Effects of Different Extraction Methods on the Structural and Functional Properties of Soluble Dietary Fibre from Sweet Potatoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SDF Extraction
2.2.1. Preparation of WT-SDF
2.2.2. Preparation of UT-SDF, UST-SDF, UET-SDF, and UMT-SDF
2.3. Structural Characterization
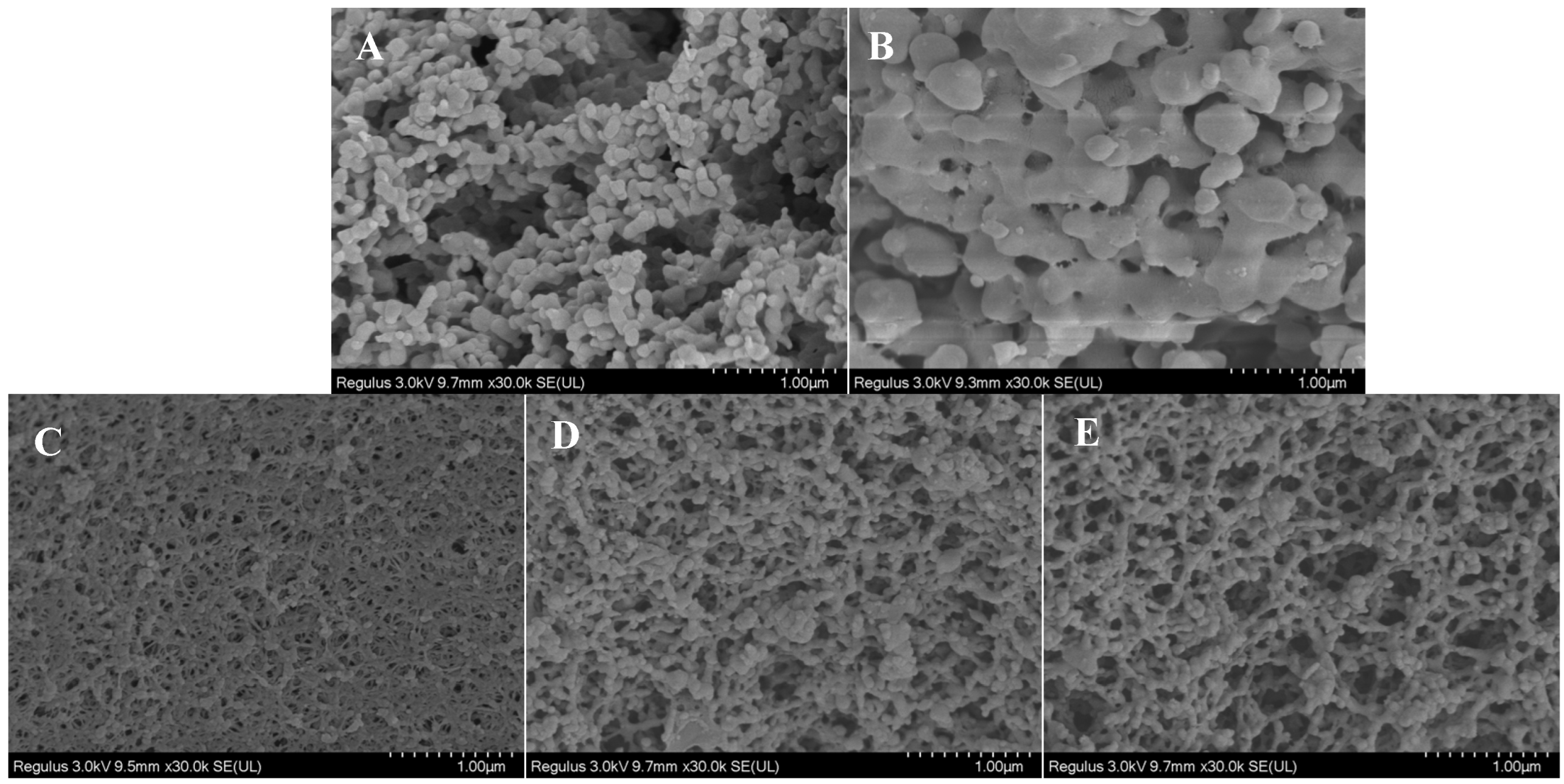
2.3.1. Scanning Electron Microscopy (SEM)
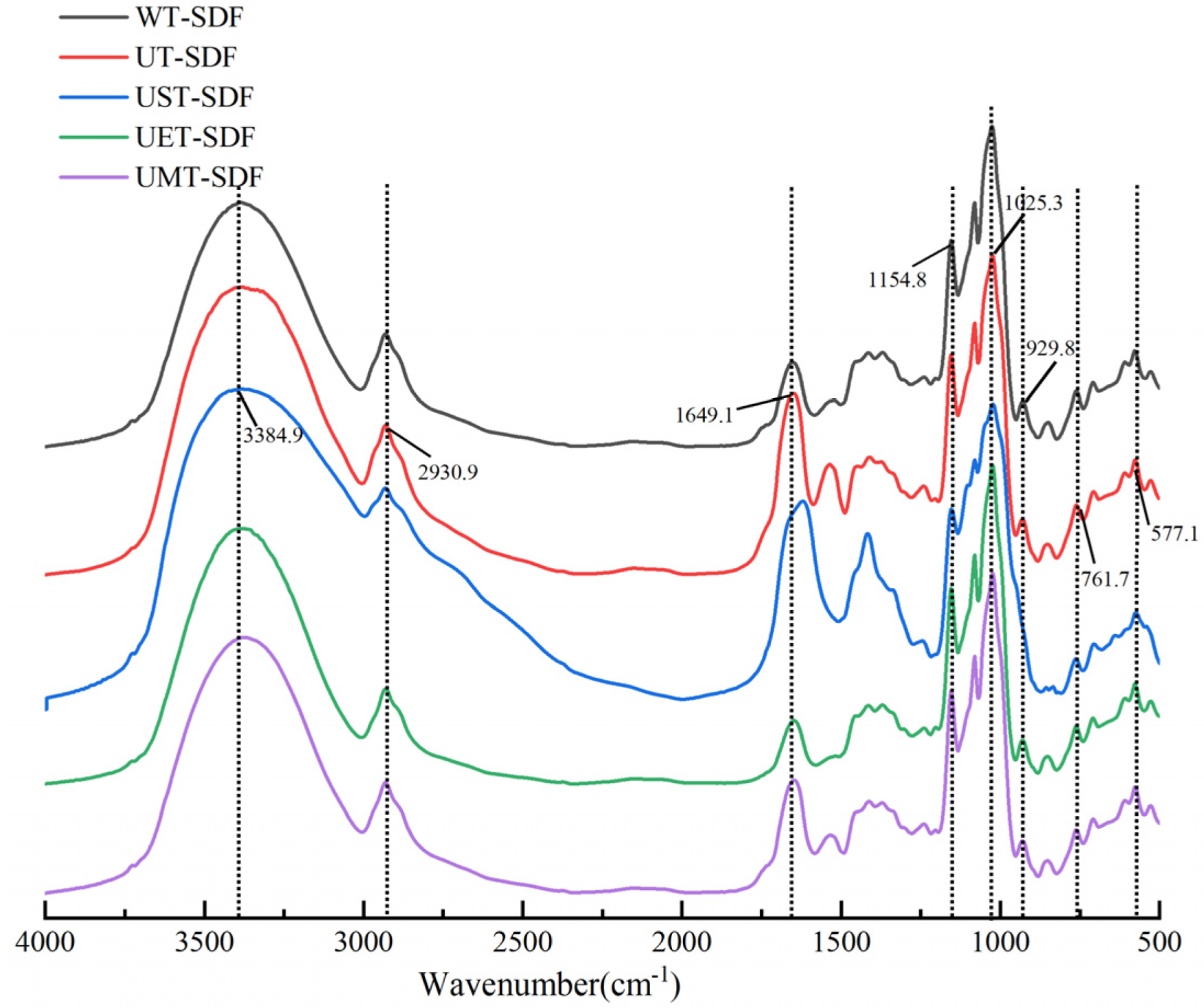
2.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
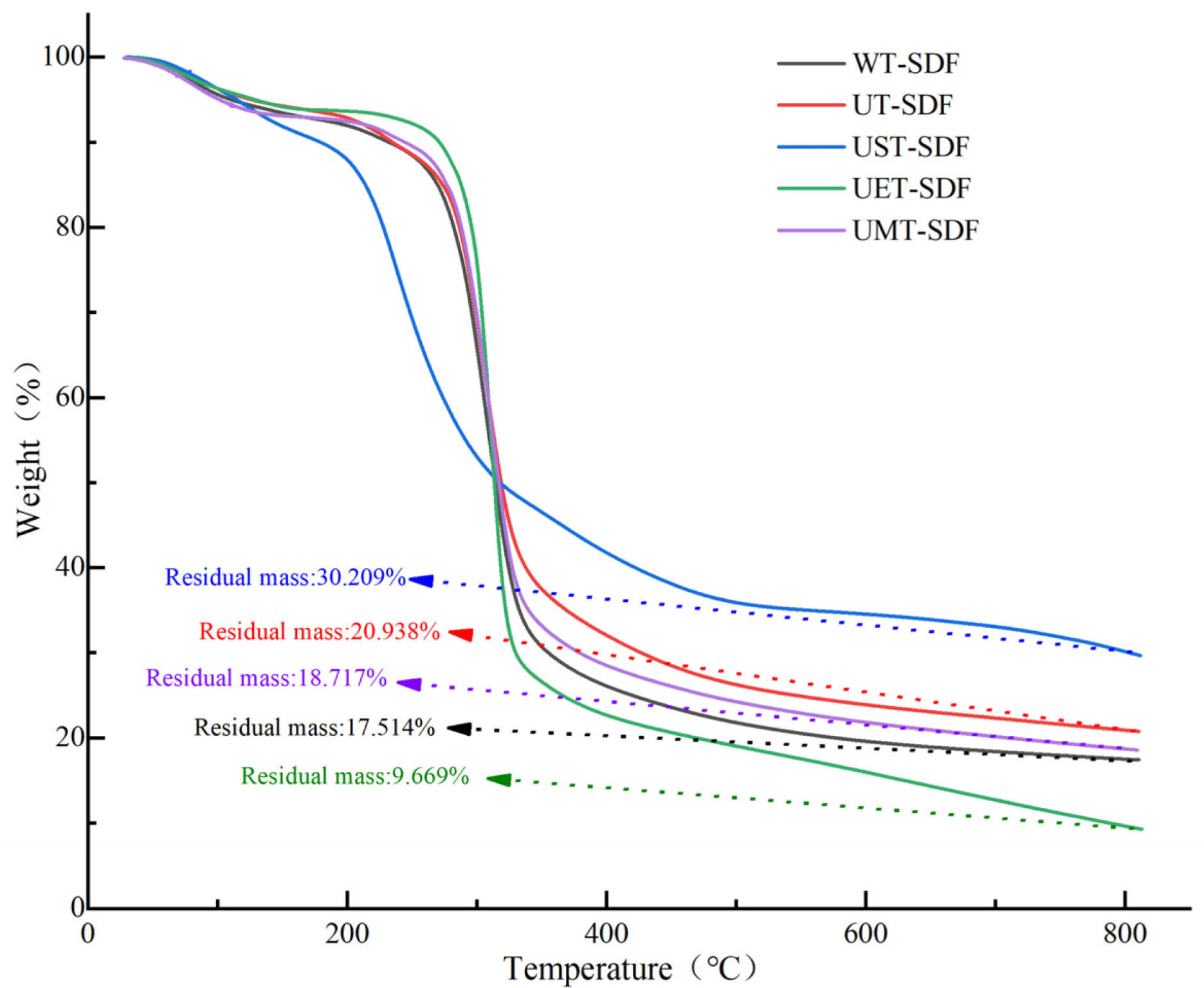
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. X-ray Diffraction (XRD)
2.3.5. Brunauer–Emmett–Teller (BET) Surface Area
2.4. Physicochemical Properties
2.4.1. Colour Analysis
2.4.2. Water-Holding Capacity (WHC)
2.4.3. Oil-Holding Capacity (OHC)
2.5. Functional Properties
2.5.1. Glucose Adsorption Capacity (GAC)
2.5.2. Glucose Dialysis Retardation Index (GDRI)
2.5.3. Sodium Cholate Adsorption Capacity (SCAC)
2.5.4. Cholesterol Adsorption Capacity (CAC)
2.5.5. Nitrite Ion Adsorption Capacity (NIAC)
2.5.6. Antioxidant Properties
2.6. Statistical Analysis
3. Results and Discussion
3.1. Structural Properties
3.1.1. SEM Observation
3.1.2. FT-IR Analysis
3.1.3. TGA
3.1.4. XRD Analysis
3.1.5. BET Surface Area and Pore Size
3.2. Physicochemical Properties
3.2.1. Extraction Yield and Colours of SDFs
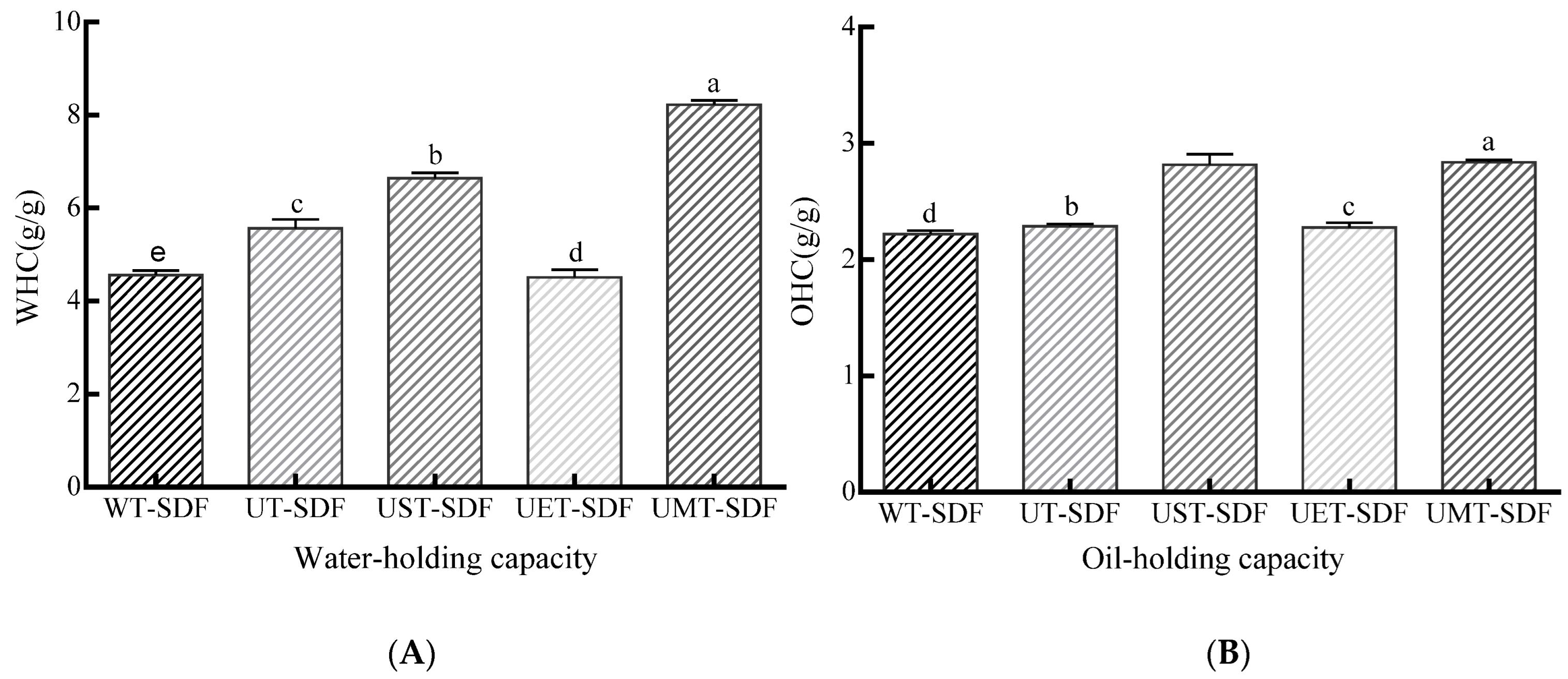
3.2.2. WHC
3.2.3. OHC
3.3. Functional Properties
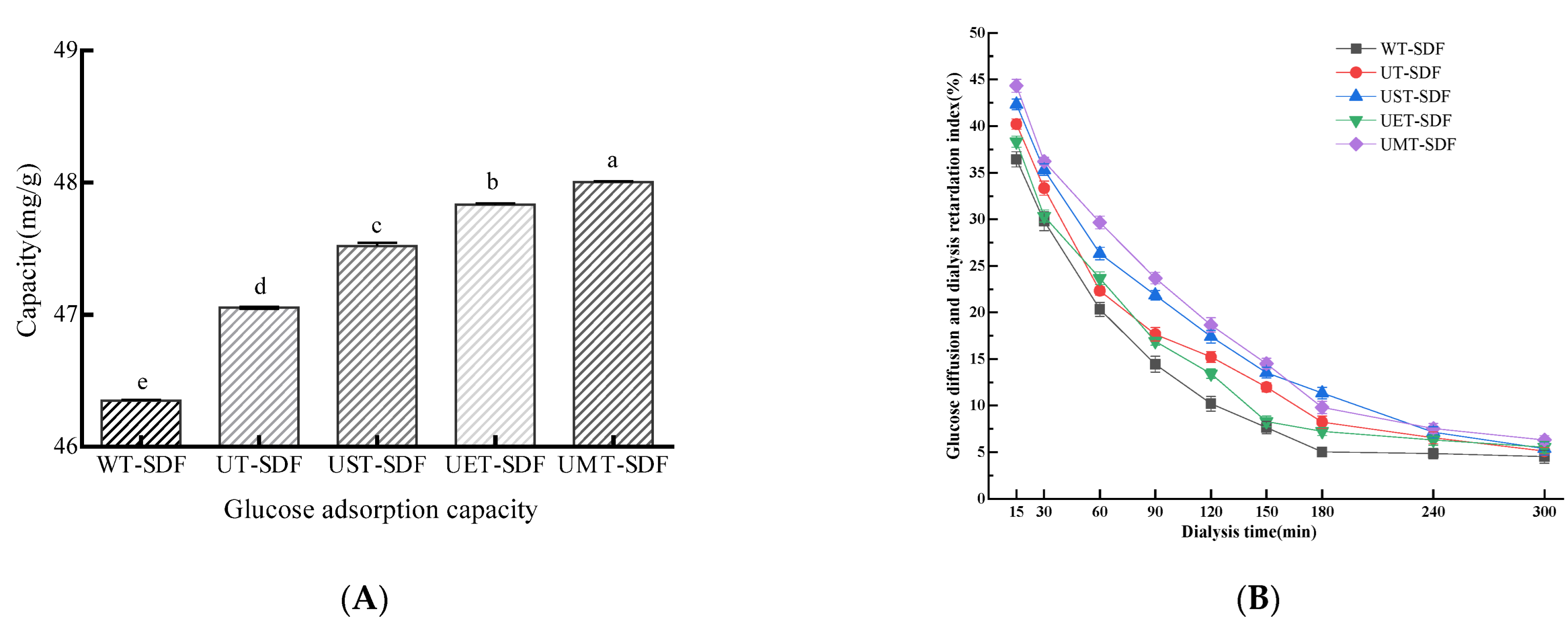
3.3.1. GAC
3.3.2. GDRI
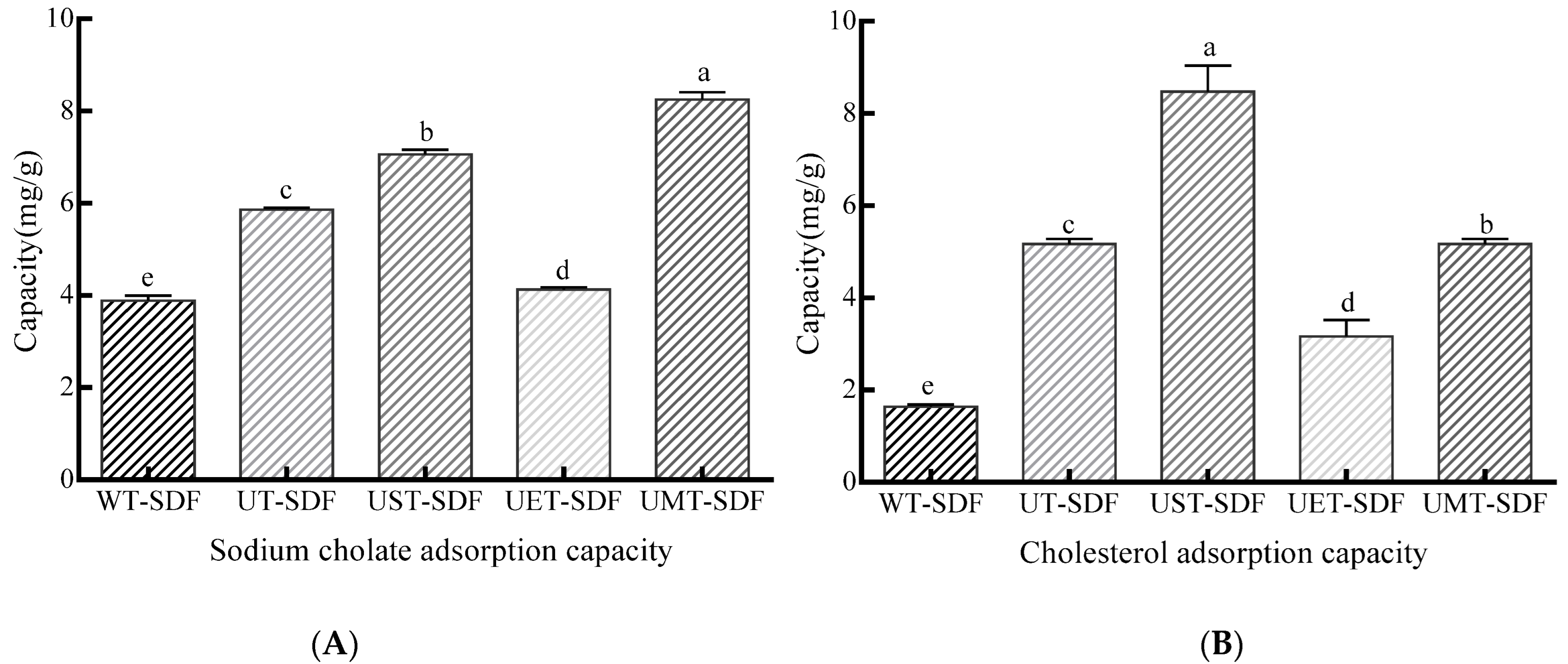
3.3.3. SCAC
3.3.4. CAC
3.3.5. NIAC
