Characteristics and Functions of Dominant Yeasts Together with Their Applications during Strong-Flavor Baijiu Brewing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis of Yeast Community, Count, Isolation, and Identification
2.2. Characterizations and Metabolic Profiles of Dominant Yeasts
2.3. Preparation of Reinforced Fuqu
2.4. Functional Verification of Dominant Yeasts via Reinforced Fuqu Application
2.5. Bioinformatics Analysis and Data Availability
2.6. Statistical Analysis
3. Results
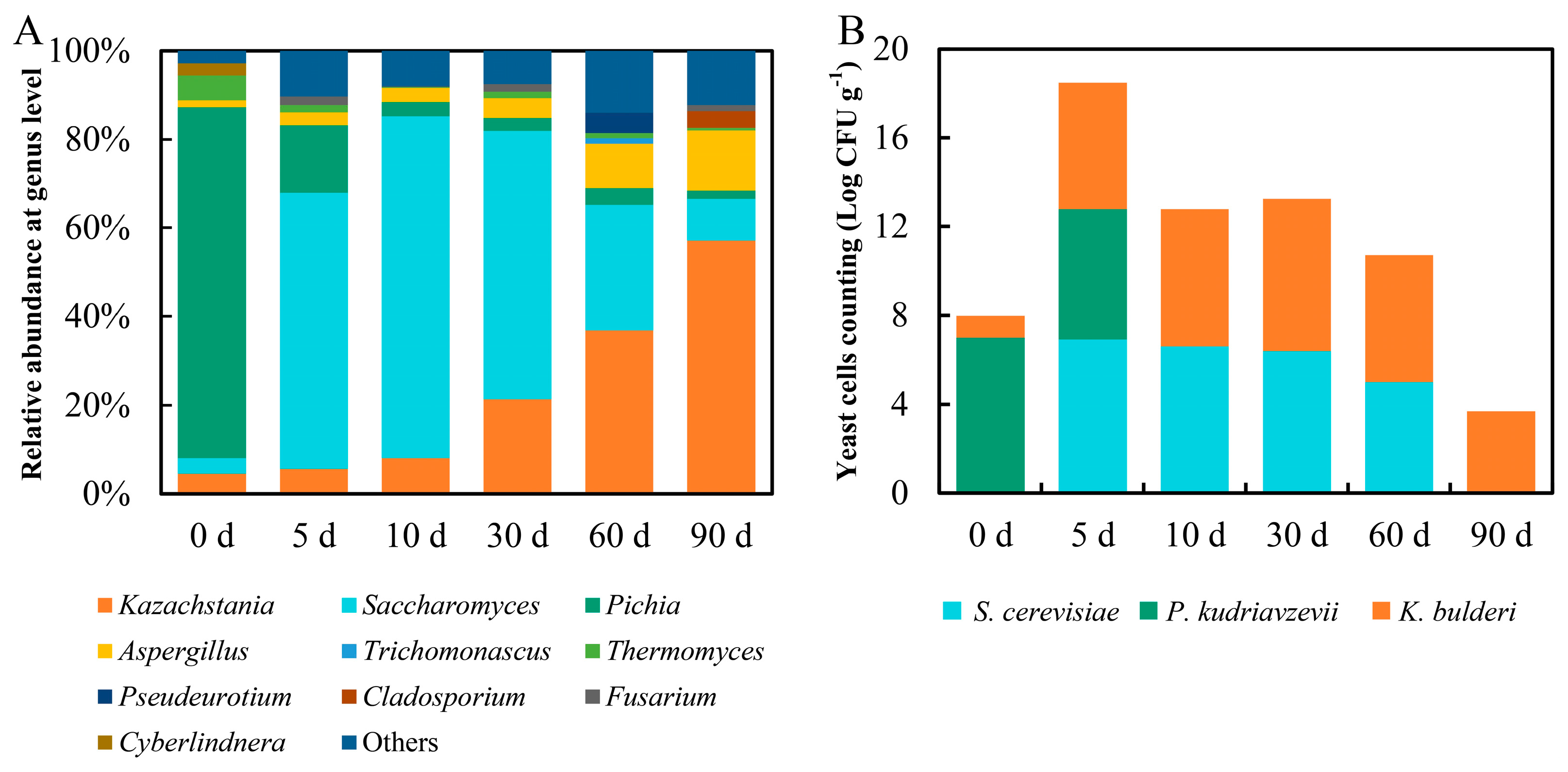
3.1. Yeast Community, Count, Isolation, and Identification
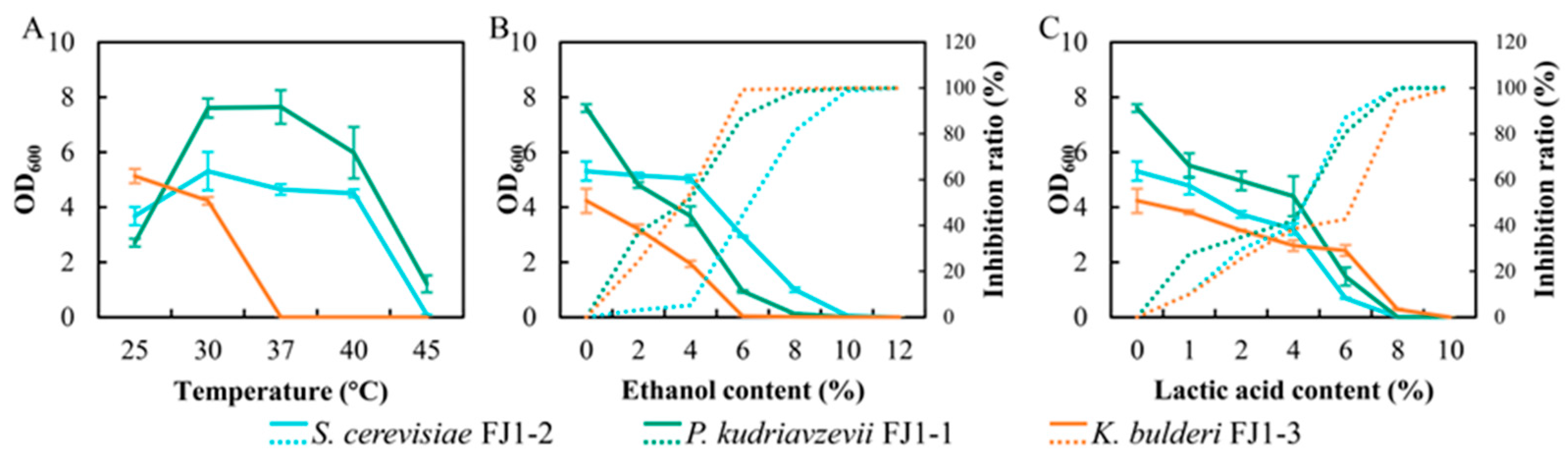
3.2. Characterizations of Dominant Yeasts
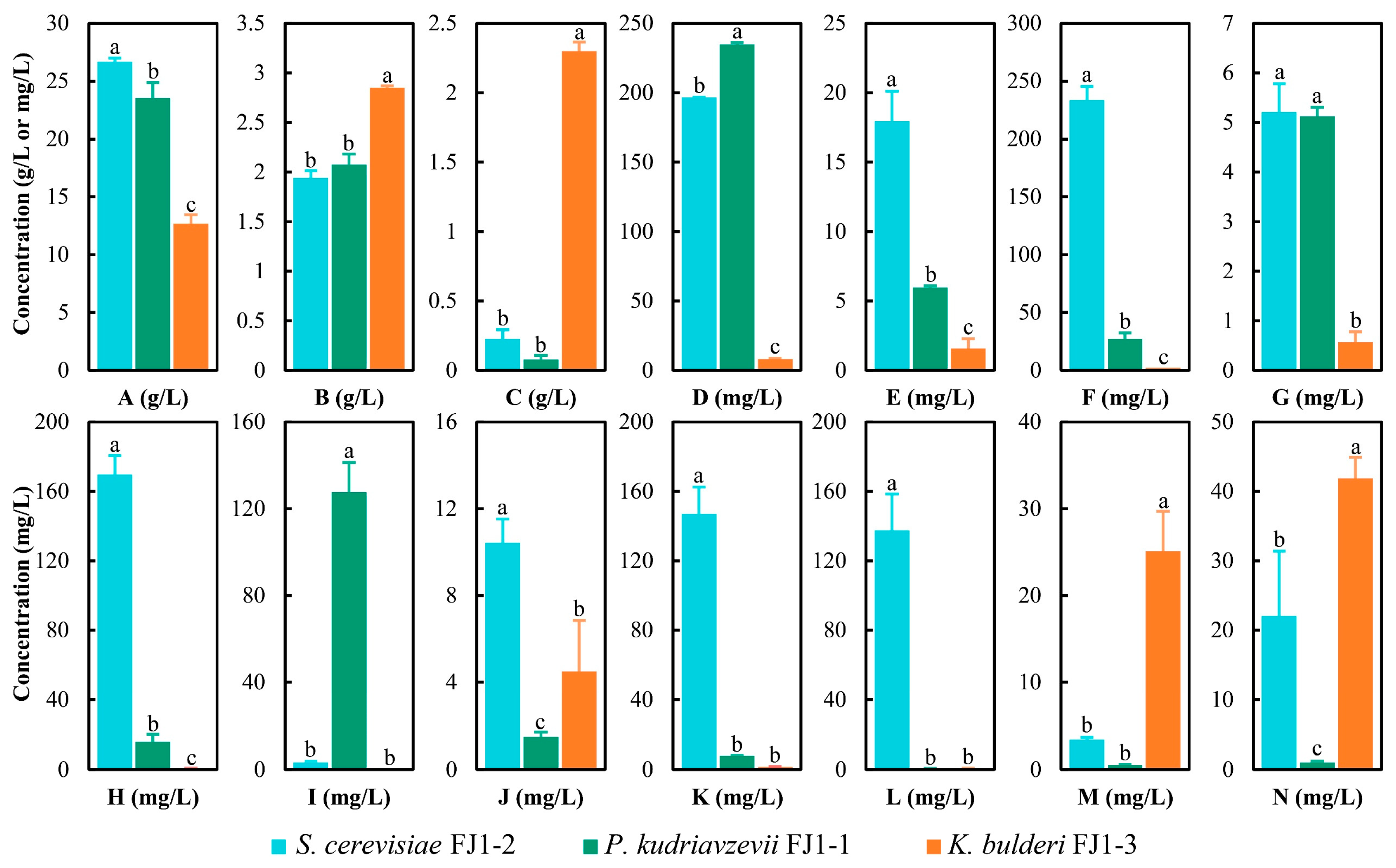
3.3. Metabolic Profiles of Dominant Yeasts
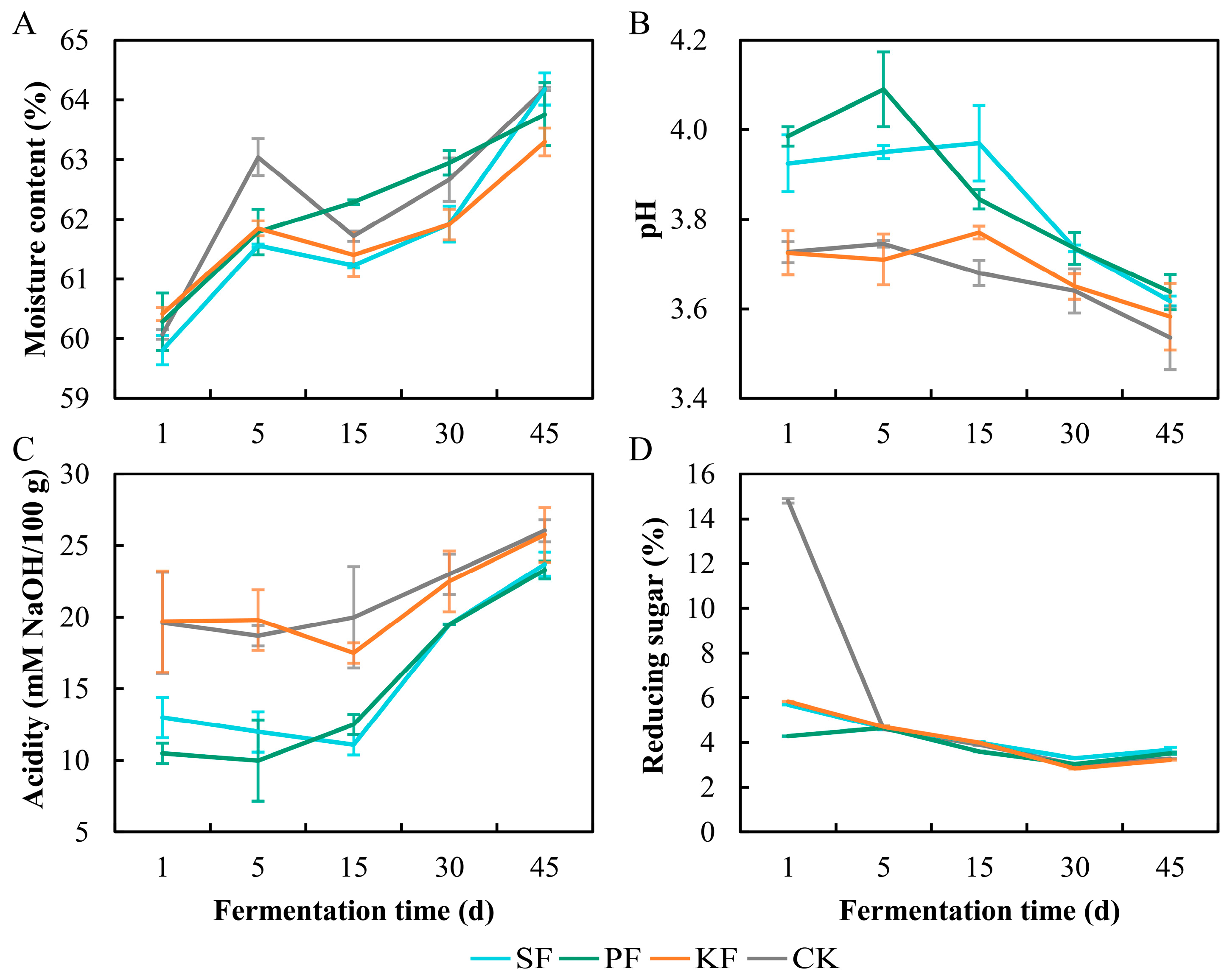
3.4. Dynamics of Physicochemical Factors during Reinforced Fuqu Application
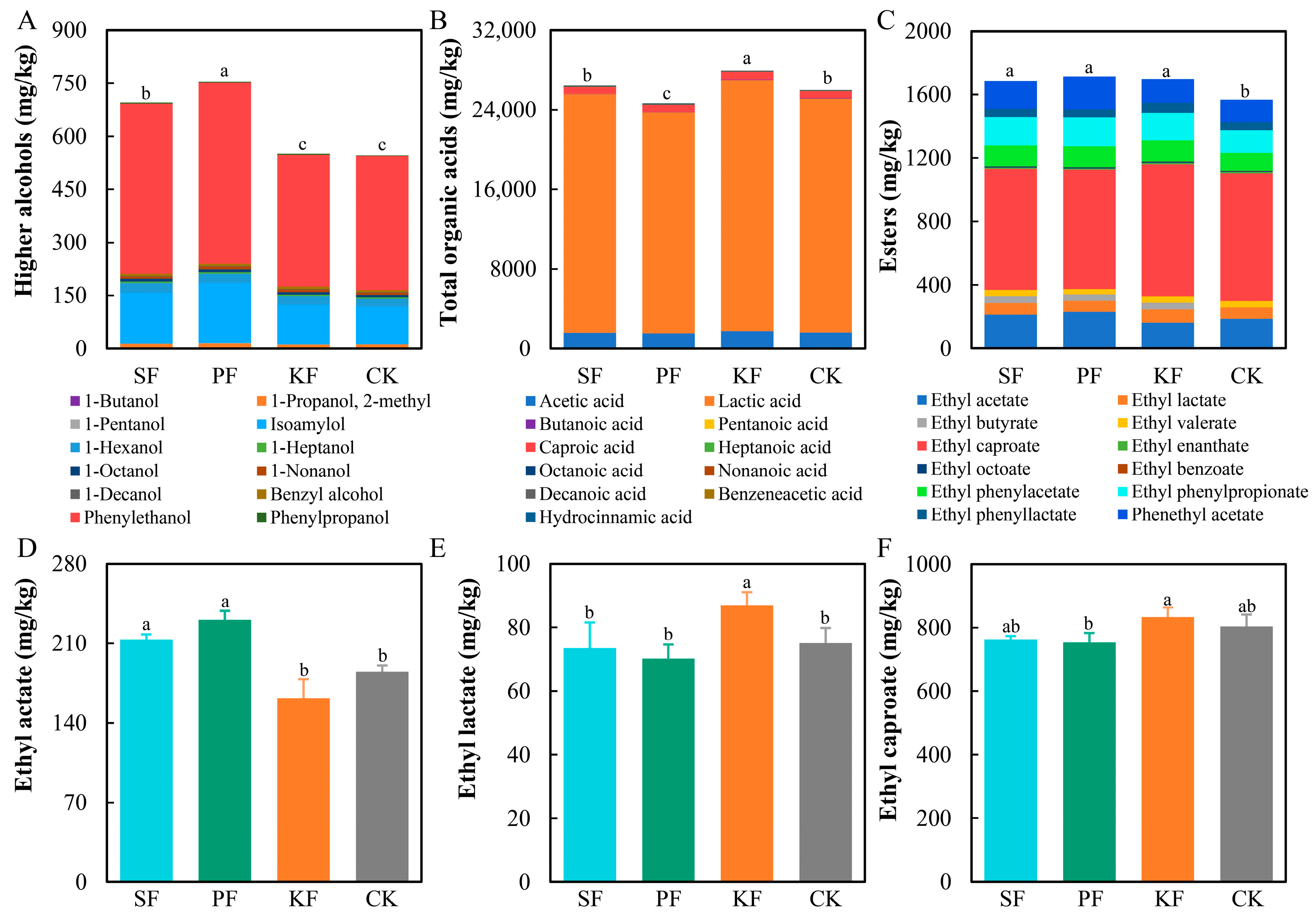
3.5. Dynamics of Flavor Substances during Reinforced Fuqu Application
3.6. Microbial Community during Reinforced Fuqu Application
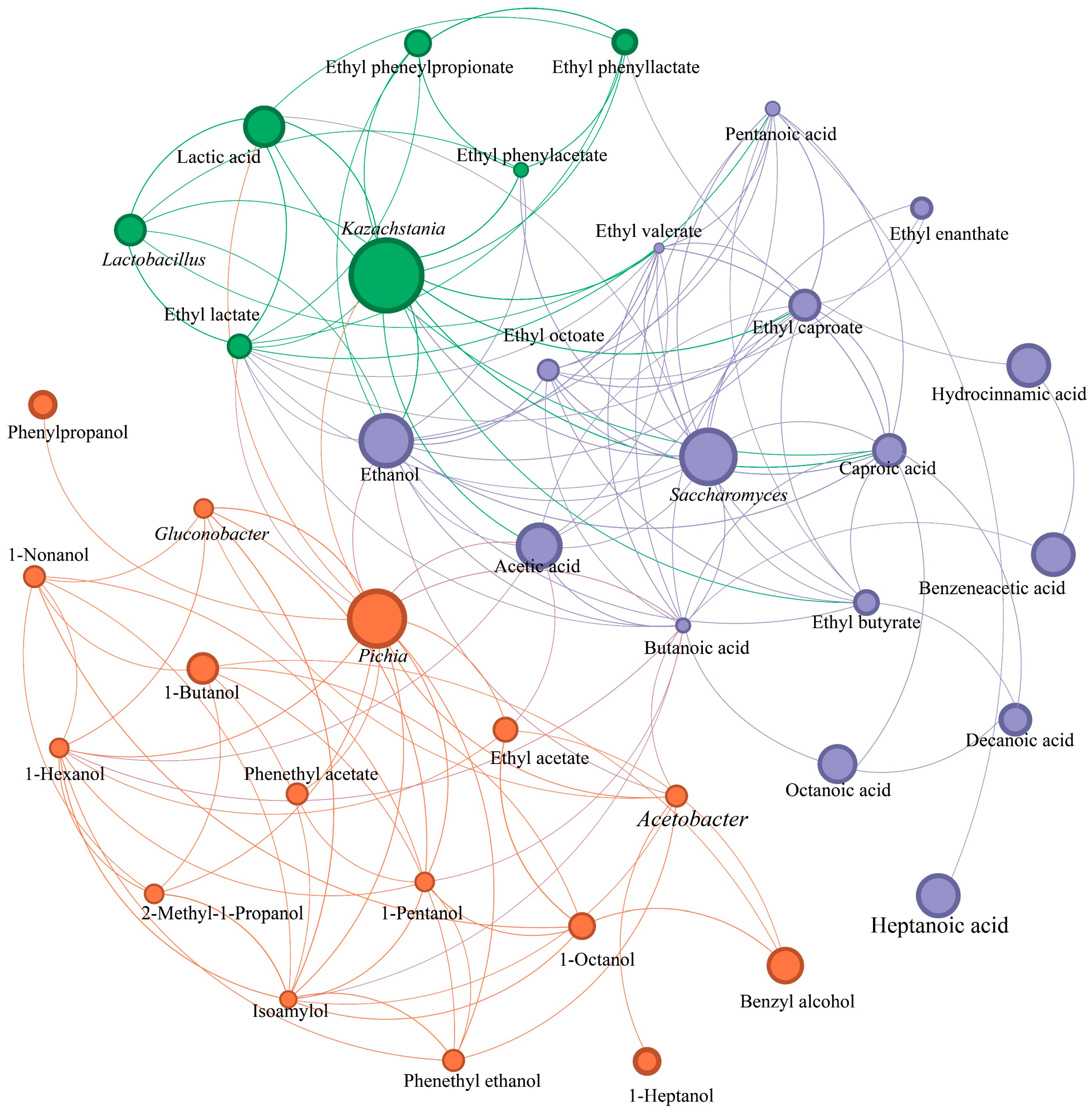
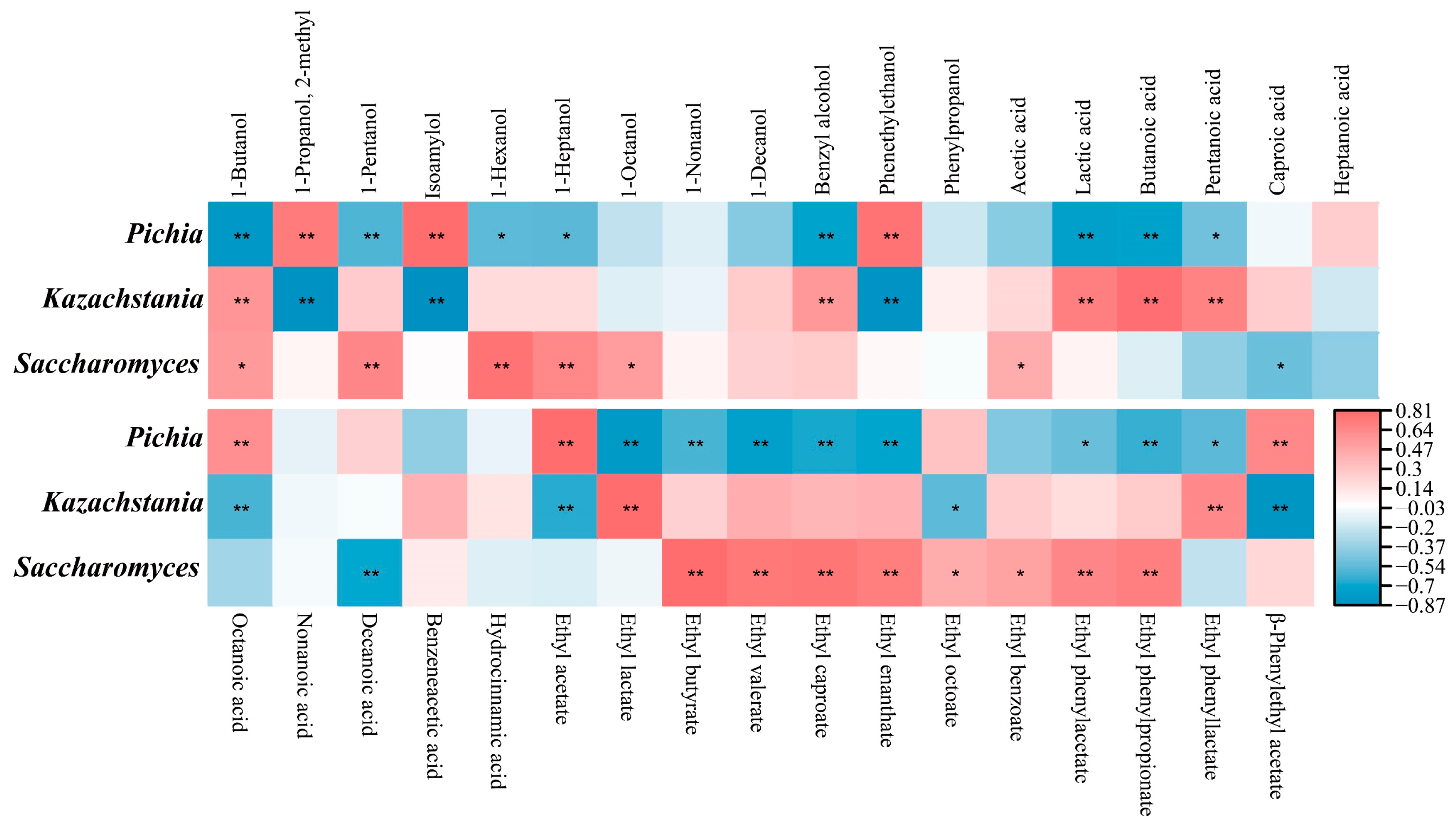
3.7. Functions Verification of Dominant Yeasts and Their Relationships to Flavor Substances
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Statistics Bureau. Baijiu Industry in China by Analysis of the Data Searching “Baijiu” in National Bureau of Statistics from Jan to Dec of 2022. 2023. Available online: https://www.stats.gov.cn/ (accessed on 25 July 2024).
