Extraction, Purification, Sulfated Modification, and Biological Activities of Dandelion Root Polysaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Extraction and Purification of DRP
2.2.1. Sulfation of DRPs
2.2.2. Structure Determination of SDRP
FTIR Analysis
High-Performance Liquid Chromatography (HPLC)
Molecular Weight Determination
Congo Red Test
NMR Analysis
Scanning Electron Microscopy Analysis
2.2.3. Biological Activity of SDRP
Antioxidant Activity Assays
- (1)
- 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging activity assay
- (2)
- Superoxide anion radical scavenging activity assay
- (3)
- Hydroxyl radical scavenging ability assay
- (4)
- Metal chelating assay
In Vitro Hypoglycemic Activity
Studies on the Value-Added Effects of Probiotics
3. Results and Discussion
3.1. Extraction of Crude DRP
3.2. Separation and Purification of DRP
3.3. Sulfation of DRP and Optimisation of Conditions
3.3.1. Single-Factor Test
3.3.2. Response Surface Analysis
3.4. Structural Characteristics
3.4.1. FT-IR Analysis and Congo Red Test
3.4.2. Monosaccharide Composition Analysis
3.4.3. Molecular Weight Analysis
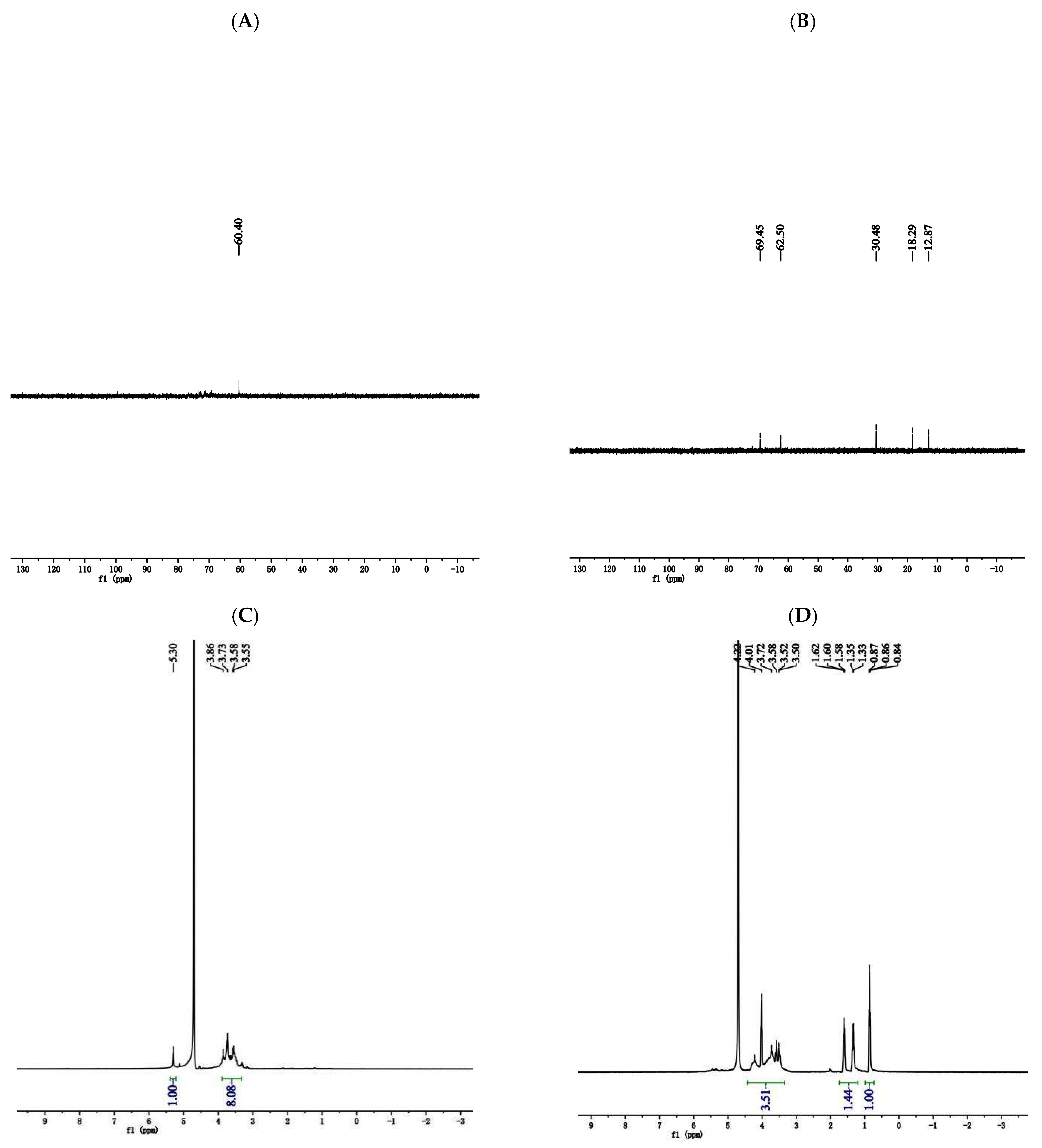
3.4.4. NMR Analysis
3.4.5. Scanning Electron Microscopy Analysis
3.5. Studies on the Biological Activity of SDRP
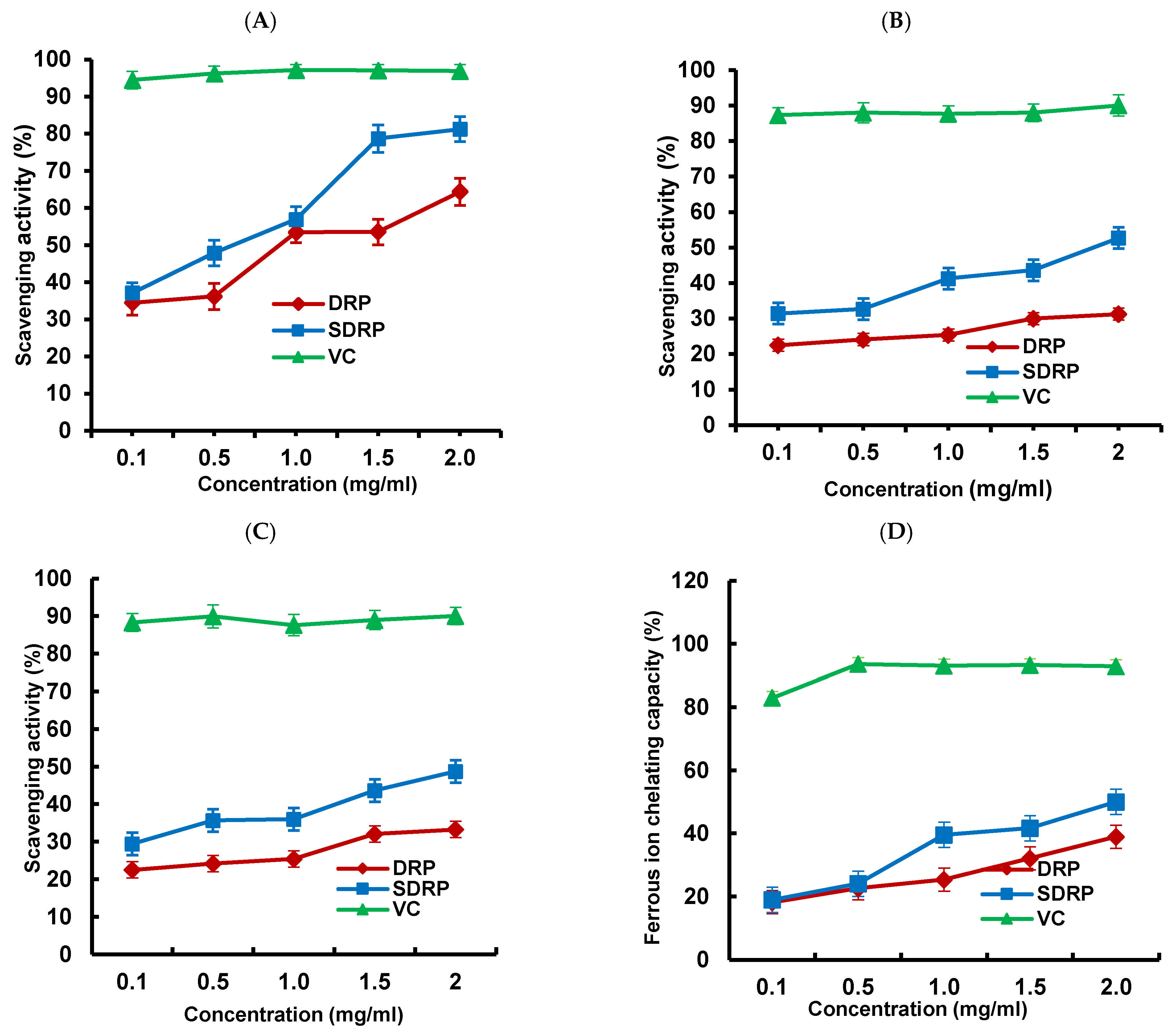
3.5.1. Antioxidant Activity Analysis
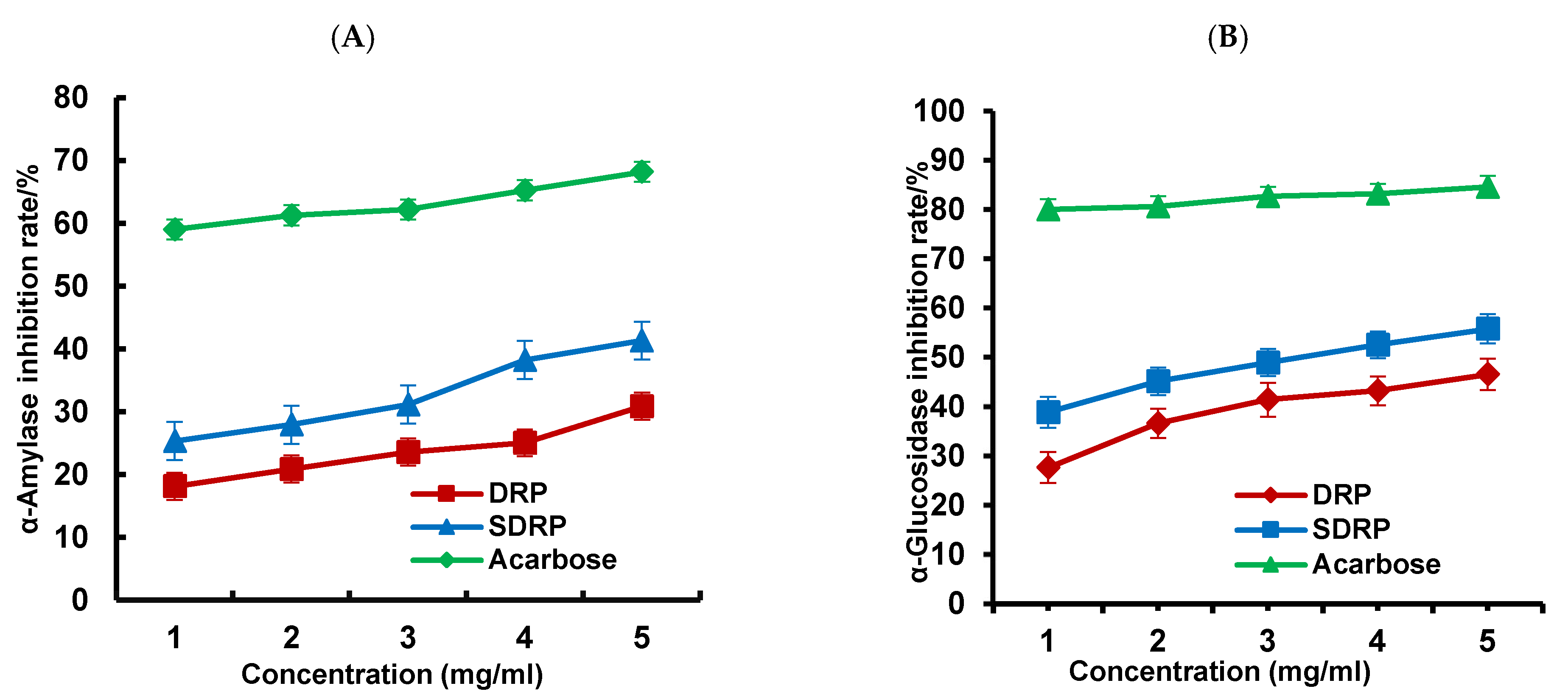
3.5.2. Study of In Vitro Hypoglycemic Activity
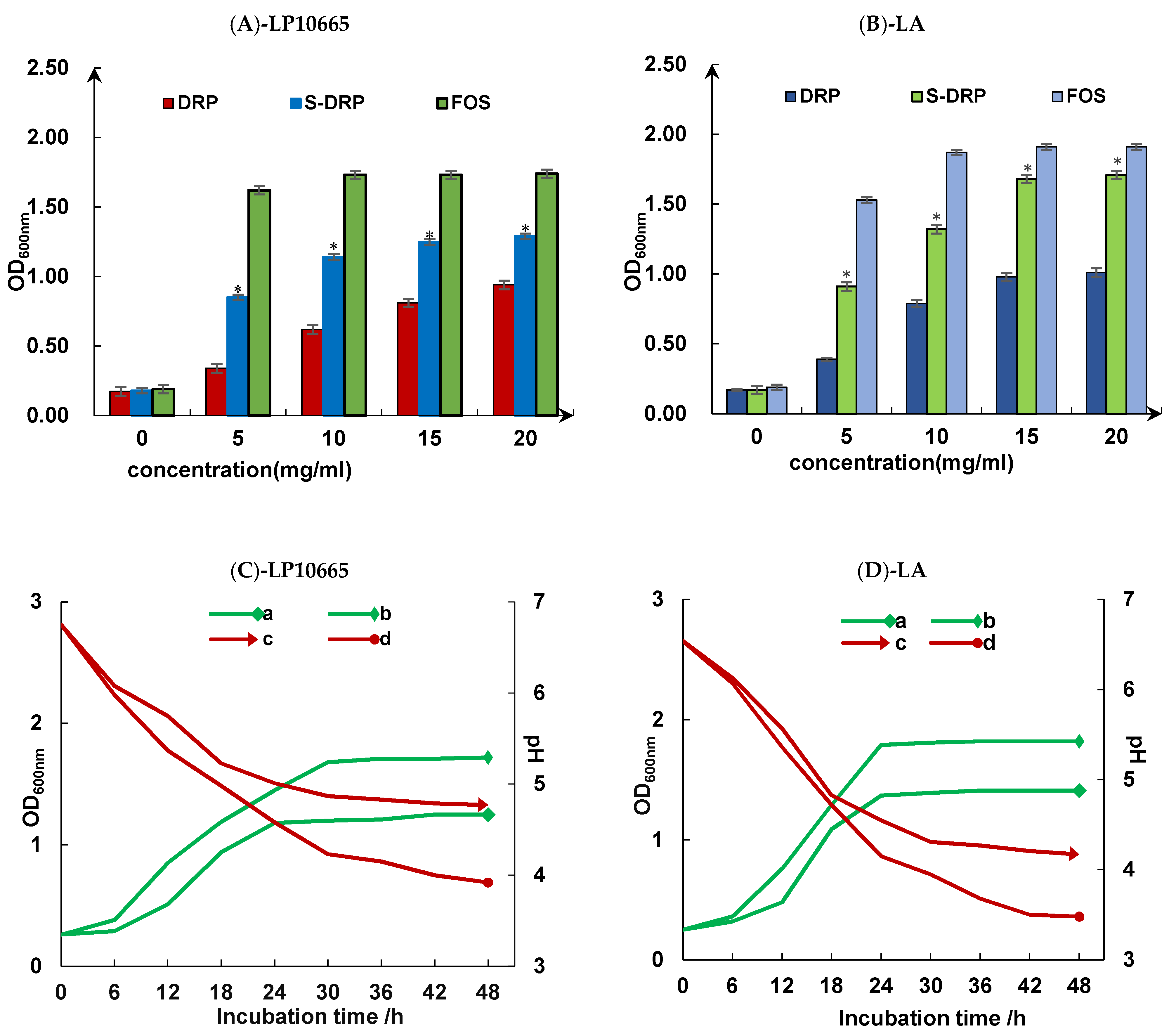
3.5.3. Studies on the Value-Added Effects of Probiotics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, M.; Ma, Y.; Wang, C.; Liu, H.; Li, Q.; Fei, M. Synthesis, Anti-Oxidant Activity, and Biodegradability of a Novel Recombinant Polysaccharide Derived from Chitosan and Lactose. Carbohydra. Polym. 2015, 118, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Bedini, E.; Laezza, A.; Parrilli, M.; Iadonisi, A. A Review of Chemical Methods for the Selective Sulfation and Desulfation of Polysaccharides. Carbohyd. Polym. 2017, 174, 1224–1239. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antioxidant Activities of Phosphorylated Pumpkin Polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-Y.; Ji, Y.-H.; Liao, A.-M.; Huang, J.-H.; Thakur, K.; Li, X.-L.; Hu, F.; Zhang, J.-G.; Wei, Z.-J. Effects of Sulfated, Phosphorylated and Carboxymethylated Modifications on the Antioxidant Activities in-Vitro of Polysaccharides Sequentially Extracted from Amana Edulis. Int. J. Bio. Macromol. 2020, 146, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Shen, M.; Wang, Z.; Xie, J. Structure, Function and Food Applications of Carboxymethylated Polysaccharides: A Comprehensive Review. Trends Food Sci. Technol. 2021, 118, 539–557. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, L.; Zhao, J.-L.; Xu, W.; Guo, Z.; Zhang, A.-Z.; Li, M.-Y. Dietary Taraxacum Mongolicum Polysaccharide Ameliorates the Growth, Immune Response, and Antioxidant Status in Association with NF-κB, Nrf2 and TOR in Jian Carp (Cyprinus Carpio Var. Jian). Aquaculture 2022, 547, 737522. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated Polysaccharides: Immunomodulation and Signaling Mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Wang, J.; Guo, H.; Zhang, J.; Wang, X.; Zhao, B.; Yao, J.; Wang, Y. Sulfated Modification, Characterization and Structure–Antioxidant Relationships of Artemisia Sphaerocephala Polysaccharides. Carbohyd. Polym. 2010, 81, 897–905. [Google Scholar] [CrossRef]
- Chen, P.; Ding, S.; Yan, Z.; Liu, H.; Tu, J.; Chen, Y.; Zhang, X. Structural Characteristic and In-Vitro Anticancer Activities of Dandelion Leaf Polysaccharides from Pressurized Hot Water Extraction. Nutrients 2023, 15, 80. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Chen, Z. Sulfated Modification of the Polysaccharides Obtained from Defatted Rice Bran and Their Antitumor Activities. Int. J. Biol. Macromol. 2009, 44, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Li, J.; Yuan, X.; Wang, Y.; Wu, K.; Kang, L.; Luo, Y.; Zhang, H.; Yuan, Z. Dandelion Polysaccharides Exert Anticancer Effect on Hepatocellular Carcinoma by Inhibiting PI3K/AKT/mTOR Pathway and Enhancing Immune Response. J. Funct. Foods 2019, 55, 263–274. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Li, C.; Liu, L.; Surendhiran, D.; Cui, H. Antibacterial Activity of PEO Nanofibers Incorporating Polysaccharide from Dandelion and Its Derivative. Carbohyd. Polym. 2018, 198, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-H.; Shen, M.-Y.; Nie, S.-P.; Li, C.; Xie, M.-Y. Decolorization of Polysaccharides Solution from Cyclocarya Paliurus (Batal.) Iljinskaja Using Ultrasound/H2O2 Process. Carbohyd. Polym. 2011, 84, 255–261. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Shang, H.; Guo, Y.; Chen, S. Extraction, Purification, Hypoglycemic and Antioxidant Activities of Red Clover (Trifolium Pratense L.) Polysaccharides. Int. J. Bio. Macromol. 2020, 148, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, M.; Gao, L.; Bao, Y.; Li, P.; Wang, M.; Shao, T.; Wang, G.; Liu, C. Optimal extraction of polysaccharides from Stevia rebaudiana roots for protection against hydrogen peroxide-induced oxidative damage in RAW264.7 cells. Nat. Prod. Res. 2023, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Meng, F.-Y.; He, Z.; Ning, Y.-L.; Li, X.-H.; Song, H.; Wang, J.; Zhou, R. Sulfated Modification of Longan Polysaccharide and Its Immunomodulatory and Antitumor Activity in Vitro. Int. J. Bio. Macromol. 2014, 67, 323–329. [Google Scholar] [CrossRef]
- Zhu, H.; Tian, L.; Zhang, L.; Bi, J.; Song, Q.; Yang, H.; Qiao, J. Preparation, Characterization and Antioxidant Activity of Polysaccharide from Spent Lentinus Edodes Substrate. Int. J. Bio. Macromol. 2018, 112, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shen, M.; Chen, Y.; Lou, Y.; Luo, R.; Chen, J.; Zhang, Y.; Li, J.; Wang, W. Optimization of the Polysaccharide Hydrolysate from Auricularia Auricula with Antioxidant Activity by Response Surface Methodology. Int. J. Bio. Macromol. 2018, 113, 543–549. [Google Scholar] [CrossRef]