3.3.6. Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussein, A.E.; Emmanuel, O.; Chinyere, A.; Majekodunmi, R.O. Utilization of sweet potato starches and flours as composites with wheat flours in the preparation of confectioneries. Afr. J. Biotechnol. 2015, 14, 17–22. [Google Scholar] [CrossRef]
- Vannini, M.; Marchese, P.; Sisti, L.; Saccani, A.; Mu, T.; Sun, H.N.; Celli, A. Integrated Efforts for the Valorization of Sweet Potato By-Products within a Circular Economy Concept: Biocomposites for Packaging Applications Close the Loop. Polymers 2021, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jia, M.Y.; Chen, J.J.; Wang, H.S.; Dong, R.H.; Nie, S.P.; Xie, M.Y.; Yu, Q. Removal of bound polyphenols and its effect on antioxidant and prebiotics properties of carrot dietary fiber. Food Hydrocoll. 2019, 93, 284–292. [Google Scholar] [CrossRef]
- Jia, M.Y.; Chen, J.J.; Liu, X.Z.; Xie, M.Y.; Nie, S.P.; Chen, Y.; Xie, J.H.; Yu, Q. Structural characteristics and functional properties of soluble dietary fiber from defatted rice bran obtained through Trichoderma viride fermentation. Food Hydrocoll. 2019, 94, 468–474. [Google Scholar] [CrossRef]
- Huang, S.Y.; He, Y.W.; Zou, Y.P.; Liu, Z. Modification of insoluble dietary fibres in soya bean okara and their physicochemical properties. Int. J. Food Sci. Technol. 2016, 50, 2606–2613. [Google Scholar] [CrossRef]
- Madenci, A.B.; Bilgiçli, N.; Türker, S. Effects of dietary fibre and antioxidant-rich ingredients on some quality characteristics of fresh and dry pasta. Qual. Assur. Saf. Crops 2018, 10, 315–324. [Google Scholar] [CrossRef]
