Influence of Cooking Technique on Bioaccessibility of Bioactive Compounds in Vegetable Lentil Soup
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Soup Formulation
2.3. Soup Preparation
2.4. Analysis
2.4.1. Vegetable Mechanical and Optical Properties
2.4.2. Total Carotenoids and Lycopene
2.4.3. Total Phenols
2.4.4. Antioxidant Capacity
2.4.5. Ascorbic Acid and vitC
2.4.6. In Vitro Digestion
2.4.7. Statistical Analysis
3. Results and Discussion
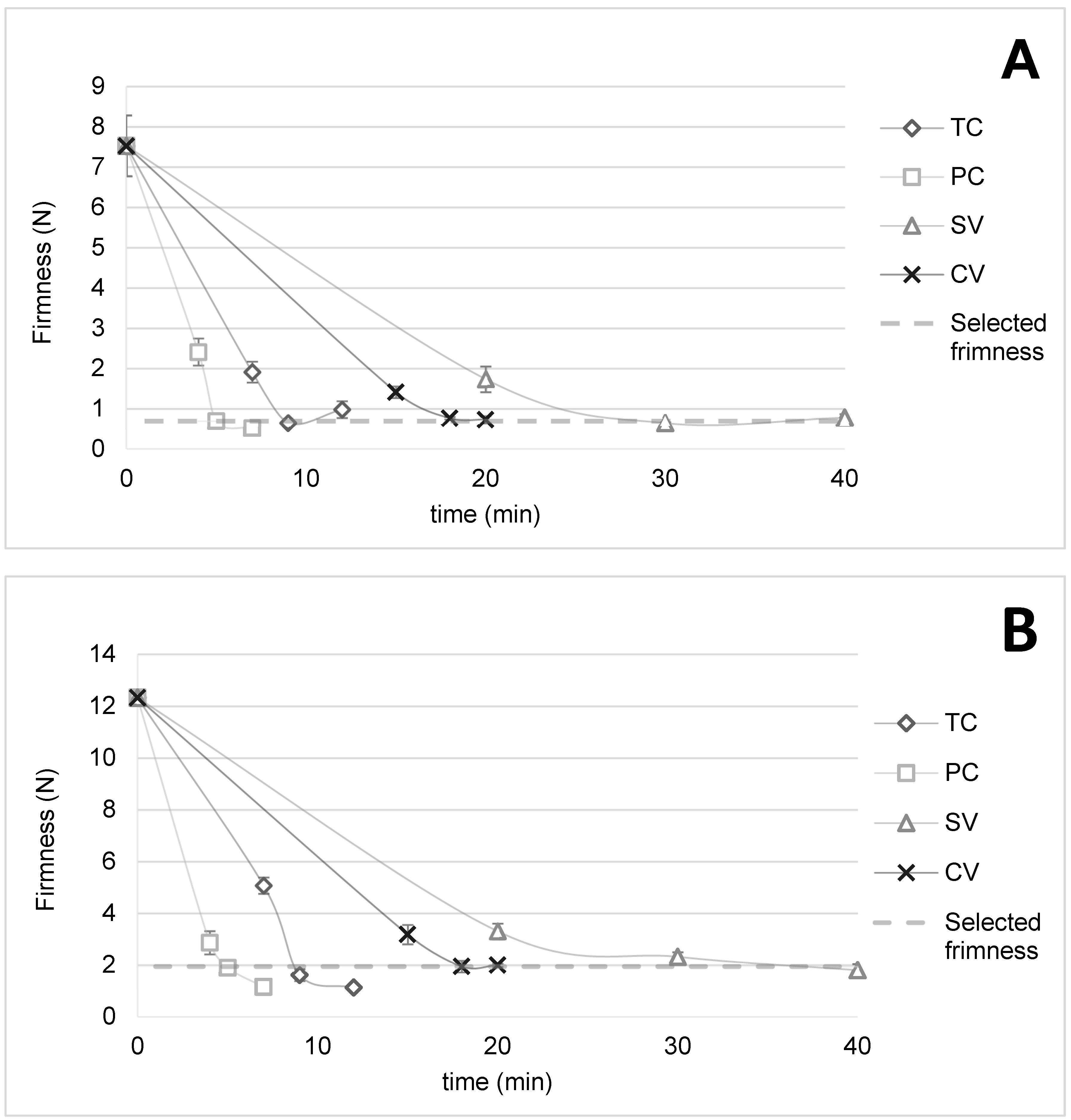
3.1. Textural Kinetics of Pumpkin and Carrot Pieces to Standardize Cooking
3.2. Effect of Cooking Method on the Physical Properties of Soup and Process Yield
3.2.1. Soups’ Physical Properties
3.2.2. Process Yield
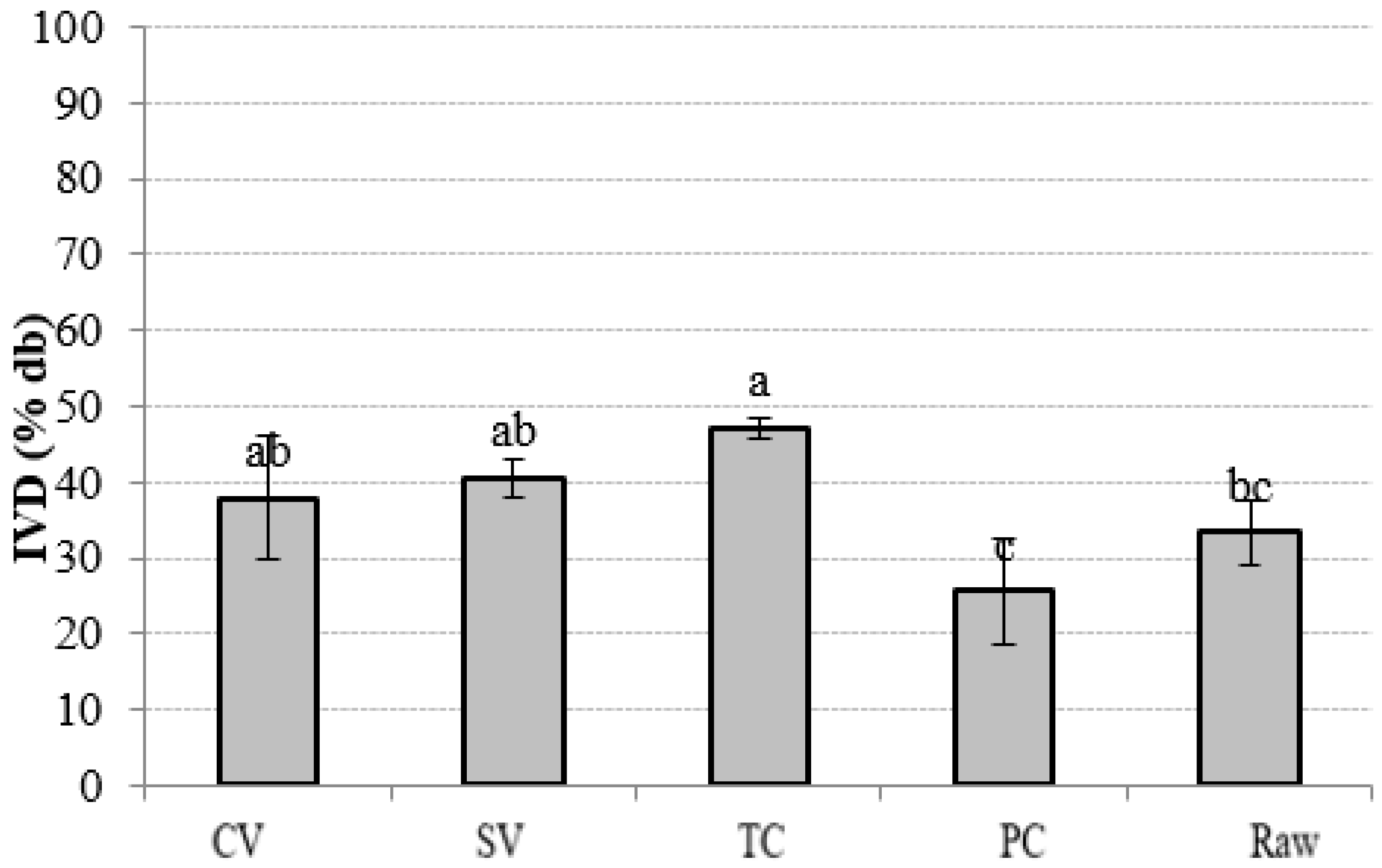
3.3. In Vitro Digestibility
3.4. Effect of Cooking Method on the Bioactive Compounds of Soup and Their Bioaccessibility
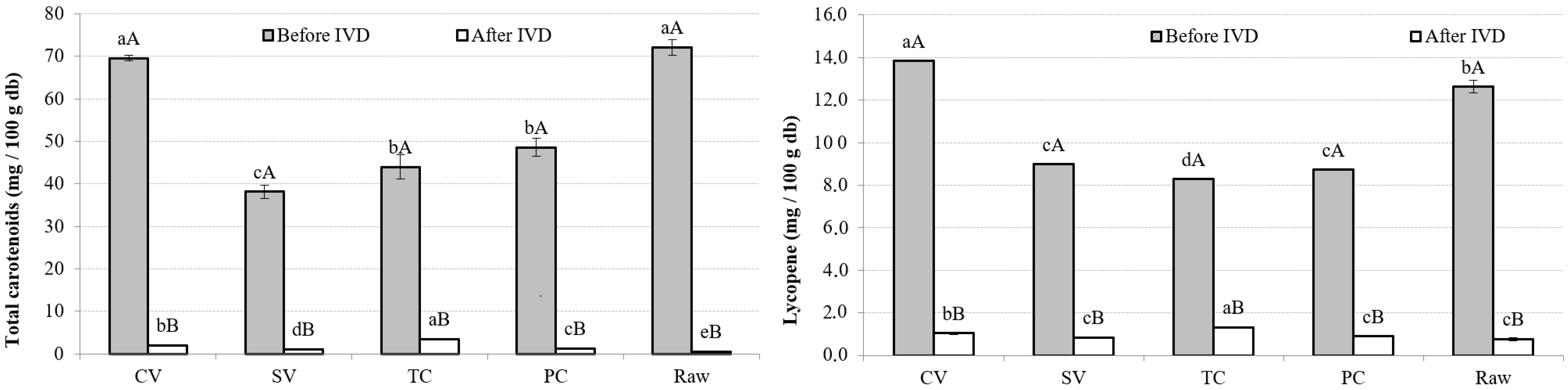
3.4.1. TotC and L
3.4.2. TP
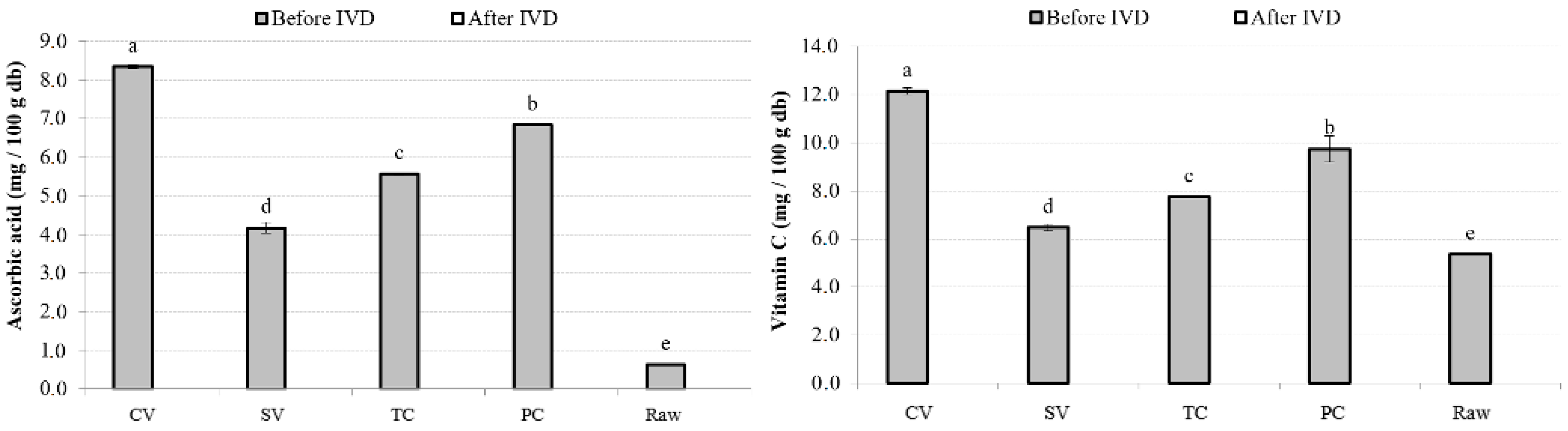
3.4.3. AA and vitC
3.4.4. AC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abalos, R.A.; Naef, E.F.; Aviles, M.V.; Gómez, M.B. Vacuum impregnation: A methodology for the preparation of a ready-to-eat sweet potato enriched in polyphenols. LWT 2020, 131, 109773. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Sous-vide cooking as a systematic approach for quality maintenance and shelf-life extension of crab lump meat. LWT 2021, 142, 111004. [Google Scholar] [CrossRef]
- Zavadlav, S.; Blažić, M.; Van de Velde, F.; Vignatti, C.; Fenoglio, C.; Piagentini, A.M.; Pirovani, M.E.; Perotti, C.M.; Bursać Kovačević, D.; Putnik, P. Sous-Vide as a Technique for Preparing Healthy and High-Quality Vegetable and Seafood Products. Foods 2020, 9, 1537. [Google Scholar] [CrossRef]
- Kathuria, D.; Dhiman, A.K.; Attri, S. Sous vide, a culinary technique for improving quality of food products: A review. Trends Food Sci. Technol. 2022, 119, 57–68. [Google Scholar] [CrossRef]
- Guillén, S.; Mir-Bel, J.; Oria, R.; Salvador, M.L. Influence of cooking conditions on organoleptic and health-related properties of artichokes, green beans, broccoli and carrots. Food Chem. 2017, 217, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Buratti, S.; Cappa, C.; Benedetti, S.; Giovanelli, G. Influence of Cooking Conditions on Nutritional Properties and Sensory Characteristics Interpreted by E-Senses: Case-Study on Selected Vegetables. Foods 2020, 9, 607. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Michalak, M.; Agellon, L.B. Importance of nutrients and nutrient metabolism on human health. Yale J. Biol. Med. 2018, 91, 95–103. [Google Scholar]
