Fish Fillet Analogue Using Formulation Based on Mushroom (Pleurotus ostreatus) and Enzymatic Treatment: Texture, Sensory, Aromatic Profile and Physicochemical Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
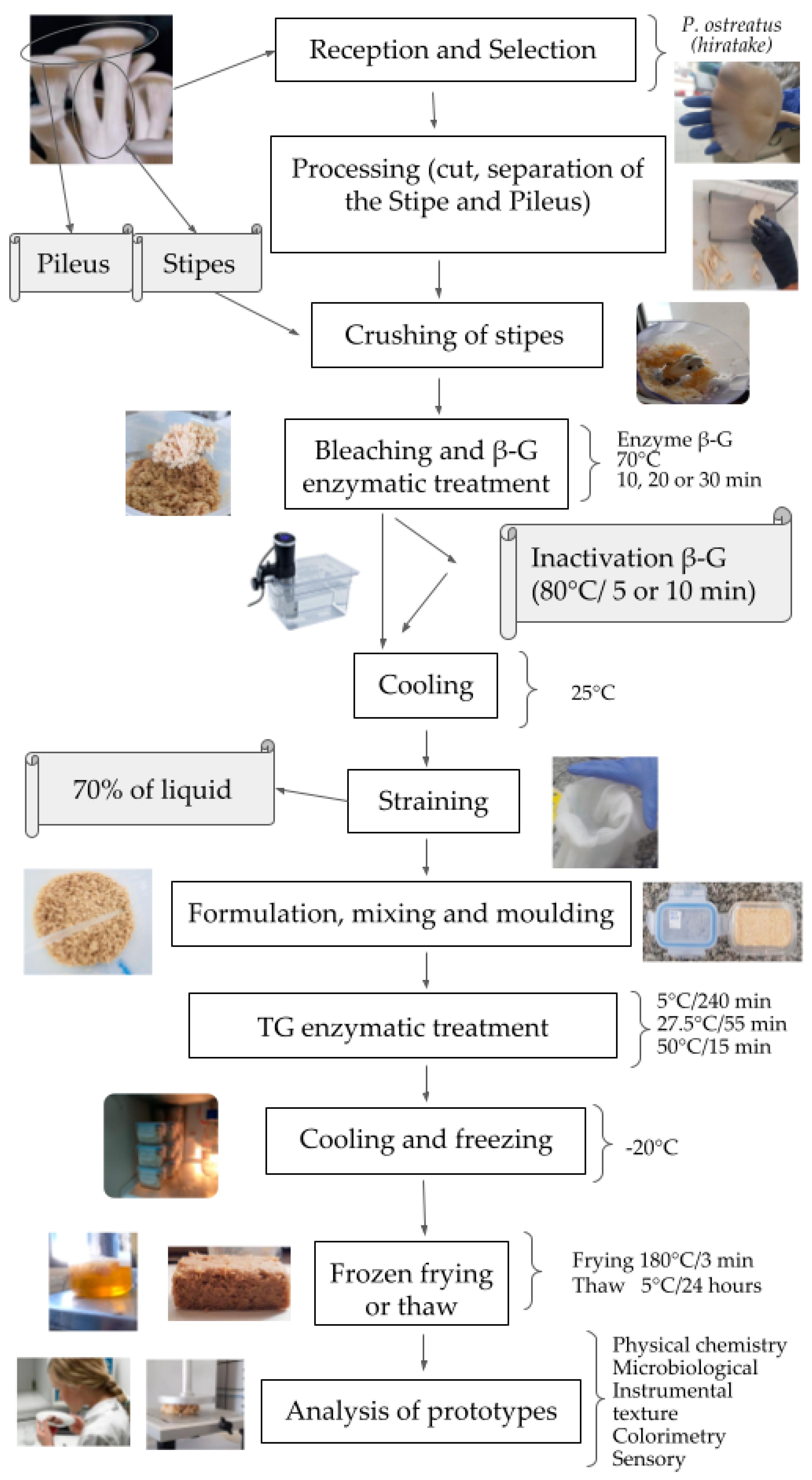
2.2. Preparation of Fish Fillet-like Analogues and Experimental Design
2.3. Analytic Methods
2.3.1. Proximate Composition
2.3.2. Instrumental Texture Profile Analysis (TPA)
2.3.3. Color Measurement
2.3.4. Sensory Analysis
2.3.5. Aromatic Profile
Headspace Solid-Phase Microextraction Combined with Gas Chromatography–Mass Spectrometry (HS-SPME-GC-MS)
E-Nose Analysis
3. Results
3.1. Proximate Composition
| Analyses | Shimeji | Hiratake | P. ostreatus | Tilapia Fillet | ||
|---|---|---|---|---|---|---|
| Integer | Integer | Pileus | Stipe | Ref. [35] | Ref. [37] | |
| Moisture (%) * | 92.64 ± 0.11 a | 92.66 ± 0.13 a | 93.01 ± 0.91 a | 92.17 ± 0.39 a | 90.65 | 77.13 |
| Ethereal extract (%) * | 0.11 ± 0.02 b | 0.05 ± 0.01 ab | 0.03 ± 0.01 a | 0.08 ± 0.04 ab | 0.15 | 2.6 |
| Crude protein (%) * | 2.07 ± 0.29 a | 1.54 ± 0.07 ab | 1.78 ± 0.29 a | 1.18 ± 0.13 b | 3.4 | 19.36 |
| Gross fiber (%) * | 0.25 ± 0.16 c | 1.69 ± 0.22 ab | 1.09 ± 0.41 a | 1.76 ± 0.14 b | 3.06 ** | - |
| Ash (%) * | 0.67 ± 0.02 c | 0.46 ± 0.01 a | 0.57 ± 0.04 b | 0.43 ± 0.02 a | 0.76 | 1.09 |
| Carbohydrates (%) * | 4.26 ± 0.22 a | 3.83 ± 0.51 a | 3.52 ± 0.82 a | 4.37 ± 0.67 a | 1.98 | - |
| pH | 6.59 ± 0.07 a | 6.44 ± 0.20 a | 6.44 ± 0.20 a | 6.42 ± 0.14 a | - | - |
| Acidity (% m/m) *** | 0.18 ± 0.01 a | 0.14 ± 0.04 a | 0.17 ± 0.06 a | 0.14 ± 0.01 a | - | - |
| Aw | 0.98 ± 0.00 a | 0.98 ± 0.01 a | 0.99 ± 0.00 a | 0.99 ± 0.00 a | - | 0.983 |
3.2. Development of a Mushroom-Based Fish Fillet-like Analogue
3.2.1. Plackett–Burman (PB20) Statistical Analysis
3.2.2. Sensory Analysis
3.3. Aromatic Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Insitutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lupetti, C.; Casselli, R. Olhar 360° Sobre o Consumidor Brasileiro e o Mercado Plant-Based 2023/2024; Tikbooks; The Good Food Institute: São Paulo, Brazil, 2024; pp. 22–32. [Google Scholar]
- Lima, M.; Costa, R.; Rodrigues, I.; Lameiras, J.; Botelho, G. A Narrative Review of Alternative Protein Sources: Highlights on Meat, Fish, Egg and Dairy Analogues. Foods 2022, 11, 2053. [Google Scholar] [CrossRef]
- Meyer, F.; Hutmacher, A.; Lu, B.; Steiger, N.; Nyström, L.; Narciso, J.O. Vegan shrimp alternative made with pink oyster and lion’s mane mushrooms: Nutritional profiles, presence of conjugated phenolic acids, and prototyping. J. Curr. Res. Food Sci. 2023, 7, 100572. [Google Scholar] [CrossRef]
- Beluhan, S.; Ranogajec, A. Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem. 2011, 124, 1076–1082. [Google Scholar] [CrossRef]
- Singh, U.; Tiwari, P.; Kelkar, S.; Kaul, D.; Tiwari, A.; Kapri, M.; Sharma, S. Edible mushrooms: A sustainable novel ingredient for meat analogs. eFood 2023, 4, e122. [Google Scholar] [CrossRef]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their Bioactive Components, Nutritional Value and Application in Functional Food Production—A Review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Vetter, J. The Mushroom Glucans: Molecules of High Biological and Medicinal Importance. Foods 2023, 12, 1009. [Google Scholar] [CrossRef]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible Mushrooms and Beta-Glucans: Impact on Human Health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef]
- Paudel, E.P.; Boom, R.M.; Haaren, E.V.; Siccama, J.; Smanl, R.G.M. Effects of cellular structure and cell wall components on water holding capacity of mushrooms. J. Food Eng. 2016, 187, 106–113. [Google Scholar] [CrossRef]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Lim, S.H.; Lee, Y.H.; Kang, H.W. Efficient Recovery of Lignocellulolytic Enzymes of Spent Mushroom Compost from Oyster Mushrooms, Pleurotus spp., and Potential Use in Dye Decolorization. Mycobiology 2013, 41, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.S. Mushroom cultivation in Brazil: Challenges and potential for growth. Ciênc. Agrotec. 2010, 34, 795–803. [Google Scholar] [CrossRef]
- Zivanovic, S.; Buescher, R. Changes in mushroom texture and cell wall composition affected by thermal processing. J. Food Sci. 2004, 69, SNQ44–SNQ49. [Google Scholar] [CrossRef]
- Zivanovic, S.; Buescher, R.; Kim, S.K. Mushroom Texture, Cell Wall Composition, Color, and Ultrastructure as Affected by pH and Temperature. J. Food Sci. 2003, 68, 1860–1865. [Google Scholar] [CrossRef]
- Buchert, J.; Cura, D.E.; Ma, H.; Gasparetti, C.; Monogioudi, E.; Faccio, G.; Mattinen, M.; Boer, H.; Partanen, R.; Selinheimo, E.; et al. Crosslinking Food Proteins for Improved Functionality. Annu. Rev. Food Sci. Technol. 2010, 1, 113–138. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef]
- Lampila, L.E. Applications and functions of food-grade phosphates. Ann. N. Y. Acad. Sci. 2013, 1301, 37–44. [Google Scholar] [CrossRef]
- Innocenzi, P. Understanding sol–gel transition through a picture. A short tutorial. J. Sol-Gel Sci. Technol. 2020, 94, 544–550. [Google Scholar] [CrossRef]
- Duarte, L.; Matte, C.R.; Bizarro, C.V.; Ayub, M.A.Z. Transglutaminases: Part I—Origins, sources, and biotechnological characteristics. World J. Microbiol. Biotechnol. 2020, 36, 15. [Google Scholar] [CrossRef]
- Rodrigues, M.I.; Iemma, A.F. Experimental Design and Process Optimization; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- StatSoft Inc. STATISTICA (Data Analysis Software System), Version 8.0.; StatSoft Inc.: Tulsa, OK, USA, 2008. [Google Scholar]
- Mazumder, M.A.R.; Sukchot, S.; Phonphimai, P.; Ketnawa, S.; Chaijan, M.; Grossmann, L.; Rawdkuen, S. Mushroom–Legume-Based Minced Meat: Physico-Chemical and Sensory Properties. Foods 2023, 12, 2094. [Google Scholar] [CrossRef]
- Fukuda, K.; Hiraga, M.; Asakuma, S.; Arai, I.; Sekikawa, M.; Urashima, T. Purification and characterization of a novel exo-beta-1,3-1,6-glucanase from the fruiting body of the edible mushroom Enoki (Flammulina velutipes). Biosci. Biotechnol. Biochem. 2008, 72, 3107–3113. [Google Scholar] [CrossRef]
- Buckow, R.; Heinz, V.; Knorr, D. Effect of High Hydrostatic Pressure-Temperature Combinations on the Activity of β-Glucanase from Barley Malt. J. Inst. Brew. 2012, 111, 282–289. [Google Scholar] [CrossRef]
