Valorization of Taioba Products and By-Products: Focusing on Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Selection Process
2.2. Data Extraction
3. Results
4. General Aspects of the Taioba Plant (Xanthosoma sagittifolium)
5. Nutritional and Functional Attributes of Taioba Leaves
6. Nutritional and Functional Behavior of the Taioba Tubers and Their Products
7. Starch from the Taioba
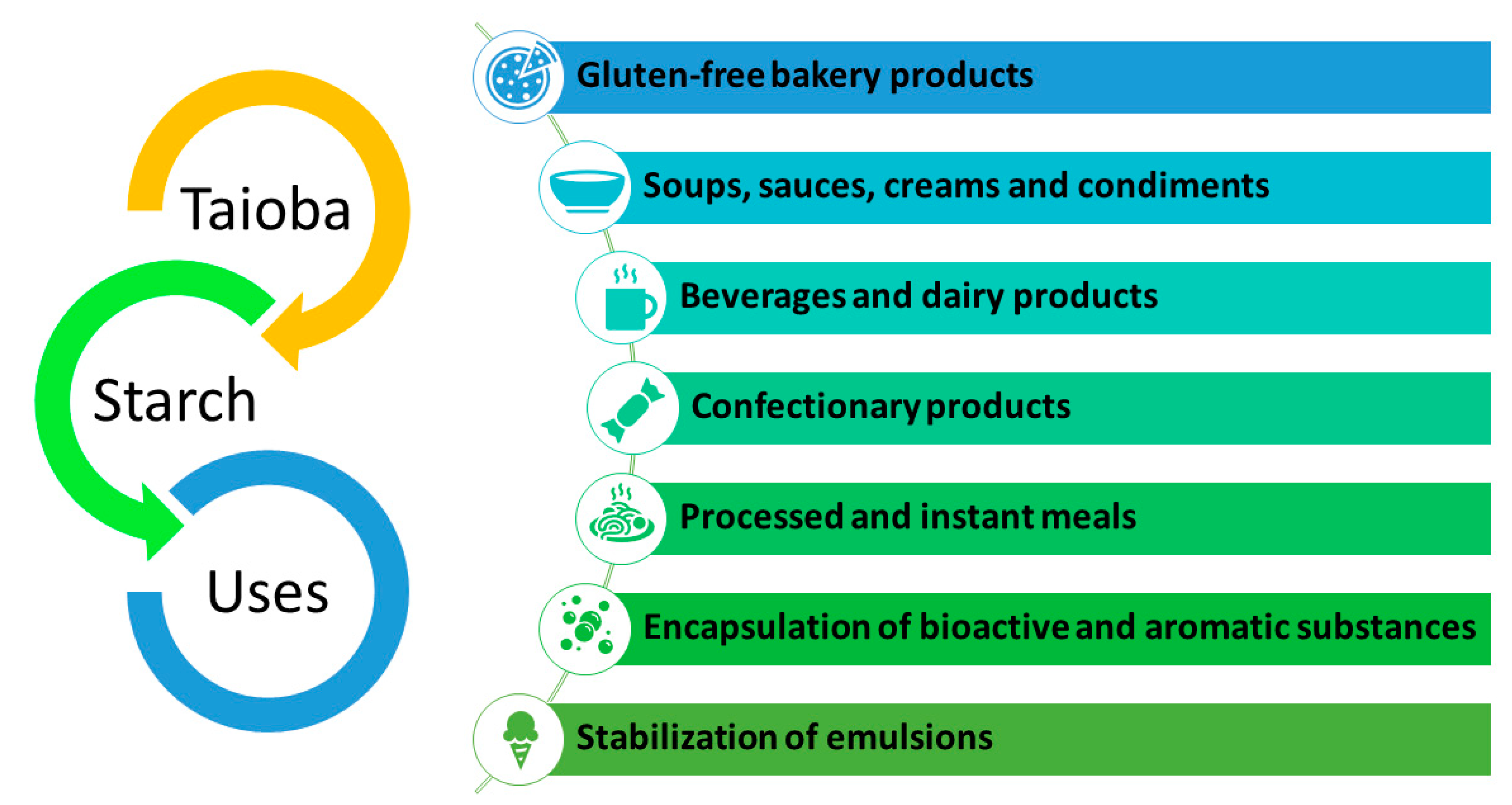
8. Technological Properties of Taioba Starch
9. Conclusions: Unlocking the Potential of Taioba
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Botrel, N.; Freitas, S.; Fonseca, M.J.O.; Melo, R.A.C.; Madeira, N. Nutritional value of unconventional leafy vegetables grown in the Cerrado Biome/Brazil. Braz. J. Food. Technol. 2020, 23, e2018174. [Google Scholar] [CrossRef]
- Cunha, M.A.; Pinto, L.C.; Santos, I.R.P.; Neves, B.M.; Cardoso, R.C.V. Neglected and Underutilized Species in the perspective of Food and Nutritional Security promotion in Brazil. Res. Soc. Dev. 2021, 10, e20610313306. [Google Scholar] [CrossRef]
- Costa, A.; Da Silva, E.C.; De Almeida, C.L.; Moreira, M.L.; Mascarenhas, M.G.; Nunes, M.T.F. Cultivation of taioba in hydroponic system (ebb and flow) using different substrates. Sci. Plena 2020, 16, 060201. [Google Scholar] [CrossRef]
- Santos, O.V.; Cunha, N.S.R.; Duarte, S.P.A.; Soares, S.D.; Costa, R.S.; Mendes, P.M.; Martins, M.G.; Nascimento, F.C.A.; Figueira, M.S.; Costa, B.E.T. Determination of bioactive compounds obtained by the green extraction of taioba leaves (Xanthosoma taioba) on hydrothermal processing. Food Sci. Technol. 2022, 42, e2242242. [Google Scholar] [CrossRef]
- Silva, A.; de Jesus Silva, A.; de Jesus Benevides, C.M. Revisão Sistemática Sobre PANC No Brasil: Aspectos Nutricionais e Medicinais. Rev. Cient. Multidisciplinar. 2022, 7, 132–151. [Google Scholar]
- Souza, P.G.; Azeredo, D.R.P.; Ayres, E.M.M. Food neophobia, risk perception and attitudes associations of Brazilian consumers towards non-conventional edible plants and research on sale promotional strategies. Food Res. Int. 2023, 163, 112628. [Google Scholar] [CrossRef]
- Araújo, S.S.; Araújo, P.S.; Giunco, A.J.; Silva, S.M.; Argandoña, E.J.S. Bromatology, Food Chemistry and Antioxidant Activity of Xanthosoma sagittifolium (L.) Schott. Emir. J. Food Agric. 2019, 31, 188–195. [Google Scholar] [CrossRef]
- Muricy, M.A.L.C.; Evangelista-Barreto, N.S. Desenvolvimento e análise sensorial de pão de forma enriquecido com concentrado proteico de peixe e farinha de taioba. In Ciência e Tecnologia de Alimentos: Pesquisa e Práticas Contemporâneas, 1st ed.; Cordeiro, C.A.M., Silva, E.M., Evangelista-Barreto, N.S., Eds.; Editora Científica Digital: São Paulo, Brazil, 2022; Volume 3, pp. 81–97. [Google Scholar]
- Bansal, S.; Sharma, M.K.; Singh, S.; Joshi, P.; Pathania, P.; Malhotra, E.V.; Rajkumar, S.; Misra, P. Histological and molecular insights in to in vitro regeneration pattern of Xanthosoma sagittifolium. Sci. Rep. 2023, 13, 5806. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.S.G.; Krupek, R.A. Caracterização morfológica, anatômica, e toxinas endógenas em Co-locasia esculenta (L.) Schott e Xanthosoma sagittifolium (L.) Schott. Luminária 2016, 18, 31–40. [Google Scholar] [CrossRef]