- Frison, E.; Smith, I.F.; Cherfas, J.; Eyzaguirre, P.; Johns, T. Using biodiversity for food, dietary diversity, better nutrition and health. S. Afr. J. Clin. Nutr. 2005, 18, 112–114. [Google Scholar] [CrossRef]
- Puwanant, M.; Boonrusmee, S.; Jaruratanasirikul, S.; Chimrung, K.; Sriplung, H. Dietary diversity and micronutrient adequacy among women of reproductive age: A cross-sectional study in Southern Thailand. BMC Nutr. 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Tomé, M.; Murcia, M.A.; Mariscal, M.; Lorenzo, M.L.; Gómez-Murcia, V.; Bibiloni, M.; Jiménez-Monreal, A.M. Evaluation of antioxidant activity and nutritional composition of flavoured dehydrated soups packaged in different formats. Reducing the sodium content. J. Food Sci. Technol. 2015, 52, 7850–7860. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lv, F.; Tian, J.; Ye, X.q.; Chen, J.; Sun, P. Domestic cooking methods affect nutrient, phytochemicals, and flavor content in mushroom soup. Food Sci. Nutr. 2019, 7, 1969–1975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lawal, O.M.; Enujiugha, V.N. Nutritional Assessment of Nigerian Ethnic Vegetable Soups (Marugbo, Tete and Ila). J. Nutr. Food Lipid Sci. 2018, 1, 32–39. [Google Scholar]
- Nguyen, T.M.; Phoukham, K.; Van Ngo, T. Formulation and quality evaluation of pearl oyster mushroom soup powder supplement with some kinds of legumes and vegetables. Acta Sci. Pol. Technol. Aliment. 2020, 19, 435–443. [Google Scholar] [PubMed]
- Mondal, I.H.; Rangan, L.; Uppaluri, R.V.S. A robust and novel methodology for the optimal targeting of leafy vegetable mix soup formulations. LWT 2020, 134, 110152. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Munoz, L.M.; Tavares, G.M.; Corredig, M. Design future foods using plant protein blends for best nutritional and technological functionality. Trends Food Sci. Technol. 2021, 113, 139–150. [Google Scholar] [CrossRef]
- Capuano, E.; Oliviero, T.; Fogliano, V.; Pellegrini, N. Role of the food matrix and digestion on calculation of the actual energy content of food. Nutr. Rev. 2018, 76, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Delaqua, D.; Carnier, R.; Cadore, S.; Sanches, V.L.; Berton, R.S.; Corbi, F.C.A.; Coscione, A.R. In vitro bioaccessibility and bioavailability of selenium in agronomic biofortified wheat. J. Food Compos. Anal. 2022, 105, 104253. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Acquistucci, R. Phenolic Compounds and Bioaccessibility Thereof in Functional Pasta. Antioxidants 2020, 9, 343. [Google Scholar] [CrossRef]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Gu, R.; Chang, X.; Bai, G.; Li, X.; Di, Y.; Liu, X.; Sun, L.; Wang, Y. Effects of household cooking methods on changes of tissue structure, phenolic antioxidant capacity and active component bioaccessibility of quinoa. Food Chem. 2021, 350, 129138. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J. In vitro bioaccessibility of minerals from microalgae-enriched cookies. Food Funct. 2020, 11, 2186–2194. [Google Scholar] [CrossRef]