- Manzi, P.; Aguzzi, A.; Pizzoferrato, L. Nutritional Value of Mushrooms Widely Consumed in Italy. Food Chem. 2001, 73, 321–325. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists—AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Bernardo, Y.A.; Rosario, D.K.A.; Monteiro, M.L.G.; Mano, S.B.; Delgado, I.F.; Conte, C.A., Jr. Texture Profile Analysis: How Parameter Settings Affect the Instrumental Texture Characteristics of Fish Fillets Stored Under Refrigeration? Food Anal. Methods 2022, 15, 144–156. [Google Scholar] [CrossRef]
- Commission Internationale de L’éclairage—CIE. Colorimetry, 2nd ed.; CIE Publication: Vienna, Austria, 1986; p. 74. [Google Scholar]
- Latimer, G.W., Jr. (Ed.) AOAC Official Method 992.30. Confirmed Total Coliform and Escherichia coli in All Foods: Substrate Supporting Disc Method (ColiComplete®). In Official Methods of Analysis of AOAC International, 22nd ed.; AOAC Publications: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Mondragón-Bernal, O.L.; Maugeri, F.F.; Alves, J.G.L.F.; Rodrigues, M.I. Synbiotic Soy Beverages: Principles and Sensory Attributes. In Handbook of Plant-Based Fermented Foods and Beverages, 2nd ed.; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2012; Volume 1, pp. 103–130. [Google Scholar]
- Li, B.; Kimatu, B.M.; Li, C.; Pei, F.; Hu, Q.; Zhao, L. Analysis of volatile compounds in L. edodes blanched by hot water and microwave. Int. J. Food Sci. Technol. 2017, 52, 1680–1689. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, X.; Zhao, M.; Liu, X.; Pang, Y.; Zhang, M. Characterization of the Key Aroma Constituents in Fried Tilapia through the Sensorics Concept. Foods 2022, 11, 494. [Google Scholar] [CrossRef]
- Mutz, Y.S.; Nunes, C.A. Use of an electronic nose based on oxide sensors metal (MOS) for analyzing specialty coffees with different roasting points. In Proceedings of the 15th SLACAN—Latin American Symposium on Food Science and Nutrition, Campinas, Brazil, 12–14 November 2023. [Google Scholar]
- Zhang, Z.; Zang, M.; Chen, J.; Zhang, K.; Wang, S.; Li, D.; Li, X.; Liu, M.; Pan, X. Effect of the mycelium of oyster mushrooms on the physical and flavor properties of a plant-based beef analogue. LWT-Food Sci. Technol. 2024, 198, 116029. [Google Scholar] [CrossRef]
- Bach, F.; Helm, C.V.; Bellettini, M.B.; Maciel, G.M.; Haminiuk, C.W. Edible mushrooms: A potential source of essential amino acids, glucans and minerals. Int. J. Food Sci. 2017, 52, 2382–2392. [Google Scholar] [CrossRef]
- Pascual-Pineda, L.A.; Hernández-Marañon, A.; Castillo-Morales, M.; Uzárraga-Salazar, R.; Rascón-Díaz, M.P.; Flores-Andrade, E. Effect of water activity on the stability of freeze-dried oyster mushroom (Pleurotus ostreatus) powder. Dry. Technol. 2021, 39, 989–1002. [Google Scholar] [CrossRef]
- Simões, M.R.; Ribeiro, C.F.A.; Ribeiro, S.C.A.; PARK, K.J.; MURR, F.E.X. Composição físico-química, microbiológica e rendimento do filé de tilápia tailandesa (Oreochromis niloticus). Food Sci. Technol. 2007, 27, 608–613. [Google Scholar] [CrossRef]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.T.; Yang, Y.C.; Li, Y.H.; Mau, J.L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef]
- Cheung, P.C.K. Mini-review on edible mushrooms as source of dietary fiber: Preparation and health benefits. Food Sci. Hum. Wellness 2013, 2, 162–166. [Google Scholar] [CrossRef]