- Jordan, R.A.; Sanjinez-Argandoña, E.J.; Ferreira, O.M.; Quequeto, W.D.; Siqueira, V.C.; Mendoza, V.S.; Flozino, G.K.M.; Sanches, I.S.; Sanches, E.S.; Antunes, B.M. Effect of drying systems and conditions on specific energy consumption and taioba (Xanthosoma sagittifolium schott) bioactive compounds. Res. Soc. Dev. 2021, 10, e21610716512. [Google Scholar] [CrossRef]
- Ramos, A.S.; Verçosa, R.M.; Teixeira, S.M.L.; Teixeira-Costa, B.E. Calcium oxalate content from two Amazonian amilaceous roots and the functional properties of their isolated starches. Food Sci. Technol. 2020, 40, 705–711. [Google Scholar] [CrossRef]
- Dereje, B. Composition, morphology and physicochemical properties of starches derived from indigenous Ethiopian tuber crops: A review. Int. J. Biol. Macromol. 2021, 187, 911–921. [Google Scholar] [CrossRef]
- Makroo, H.A.; Naqash, S.; Saxena, J.; Sharma, S.; Majid, D.; Dar, B.N. Recovery and characteristics of starches from unconventional sources and their potential applications: A review. Appl. Food Res. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Maia, A.G.; Melo, N.G.M.; Dantas, L.O.; Souza, R.P.; Moreno, M.N.; Marinho, N.M.V.; Martim, S.R. Chips of Dioscorea bulbifera: An innovative alternative for the technological processing of non-conventional food plants from Amazon. Res. Soc. Dev. 2021, 10, e284101523052. [Google Scholar] [CrossRef]
- Pires, M.B.; Amante, R.E.; Petkowicz, C.L.O.; Esmerino, E.A.; Rodrigues, A.M.C.; Silva, L.H.M. Impact of extraction methods and genotypes on the properties of starch from peach palm (Bactris gasipaes Kunth) fruits. LWT-Food Sci. Technol. 2021, 150, 111983. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372. [Google Scholar] [CrossRef]
- Galiazzi, M.C.; Sousa, R.S. O que é isso que se mostra: O fenômeno na análise textual discursiva? Atos Pesqui. Educ. 2020, 15, 1167–1184. [Google Scholar] [CrossRef]
- Pereira, N.C.T.C.; Alburquerque, I.N.; Costa, K.H.; Borges, P.L.; Nunes, R.K.S.; Almeida, E.B.; Guedes, M.R.; Heen, R. Ações de educação alimentar e nutricional com grupos em vulnerabilidade social: Relato de experiência. Rev. Ciênc. Plur. 2020, 6, 170–191. [Google Scholar] [CrossRef]
- Bezerra, J.A.; Brito, M.M. Potencial nutricional e antioxidantes das Plantas alimentícias não convencionais (PANCs) e o uso na alimentação: Revisão. Res. Soc. Dev. 2020, 9, e369997159. [Google Scholar] [CrossRef]
- Minello, L.; Sartori, V.C.; Touguinha, L.B.A.; Agostini, F.; Silva, S.M.; Salvador, M. Comparative study of different methods of extracting bioactive compounds from wild edible plants (WEP). Res. Soc. Dev. 2021, 10, e190101724210. [Google Scholar] [CrossRef]
- Silva, G.M.; Rocha, N.C.; Souza, B.K.M.; Amaral, M.P.C.; Cunha, N.S.R.; Moraes, L.V.S.; Gemaque, E.M.; Dutra, C.D.T.; Moura, J.S.; Mendes, P.M.O. Potencial das plantas alimentícias não convencionais (PANC): Uma revisão de literatura / The potential of unconventional food plants (PANC): A literature review. Braz. J. Dev. 2022, 8, 14838–14853. [Google Scholar] [CrossRef]
- Hakim, L.; Triwitono, P.; Marseno, D.W. Microwave treatment to optimize physicochemical properties of modified Busil (Xanthosoma sagittifolium) starch. Food Res. 2022, 6, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, J.R.J.; Correa, J.L.G.; Resende, N.S.; Balbinoti, T.C.V.; Gatti, I.P.; Mendonça, K.S. Ethanol pretreatment in taioba leaves during vacuum drying. Food Sci. Technol. 2021, 45, e020421. [Google Scholar] [CrossRef]