- Moczkowska, M.; Karp, S.; Niu, Y.; Niu, Y.; Kurek, M.A. Enzymatic, enzymatic-ultrasonic and alkaline extraction of soluble dietary fibre from flaxseed-A physicochemical approach. Food Hydrocoll. 2019, 90, 105–112. [Google Scholar] [CrossRef]
- Dong, W.J.; Wang, D.D.; Hu, R.S.; Long, Y.Z.; Lv, L.S. Chemical composition, structural and functional properties of soluble dietary fiber obtained from coffee peel using different extraction methods. Food Res. Int. 2020, 136, 109497. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Shi, Q.K.; Zhao, C.B.; Yin, H.H.; Xu, X.Y.; Wu, Y.Z.; Cao, Y.; Zhang, H.; Liu, J.S. Effects of ultrasonic modification on the physicochemical properties and structure of oat dietary fiber. Food Sci. 2020, 41, 130–136. [Google Scholar]
- Peerajit, P.; Chiewchan, N.; Devahastin, S. Effects of pretreatment methods on health-related functional properties of high dietary fiber powder from lime residues. Food Chem. 2012, 132, 1891–1898. [Google Scholar] [CrossRef]
- Seguenka, B.; Nascimento, L.H.D.; Feiden, T.; Fernandes, I.A.; Magro, J.D.; Junges, A.; Valduga, E.; Steffens, J. Ultrasound-assisted extraction and concentration of phenolic compounds from jabuticaba sabará (Plinia peruviana (Poir.) Govaerts) peel by nanofiltration membrane. Food Chem. 2024, 453, 139690. [Google Scholar] [CrossRef]
- İşçimen, E.M.; Hayta, M. Optimisation of ultrasound assisted extraction of rice bran proteins: Effects on antioxidant and antiproliferative properties. Qual. Assur. Saf. Crops 2018, 10, 165–174. [Google Scholar] [CrossRef]