- Li, Q.; Wang, W.; Zhu, Y.; Chen, Y.; Zhang, W.; Yu, P.; Mao, G.; Zhao, T.; Feng, W.; Yang, L.; et al. Structural Elucidation and Antioxidant Activity a Novel Se-Polysaccharide from Se-Enriched Grifola Frondosa. Carbohyd. Polym. 2017, 161, 42–52. [Google Scholar] [CrossRef]
- Zheng, Y.; Yan, J.; Cao, C.; Liu, Y.; Yu, D.; Liang, X. Application of Chromatography in Purification and Structural Analysis of Natural Polysaccharides: A Review. J. Sep. Sci. 2023, 46, e2300368. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, M. Purification and Structural Characterization of Polysaccharides Isolated from Auricularia Cornea Var. Li. Carbohyd. Polym. 2020, 230, 115680. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, Y.; Tian, Y.; Xu, Q.; Liu, S.; Li, H.; Cheng, K.; Yuan, J.; Liu, H.; Zhu, P. Astragalus polysaccharide alleviates alcoholic-induced hepatic fibrosis by inhibiting polymerase I and transcript release factor and the TLR4/JNK/NF-κB/MyD88 pathway. J. Ethnopharmacol. 2023, 314, 116662. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Tan, H.; Zhao, T.; Cao, J.; Wang, F. Sulfation of a Polysaccharide Obtained from Phellinus Ribis and Potential Biological Activities of the Sulfated Derivatives. Carbohyd. Polym. 2009, 77, 370–375. [Google Scholar] [CrossRef]
- Xie, J.-H.; Wang, Z.-J.; Shen, M.-Y.; Nie, S.-P.; Gong, B.; Li, H.-S.; Zhao, Q.; Li, W.-J.; Xie, M.-Y. Sulfated Modification, Characterization and Antioxidant Activities of Polysaccharide from Cyclocarya Paliurus. Food Hydrocoll. 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Duan, S.; Zhao, M.; Wu, B.; Wang, S.; Yang, Y.; Xu, Y.; Wang, L. Preparation, Characteristics, and Antioxidant Activities of Carboxymethylated Polysaccharides from Blackcurrant Fruits. Int. J. Bio. Macromol. 2020, 155, 1114–1122. [Google Scholar] [CrossRef]
- Li, B.; Zhang, N.; Wang, D.X.; Jiao, L.; Tan, Y.; Wang, J.; Li, H.; Wu, W.; Jiang, D.C. Structural Analysis and Antioxidant Activities of Neutral Polysaccharide Isolated from Epimedium Koreanum Nakai. Carbohyd. Polym. 2018, 196, 246–253. [Google Scholar] [CrossRef]