- Benevides, C.M.; Silva, H.B.M.; Lopes, M.V.; Montes, S.S.; Silva, A.L.S.; Matos, R.A.; Junior, A.F.S.; Souza, A.C.S.; Bezerra, M.A. Multivariate analysis for the quantitative characterization of bioactive compounds in “Taioba” (Xanthosoma sagittifolium) from Brazil. J. Food Meas. Charact. 2020, 16, 1901–1910. [Google Scholar] [CrossRef]
- Farias, F.A.C.; Moretti, M.M.S.; Costa, M.S.; Júnior Bordignon, S.E.; Cavalcante, K.S.B.; Boscolo, M.; Gomes, E.; Franco, C.M.L.; Silva, R. Structural and physicochemical characteristics of taioba starch in comparison with cassava starch and its potential for ethanol production. Ind. Crops Prod. 2020, 157, 112825. [Google Scholar] [CrossRef]
- Souza, B.J.; Duarte, L.S.F.; Lima, C.L.S.; Silva, A.P.P.; Moura, L.F.W.G.; Mendes, F.N.P.; Veira, I.G.P.; Bonilla, O.H.; Nascimento, N.R.F.; Guedes, M.I.F. Potencial hipocolesterolêmico e antioxidante da folha de Taioba (Xanthosoma sagittifolium (L.) Schott) em camundongos dislipidêmicos induzidos por dieta. Nutrivisa 2024, 11, e12562. [Google Scholar] [CrossRef]
- Moura, H.F.S.; Dias, F.S.; Souza, L.B.S.; Magalhães, B.E.A.; Tannus, C.A.; Carvalho, W.C.; Brandão, G.C.; Santos, W.N.L.; Korn, M.G.A.; Santos, D.C.M.B.; et al. Evaluation of multielement/proximate composition and bioactive phenolics contents of unconventional edible plants from Brazil using multivariate analysis techniques. Food Chem. 2021, 363, 129995. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, A.F.L.; Pinheiro, A.P.O.; Narita, I.M.P.; Dalla Villa, R.; Paiva de Oliveira, A. Composição centesimal e bioacessibilidade in vitro de ferro em folhas de duas espécies olerícolas não-convencionais. ESAFIOS-Rev. Interdiscip. Univ. Fed. Tocantins 2023, 10, 1–14. [Google Scholar] [CrossRef]
- Silva, C.M.; Martins, J.B.C.; Abreu, C.R.C.; Silva, D.M. Vitamina C para aumento da imunidade: Efeitos benéficos e efeitos adversos. Rev. JRG Estud. Acad. 2022, 5, 5. [Google Scholar] [CrossRef]
- Espinosa-Solis, V.; Zamudio-Flores, P.B.; Espino-Díaz, M.; Vela-Gutiérrez, G.; Rendón-Villalobos, J.R.; Hernández-González, M.; Hernández-Centeno, F.; López-De-La-Peña, H.Y.; Salgado-Delgado, R.; Ortega-Ortega, A. Physicochemical Characterization of Resistant Starch Type-III (RS3) Obtained by Autoclaving Malanga (Xanthosoma sagittifolium) Flour and Corn Starch. Molecules 2021, 26, 4006. [Google Scholar] [CrossRef]
- Santos, P.P.A.; Ferrari, G.S.; Rosa, M.S.; Almeida, K.; Araújo, L.A.; Pereira, M.H.C.; Wanderley, M.E.F.; Morato, P.N. Desenvolvimento e caracterização de sorvete funcional de alto teor proteico com ora-pro-nóbis (Pereskia aculeata Miller) e inulina. Braz. J. Food Technol. 2022, 25, e2020129. [Google Scholar] [CrossRef]
- Gonçalves, J.; Silva, G.C.O.; Carlos, L.A. Compostos bioativos em flores comestíveis. Perspect. Online Biol. Saúde 2019, 9, 11–20. [Google Scholar] [CrossRef]
- Avellar, G.S.; Andrade, R.M.; Brito, L.M.; Carlos, L.D.A.; Clarete, E. Compostos bioativos presentes em hortaliças não tradicionais cultivadas em hortas urbanas de Sete Lagoas-MG. Cad. Agroecol. 2018, 13, 1–5. Available online: https://cadernos.aba-agroecologia.org.br/cadernos/article/view/190 (accessed on 13 September 2023).