- Xu, Y.; Zhao, J.; Liu, X.; Zhang, C.; Zhao, Z.; Li, X.; Sun, B. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 130920. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tan, G.; Chen, Q.; Dong, W.; Chen, P.; Cai, K.; Hu, Y.; Zhang, W.; Peng, N.; Liang, Y.; et al. Detection of viable and total fungal community in zaopei of Chinese strong-flavor baijiu using PMA combined with qPCR and HTS based on ITS2 region. BMC Microbiol. 2021, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Zou, W.; Zhao, C.; Luo, H. Diversity and Function of Microbial Community in Chinese Strong-Flavor Baijiu Ecosystem: A Review. Front. Microbiol. 2018, 9, 671. [Google Scholar] [CrossRef]
- He, Y.; Liu, Z.; Qian, M.; Yu, X.; Xu, Y.; Chen, S. Unraveling the chemosensory characteristics of strong-aroma type Baijiu from different regions using comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry and descriptive sensory analysis. Food Chem. 2020, 331, 127335. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, B.; Fan, G.; Teng, C.; Xiong, K.; Zhu, Y.; Li, J.; Li, X. The brewing process and microbial diversity of strong flavour Chinese spirits: A review. J. Inst. Brew. 2017, 123, 5–12. [Google Scholar] [CrossRef]
- Qian, W.; Lu, Z.-M.; Chai, L.-J.; Zhang, X.-J.; Li, Q.; Wang, S.-T.; Shen, C.-H.; Shi, J.-S.; Xu, Z.-H. Cooperation within the microbial consortia of fermented grains and pit mud drives organic acid synthesis in strong-flavor Baijiu production. Food Res. Int. 2021, 147, 110449. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, W.; Gao, J.; Ren, C.; Xu, Y. Revealing the Characteristics of Glucose- and Lactate-Based Chain Elongation for Caproate Production by Caproicibacterium lactatifermentans through Transcriptomic, Bioenergetic, and Regulatory Analyses. mSystems 2022, 7, e00534-22. [Google Scholar] [CrossRef]