- Egger, L.; Ménard, O.; Delgado-Andrade, C.; Alvito, P.; Assunção, R.; Balance, S.; Barberá, R.; Brodkorb, A.; Cattenoz, T.; Clemente, A.; et al. The harmonized INFOGEST in vitro digestion method: From knowledge to action. Food Res. Int. 2016, 88, 217–225. [Google Scholar] [CrossRef]
- Igual, M.; Păucean, A.; Vodnar, D.C.; García-Segovia, P.; Martínez-Monzó, J.; Chiş, M.S. In Vitro Bioaccessibility of Bioactive Compounds from Rosehip-Enriched Corn Extrudates. Molecules 2022, 27, 1972. [Google Scholar] [CrossRef]
- Igual, M.; Fernandes, Â.; Dias, M.I.; Pinela, J.; García-Segovia, P.; Martínez-Monzó, J.; Barros, L. The In Vitro Simulated Gastrointestinal Digestion Affects the Bioaccessibility and Bioactivity of Beta vulgaris Constituents. Foods 2023, 12, 338. [Google Scholar] [CrossRef]
- Iborra-Bernad, C.; García-Segovia, P.; Martínez-Monzó, J. Effect of vacuum cooking treatment on physicochemical and structural characteristics of purple-flesh potato. Int. J. Food Sci. Technol. 2014, 49, 943–951. [Google Scholar] [CrossRef]
- Rondanelli, M.; Daglia, M.; Meneghini, S.; Di Lorenzo, A.; Peroni, G.; Faliva, M.A.; Perna, S. Nutritional advantages of sous-vide cooking compared to boiling on cereals and legumes: Determination of ashes and metals content in ready-to-eat products. Food Sci. Nutr. 2017, 5, 827–833. [Google Scholar] [CrossRef]
- Cui, Z.K.; Yan, H.; Manoli, T.; Mo, H.Z.; Bi, J.C.; Zhang, H. Advantages and challenges of sous vide cooking. Food Sci. Technol. Res. 2021, 27, 25–34. [Google Scholar] [CrossRef]
- Fabbri, A.D.T.; Crosby, G.A. A review of the impact of preparation and cooking on the nutritional quality of vegetables and legumes. Int. J. Gastron. Food Sci. 2016, 3, 2–11. [Google Scholar] [CrossRef]
- García-Segovia, P.; Andrés-Bello, A.; Martínez-Monzó, J. Effect of cooking method on mechanical properties, color and structure of beef muscle (M. pectoralis). J. Food Eng. 2007, 80, 813–821. [Google Scholar] [CrossRef]
- Andrés-Bello, A.; García-Segovia, P.; Martínez-Monzó, J. Effects of Vacuum Cooking (Cook-Vide) on the Physical-Chemical Properties of Sea Bream Fillets (Sparus aurata). J. Aquat. Food Prod. Technol. 2009, 18, 79–89. [Google Scholar] [CrossRef]
- Baldwin, D.E. Sous vide cooking: A review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- Iborra-Bernad, C.; Tárrega, A.; García-Segovia, P.; Martínez-Monzó, J. Comparison of Vacuum Treatments and Traditional Cooking Using Instrumental and Sensory Analysis. Food Anal. Methods 2014, 7, 400–408. [Google Scholar] [CrossRef]