- Moura, I.O.; Santana, C.C.; Lourenço, Y.R.F.; Souza, M.F.; Silva, A.R.S.T.; Dolabella, S.S.; de Oliveira e Silva, A.M.; Oliveira, T.B.; Duarte, M.C.; Faraoni, A.S. Chemical Characterization, Antioxidant Activity and Cytotoxicity of the Unconventional Food Plants: Sweet Potato (Ipomoea batatas (L.) Lam.) Leaf, Major Gomes (Talinum paniculatum (Jacq.) Gaertn.) and Caruru (Amaranthus deflexus L.). Waste Biomass Valor. 2021, 12, 2407–2431. [Google Scholar] [CrossRef]
- Campos, R.A.S.; Junior, S.S.; Gonçalves, G.G.; Neves, L.G.; Gusmão, S.A.L.; Vianello, F.; Lima, G.P.P. Changes in bioactive compounds in spiny coriander leaves in response to inflorescence pruning at different growth stages. Sci. Hortic. 2019, 245, 250–257. [Google Scholar] [CrossRef]
- Nörnberg, M.L.; Pinheiro, P.N.; Nascimento, T.C.; Fernandes, A.S.; Nörnberg, M.F.B.L.; Lopes, E.J.; Zepka, L.Q.; Nörnberg, J.L. Compostos bioativos em manteigas: Carotenoides e ácidos graxos. Braz. J. Dev. 2022, 8, 10270–10288. [Google Scholar] [CrossRef]
- Filho, A.F.S.; Silva, D.A.; Silva, L.H.M.; Rodrigues, A.M.C. Evaluation of ultrasound-assisted extraction of bioactive compounds from amazonian chicory (Eryngium foetidum L.) and cariru (Talinum triangulare Jacq. Willd) leaves. Braz. J. Dev. 2021, 7, 118256–118270. [Google Scholar] [CrossRef]
- Petterson, A.R.; Lima, V.A.; Anaissi, F.J. Clorofila extraída de resíduo industrial da erva-mate (Llex paraguaiensis) uma possibilidade de economia circular. Quim. Nova 2022, 7, 767–776. [Google Scholar] [CrossRef]
- Arnoso, B.J.M.; Costa, G.F.; Schmidt, B. Biodisponibilidade e classificação de compostos fenólicos. Rev. Nutr. 2019, 18, 39–48. [Google Scholar] [CrossRef]
- Vargas, G.C.; Andrade, E.H.B. Estudo da atividade antioxidante dos compostos fenólicos na medicina preventiva: Revisão de literatura. Visão Acad. 2022, 23, 55–64. [Google Scholar] [CrossRef]
- Markusse, D.; Marcel, N.R.; Aboubakar, X.; Nicolas, N.Y.; Joël, S.; Moses, M.F.C. Production, physicochemical and sensory characterization of cocoyam mixed flours and pastes (achu). J. Food Meas. Charact. 2018, 12, 1242–1252. [Google Scholar] [CrossRef]
- Ndabikunze, B.K.; Talwana, H.A.L.; Mongi, R.J.; Issa-Zacharia, A.; Serem, A.K.; Palapala, V.; Nandi, J.O.M. Proximate and mineral composition of cocoyam (Colocasia esculenta L. and Xanthosoma sagittifolium L.) grown along the Lake Victoria Basin in Tanzania and Uganda. Afr. J. Food Sci. 2011, 5, 248–254. [Google Scholar] [CrossRef]
- Wada, E.; Feyissa, T.; Tesfaye, K. Proximate, Mineral and Antinutrient Contents of Cocoyam (Xanthosoma sagittifolium (L.) Schott) from Ethiopia. Int. J. Food Sci. 2019, 2019, 8965476. [Google Scholar] [CrossRef]
- Cukier, C.; Cukier, V. Micronutrientes. In Macro e Micronutrientes em Nutrição Clínica, 1st ed.; Editora Manole: Barueri, Brazil, 2020; Volume 1, pp. 433–599. [Google Scholar]
- Silva, J.C.; Santos, G.M.; Nunes, M.I.L.B.; Melo, P.K.M.; Silva Júnior, R.R.; Azevedo Filho, F.M.; Medeiros, A.D.F.F.; Vasconcelos Filho, F.S.L. The benefits of magnesium in exercise practitioners: Integrative literature review study. Res. Soc. Dev. 2021, 10, e35101119253. [Google Scholar] [CrossRef]
- Melo, S.R.S.; Santos, L.R.; Silva, T.M.; Cardoso, B.E.P.; Araújo, D.S.C.; Sousa, T.G.V.; Sousa, M.P.; Severo, J.S.; Marreiro, D.N. Suplementação com magnésio sobre a performance de atletas: Uma revisão sistemática Magnesium supplementation on the performance of healthy athletes: A systematic review. Res. Soc. Dev. 2020, 9, e117911754. [Google Scholar] [CrossRef]
- Lima, E.F.C.; Formiga, L.M.F.; Silva, D.M.C.; Feitosa, L.M.H.; Araújo, A.K.S.; Leal, S.R. Ingestão alimentar de cálcio e vitamina D em idosos. Rev. Enferm. 2019, 87, 1–8. [Google Scholar] [CrossRef]