- Yao, H.-Y.-Y.; Wang, J.-Q.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. A Review of NMR Analysis in Polysaccharide Structure and Conformation: Progress, Challenge and Perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef]
- Kan, Y.; Chen, T.; Wu, Y.; Wu, J. Antioxidant Activity of Polysaccharide Extracted from Ganoderma Lucidum Using Response Surface Methodology. Int. J. Bio. Macromol. 2015, 72, 151–157. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Liu, Y. Characterization and Antioxidant Activities of Polysaccharides from Thirteen Boletus Mushrooms. Int. J. Bio. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; He, X.; Wang, S.; Cao, S.; Xia, Z.; Xian, H.; Qin, L.; Mao, W. Structure and Anticoagulant Property of a Sulfated Polysaccharide Isolated from the Green Seaweed Monostroma Angicava. Carbohyd. Polym. 2017, 159, 195–206. [Google Scholar] [CrossRef]
- Peng, L.; Li, H.; Meng, Y. Layer-by-Layer Structured Polysaccharides-Based Multilayers on Cellulose Acetate Membrane: Towards Better Hemocompatibility, Antibacterial and Antioxidant Activities. Appl. Surf. Sci. 2017, 401, 25–39. [Google Scholar] [CrossRef]
- Liu, D.; Sun, Q.; Xu, J.; Li, N.; Lin, J.; Chen, S.; Li, F. Purification, Characterization, and Bioactivities of a Polysaccharide from Mycelial Fermentation of Bjerkandera Fumosa. Carbohyd. Polym. 2017, 167, 115–122. [Google Scholar] [CrossRef]
- Zhai, K.; Wang, W.; Zheng, M.; Khan, G.; Wang, Q.; Chang, J.; Dong, Z.; Zhang, X.; Duan, H.; Gong, Z.; et al. Protective effects of Isodon Suzhouensis extract and glaucocalyxin A on chronic obstructive pulmonary disease through SOCS3–JAKs/STATs pathway. Food Front. 2023, 4, 511–523. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X. Hypoglycemic Activity in Vitro of Polysaccharides from Camellia Oleifera Abel. Seed Cake. Int. J. Bio. Macromol. 2018, 115, 811–819. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.W.; Ranasinghe, P.; Peiris, L.D.C. In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva. Molecules 2019, 24, 3708. [Google Scholar] [CrossRef]