- Raya, M.; Shalaby, M.; Hafez, S.; Hamouda, A. Chemical composition and nutritional potential of some mushroom varieties cultivated in Egypt. J. Food Dairy Sci. 2014, 5, 421–434. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T. Beta-glucans in higher fungi and their health effects. Nutr. Rev. 2009, 67, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Guha, A.K. A comprehensive analysis of the nutritional quality of edible mushroom Pleurotus sajor-caju grown in deproteinized whey medium. LWT-Food Sci. Technol. 2015, 61, 339–345. [Google Scholar] [CrossRef]
- Fidanza, M.; Sanford, D.; Beyer, D.; Aurentz, D. Analysis of Fresh Mushroom Compost. HortTechnology 2010, 20, 449–453. [Google Scholar] [CrossRef]
- Boonsiriwit, A.; Xiao, Y.; Kathuria, A.; Lee, Y.S. Effect of moisture-controlled packaging treatment with acid-modified expanded vermiculite-calcium chloride on the quality of fresh mushrooms (Agaricus bisporus) during low-temperature storage. J. Sci. Food Agric. 2022, 102, 3029–3037. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, S.; Yang, X.; Li, L.; Hao, S.; Cen, J.; Zhang, H. Effects of Ozonated Water Treatment on Physico-chemical, Microbiological and Sensory Characteristics Changes of Nile Tilapia (Oreochromis niloticus) Fillets during Storage in Ice. Ozone Sci. Eng. 2019, 42, 408–419. [Google Scholar] [CrossRef]
- Tyl, C.; Sadler, G.D. pH and Titratable Acidity. In Food Analysis-Food Science Text Series, 5th ed.; Nielsen, S.S., Ed.; Springer: New York, NY, USA, 2017; pp. 389–406. [Google Scholar] [CrossRef]
- Lee, J.S.; Oh, H.; Choi, I.; Yoon, C.S.; Han, J. Physico-chemical characteristics of rice protein-based novel textured vegetable proteins as meat analogues produced by low-moisture extrusion cooking technology. LWT-Food Sci. Technol. 2022, 157, 113056. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, W.; Tao, N.; Jin, Y.; Feng, Y.; Jin, Y. Effect of ratio of oil to sample on the quality of fried fish (Pseudorasbora parva). J. Food Process. Preserv. 2021, 45, e15712. [Google Scholar] [CrossRef]
- Yuliarti, O.; Kovis, T.J.K.; Yi, N.J. Structuring the meat analogue by using plant-based derived composites. J. Food Eng. 2021, 288, 110138. [Google Scholar] [CrossRef]
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, C.; Wang, N.; Zheng, Q.-W.; Ye, Z.-W.; Wei, T.; Zhong, J.-R.; Guo, L.-Q.; Lin, J.-F. Development of Flammulina velutipes-based meat analogs with tunable physicochemical, structural, and sensory properties. Int. J. Food Eng. 2023, 19, 177–186. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Interventions of two-stage thermal sous-vide cooking on the toughness of beef semitendinosus. Meat Sci. 2019, 157, 107882. [Google Scholar] [CrossRef] [PubMed]
- León, K.; Mery, D.; Pedreschi, F.; León, J. Color measurement in L*a*b* units from RGB digital images. Food Res. Int. 2006, 39, 1084–1091. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Goh, A.T.; Stieger, M.; Forde, C.G. Correlation of Instrumental Texture Properties from Textural Profile Analysis (TPA) with Eating Behaviours and Macronutrient Composition for a Wide Range of Solid Foods. Food Funct. 2018, 9, 5301–5312. [Google Scholar] [CrossRef] [PubMed]
- Kaleda, A.; Talvistu, K.; Vaikma, H.; Tammik, M.-L.; Rosenvald, S.; Vilu, R. Physicochemical, textural, and sensorial properties of fibrous meat analogs from oat-pea protein blends extruded at different moistures, temperatures, and screw speeds. Future Foods 2021, 4, 100092. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Zhu, S.; Wang, Q. Texturisation Behaviour of Peanut–SoyBean/Wheat Protein Mixtures during High Moisture Extrusion Cooking. Int. J. Food Sci. Tech. 2018, 53, 2535–2541. [Google Scholar] [CrossRef]
- Dobson, S.; Pensini, E.; Dupuis, J.; Yada, R.; Marangoni, A. Synergistic interactions between pea protein isolate and rapid-swelling starch. Food Hydrocoll. 2023, 142, 108753. [Google Scholar] [CrossRef]
- Joshi, M.; Aldred, P.; Panozzo, J.; Kasapis, S.; Adhikari, B. Rheological and microstructural characteristics of lentil starch–lentil protein composite pastes and gels. Food Hydrocoll. 2014, 35, 226–237. [Google Scholar] [CrossRef]
- Li, P.; Wu, G.; Yang, D.; Zhang, H.; Qi, X.; Jin, Q.; Wang, X. Effect of multistage process on the quality, water and oil distribution and microstructure of French fries. Food Res. Int. 2020, 137, 109229. [Google Scholar] [CrossRef]
- Stephan, A.; Ahlborn, J.; Zajul, M.; Zorn, H. Edible mushroom mycelia of Pleurotus sapidus as novel protein sources in a vegan boiled sausage analog system: Functionality and sensory tests in comparison to commercial proteins and meat sausages. Eur. Food Res. Technol. 2018, 244, 913–924. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, M.; Adhikari, B. The inactivation kinetics of polyphenol oxidase in mushroom (Agaricus bisporus) during thermal and thermosonic treatments. Ultrason. Sonochem. 2013, 20, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, W.; Zeng, X.; Xi, Y.; Li, Y.; Hui, B.; Li, J. Characterization of the Major Odor-Active Off-Flavor Compounds in Normal and Lipoxygenase-Lacking Soy Protein Isolates by Sensory-Directed Flavor Analysis. J. Agric. Food Chem. 2023, 71, 8129–8139. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Bouchon, P.; Hollins, P.; Pearson, M.; Pyle, D.L.; Tobin, M.J. Oil Distribution in Fried Potatoes Monitored by Infrared Microspectroscopy. J. Food Sci. 2006, 66, 918–923. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Fan, L. Effect of Pore Characteristics on Oil Absorption Behavior during Frying of Potato Chips. Innov. Food Sci. Emerg. Technol. 2020, 66, 102508. [Google Scholar] [CrossRef]
- Muhammad, R.; Mohd, A.I.; Ahmad, R.; Hanan, F. Psychological Factors on Food Neophobia among the Young Culinarian in Malaysia: Novel Food Preferences. Procedia Soc. Behav. Sci. 2016, 222, 358–366. [Google Scholar] [CrossRef]
- FlavorNet: Database of Aroma Compounds. Available online: https://www.flavornet.org/flavornet.html (accessed on 29 May 2024).
- PubChem: Open Chemistry Database. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 29 May 2024).
- The Good Scents Company. Good Scents Data: Aroma Chemical Information. Available online: https://www.thegoodscentscompany.com/data/rw1155991.html (accessed on 29 May 2024).
- Zhang, Z.-M.; Wu, W.-W.; Li, G.-K. A GC—MS Study of the Volatile Organic Composition of Straw and Oyster Mushrooms During Maturity and its Relation to Antioxidant Activity. J. Chromatogr. Sci. 2008, 46, 690–696. [Google Scholar] [CrossRef]
- De Maria, C.A.B.; Moreira, R.F.A.; Trugo, L.C. Componentes voláteis do café torrado. Parte I: Compostos heterocíclicos. Quim. Nova 1999, 22, 209–217. [Google Scholar] [CrossRef]