- Oliveira, F.C.H.A.; Mendes, G.A.; Silva, W.M.B.; Nascimento, G.H.M.; Alves, D.R.; Pinheiro, S.O. Íon zinco e ácido ascórbico, uma combinação de seus efeitos imunológicos na prevenção da COVID-19: Um referencial teórico. In Opens Science Research II, 2nd ed.; Editora Científica: São Paulo, Brazil, 2022; Volume 2, pp. 594–603. [Google Scholar]
- Oliveira, H.A.B.; Anunciação, P.C.; Silva, B.P.; Souza, A.M.N.; Pinheiro, S.S.; Lucia, C.M.D.; Cardoso, L.M.; Castro, L.C.V.; Pinheiro-Sant’Ana, H.M. Nutritional value of non-conventional vegetables prepared by family farmers in rural communities. Cienc. Rural. 2019, 49, e20180918. [Google Scholar] [CrossRef]
- Morais, A.F.; Canapá, L.B.S.; Silveira, M.F.A.; Vera, R. Production and characterization of tannia rhizome flour. Sci. Electron. Arch. 2020, 13, 67–73. [Google Scholar] [CrossRef]
- Rosida, D.F.; Sarofa, U.; Aliffauziah, D. Characteristics of non-gluten noodles from modified cocoyam (Xanthosoma sagittifolium) and porang (Amorphophallus oncophyllus). Ital. J. Food Sci. 2022, 34, 13–23. [Google Scholar] [CrossRef]
- Adedeji, T.O.; Oluwalana, I.B. Development and Quality Evaluation of a Non-Alcoholic Beverage from Cocoyam (Xanthosoma sagittifolium and Colocasia esculenta). Niger. Food J. 2014, 32, 10–20. [Google Scholar] [CrossRef]
- Palomino, C.; Molina, Y.; Pérez, E. Atributos físicos y composición química de harinas y almidones de los tubérculos de Colocasia esculenta (L.) Schott. y Xanthosoma sagittifolium (L.) Schott. Rev. Fac. Agron. 2010, 36, 58–66. [Google Scholar]
- Odeku, O.A. Potentials of tropical starches as pharmaceutical excipients: A review. Starch/Staerke 2013, 65, 89–106. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R.A. Physical Properties of Thermoplastic Starch Derived from Natural Resources and Its Blends: A Review. Polymers 2021, 13, 1396. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Li, C.; Yu, W.; Gilbet, R.G.; Li, E. How amylose molecular fine structure of rice starch affects functional properties. Carbohydr. Polym. 2018, 204, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Majzoobi, M.; Farahnaky, A. Granular cold-water swelling starch; properties, preparation and applications, a review. Food Hydrocoll. 2021, 111, 106393. [Google Scholar] [CrossRef]
- Fuentes, C.; Castañeda, R.; Rengel, F.; Peñarrieta, J.M.; Nilsson, L. Characterization of molecular properties of wheat starch from three different types of breads using asymmetric flow field-flow fractionation (AF4). Food Chem. 2019, 298, 125090. [Google Scholar] [CrossRef]
- Szwengiel, A.; Lewandowicz, G.; Górecki, A.R.; Błaszczak, W. The effect of high hydrostatic pressure treatment on the molecular structure of starches with different amylose content. Food Chem. 2018, 240, 51–58. [Google Scholar] [CrossRef]
- Apriyanto, A.; Compart, J.; Fettke, J. A review of starch, a unique biopolymer–Structure, metabolism and in planta modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Saleh, A.S.M.; Zhang, B.; Zhao, K.; Xiangzhen, G.; Zhang, Q.; Li, W. Changes in structural, physicochemical, and digestive properties of normal and waxy wheat starch during repeated and continuous annealing. Carbohydr. Polym. 2020, 247, 116675. [Google Scholar] [CrossRef]
- Borba, V.S.; Silveira, C.O.; Alves, J.B.; Gropelli, V.M.; Badiale-Furlong, E. Modificações do amido e suas implicações tecnológicas e nutricionais. In Ciência e Tecnologia de Alimentos: Pesquisa e Práticas Contemporâneas, 1st ed.; Cordeiro, C.A.M., Silva, E.M., Silva, B.A., Eds.; Editora Científica Digital: São Paulo, Brazil, 2021; Volume 1, pp. 428–457. [Google Scholar]