- Yin, X.; You, Q.; Jiang, Z. Optimization of Enzyme Assisted Extraction of Polysaccharides from Tricholoma Matsutake by Response Surface Methodology. Carbohyd. Polym. 2011, 86, 1358–1364. [Google Scholar] [CrossRef]
- Campbell, C.; Nanjundaswamy, A.K.; Njiti, V.; Xia, Q.; Chukwuma, F. Value-Added Probiotic Development by High-Solid Fermentation of Sweet Potato with Saccharomyces Boulardii. Food Sci. Nutr. 2017, 5, 633–638. [Google Scholar] [CrossRef]
- Fathy, H.M.; Abd El-Maksoud, A.A.; Cheng, W.; Elshaghabee, F.M.F. Value-Added Utilization of Citrus Peels in Improving Functional Properties and Probiotic Viability of Acidophilus-Bifidus-Thermophilus (ABT)-Type Synbiotic Yoghurt during Cold Storage. Foods 2022, 11, 2677. [Google Scholar] [CrossRef] [PubMed]
- Bisson, G.; Marino, M.; Poletti, D.; Innocente, N.; Maifreni, M. Turbidimetric Definition of Growth Limits in Probiotic Lactobacillus Strains from the Perspective of an Adaptation Strategy. J. Dairy Sci. 2021, 104, 12236–12248. [Google Scholar] [CrossRef] [PubMed]
- Darabdhara, G.; Boruah, P.K.; Das, M.R. Colorimetric Determination of Glucose in Solution and via the Use of a Paper Strip by Exploiting the Peroxidase and Oxidase Mimicking Activity of Bimetallic Cu-Pd Nanoparticles Deposited on Reduced Graphene Oxide, Graphitic Carbon Nitride, or MoS2 Nanosheets. Microchim. Acta 2018, 186, 13. [Google Scholar] [CrossRef]
- Caputo, H.E.; Straub, J.E.; Grinstaff, M.W. Design, Synthesis, and Biomedical Applications of Synthetic Sulphated Polysaccharides. Chem. Soc. Rev. 2019, 48, 2338–2365. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.-N.; Nguyen, T.-V.-A.; Mai, D.-T.; Tran, N.-M.-A.; Nguyen, N.-H.; Vo, G.V.; Duong, T.-H.; Truong Nguyen, H. Alpha-Glucosidase Inhibitors from Nervilia Concolor, Tecoma Stans, and Bouea Macrophylla. Saudi J. Biol. Sci. 2022, 29, 1029–1042. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, L.; Xu, C.; Jiang, H.; Zhu, C.; Sun, L.; Sun, C.; Li, X. α-Glucosidase Inhibitors from Chinese Bayberry (Morella Rubra Sieb. et Zucc.) Fruit: Molecular Docking and Interaction Mechanism of Flavonols with Different B-Ring Hydroxylations. RSC Adv. 2020, 10, 29347–29361. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Sreeja, P.S.; Yang, X. The Antioxidant Properties of Mushroom Polysaccharides Can Potentially Mitigate Oxidative Stress, Beta-Cell Dysfunction and Insulin Resistance. Front. Pharmacol. 2022, 13, 874474. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, Y. Ultrasound-Assisted Polysaccharide Extraction from Cercis Chinensis and Properites, Antioxidant Activity of Polysaccharide. Ultrason. Sonochem. 2023, 96, 106422. [Google Scholar] [CrossRef]
- Di Stefano, E.; Oliviero, T.; Udenigwe, C.C. Functional Significance and Structure–Activity Relationship of Food-Derived α-Glucosidase Inhibitors. Curr. Opin. Food Sci. 2018, 20, 7–12. [Google Scholar] [CrossRef]
- Li, Z.-W.; Du, Z.-M.; Wang, Y.-W.; Feng, Y.-X.; Zhang, R.; Yan, X.-B. Chemical Modification, Characterization, and Activity Changes of Land Plant Polysaccharides: A Review. Polymers 2022, 14, 4161. [Google Scholar] [CrossRef]
- Thilagam, E.; Parimaladevi, B.; Kumarappan, C.; Chandra Mandal, S. α-Glucosidase and α-Amylase Inhibitory Activity of Senna Surattensis. J. Acupunct. Meridian 2013, 6, 24–30. [Google Scholar] [CrossRef]

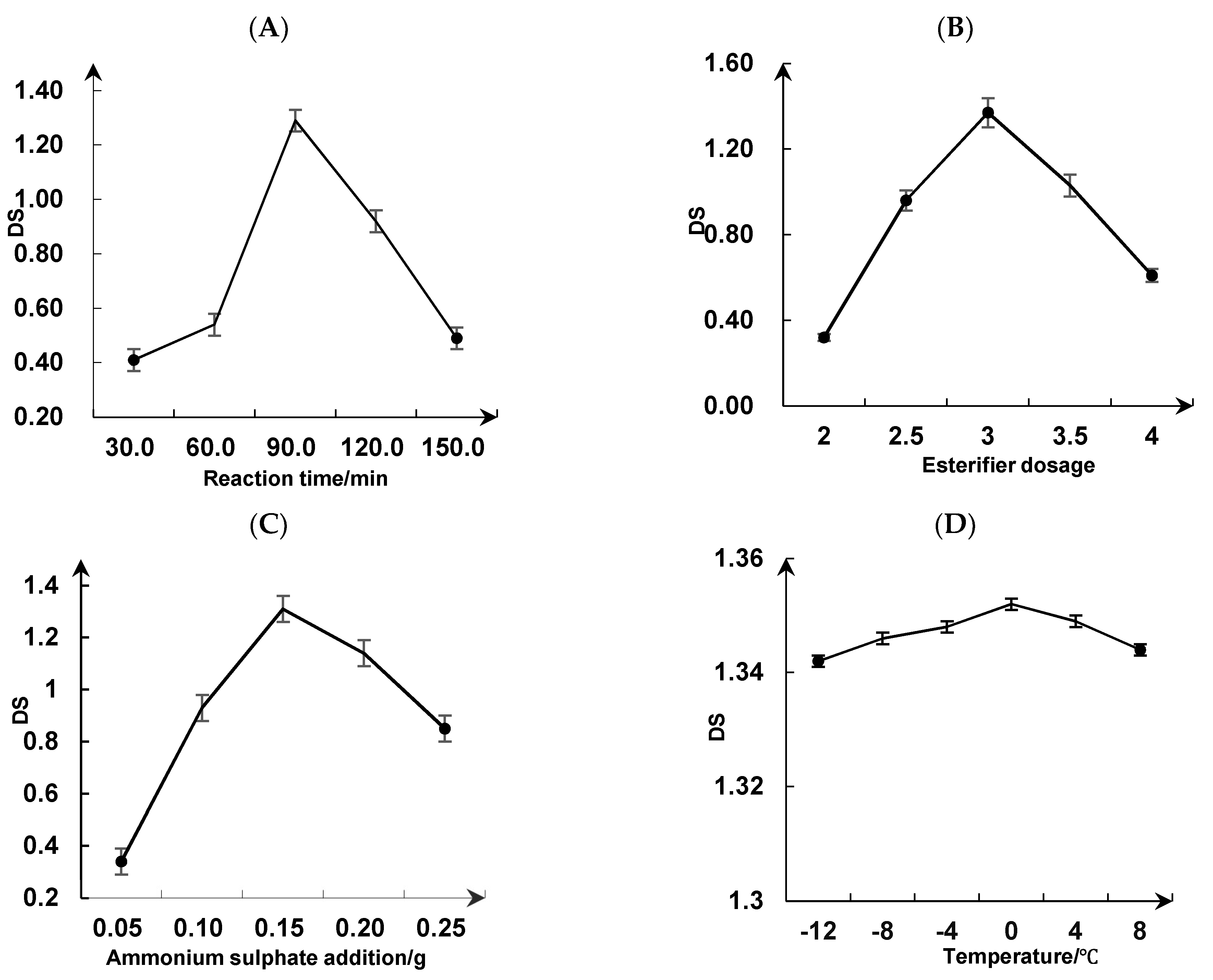
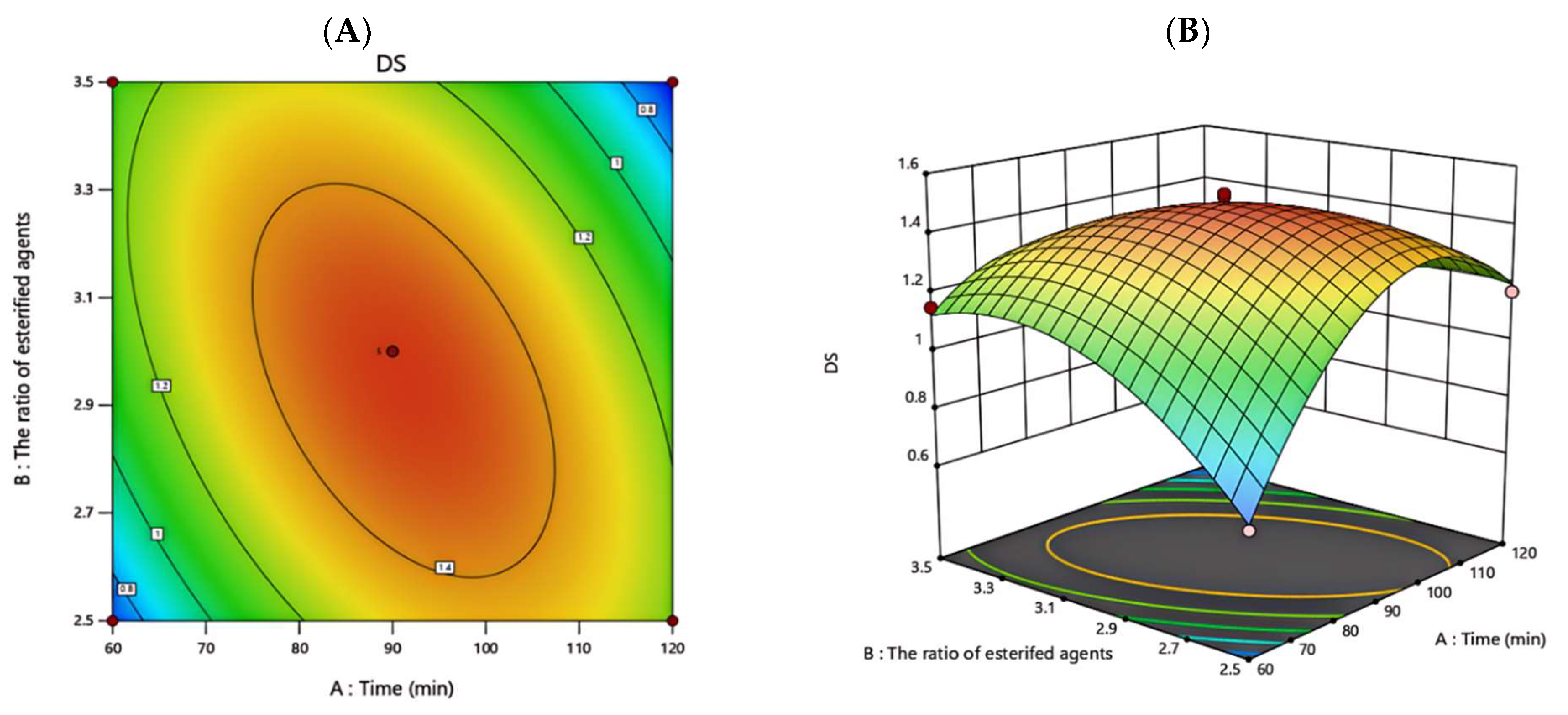
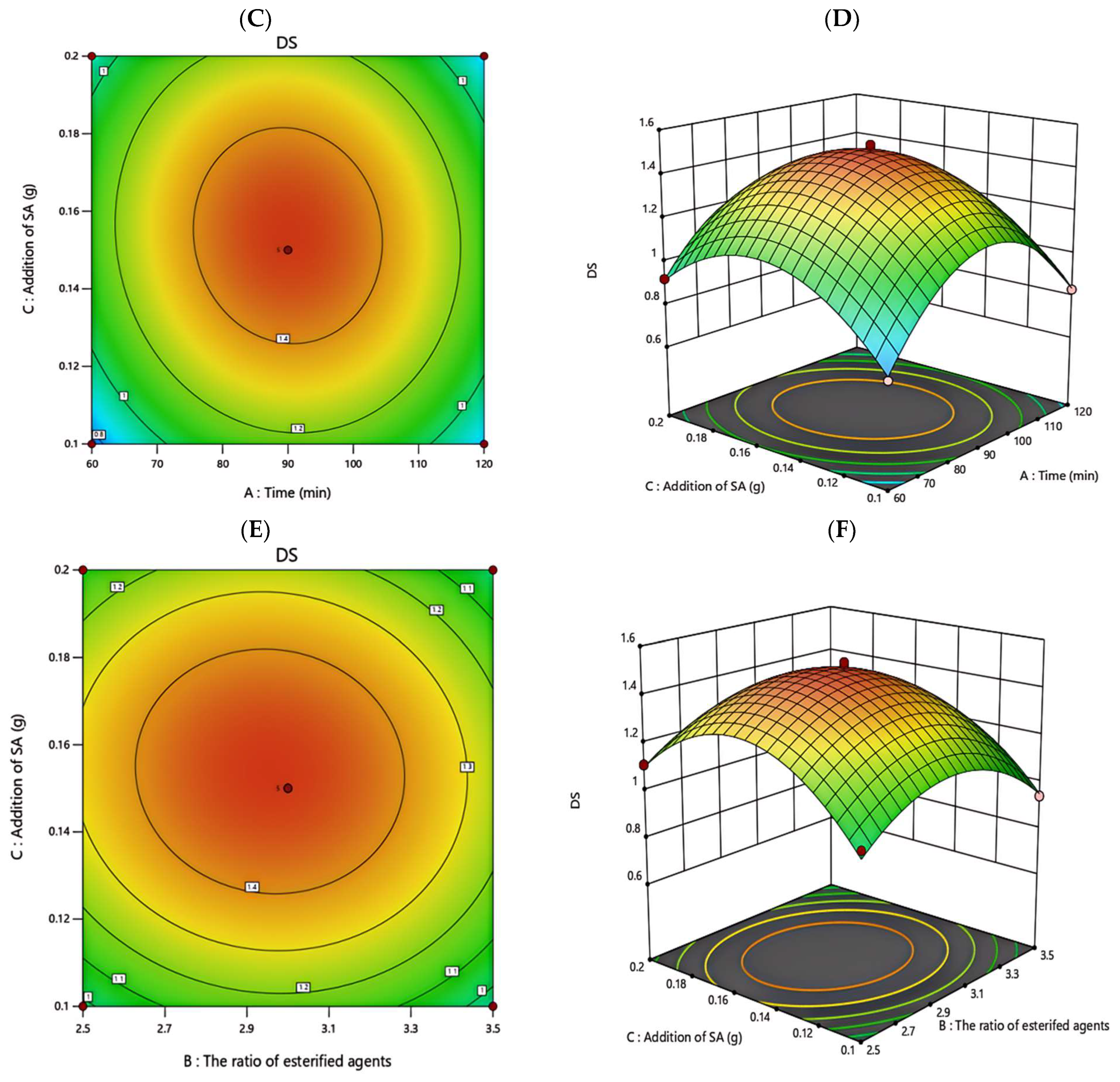
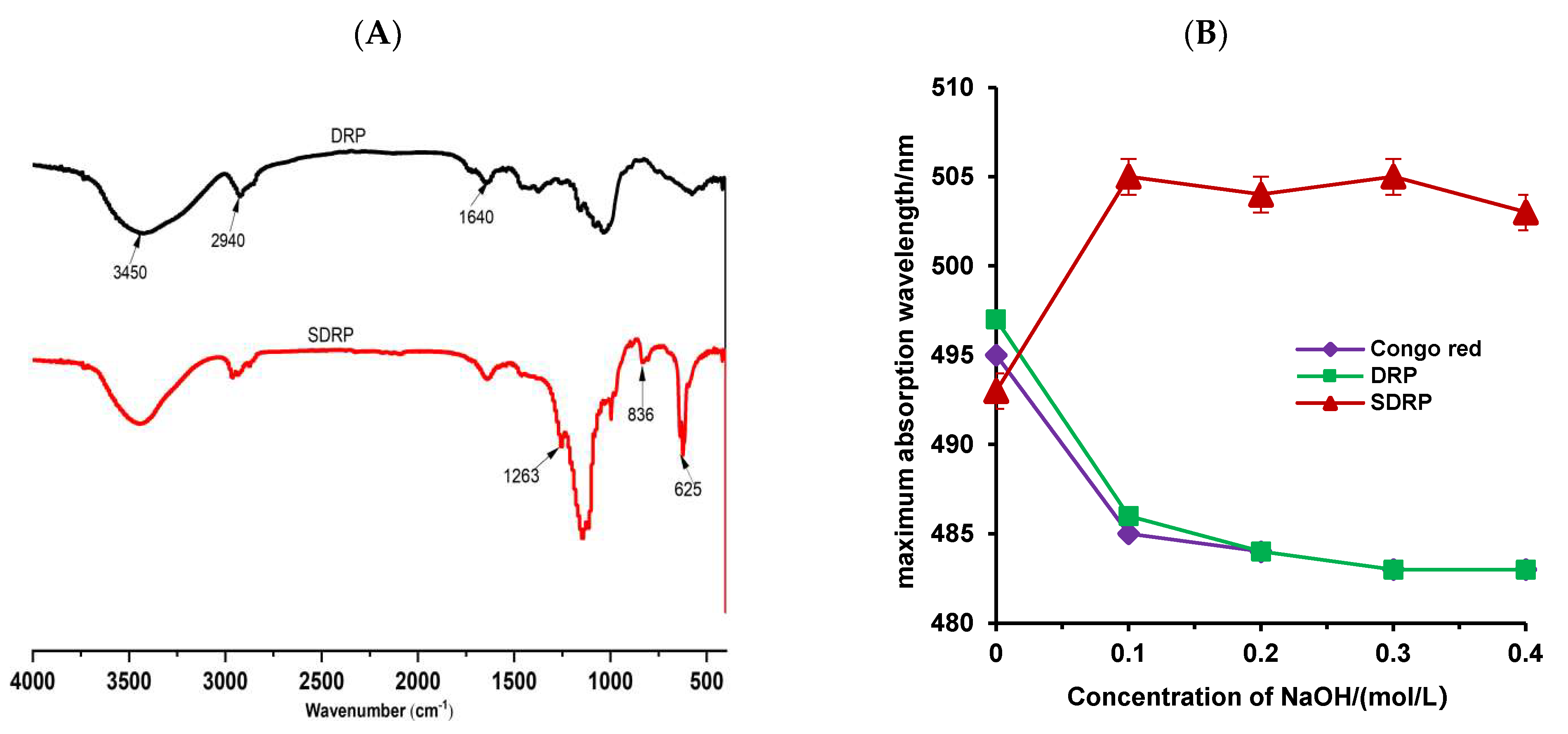



| Factor | Encoding | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Reaction time (min) | A | 60 | 90 | 120 |
| Ratio of esterifier dosage | B | 2.5 | 3 | 3.5 |
| Sodium sulfate addition (g) | C | 1.0 | 1.5 | 2.0 |
| Number | A (min) | B | C (g) | DS |
|---|---|---|---|---|
| 1 | 60 | 2.5 | 0.15 | 0.68 |
| 2 | 120 | 2.5 | 0.15 | 1.17 |
| 3 | 60 | 3.5 | 0.15 | 1.15 |
| 4 | 120 | 3.5 | 0.15 | 0.65 |
| 5 | 60 | 3 | 0.1 | 0.74 |
| 6 | 120 | 3 | 0.1 | 0.83 |
| 7 | 60 | 3 | 0.2 | 0.92 |
| 8 | 120 | 3 | 0.2 | 0.86 |
| 9 | 90 | 2.5 | 0.1 | 1.01 |
| 10 | 90 | 3.5 | 0.1 | 0.94 |