- Akiyama, M.; Murakami, K.; Ikeda, M.; Iwatsuki, K.; Kokubo, S.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H.; Tanaka, K. Characterization of Flavor Compounds Released During Grinding of Roasted Robusta Coffee Beans. Food Sci. Technol. Res. 2005, 11, 298–307. [Google Scholar] [CrossRef]
- Baltes, W.; Bochmann, G. Model reactions on roast aroma formation. 1. Reaction of serine and threonine with sucrose under the conditions on coffee roasting and identification of new coffee aroma compounds. J. Agric. Food Chem. 1987, 35, 340–346. [Google Scholar] [CrossRef]
- Choi, Y.; Chae, J.; Kim, S.; Shin, E.C.; Choi, G.; Kim, D.; Cho, S. Surimi for snacks: Physicochemical and sensory properties of fried fish snacks prepared from surimi of different fish species. Fish. Aquat. Sci. 2023, 26, 145–157. [Google Scholar] [CrossRef]
- Pires, C.V.; Oliveira, M.G.A.; Rosa, J.C.; Costa, N.M.B. Qualidade nutricional e escore químico de aminoácidos de diferentes fontes protéicas. Food Sci. Technol. 2006, 26, 179–187. [Google Scholar] [CrossRef]
- Handzlik, M.K.; Metallo, C.M. Sources and Sinks of Serine in Nutrition, Health, and Disease. Annu. Rev. Nutr. 2023, 43, 123–151. [Google Scholar] [CrossRef] [PubMed]
- Radványi, D. Smelling the difference: Separation of healthy and infected button mushrooms via microbial volatile organic compounds. Heliyon 2022, 9, e12703. [Google Scholar] [CrossRef]


| Variables | Code | Real Values | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| β-G concentration * (%) | x1 | 0 | 0.3 | 0.6 |
| Time with β-G (min) | x2 | 10 | 20 | 30 |
| Concentration TG ** (% w/w) | x3 | 0.1 | 0.55 | 1 |
| TG temperature/time binomial (°C/min) | x4 | 5/240 | 27.5/55 | 50/15 |
| SPI *** (% w/w) | x5 | 5 | 7.5 | 10 |
| Oat flour—OF (% w/w) | x6 | 0 | 2.5 | 5 |
| Glutamine—Gt (% w/w) | x7 | 0 | 1 | 2 |
| Monosodium Glutamate—MG (% w/w) | x8 | 0 | 1 | 2 |
| Acacia gum—AG (% w/w) | x9 | 0 | 5 | 10 |
| Cassava starch—CS (% w/w) | x10 | 0 | 0.5 | 1 |
| Coconut oil—CO (% w/w) | x11 | 0 | 0.5 | 1 |
| Soybean oil—SO (% w/w) | x12 | 0 | 0.5 | 1 |
| Sodium tripolyphosphate—ST (% w/w) | x13 | 0 | 0.1 | 0.2 |
| β-G inactivation (min) | x14 | 0 | 5 | 10 |
| Coded Matrix PB20 | PB 20 Response Matrix—Fried Prototypes | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treat. | x1 | x2 | x3 | x4 | x5 | x6 | x7 | x8 | x9 | x10 | x11 | x12 | x13 | x14 | Hard | Elas | Coe | Gum | Che | Res | L* | a* | b* | aw | pH |
| 1 | 1 | −1 | 1 | 1 | −1 | −1 | −1 | −1 | 1 | −1 | 1 | −1 | 1 | 1 | 234 | 0.32 | 0.27 | 64 | 21 | 0.1 | 43 | 7 | 17 | 0.9 | 6.5 |
| 2 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | −1 | −1 | 1 | −1 | 1 | −1 | 1 | 321 | 0.4 | 0.33 | 105 | 41 | 0.1 | 48 | 8 | 21 | 0.9 | 6.7 |
| 3 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | −1 | −1 | 1 | −1 | 1 | −1 | 363 | 0.41 | 0.32 | 115 | 46 | 0.1 | 52 | 7 | 21 | 0.8 | 6.7 |
| 4 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | −1 | −1 | 1 | −1 | 1 | 402 | 0.39 | 0.31 | 126 | 50 | 0.1 | 43 | 10 | 21 | 0.8 | 6.9 |
| 5 | 1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | −1 | −1 | 1 | −1 | 220 | 0.49 | 0.38 | 82 | 40 | 0.2 | 50 | 6 | 20 | 0.9 | 7 |
| 6 | 1 | 1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | −1 | −1 | 1 | 629 | 0.35 | 0.26 | 164 | 58 | 0.1 | 45 | 7 | 19 | 0.8 | 6.5 |
| 7 | 1 | 1 | 1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | −1 | −1 | 381 | 0.23 | 0.23 | 90 | 22 | 0.1 | 46 | 6 | 16 | 0.8 | 7.1 |
| 8 | 1 | 1 | 1 | 1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | −1 | 205 | 0.36 | 0.28 | 52 | 18 | 0.1 | 44 | 8 | 21 | 0.8 | 7.1 |
| 9 | −1 | 1 | 1 | 1 | 1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 373 | 0.36 | 0.29 | 107 | 38 | 0.1 | 43 | 9 | 24 | 0.8 | 6.8 |
| 10 | 1 | −1 | 1 | 1 | 1 | 1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | 372 | 0.29 | 0.25 | 93 | 28 | 0.1 | 37 | 7 | 14 | 0.7 | 6.6 |
| 11 | −1 | 1 | −1 | 1 | 1 | 1 | 1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | 283 | 0.36 | 0.27 | 75 | 25 | 0.1 | 52 | 6 | 18 | 0.9 | 7 |
| 12 | 1 | −1 | 1 | −1 | 1 | 1 | 1 | 1 | −1 | −1 | 1 | 1 | −1 | 1 | 403 | 0.33 | 0.26 | 105 | 36 | 0.1 | 48 | 5 | 15 | 0.8 | 7.1 |
| 13 | −1 | 1 | −1 | 1 | −1 | 1 | 1 | 1 | 1 | −1 | −1 | 1 | 1 | −1 | 559 | 0.27 | 0.22 | 124 | 33 | 0.1 | 50 | 8 | 20 | 0.9 | 6.8 |
| 14 | −1 | −1 | 1 | −1 | 1 | −1 | 1 | 1 | 1 | 1 | −1 | −1 | 1 | 1 | 291 | 0.32 | 0.31 | 91 | 30 | 0.1 | 43 | 6 | 15 | 0.7 | 7.1 |
| 15 | −1 | −1 | −1 | 1 | −1 | 1 | −1 | 1 | 1 | 1 | 1 | −1 | −1 | 1 | 233 | 0.37 | 0.3 | 68 | 26 | 0.1 | 49 | 6 | 18 | 0.9 | 6.8 |
| 16 | −1 | −1 | −1 | −1 | 1 | −1 | 1 | −1 | 1 | 1 | 1 | 1 | −1 | −1 | 276 | 0.32 | 0.27 | 74 | 26 | 0.1 | 52 | 6 | 20 | 0.7 | 7.1 |
| 17 | 1 | −1 | −1 | −1 | −1 | 1 | −1 | 1 | −1 | 1 | 1 | 1 | 1 | −1 | 725 | 0.31 | 0.25 | 181 | 58 | 0.1 | 42 | 7 | 13 | 0.9 | 6.9 |
| 18 | 1 | 1 | −1 | −1 | −1 | −1 | 1 | −1 | 1 | −1 | 1 | 1 | 1 | 1 | 165 | 0.3 | 0.26 | 42 | 13 | 0.1 | 49 | 6 | 17 | 0.8 | 6.8 |
| 19 | −1 | 1 | 1 | −1 | −1 | −1 | −1 | 1 | −1 | 1 | −1 | 1 | 1 | 1 | 168 | 0.33 | 0.29 | 51 | 18 | 0.1 | 56 | 6 | 21 | 0.7 | 6.9 |
| 20 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 155 | 0.37 | 0.32 | 49 | 19 | 0.2 | 43 | 9 | 16 | 0.9 | 7.1 |
| 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 482 | 0.33 | 0.27 | 99 | 41 | 0.1 | 52 | 8 | 22 | 0.8 | 7.1 |
| 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 417 | 0.32 | 0.25 | 140 | 32 | 0.1 | 45 | 7 | 19 | 0.8 | 7 |
| 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 375 | 0.34 | 0.26 | 165 | 37 | 0.1 | 48 | 6 | 17 | 0.8 | 6.9 |
| Tilapia | 1507 | 0.57 | 0.37 | 560 | 318 | 0.1 | 40 | 14 | 21 | 1 | 6.6 | ||||||||||||||
| Hard | Elas | Coe | Gum | Che | Res | L* | a* | b* | aw | pH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ef | p | Ef | p | Ef | p | Ef | p | Ef | p | Ef | p | Ef | p | Ef | p | Ef | p | Ef | p | Ef | p | |
| Mean | 338 | 0.00 | 0.30 | 0.00 | 0.3 | 0.00 | 99 | 0.00 | 36 | 0.00 | 0.10 | 0.00 | 46.8 | 0.00 | 6.9 | 0.00 | 18.32 | 0.00 | 0.81 | 0.00 | 6.87 | 0.00 |
| Curv. | 174 | 0.40 | 0.00 | 0.60 | 0.00 | 0.20 | 71 | 0.20 | 1 | 1.00 | 0.00 | 0.10 | 3.4 | 0.61 | 0.5 | 0.72 | 2.26 | 0.46 | −0.01 | 0.82 | 0.28 | 0.09 |
| x1 | 55 | 0.40 | 0.00 | 0.50 | 0.00 | 0.40 | −1 | 1.00 | −1 | 0.90 | 0.00 | 0.10 | −3.4 | 0.19 | −0.4 | 0.44 | −2.10 | 0.09 | 0.01 | 0.58 | −0.11 | 0.08 |
| x2 | 14 | 0.80 | 0.00 | 0.40 | 0.00 | 0.20 | 19 | 0.30 | 5 | 0.40 | 0.00 | 0.30 | 3.5 | 0.17 | 0.1 | 0.78 | 2.57 | 0.04 | 0.01 | 0.80 | −0.06 | 0.26 |
| x3 | −37 | 0.60 | 0.00 | 0.30 | 0.00 | 0.70 | −3 | 0.90 | −2 | 0.80 | 0.00 | 0.70 | −2.5 | 0.32 | 0.3 | 0.57 | 0.47 | 0.67 | −0.06 | 0.02 | 0.03 | 0.61 |