- Zeng, C.; Zeng, X.; Xia, S.; Ye, G. Caproicibacterium argilliputei sp. nov., a novel caproic acid producing anaerobic bacterium isolated from pit clay. Int. J. Syst. Evol. Microbiol. 2024, 74, 6246. [Google Scholar] [CrossRef]
- Tu, W.; Cao, X.; Cheng, J.; Li, L.; Zhang, T.; Wu, Q.; Xiang, P.; Shen, C.; Li, Q. Chinese Baijiu: The Perfect Works of Microorganisms. Front. Microbiol. 2022, 13, 919044. [Google Scholar] [CrossRef]
- Wang, H.; Gu, Y.; Zhou, W.; Zhao, D.; Qiao, Z.; Zheng, J.; Gao, J.; Chen, X.; Ren, C.; Xu, Y. Adaptability of a Caproate-Producing Bacterium Contributes to Its Dominance in an Anaerobic Fermentation System. Appl. Environ. Microbiol. 2021, 87, e01203-21. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Liu, P.; Chang, X.; Yin, H.; Cheng, L.; Teng, C.; Gong, Y.; Li, X. Isolation and Identification of a High-Yield Ethyl Caproate-Producing Yeast From Daqu and Optimization of Its Fermentation. Front. Microbiol. 2021, 12, 663744. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Hou, X.; Tian, Q.; Hui, M. Gene Mining and Flavour Metabolism Analyses of Wickerhamomyces anomalus Y-1 Isolated From a Chinese Liquor Fermentation Starter. Front. Microbiol. 2022, 13, 891387. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wu, Q.; Xu, Y. Comparative studies on the fermentation performance of autochthonous Saccharomyces cerevisiae strains in Chinese light-fragrant liquor during solid-state or submerged fermentation. J. Appl. Microbiol. 