- Schmiele, M.; Sampaio, U.M.; Clerici, M.T.P.S. Basic Principles: Composition and Properties of Starch. In Starches for Food Application. Chemical, Technological and Health Properties, 1st ed.; Clerici, M.S., Schmiele, M., Eds.; Editora Academic Press: Cambridge, MA, USA, 2018; Volume 1, pp. 10–60. [Google Scholar]
- Wang, S.; Chao, C.; Cai, J.; Niu, B.; Copeland, L.; Wang, S. Starch–lipid and starch–lipid–protein complexes: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1056–1079. [Google Scholar] [CrossRef]
- Cesar, A.L.T.M.S.; Cheim, L.M.G.; Rossignoli, P.A.; Rodrigues, L.J.; Silva, F.F.; Takeuchi, K.P.; Carvalho, D.M.; Faria, A.M.M. Physical chemicals and rheologicals characterization of corn starch (Zea mays L.) landraces popcorn. Res. Soc. Dev. 2021, 10, e402101321394. [Google Scholar] [CrossRef]
- Takeiti, C.Y.; Reis, R.C.; Carvalho, C.W.P.; Viana, E.S.; Oliveira, N.A.; Neta, P.J.; Oliveira, L.A. Propriedades Tecnológicas de Amidos Isolados de Plátanos e Bananas da Coleção de Germoplasma da Embrapa. Embrapa Agroind. Aliment. 2020, 36, 5–26. [Google Scholar]
- Felisberto, M.H.F.; Beraldo, A.L.; Costa, M.S.; Boas, F.B.; Franco, C.M.L.; Clerici, M.T.P.S. Characterization of young bamboo culm starch from Dendrocalamus asper. Food Res. Int. 2019, 124, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Barros, D.R.; Carvalho, A.P.M.G.; Silva, E.O.; Sampaio, U.M.; Souza, S.M.; Sanches, E.A.; Sant’ana, A.S.; Clerici, M.T.P.S.; Campelo, P.H. Ariá (Goeppertia allouia) Brazilian Amazon tuber as a non-conventional starch source for foods. Int. J. Biol. Macromol. 2021, 168, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.S.C.; Martim, S.R.; Souza, R.A.T.; Machado, A.R.G.; Teixeira, L.S.; Sousa, L.B.S.; Vasconcellos, M.C.; Teixeira, M.F.S. Extração e caracterização de amido de espécies de Dioscorea cultivadas na Amazônia. Bol. Mus. Para. Emílio Goeldi. Sér. Ciênc. Nat. 2019, 14, 439–452. [Google Scholar] [CrossRef]
- Ferronatto, A.N.; Rossi, R.C.; Cappellari, F. Resistant Starch: Functional Food Alternative to Glucose Homeostasis, Decrease of Fat Profile and Gut Microbiota Modulation. Rev. Saúde Desenvolv. 2020, 8, 109–120. [Google Scholar] [CrossRef]
- Cândido, H.T.; Molha, N.Z.; Eburneo, J.A.M.; Leonel, M. Minerals And Resistant Starch In Red Banana Flours ‘São Domingos’ Triploid (AAA) Minerales Y Almidón Resistente En Harinas De Plátano Rojo Triploide ‘São Domingos’ (AAA). Res. Soc. Dev. 2021, 10, e1810413860. [Google Scholar] [CrossRef]
- Alves, A.A.; Pigoso, A.A.; Chang, Y.K.; Tagliapietra, B.L.; Schmiele, M.; Campelo, P.H.; Clerici, M.T.P.S. Changes induced by diet with different concentrations of resistant starch in the metabolism of carbohydrates and lipids in Wistar rats. Res. Soc. Dev. 2021, 10, e18110716448. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Xi, H.; Xu, J.; Deng, D.; Huang, G. Effects of soluble dietary fiber on the crystallinity, pasting, rheological, and morphological properties of corn resistant starch. LWT-Food Sci. Technol. 2019, 111, 632–639. [Google Scholar] [CrossRef]
- Oliveira, L.A.; Souza, J.M.L.; Matos, M.F.R.; Alvares, V.S. Farinha de mandioca: Alimento fonte de fibras e amido resistente. In Embrapa Mandioca e Fruticultura, 1st ed.; Embrapa: Brasília, Brazil, 2021; Volume 1, pp. 09–19. [Google Scholar]
- Öztürk, S.; Mutlu, S. Physicochemical Properties, Modifications, and Applications of Resistant Starches. In Starches for Food Application: Chemical, Technological and Health Properties, 1st ed.; Clerici, M.S., Schmiele, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 1, pp. 297–332. [Google Scholar]