| x4 | −35 | 0.60 | 0.03 | 0.08 | 0.00 | 0.40 | 3 | 0.90 | 4 | 0.50 | 0.00 | 0.10 | −1.7 | 0.49 | 0.8 | 0.14 | 2.18 | 0.08 | 0.04 | 0.06 | −0.11 | 0.07 |
| x5 | 30 | 0.60 | 0.03 | 0.08 | 0.00 | 0.20 | 33 | 0.08 | 17 | 0.03 | 0.00 | 0.40 | 0.4 | 0.86 | −0.8 | 0.15 | 0.52 | 0.64 | −0.04 | 0.09 | −0.03 | 0.57 |
| x6 | 194 | 0.01 | 0.00 | 0.20 | −0.03 | 0.04 | 38 | 0.05 | 9 | 0.20 | −0.02 | 0.02 | −0.9 | 0.70 | −0.2 | 0.62 | −1.74 | 0.14 | 0.02 | 0.33 | −0.07 | 0.19 |
| x7 | −39 | 0.60 | 0.00 | 0.40 | 0.00 | 0.50 | −9 | 0.60 | −4 | 0.60 | 0.00 | 0.30 | 1.7 | 0.47 | −0.5 | 0.28 | −0.15 | 0.89 | −0.04 | 0.10 | 0.26 | 0.00 |
| x8 | 85 | 0.20 | 0.00 | 0.60 | 0.00 | 0.90 | 9 | 0.60 | 3 | 0.60 | 0.00 | 0.70 | 0.5 | 0.83 | −0.6 | 0.23 | 0.41 | 0.71 | −0.01 | 0.77 | 0.06 | 0.27 |
| x9 | 27 | 0.70 | −0.06 | 0.00 | −0.03 | 0.04 | −8 | 0.60 | −11 | 0.10 | −0.04 | 0.00 | −2.2 | 0.37 | −0.4 | 0.37 | −0.80 | 0.47 | −0.04 | 0.07 | −0.16 | 0.02 |
| x10 | −25 | 0.70 | 0.00 | 0.10 | 0.00 | 0.40 | −16 | 0.40 | −10 | 0.20 | 0.00 | 0.40 | 0.4 | 0.86 | −1.0 | 0.07 | −1.24 | 0.28 | −0.04 | 0.09 | 0.12 | 0.06 |
| x11 | −24 | 0.70 | 0.00 | 1.00 | 0.00 | 0.40 | −7 | 0.70 | −1 | 0.90 | 0.00 | 0.30 | 1.1 | 0.63 | −0.6 | 0.27 | 0.14 | 0.90 | 0.01 | 0.80 | 0.00 | 0.99 |
| x12 | 77 | 0.30 | 0.00 | 0.10 | 0.00 | 0.20 | −10 | 0.60 | −8 | 0.30 | −0.02 | 0.05 | 0.4 | 0.87 | 0.4 | 0.42 | 0.60 | 0.59 | −0.02 | 0.29 | −0.05 | 0.39 |
| x13 | 0 | 1.00 | 0.00 | 0.70 | 0.00 | 0.80 | 7 | 0.70 | 4 | 0.60 | 0.00 | 0.40 | 1.2 | 0.60 | −0.9 | 0.09 | −1.56 | 0.18 | 0.01 | 0.55 | −0.08 | 0.18 |
| x14 | −50 | 0.50 | 0.00 | 0.70 | 0.00 | 0.70 | −18 | 0.30 | −7 | 0.30 | 0.00 | 0.50 | 1.6 | 0.50 | −0.4 | 0.40 | −0.14 | 0.90 | −0.01 | 0.58 | −0.08 | 0.16 |
| Treatment | Appearance | Texture | Aroma | Flavor | Global Impression |
|---|---|---|---|---|---|
| 6 | 3.1 ± 2.3 a | 3.4 ± 2.3 a | 3.1 ± 2.4 a | 2.0 ± 2.0 a | 2.9 ± 2.2 a |
| 17 | 5.5 ± 2.3 c | 4.5 ± 2.6 b | 3.5 ± 2.3 a | 3.0 ± 2.4 b | 4.0 ± 2.6 b |
| 21 (CP) | 4.3 ± 2.7 b | 3.3 ± 2.4 a | 3.3 ± 2.3 a | 2.0 ± 1.9 a | 3.0 ± 2.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, N.T.F.; Venancio, A.R.; Martos, E.T.; Oliveira, A.C.G.; Oliveira, A.A.A.; Mutz, Y.d.S.; Nunes, C.A.; Mondragón-Bernal, O.L.; Alves, J.G.L.F. Fish Fillet Analogue Using Formulation Based on Mushroom (Pleurotus ostreatus) and Enzymatic Treatment: Texture, Sensory, Aromatic Profile and Physicochemical Characterization. Foods 2024, 13, 2358. https://doi.org/10.3390/foods13152358
Silva NTF, Venancio AR, Martos ET, Oliveira ACG, Oliveira AAA, Mutz YdS, Nunes CA, Mondragón-Bernal OL, Alves JGLF. Fish Fillet Analogue Using Formulation Based on Mushroom (Pleurotus ostreatus) and Enzymatic Treatment: Texture, Sensory, Aromatic Profile and Physicochemical Characterization. Foods. 2024; 13(15):2358. https://doi.org/10.3390/foods13152358
Chicago/Turabian StyleSilva, Nayara Thalita Ferreira, Andreia Reis Venancio, Emerson Tokuda Martos, Ana Clara Gomes Oliveira, Ana Alice Andrade Oliveira, Yhan da Silva Mutz, Cleiton Antonio Nunes, Olga Lucía Mondragón-Bernal, and José Guilherme Lembi Ferreira Alves. 2024. "Fish Fillet Analogue Using Formulation Based on Mushroom (Pleurotus ostreatus) and Enzymatic Treatment: Texture, Sensory, Aromatic Profile and Physicochemical Characterization" Foods 13, no. 15: 2358. https://doi.org/10.3390/foods13152358