- Agiang, M.A.; Umoh, I.B.; Essien, A.I.; Eteng, M.U. Nutrient changes and antinutrient contents of beniseed and beniseed soup during cooking using a Nigerian traditional method. Pak. J. Biol. Sci. 2010, 13, 1011–1015. [Google Scholar] [CrossRef][Green Version]
- Kala, A.; Prakash, J. The comparative evaluation of the nutrient composition and sensory attributes of four vegetables cooked by different methods. Int. J. Food Sci. Technol. 2006, 41, 163–171. [Google Scholar] [CrossRef]
- Iborra-Bernad, C.; García-Segovia, P.; Martínez-Monzó, J. Physico-Chemical and Structural Characteristics of Vegetables Cooked Under Sous-Vide, Cook-Vide, and Conventional Boiling. J. Food Sci. 2015, 80, E1725–E1734. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Santi, S.; Paciulli, M.; Ganino, T.; Pellegrini, N.; Visconti, A.; Vitaglione, P.; Barbanti, D.; Chiavaro, E. Comparison of physical, microstructural and antioxidative properties of pumpkin cubes cooked by conventional, vacuum cooking and sous vide methods. J. Sci. Food Agric. 2021, 101, 2534–2541. [Google Scholar] [CrossRef]
- Olives Barba, A.I.; Cámara Hurtado, M.; Sánchez Mata, M.C.; Fernández Ruiz, V.; López Sáenz De Tejada, M. Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Stability of micronutrients and phytochemicals of grapefruit jam as affected by the obtention process. Food Sci. Technol. Int. 2016, 22, 203–212. [Google Scholar] [PubMed]
- Igual, M.; Sampedro, F.; Martínez-Navarrete, N.; Fan, X. Combined osmodehydration and high pressure processing on the enzyme stability and antioxidant capacity of a grapefruit jam. J. Food Eng. 2013, 114, 514–521. [Google Scholar]
- Igual, M.; Cebadera, L.; Cámara, R.M.A.; Agudelo, C.; Martínez-Navarrete, N.; Cámara, M. Novel Ingredients Based on Grapefruit Freeze-Dried Formulations: Nutritional and Bioactive Value. Foods 2019, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Plaza, L.; De Ancos, B.; Cano, M.P. Quantitative bioactive compounds assessment and their relative contribution to the antioxidant capacity of commercial orange juices. J. Sci. Food Agric. 2003, 83, 430–439. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Arilla, E.; García-Segovia, P.; Martínez-Monzó, J.; Codoñer-Franch, P.; Igual, M. Effect of Adding Resistant Maltodextrin to Pasteurized Orange Juice on Bioactive Compounds and Their Bioaccessibility. Foods 2021, 10, 1198. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Khouzam, R.B.; Pohl, P.; Lobinski, R. Bioaccessibility of essential elements from white cheese, bread, fruit and vegetables. Talanta 2011, 86, 425–428. [Google Scholar] [CrossRef]