2017, 122, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, K.; Chen, M.; Fan, J.; Han, S.; Hou, J.; Chi, L.; Liu, Y.; Wei, T. Profiling the composition and metabolic activities of microbial community in fermented grain for the Chinese strong-flavor Baijiu production by using the metatranscriptome, high-throughput 16S rRNA and ITS gene sequencings. Food Res. Int. 2020, 138, 109765. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, L.; Wu, Z.; Zhang, W.; Deng, Y.; Zhong, F.; Li, J. Analysis of the fungi community in multiple- and single-grains Zaopei from a Luzhou-flavor liquor distillery in western China. World J. Microbiol. Biotechnol. 2011, 27, 1869–1874. [Google Scholar] [CrossRef]
- You, L.; Zhao, D.; Zhou, R.; Tan, Y.; Wang, T.; Zheng, J. Distribution and function of dominant yeast species in the fermentation of strong-flavor baijiu. World J. Microbiol. Biotechnol. 2021, 37, 26. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Shen, H.; Liu, H.; Song, F.; Li, P.; Peng, N.; Liang, Y.; Zhao, S. Unraveling the microbial community and succession during zha-chili fermentation and their relationships with flavor formation. Food Res. Int. 2022, 157, 111239. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Shen, H.; Yang, Q.; Chen, S.; Dun, Y.; Liang, Y.; Zheng, J.; Zhao, S. Deciphering Succession and Assembly Patterns of Microbial Communities in a Two-Stage Solid-State Fermentation System. Microbiol. Spectr. 2021, 9, e00718-21. [Google Scholar] [CrossRef]
- Dong, W.; Yang, Q.; Liao, Y.; Liu, Y.; Hu, Y.; Peng, N.; Liang, Y.; Zhao, S. Characterisation and comparison of the microflora of traditional and pure culture xiaoqu during the baijiu liquor brewing process. J. Inst. Brew. 2020, 126, 213–220. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fan, G.; Fu, Z.; Wang, W.; Xu, Y.; Teng, C.; Zhang, C.; Yang, R.; Sun, B.; Li, X. Effects of fortification of Daqu with various yeasts on microbial community structure and flavor metabolism. Food Res. Int. 2020, 129, 108837. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Zhang, Y.; Lu, N.; Shi, C.; Yan, S. Yeasts from Chinese strong flavour Daqu samples: Isolation and evaluation of their potential for fortified Daqu production. AMB Express 2021, 11, 176. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, J.; He, G. Effect of microbial interaction on flavor quality in Chinese baijiu fermentation. Front. Nutr. 2022, 9, 960712. [Google Scholar] [CrossRef] [PubMed]
- Bordet, F.; Joran, A.; Klein, G.; Roullier-Gall, C.; Alexandre, H. Yeast–Yeast Interactions: Mechanisms, Methodologies and Impact on Composition. Microorganisms 2020, 8, 600. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, L.; Xu, Y. Yeast community associated with the solid state fermentation of traditional Chinese Maotai-flavor liquor. Int. J. Food Microbiol. 2013, 166, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fan, G.; Li, X.; Fu, Z.; Liang, X.; Sun, B. Application of Wickerhamomyces anomalus in Simulated Solid-State Fermentation for Baijiu Production: Changes of Microbial Community Structure and Flavor Metabolism. Front. Microbiol. 2020, 11, 598758. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Du, H.; Xu, Y. Cooperative Response of Pichia kudriavzevii and Saccharomyces cerevisiae to Lactic Acid Stress in Baijiu Fermentation. J. Agric. Food Chem. 2020, 68, 4903–4911. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, Z.; Tang, Y.; Hu, C.; Sun, Q.; Morimura, S.; Kida, K. Analysis of the Fungal Community in Zaopei During the Production of Chinese Luzhou-flavour Liquor. J. Inst. Brew. 2007, 113, 21–27. [Google Scholar] [CrossRef]
- Xu, Y.; Zhi, Y.; Wu, Q.; Du, R.; Xu, Y. Zygosaccharomyces bailii Is a Potential Producer of Various Flavor Compounds in Chinese Maotai-Flavor Liquor Fermentation. Front. Microbiol. 2017, 8, 2609. [Google Scholar] [CrossRef]
- You, L.; Wang, T.; Yang, Z.; Feng, S. Performance of indigenous yeasts in the processing of Chinese strong-flavoured liquor during spontaneous mixed solid-state or submerged fermentation. J. Inst. Brew. 2015, 121, 295–303. [Google Scholar] [CrossRef]
- Bardi, L.; Cocito, C.; Marzona, M. Saccharomyces cerevisiae cell fatty acid composition and release during fermentation without aeration and in absence of exogenous lipids. Int. J. Food Microbiol. 1999, 47, 133–140. [Google Scholar] [CrossRef]
- Nieto-Sarabia, V.; Ballinas-Cesatti, C.; Melgar-Lalanne, G.; Cristiani-Urbina, E.; Morales-Barrera, L. Isolation, identification, and kinetic and thermodynamic characterization of a Pichia kudriavzevii yeast strain capable of fermentation. Food Bioprod. Process. 2022, 131, 109–124. [Google Scholar] [CrossRef]
- Chu, Y.; Li, M.; Jin, J.; Dong, X.; Xu, K.; Jin, L.; Qiao, Y.; Ji, H. Advances in the Application of the Non-Conventional Yeast Pichia kudriavzevii in Food and Biotechnology Industries. J. Fungi 2023, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Harth, H.; Van Kerrebroeck, S.; Leroy, F. Yeast diversity of sourdoughs and associated metabolic properties and functionalities. Int. J. Food Microbiol. 