- Castro, D.S.; Moreira, I.S.; Melo, B.A.; Gomes, J.P.; Silva, W.P. Sensorial assessment of added ketchup of pitomba seed starch. Res. Soc. Dev. 2020, 9, e985986774. [Google Scholar] [CrossRef]
- Omoregie, E.H. Chemical Properties of Starch and Its Application in the Food Industry. In Chemical Properties of Starch; Intech Open: London, UK, 2020; Volume 32, pp. 137–144. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; Oliveira, R.A. Effect of incorporation of blackberry particles on the physicochemical properties of edible films of arrowroot starch. Dry. Technol. 2018, 37, 448–457. [Google Scholar] [CrossRef]
- Okyere, A.Y.; Rajendran, S.; Annor, G.A. Cold plasma technologies: Their effect on starch properties and industrial scale-up for starch modification. Curr. Res. Food Sci. 2022, 5, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Jesus, B.B.S.; Santana, K.S.L.; Oliveira, V.J.S.; Carvalho, M.J.S.; Almeida, W.A.B. PANCs—Plantas alimentícias não convencionais, benefícios nutricionais, potencial econômico e resgate da cultura: Uma revisão sistemática. Enciclopédia Biosfera 2020, 17, 309–322. [Google Scholar] [CrossRef]
- Carvalho, A.; Rosas, A.; Barros, D.; Laranjeira, R.; Dias, J.; Lima Júnior, E.; Nascimento, K.; Araújo, S.; Assis, T.; Campelo, P. Análise físico-quimico do amido de ária (Goeppertia allouia (AUBL.) Borchs. & S. Suárez). In Avanços em Ciência e Tecnologia de Alimentos, 1st ed.; Verruck, S., Ed.; Editora Científica Digital: São Paulo, Brazil, 2021; Volume 1, pp. 255–265. [Google Scholar]
- Moura, C.V.R.; Sousa, D.C.; Moura, E.M.; Araújo, E.C.E.; Sittolin, I.M. New biodegradable composites from starch and fibers of the babassu coconut. Polímeros 2021, 31, e2021007. [Google Scholar] [CrossRef]
- Felisberto, M.H.F.; Beraldo, A.L.; Sentone, D.T.; Klosterhoff, R.R.; Clerici, M.T.P.S.; Cordeiro, L.M.C. Young culm of Dendrocalamus asper, Bambusa tuldoides and B. vulgaris as source of hemicellulosic dietary fibers for the food industry. Food Res. Int. 2021, 140, 109866. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, G.R.; Crema, N.M.; Zuge, L.C.B.; Ribeiro, L.F. Physical and chemical evaluation of husk and pulp flours of Dioscorea bulbifera L. with the possibility of application in bakery. Braz. J. Dev. 2020, 6, 96201–96211. [Google Scholar] [CrossRef]
- Farias, D.P.; Araújo, F.F.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; Catharino, R.R.; Pastore, G.M. Distribution of nutrientes and functional potential in fractions of Eugenia pyriformis: Na underutilized native Brazilian fruit. Food Res. Int. 2020, 137, 109522. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Romanini, E.B.; Silva, E.; Pilau, E.J.; Costa, S.C.; Madrona, G.S. Uvaia (Eugenia pyriformis Cambess) residue as a source of antioxidants: An approach to ecofriendly extraction. LWT-Food Sci. Technol. 2021, 138, 110785. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tapia-Blácido, D.R. Isolation and characterization of starch from babassu mesocarp. Food Hydrocoll. 2017, 55, 47–55. [Google Scholar] [CrossRef]
- Tresina, P.S.; Doss, A.; Mohan, V.R. Nutritional and antinutritional assessment of some underutilized corms, rhizomes and tubers. Trop. Subtrop. Agroecosyst. 2020, 23, 1–11. [Google Scholar] [CrossRef]


| Nutritional Composition (g 100 g−1) | Souza et al. [27] | Moura et al. [28] | Siqueira et al. [29] | ||
|---|---|---|---|---|---|
| Raw | Cooked | ||||
| Moisture * | 82.00 | 92.00 | 86.58 | 90.77 | 85.00 ± 0.11 |
| Minerals | ND ** | ND ** | 1.74 | 1.57 | 3.45 ± 0.71 |
| Lipids | 0.20 | 0.20 | 0.62 | 0.51 | 0.75 ± 0.05 |