- Grave, L.C.; McArdle, R.N.; Gohlke, J.R.; Labavitch, J.M. Impact of Heating on Carrot Firmness: Changes in Cell Wall Components. J. Agric. Food Chem. 1994, 42, 2900–2906. [Google Scholar] [CrossRef]
- Koç, M.; Baysan, U.; Devseren, E.; Okut, D.; Atak, Z.; Karataş, H.; Kaymak-Ertekin, F. Effects of different cooking methods on the chemical and physical properties of carrots and green peas. Innov. Food Sci. Emerg. Technol. 2017, 42, 109–119. [Google Scholar] [CrossRef]
- Iborra-Bernad, C.; Philippon, D.; García-Segovia, P.; Martínez-Monzó, J. Optimizing the texture and color of sous-vide and cook-vide green bean pods. LWT-Food Sci. Technol. 2013, 51, 507–513. [Google Scholar] [CrossRef]
- Trejo Araya, X.I.; Smale, N.; Zabaras, D.; Winley, E.; Forde, C.; Stewart, C.M.; Mawson, A.J. Sensory perception and quality attributes of high pressure processed carrots in comparison to raw, sous-vide and cooked carrots. Innov. Food Sci. Emerg. Technol. 2009, 10, 420–433. [Google Scholar] [CrossRef]
- Oey, I.; Lille, M.; Van Loey, A.; Hendrickx, M. Effect of high-pressure processing on colour, texture and flavour of fruit- and vegetable-based food products: A review. Trends Food Sci. Technol. 2008, 19, 320–328. [Google Scholar] [CrossRef]
- Murador, D.C.; da Cunha, D.T.; de Rosso, V.V. Effects of cooking techniques on vegetable pigments: A meta-analytic approach to carotenoid and anthocyanin levels. Food Res. Int. 2014, 65, 177–183. [Google Scholar] [CrossRef]
- da Silva, M.d.F.G.; de Sousa, P.H.M.; Figueiredo, R.W.; Gouveia, S.T.; Lima, J.S.S. Cooking effects on bioactive compounds and sensory acceptability in pumpkin (Cucurbita moschata cv. Leite). Rev. Ciênc. Agron. 2019, 50, 394–401. [Google Scholar]
- Boisen, S.; Fernández, J.A. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed Sci. Technol. 1997, 68, 277–286. [Google Scholar] [CrossRef]
- Duijsens, D.; Gwala, S.; Pallares, A.P.; Pälchen, K.; Hendrickx, M.; Grauwet, T. How postharvest variables in the pulse value chain affect nutrient digestibility and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5067–5096. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances, 10th ed.; National Academies Press: Washington, DC, USA, 1989. [Google Scholar]
- Sandberg, A.S. Methods and options in vitro dialyzability; benefits and limitations. Int. J. Vitam. Nutr. Res. 2005, 75, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Changes in vitamin C, phenolic, and carotenoid profiles throughout in vitro gastrointestinal digestion of a blended fruit juice. J. Agric. Food Chem. 2013, 61, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- López-Gámez, G.; Elez-Martínez, P.; Quiles-Chuliá, A.; Martín-Belloso, O.; Hernando-Hernando, I.; Soliva-Fortuny, R. Effect of pulsed electric fields on carotenoid and phenolic bioaccessibility and their relationship with carrot structure. Food Funct. 2021, 12, 2772–2783. [Google Scholar] [CrossRef]