2016, 239, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hou, Y.; Chen, H.; Wang, J.; Zhang, C.; Zhao, Z.; Ao, R.; Huang, H.; Hong, J.; Zhao, D.; et al. “Key Factor” for Baijiu Quality: Research Progress on Acid Substances in Baijiu. Foods 2022, 11, 92959. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhong, H.; Zhao, D.; Du, H.; Xu, Y. Succession rate of microbial community causes flavor difference in strong-aroma Baijiu making process. Int. J. Food Microbiol. 2019, 311, 108350. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Lin, Y.; Chen, K.; Ou, M.; Zhang, J. Physicochemical Factors Affecting Microbiota Dynamics During Traditional Solid-State Fermentation of Chinese Strong-Flavor Baijiu. Front. Microbiol. 2020, 11, 2090. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Yang, F.; Wang, H.; Wang, L. Yeasts and their importance to the flavour of traditional Chinese liquor: A review. J. Inst. Brew. 2020, 125, 214–221. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Q.; Chen, D.; Fu, B.; Zhang, Y.; Zhang, Y.; Xia, X.; Peng, N.; Liang, Y.; Zhao, S. Study on microbial communities and higher alcohol formations in the fermentation of Chinese Xiaoqu Baijiu produced by traditional and new mechanical technologies. Food Res. Int. 2021, 140, 109876. [Google Scholar] [CrossRef]
- Li, R.; Xu, M.; Zheng, J.; Liu, Y.; Sun, C.; Wang, H.; Guo, X.; Xiao, D.; Wu, X.; Chen, Y. Application Potential of Baijiu Non-Saccharomyces Yeast in Winemaking Through Sequential Fermentation With Saccharomyces cerevisiae. Front. Microbiol. 2022, 13, 902597. [Google Scholar]