| 11 | 90 | 2.5 | 0.2 | 1.11 |
| 12 | 90 | 3.5 | 0.2 | 0.96 |
| 13 | 90 | 3 | 0.15 | 1.46 |
| 14 | 90 | 3 | 0.15 | 1.51 |
| 15 | 90 | 3 | 0.15 | 1.45 |
| 16 | 90 | 3 | 0.15 | 1.48 |
| 17 | 90 | 3 | 0.15 | 1.52 |
| Origin of Variance | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 1.46 | 9 | 0.1623 | 132.95 | <0.0001 | ** |
| A | 0.0000 | 1 | 0.0000 | 0.0410 | 0.8454 | |
| B | 0.0091 | 1 | 0.0091 | 7.46 | 0.0292 | * |
| C | 0.0136 | 1 | 0.0136 | 11.15 | 0.0124 | * |
| AB | 0.2450 | 1 | 0.2450 | 200.72 | <0.0001 | ** |
| AC | 0.0056 | 1 | 5.6 × 10−3 | 4.61 | 0.0690 | |
| BC | 0.0016 | 1 | 1.6 × 10−3 | 1.31 | 0.2899 | |
| A2 | 0.5749 | 1 | 0.5749 | 470.92 | <0.0001 | ** |
| B2 | 0.1718 | 1 | 0.1718 | 140.74 | <0.0001 | ** |
| C2 | 0.3231 | 1 | 0.3231 | 264.66 | <0.0001 | ** |
| Residual | 0.0085 | 7 | 1.2 × 10−3 | |||
| Lack of fit | 0.0048 | 3 | 1.6 × 10−3 | 1.73 | 0.2986 | |
| Pure error | 0.0037 | 4 | 9 × 10−4 | |||
| Cor. total | 1.47 | 16 |
| Sample | Peak Number | Retention Time/min | Relative Molecular Mass | Relative Peak Area (%) |
|---|---|---|---|---|
| DRP | 1 | 18.967 | 40,644 | 23.57 |
| 2 | 20.299 | 965 | 76.43 | |
| SDRP | 1 | 18.950 | 13,673 | 14.46 |
| 2 | 20.729 | 446 | 28.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Li, N.; Dong, Z.; Yin, Q.; Zhou, T.; Zhu, L.; Yan, H.; Chen, Z.; Zhai, K. Extraction, Purification, Sulfated Modification, and Biological Activities of Dandelion Root Polysaccharides. Foods 2024, 13, 2393. https://doi.org/10.3390/foods13152393
Wu X, Li N, Dong Z, Yin Q, Zhou T, Zhu L, Yan H, Chen Z, Zhai K. Extraction, Purification, Sulfated Modification, and Biological Activities of Dandelion Root Polysaccharides. Foods. 2024; 13(15):2393. https://doi.org/10.3390/foods13152393
Chicago/Turabian StyleWu, Xiao, Na Li, Zeng Dong, Qin Yin, Tong Zhou, Lixiang Zhu, Hanxi Yan, Ziping Chen, and Kefeng Zhai. 2024. "Extraction, Purification, Sulfated Modification, and Biological Activities of Dandelion Root Polysaccharides" Foods 13, no. 15: 2393. https://doi.org/10.3390/foods13152393