- Veda, S.; Platel, K.; Srinivasan, K. Enhanced bioaccessibility of β-carotene from yellow-orange vegetables and green leafy vegetables by domestic heat processing. Int. J. Food Sci. Technol. 2010, 45, 2201–2207. [Google Scholar] [CrossRef]
- De Ancos, B.; Cilla, A.; Barberá, R.; Sánchez-Moreno, C.; Cano, M.P. Influence of orange cultivar and mandarin postharvest storage on polyphenols, ascorbic acid and antioxidant activity during gastrointestinal digestion. Food Chem. 2017, 225, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Yaman, M.; Çatak, J.; Uğur, H.; Gürbüz, M.; Belli, İ.; Tanyıldız, S.N.; Yıldırım, H.; Cengiz, S.; Yavuz, B.B.; Kişmiroğlu, C.; et al. The bioaccessibility of water-soluble vitamins: A review. Trends Food Sci. Technol. 2021, 109, 552–563. [Google Scholar] [CrossRef]
- de Castro, N.T.; de Alencar, E.R.; Zandonadi, R.P.; Han, H.; Raposo, A.; Ariza-Montes, A.; Araya-Castillo, L.; Botelho, R.B.A. Influence of Cooking Method on the Nutritional Quality of Organic and Conventional Brazilian Vegetables: A Study on Sodium, Potassium, and Carotenoids. Foods 2021, 10, 1782. [Google Scholar] [CrossRef]
- Lemmens, L.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. Particle size reduction leading to cell wall rupture is more important for the β-carotene bioaccessibility of raw compared to thermally processed carrots. J. Agric. Food Chem. 2010, 58, 12769–12776. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B.; Kimura, M.; Amaya-Farfan, J. Fontes Brasileiras de Carotenóides. Tabela Brasileira de Composição de Carotenoides em Alimentos; MMA/SBF: Brasília, Brazil, 2008; Volume 1, pp. 58–59. [Google Scholar]
- Van Het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G.A.J. Dietary Factors That Affect the Bioavailability of Carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef]
- Hedrén, E.; Diaz, V.; Svanberg, U. Estimation of carotenoid accessibility from carrots determined by an in vitro digestion method. Eur. J. Clin. Nutr. 2002, 56, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.M.; Rosado, G.P. New conversion factors of provitamin A carotenoids. Food Sci. Technol. 2005, 25, 571–578. [Google Scholar] [CrossRef]
- Hornero-Méndez, D.; Mínguez-Mosquera, M.I. Bioaccessibility of carotenes from carrots: Effect of cooking and addition of oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 407–412. [Google Scholar] [CrossRef]
- Ghasemi Baghabrishami, R.; Goli, S.A.H. Tomato seed oil-enriched tomato juice: Effect of oil addition type and heat treatment on lycopene bioaccessibility and oxidative stability. Food Chem. 2023, 402, 134217. [Google Scholar] [CrossRef]
- Liang, X.; Yan, J.; Guo, S.; McClements, D.J.; Ma, C.; Liu, X.; Liu, F. Enhancing lycopene stability and bioaccessibility in homogenized tomato pulp using emulsion design principles. Innov. Food Sci. Emerg. Technol. 2021, 67, 102525. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; McClements, D.J. Enhancement of lycopene bioaccessibility from tomato juice using excipient emulsions: Influence of lipid droplet size. Food Chem. 2016, 210, 295–304. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P.; Elez-Martínez, P.; Martín-Belloso, O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J. Funct. Foods 2015, 14, 33–43. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Sytařová, I.; Orsavová, J.; Snopek, L.; Mlček, J.; Byczyński, Ł.; Mišurcová, L. Impact of phenolic compounds and vitamins C and E on antioxidant activity of sea buckthorn (Hippophaë rhamnoides L.) berries and leaves of diverse ripening times. Food Chem. 2020, 310, 125784. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- López-Hernández, A.A.; Ortega-Villarreal, A.S.; Vázquez Rodríguez, J.A.; López-Cabanillas Lomelí, M.; González-Martínez, B.E. Application of different cooking methods to improve nutritional quality of broccoli (Brassica oleracea var. italica) regarding its compounds content with antioxidant activity. Int. J. Gastron. Food Sci. 2022, 28, 100510. [Google Scholar] [CrossRef]
- Hagos, M.; Chandravanshi, B.S.; Redi-Abshiro, M.; Yaya, E.E. Determination of total phenolic, total flavonoid, ascorbic acid contents and antioxidant activity of pumpkin flesh, peel and seeds. Bull. Chem. Soc. Ethiop. 2023, 37, 1093–1108. [Google Scholar] [CrossRef]


| TC | PC | SV | CV | |
|---|---|---|---|---|
| Cooking temperature | 100 °C | 120 °C | 90 °C | 90 °C |
| Cooking time | 9 min | 5 min | 30 min | 18 min |
| Material in contact with the sample and cooking media state | Sample in contact with boiling water | Sample in contact with heating boiling water | Sample inside of a vacuum-sealed pouch surrounded by liquid hot water | Sample in contact with boiling water |
| Vegetable | Cooking Method | Firmness (N) |
| Pumpkins | TC (9 min) | 0.65 (0.07) b |
| PC (5 min) | 0.70 (0.08) b | |
| SV (30 min) | 0.64 (0.03) b | |
| CV (18 min) | 0.77 (0.03) a | |
| Carrots | TC (9 min) | 1.6 (0.2) c |
| PC (5 min) | 1.90 (0.18) b | |
| SV (30 min) | 2.3 (0.2) a | |
| CV (18 min) | 1.94 (0.18) b |
| Vegetable | Cooking Method | a* | b* | L* | C* | h* | ΔE* Raw | ΔE* TC |
|---|---|---|---|---|---|---|---|---|
| Pumpkins | Raw | 19 (1.9) a | 45 (3.7) a | 51.5 (1.67) a | 49 (4.1) a | 1.18 (0.020) b | 18.2 (3.22) a | |
| TC | 10.9 (0.47) c | 35.4 (0.90) b | 38.3 (1.43) b | 37.1 (0.94) b | 1.271 (0.0103) a | 18.04 (1.023) b | ||
| PC | 12.6 (0.45) b | 37 (3.0) b | 39.3 (1.08) b | 39 (2.9) b | 1.24 (0.027) a | 16.0 (1.48) b | 3.7 (1.26) c | |
| SV | 11 (1.8) c | 26 (4.8) c | 34 (3.1) d | 28 (4.9) c | 1.16 (0.050) b | 27 (3.7) a | 11 (3.2) b | |
| CV | 9.27 (1.68) d | 27 (3.6) c | 36.0 (1.70) c | 29 (3.0) c | 1.24 (0.064) b | 25 (2.9) a | 9 (3.3) b | |
| Carrots | Raw | 28 (3.0) a | 36 (3.1) a | 48 (2.0) a | 45 (3.7) a | 0.91 (0.042) b | 14.7 (1.94) b | |
| TC | 20 (1.9) b | 30 (4.2) b | 38 (2.5) c | 36 (3.7) c | 0.99 (0.077) a | 15 (2.9) b | ||
| PC | 20.7 (1.53) b | 34 (4.6) a | 40.9 (0.60) b | 40 (4.4) b | 1.02 (0.052) a | 11 (2.4) c | 6 (2.2) c | |
| SV | 14.9 (1.40) c | 18 (2.6) c | 26.8 (1.71) d | 23 (2.4) d | 0.88 (0.075) b | 31 (1.7) a | 18 (1.9) a | |
| CV | 21.4 (0.84) b | 35.9 (1.84) a | 39.1 (1.14) c | 41.8 (1.67) b | 1.03 (0.028) a | 11.60 (1.139) c | 6.2 (1.64) c |
| TotC Bioaccessibility (%) | Lycopene Bioaccessibility (%) | TP Bioaccessibility (%) | |
|---|---|---|---|
| CV | 2.84 (0.14) b | 7.5 (0.5) c | 21.86 (1.02) c |
| SV | 2.83 (0.05) b | 9.28 (0.15) b | 21.01 (0.13) d |
| TC | 8.00 (0.14) a | 15.8 (0.4) a | 22.7 (0.4) b |
| PC | 2.63 (0.07) b | 10.2 (0.3) b | 20.89 (0.15) d |
| Raw | 0.78 (0.16) c | 6.1 (0.6) d | 24.52 (0.17) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargha, S.; Igual, M.; Miraballes, M.; Gámbaro, A.; García-Segovia, P.; Martínez-Monzó, J. Influence of Cooking Technique on Bioaccessibility of Bioactive Compounds in Vegetable Lentil Soup. Foods 2024, 13, 2405. https://doi.org/10.3390/foods13152405
Vargha S, Igual M, Miraballes M, Gámbaro A, García-Segovia P, Martínez-Monzó J. Influence of Cooking Technique on Bioaccessibility of Bioactive Compounds in Vegetable Lentil Soup. Foods. 2024; 13(15):2405. https://doi.org/10.3390/foods13152405
Chicago/Turabian StyleVargha, Sofía, Marta Igual, Marcelo Miraballes, Adriana Gámbaro, Purificación García-Segovia, and Javier Martínez-Monzó. 2024. "Influence of Cooking Technique on Bioaccessibility of Bioactive Compounds in Vegetable Lentil Soup" Foods 13, no. 15: 2405. https://doi.org/10.3390/foods13152405
APA StyleVargha, S., Igual, M., Miraballes, M., Gámbaro, A., García-Segovia, P., & Martínez-Monzó, J. (2024). Influence of Cooking Technique on Bioaccessibility of Bioactive Compounds in Vegetable Lentil Soup. Foods, 13(15), 2405. https://doi.org/10.3390/foods13152405