- Qian, Y.L.; An, Y.; Chen, S.; Qian, M.C. Characterization of Qingke Liquor Aroma from Tibet. J. Agric. Food Chem. 2019, 67, 13870–13881. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Teng, C.; Xu, D.; Fu, Z.; Liu, P.; Wu, Q.; Yang, R.; Li, X. Improving Ethyl Acetate Production in Baijiu Manufacture by Wickerhamomyces anomalus and Saccharomyces cerevisiae Mixed Culture Fermentations. BioMed Res. Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]


| Group Sets | Parameters |
|---|---|
| CK group | 20 kg brewed grains + 2.0 kg Daqu |
| SF group | 20 kg brewed grains + 1.8 kg Daqu + 0.2 kg SF |
| PF group | 20 kg brewed grains + 1.8 kg Daqu + 0.2 kg PF |
| KF group | 20 kg brewed grains + 1.8 kg Daqu + 0.2 kg KF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Zeng, Y.; Ma, J.; Cai, K.; Guo, T.; Tan, G.; Yu, X.; Hu, Y.; Peng, N.; Zhao, S. Characteristics and Functions of Dominant Yeasts Together with Their Applications during Strong-Flavor Baijiu Brewing. Foods 2024, 13, 2409. https://doi.org/10.3390/foods13152409
Dong W, Zeng Y, Ma J, Cai K, Guo T, Tan G, Yu X, Hu Y, Peng N, Zhao S. Characteristics and Functions of Dominant Yeasts Together with Their Applications during Strong-Flavor Baijiu Brewing. Foods. 2024; 13(15):2409. https://doi.org/10.3390/foods13152409
Chicago/Turabian StyleDong, Weiwei, Yulun Zeng, Jiyuan Ma, Kaiyun Cai, Tingting Guo, Guangxun Tan, Xiang Yu, Yuanliang Hu, Nan Peng, and Shumiao Zhao. 2024. "Characteristics and Functions of Dominant Yeasts Together with Their Applications during Strong-Flavor Baijiu Brewing" Foods 13, no. 15: 2409. https://doi.org/10.3390/foods13152409