- Backes, E.; Kato, C.G.; Corrêa, R.C.G.; Moreira, R.F.P.M.; Peralta, R.A.; Barros, L.; Ferreira, I.G.F.R.; Zanin, G.M.; Bracht, A.; Peralta, R.M. Laccases in food processing: Current status, bottlenecks and perspectives. Trends Food Sci. Technol. 2021, 115, 445–460. [Google Scholar] [CrossRef]
- Wei, E.W.; Yang, R.; Zhao, H.P.; Wang, P.H.; Zhao, S.Q.; Zhai, W.C.; Zhang, Y.; Zhou, H.L. Microwave-assisted extraction releases the antioxidant polysaccharides from seabuckthorn (Hippophae rhamnoides L.) berries. Int. J. Biol. Macromol. 2019, 123, 280–290. [Google Scholar] [CrossRef]
- Zhang, W.M.; Zeng, G.G.; Pan, Y.G.; Chen, W.X.; Huang, W.X.; Chen, H.M.; Li, Y.S. Properties of soluble dietary fiber polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohyd. Polym. 2017, 172, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Du, X.J.; Wang, L.; Huang, X.; Jing, H.J.; Ye, X.; Gao, W.; Bai, X.P.; Wang, H.X. Effects of different extraction methods on structure and properties of soluble dietary fiber from defatted coconut flour. LWT-Food Sci. Technol. 2021, 143, 111031. [Google Scholar] [CrossRef]
- Ma, S.; Ren, B.; Diao, Z.J.; Chen, Y.W.; Qiao, Q.L.; Liu, X.B. Physicochemical properties and intestinal protective effect of ultra-micro ground insoluble dietary fibre from carrot pomace. Food Funct. 2016, 7, 3902–3909. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.G.; Li, N.; Xia, Q.; Hou, Y.W.; Xu, G.N. Comparisons of three modifications on structural, rheological and functional properties of soluble dietary fibers from tomato peels. LWT-Food Sci. Technol. 2018, 88, 56–63. [Google Scholar] [CrossRef]
- Qi, J.; Li, Y.; Masamba, K.G.; Shoemaker, C.F.; Zhong, F.; Majeed, H.; Ma, J.G. The effect of chemical treatment on the in vitro hypoglycemic properties of rice bran insoluble dietary fiber. Food Hydrocoll. 2016, 52, 699–706. [Google Scholar] [CrossRef]
- Benitez, V.; Rebollo-Hernanz, M.; Hernanz, S.; Chantres, S.; Aguilera, Y.; Martin-Cabrejas, M.A. Coffee parchment as a new dietary fiber ingredient: Functional and physiological characterization. Food Res. Int. 2019, 122, 105–113. [Google Scholar] [CrossRef]
- Gan, J.P.; Huang, Z.Y.; Yu, Q.; Peng, G.Y.; Chen, Y.; Xie, J.H.; Nie, S.P.; Xie, M.Y. Microwave assisted extraction with three modifications on structural and functional properties of soluble dietary fibers from grapefruit peel. Food Hydrocoll. 2020, 101, 105549. [Google Scholar] [CrossRef]
- Zhu, Y.; Chu, J.X.; Lu, Z.X.; Lv, F.X.; Bie, X.M.; Zhang, C.; Zhao, H.Z. Physicochemical and functional properties of dietary fiber from foxtail millet (Setaria italic) bran. J. Cereal Sci. 2018, 79, 456–461. [Google Scholar] [CrossRef]
- Ma, M.M.; Mu, T.H. Modification of deoiled cumin dietary fiber with laccase and cellulase under high hydrostatic pressure. Carbohyd. Polym. 2016, 136, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.G.; Jiao, Q.; Yuan, F.; Gao, Y.X. In vitro binding capacities and physicochemical properties of soluble fiber prepared by microfluidization pretreatment and cellulase hydrolysis of peach pomace. LWT-Food Sci. Technol. 2015, 63, 677–684. [Google Scholar] [CrossRef]