| Proteins | 3.00 | 2.80 | 3.05 | 3.08 | 4.15 ± 0.02 |
| Carbohydrates | 2.60 | 2.90 | 4.12 | 4.07 | 8.17 ± 0.15 |
| Fibers | 11.00 | 1.10 | 3.89 | ND ** | ND ** |
| Calorific value (kcal 100 g−1) | 24.20 | 24.60 | 34.26 | ND ** | ND ** |
| Bioactive Compounds | Araújo et al. [7] | Jordan et al. [11] | Avelar [34] |
|---|---|---|---|
| Ascorbic acid (mg 100 g−1) | 87 ± 0.79 | 561.60 ± 0.73 | 58.30 ± 0.82 |
| Carotenoids (mg g−1) | 83.19 ± 0.54 | ND * | 36.05 |
| Total chlorophyll (mg 100 g−1) | 8.94 ± 0.02 | 383.22 ± 0.01 | 272.81 |
| Phenolic compounds (mg GAE g−1) | 5.33 ± 0.18 | 83.20 ± 0.82 | 2.61 |
| Flavonoids (mg 100 g−1) | ND * | ND * | 55.00 |
| Micronutrients (mg 100 g−1) | Botrel et al. [1] | Ndabikunze [43] | Wada [44] |
|---|---|---|---|
| Sodium | 1.05 | 8.39 ± 0.22 | 29.22 ± 1.44 |
| Potassium | 302.86 | 760.21 ± 29.00 | 1085.70 ± 32.10 |
| Magnesium | 0.32 | 0.783 ± 0.05 | 2.48 ± 0.19 |
| Calcium | 77.63 | 76.66 ± 11.70 | 56.57 ± 1.50 |
| Manganese | 23.82 | 69.53 ± 4.90 | 78.77 ± 0.67 |
| Iron | 1.17 | 3.28 ± 0.19 | 8.20 ± 0.60 |
| Zinc | 0.21 | 1.35 ± 0.13 | 3.07 ± 0.10 |
| Copper | 0.10 | 0.43 ± 0.45 | 1.04 ± 0.08 |
| Phosphor | ND * | 277.76 ± 9.015 | 120.93 ± 2.07 |
| Source | Moisture (g 100 g−1) | Lipids (g 100 g−1) | Minerals (g 100 g−1) | Proteins (g 100 g−1) | Apparent Amylose (g 100 g−1) | Starch Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| Bactris gasipaes | 10.63 ± 0.20–12.69 ± 0.21 | 0.47 ± 0.03–1.04 ± 0.06 | 0.11 ± 0.03–0.15 ± 0.01 | 0.35 ± 0.04–0.49 ± 0.02 | 1.07 ± 0.06–3.99 ± 0.01 | 28.25 ± 3.19–61.70 ± 1.91 | [16] |
| Dendrocalamus asper | 5.88 ± 0.4–6.88 ± 0.65 | 0.21 ± 0.04–0.46 ± 0.08 | 0.90 ± 0.01–1.13 ± 0.02 | 1.76 ± 0.02–2.17 ± 0.03 | 12.03 ± 0–29.71 ± 0 | 11.06 ± 1.90–15.19 ± 1.47 | [68] |
| 3.73 ± 0.09–4.65 ± 0.13 | 0.19 ± 0.12–0.22 ± 0.03 | 0.36 ± 0.01–0.72 ± 0.02 | 0.77 ± 0.02–1.04 ± 0.03 | ND * | 1.01 ± 0.17–3.94 ± 0.07 | [84] | |
| Dioscorea alata and D. altissima. | 9.67 ± 0.09–13.46 ± 0.01 | 0.57 ± 0.03–0.83 ± 0.04 | 0.06 ± 0.00–0.11 ± 0.02 | 0.77 ± 0.00–0.80 ± 0.01 | 17.91 ± 0.01–19.15 ± 0.01 | 7.76–8.57 | [70] |
| Dioscorea bulbifera | 10.43 ± 0.02–12.06 ± 0.01 | 0.85 ± 0.13–1.63 ± 0.58 | 2.14 ± 0.03–2.70 ± 0.03 | 13.83 ± 0.29–16.53 ± 0.26 | ND * | 57.77 ± 3.73–75.70 ± 2.92 | [85] |
| Eugenia pyriformis | 91.75 ± 0.13 | 2.95 ± 0.17 | 3.63 ± 0.14 | 17.85 ± 0.23 | ND * | ND * | [86] |
| 76.0 ± 1.0 | 0.35 ± 0.006 | 0.31 ± 0.015 | 2.66 ± 0.006 | ND * | ND * | [87] | |
| Goeppertia allouia | 7.02 ± 0.38 | 0.028 ± 0.01 | 0.03 ± 0.02 | 0.026 ± 0.4 | ND * | 13.145 ± 0.06 | [82] |
| 8.45 ± 0.04 | 0.39 ± 0.01 | 0.15 ± 0.02 | 2.4 ± 0.1 | 38.6 ± 1.6 | ND * | [70] | |
| Orbignya speciosa | 6.35 ± 0.14 | 0.72 ± 0.03 | 0.31 ± 0.01 | 1.02 ± 0.06 | 36.51 ± 1.02 | ND * | [83] |
| 15.1 ± 1.6 | 1.8 ± 0.4 | 1.1 ± 0.1 | 1.4 ± 0.1 | 36.6 ± 0.5 | ND * | [88] | |
| Xanthosoma sagittifolium | 72.16 ± 0.76 | 6.32 ± 0.12 | 4.68 ± 0.08 | 9.68 ± 0.05 | ND * | ND * | [89] |
| 77.1 ± 2.82 | 0.2 ± 0.06 | 1.1 ± 0.09 | 4.4 ± 0.42 | ND * | ND * | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Paula de Almeida Duarte, S.; Teixeira-Costa, B.E.; do Rosário, R.C.; Amante, E.R.; Pires, M.B.; dos Santos, O.V. Valorization of Taioba Products and By-Products: Focusing on Starch. Foods 2024, 13, 2415. https://doi.org/10.3390/foods13152415
de Paula de Almeida Duarte S, Teixeira-Costa BE, do Rosário RC, Amante ER, Pires MB, dos Santos OV. Valorization of Taioba Products and By-Products: Focusing on Starch. Foods. 2024; 13(15):2415. https://doi.org/10.3390/foods13152415
Chicago/Turabian Stylede Paula de Almeida Duarte, Samanta, Bárbara E. Teixeira-Costa, Rosely Carvalho do Rosário, