 1816 | 23726-93-4 | 0.087 a ± 0.012 | NQ | 0.275 b ± 0.040 | 0.333 c ± 0.058 | Apple, rose, honey, tobacco, sweet |
| 65 | Ethyl laurate | 1819 | 106-33-2 | 0.159 a ± 0.027 | 0.219 b ± 0.031 | 0.019 c ± 0.003 | NQ | Sweet, waxy, floral, soapy, clean |
| 66 | Geraniol | 1821 | 106-24-1 | 0.103 a ± 0.027 | 0.054 b ± 0.009 | 0.066 c ± 0.010 | 0.095 d ± 0.012 | Sweet, floral, fruity, rose, waxy, citrus |
| 67 | Geranylacetone | 1825 | 689-67-8 | 0.170 a ± 0.030 | 0.068 b ± 0.011 | 0.075 b ± 0.013 | NQ | Fresh, rose, leaf, floral, green, magnolia, aldehydic, fruity |
| 68 | Trimethyl pentanyl diisobutyrate | 1828 | 6846-50-0 | NQ | 0.077 a ± 0.013 | 0.039 b ± 0.006 | NQ | NF |
| 69 | Ethyl hydrocinnamate | 1930 | 2021-28-5 | 0.070 a ± 0.010 | 0.108 b ± 0.017 | 0.072 a ± 0.013 | 0.068 a ± 0.013 | Hyacinth, rose, honey, fruity, rum |
| 70 | Butylated hydroxytoluene | 1936 | 128-37-0 | NQ | 0.013 a ± 0.002 | 0.486 b ± 0.071 | NQ | Mild, phenolic, camphor |
| 71 | DMS | 767 | 75-18-3 | 0.019 ba ± 0.003 | 0.085 c ± 0.011 | 0.010 a ± 0.001 | 0.025 b ± 0.004 | Sulfury, onion, sweet, corn, vegetable, cabbage, tomato, green, radish |
| 72 | Heptyl acetate | 1356 | 112-06-1 | 0.066 a ± 0.012 | 0.113 b ± 0.017 | 0.183 c ± 0.030 | 0.134 bc ± 0.023 | Fresh, green, rum, ripe, fruit, pear, apricot, woody |
| 73 | Benzyl alcohol | 1571 | 100-51-6 | 0.033 a ± 0.005 | 0.077 b ± 0.012 | 0.117 c ± 0.020 | NQ | Floral, rose, phenolic, balsamic |
| 74 | Isoamyl octanoate | 1725 | 2035-99-6 | 0.101 a ± 0.017 | 0.302 b ± 0.048 | NQ | 0.015 c ± 0.002 | Sweet, oily, fruity, green, soapy, pineapple, coconut |
| 75 | Phenethyl acetate | 1813 | 103-45-7 | 2.611 a ± 0.415 | 8.201 cb ± 1.311 | 6.086 b ± 0.985 | 9.984 c ± 1.837 | Floral, rose, sweet, honey, fruity, tropical, |
| 76 | Phenethyl alcohol | 1941 | 60-12-8 | 9.061 a ± 1.364 | 12.49 b ± 2.074 | 9.913 a ± 1.693 | 10.503 ab ± 1.795 | Floral rose, dried rose, flower, rose water |
| 77 | 2,4-Di-t-butylphenol | 2341 | 96-76-4 | 0.127 a ± 0.016 | 0.357 b ± 0.063 | 0.125 a ± 0.020 | 0.105 c ± 0.018 | Phenolic |
| Aging-Related Substance Content (µg/L) | ||||||
|---|---|---|---|---|---|---|
| 2-Furaldehyde | Acetaldehyde | Isobutyraldehyde | Valeraldehyde | Phenylacetaldehyde | Total Aldehyde | |
| Unaged commercially available lager (CAL1) | NQ | 3.04 ± 0.413 | 38.385 ± 3.125 | 5.386 ± 0.281 | 6.133 ± 0.818 | 52.944 b ± 4.696 |
| Aged commercially available lagers (CAL1) | 66.887 ± 8.423 | 75.414 ± 9.052 | 49.914 ± 5.747 | 17.257 ± 1.425 | 8.996 ± 0.755 | 218.468 a ± 25.402 |
| Unaged commercially available lager (CAL2) | NQ | 7.08 ± 0.629 | 15.394 ± 1.724 | 16.841 ± 2.218 | 4.823 ± 0.293 | 44.138 b ± 4.864 |
| Unaged commercially available lager (CAL2) | 48.792 ± 5.466 | 92.348 ± 10.133 | 38.266 ± 2.635 | 25.978 ± 3.935 | 12.759 ± 0.828 | 218.143 a ± 22.997 |
| Unaged LS | NQ | 28.592 ± 3.981 | 2.7126 ± 0.416 | 14.197 ± 1.325 | 1.861 ± 0.252 | 47.366 b ± 5.78 |
| Aged LS | 11.412 ± 2.793 | 77.862 ± 9.051 | 8.758 ± 1.520 | 13.886 ± 2.047 | 10.744 ± 0.973 | 122.662 c ± 16.384 |
| Unaged OWBL | NQ | 69.306 ± 7.248 | 7.872 ± 0.923 | 9.162 ± 1.325 | 3.743 ± 0.287 | 90.083 d ± 11.125 |
| Aged OWBL | 22.503 ± 2.83 | 139.78 ± 18.723 | 14.657 ± 1.937 | 16.035 ± 1.692 | 9.622 ± 0.983 | 202.597 a ± 26.165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, H.; Jia, Y.; Liu, C.; Xu, J.; Xie, C.; Li, K.; Huang, K.; Hang, F. A Preliminary Study on the Effect of Adding Sugarcane Syrup on the Flavor of Barley Lager Fermentation. Foods 2024, 13, 2339. https://doi.org/10.3390/foods13152339
Lv H, Jia Y, Liu C, Xu J, Xie C, Li K, Huang K, Hang F. A Preliminary Study on the Effect of Adding Sugarcane Syrup on the Flavor of Barley Lager Fermentation. Foods. 2024; 13(15):2339. https://doi.org/10.3390/foods13152339
Chicago/Turabian StyleLv, Hechao, Yusheng Jia, Chaoyi Liu, Jia Xu, Caifeng Xie, Kai Li, Kai Huang, and Fangxue Hang. 2024. "A Preliminary Study on the Effect of Adding Sugarcane Syrup on the Flavor of Barley Lager Fermentation" Foods 13, no. 15: 2339. https://doi.org/10.3390/foods13152339
APA StyleLv, H., Jia, Y., Liu, C., Xu, J., Xie, C., Li, K., Huang, K., & Hang, F. (2024). A Preliminary Study on the Effect of Adding Sugarcane Syrup on the Flavor of Barley Lager Fermentation. Foods, 13(15), 2339. https://doi.org/10.3390/foods13152339