- Wang, H.O.; Liu, S.H.; Zhou, X.J.; Yang, X.Y.; Gao, Q.; Tanokura, M.; Xue, Y.L. Treatment with hydrogen peroxide improves the physicochemical properties of dietary fibres from Chinese yam peel. Int. J. Food Sci. Technol. 2020, 55, 1289–1297. [Google Scholar] [CrossRef]
- Hua, M.; Lu, J.X.; Qu, D.; Liu, C.; Zhang, L.; Li, S.S.; Chen, J.B.; Sun, Y.S. Structure, physicochemical properties and adsorption function of insoluble dietary fiber from ginseng residue: A potential functional ingredient. Food Chem. 2019, 286, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wen, X.Y.; Zhang, X.Q.; Pu, H.M.; Kan, J.; Jin, C.H. Extraction, characterization and in vitro antioxidant activity of polysaccharides from black soybean. Int. J. Biol. Macromol. 2015, 72, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Zhang, H.B.; Yi, C.P.; Quan, K.; Lin, B.P. Chemical composition, structure, physicochemical and functional properties of rice bran dietary fiber modified by cellulase treatment. Food Chem. 2021, 342, 128352. [Google Scholar] [CrossRef] [PubMed]
- Gil-López, D.I.L.; Lois-Correa, J.A.; Sánchez-Pardo, M.E.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Rodríguez-Salazar, A.E.; Orta-Guzmán, V.N. Production of dietary fibers from sugarcane bagasse and sugarcane tops using microwave-assisted alkaline treatments. Ind. Crops Prod. 2019, 135, 159–169. [Google Scholar] [CrossRef]
- Nasseri, R.; Ngunjiri, R.; Moresoli, C.; Yu, A.P.; Yuan, Z.S.; Xu, C.B. Poly (lactic acid)/acetylated starch blends: Effect of starch acetylation on the material properties. Carbohyd. Polym. 2020, 229, 115453. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Schneeman, B.O. Fiber, inulin and oligofructose: Similarities and differences. J. Nutr. 1999, 129 (Suppl. S7), 1424S. [Google Scholar] [CrossRef] [PubMed]
- Miehle, E.; Hass, M.; Bader-Mittermaier, S.; Eisner, P. The role of hydration properties of soluble dietary fibers on glucose diffusion. Food Hydrocoll. 2022, 131, 107822. [Google Scholar] [CrossRef]
- Siddiqui, H.; Sultan, Z.; Yousuf, O.; Malik, M.; Younis, K. A review of the health benefits, functional properties, and ultrasound-assisted dietary fiber extraction. Bioact. Carbohydr. Diet. Fibre 2023, 30, 100356. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Zhong, F.; Mothibe, K.J. In vitro hypoglycemic and cholesterol lowering effects of dietary fiber prepared from cocoa (Theobroma cacao L.) shells. Food Funct. 2012, 3, 1044–1050. [Google Scholar] [CrossRef]
- Yan, X.G.; Ye, G.; Chen, Y. Blasting extrusion processing: The increase of soluble dietary fiber content and extraction of soluble-fiber polysaccharides from wheat bran. Food Chem. 2015, 180, 106–115. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Xiong, Q.; Yu, Y.; Peng, L.C. Sulfated modification, characterization, and potential bioactivities of polysaccharide from the fruiting bodies of Russula virescens. Int. J. Biol. Macromol. 2020, 154, 1438–1447. [Google Scholar] [CrossRef]

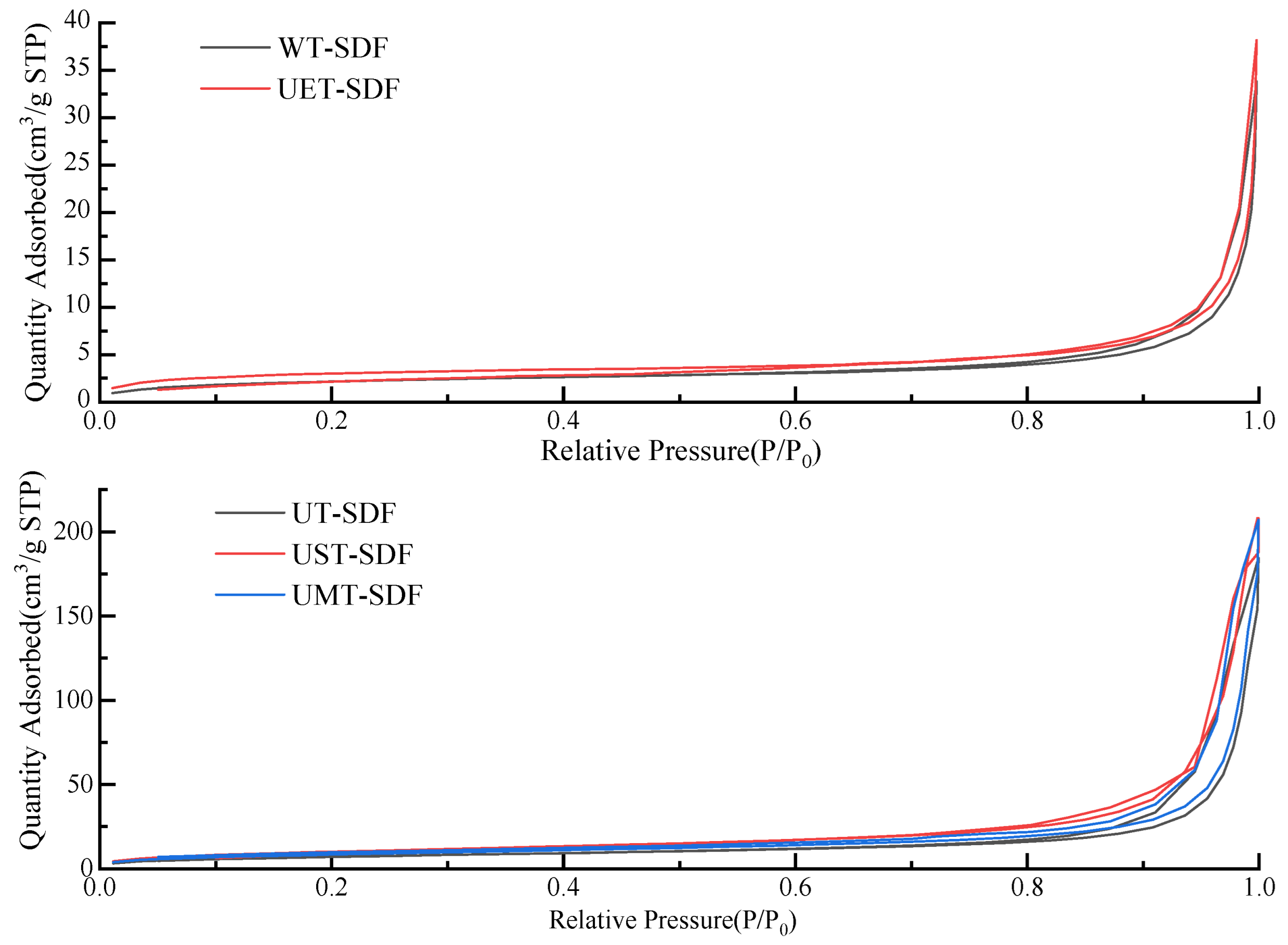


| Sample | Specific Surface Area (m2/g) | Pore Size (nm) | L* | a* | b* | Extraction Yield (g/100 g) |
|---|---|---|---|---|---|---|
| WT-SDF | 7.95 | 17.25 | 84.53 ± 0.46 b | 12.27 ± 0.19 a | 13.20 ± 0.14 b | 3.80 ± 0.20 e |
| UT-SDF | 26.66 | 28.33 | 83.23 ± 2.44 c | 11.06 ± 0.08 b | 11.83 ± 0.34 c | 6.27 ± 0.25 d |
| UST-SDF | 37.86 | 26.16 | 72.20 ± 1.69 e | 4.77 ± 0.05 e | 17.83 ± 0.29 a | 10.03 ± 0.61 a |
| UET-SDF | 10.57 | 15.33 | 88.73 ± 0.95 a | 8.93 ± 0.19 c | 10.57 ± 0.29 e | 7.83 ± 0.12 c |
| UMT-SDF | 31.38 | 27.94 | 83.20 ± 0.50 d | 5.07 ± 0.12 d | 10.67 ± 0.38 d | 8.30 ± 0.30 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhu, R.; Zhang, N.; Zhao, W.; Wang, C. Effects of Different Extraction Methods on the Structural and Functional Properties of Soluble Dietary Fibre from Sweet Potatoes. Foods 2024, 13, 2395. https://doi.org/10.3390/foods13152395
Yang L, Zhu R, Zhang N, Zhao W, Wang C. Effects of Different Extraction Methods on the Structural and Functional Properties of Soluble Dietary Fibre from Sweet Potatoes. Foods. 2024; 13(15):2395. https://doi.org/10.3390/foods13152395
Chicago/Turabian StyleYang, Liuqing, Rongan Zhu, Ning Zhang, Wenya Zhao, and Chuyan Wang. 2024. "Effects of Different Extraction Methods on the Structural and Functional Properties of Soluble Dietary Fibre from Sweet Potatoes" Foods 13, no. 15: 2395. https://doi.org/10.3390/foods13152395
APA StyleYang, L., Zhu, R., Zhang, N., Zhao, W., & Wang, C. (2024). Effects of Different Extraction Methods on the Structural and Functional Properties of Soluble Dietary Fibre from Sweet Potatoes. Foods, 13(15), 2395. https://doi.org/10.3390/foods13152395







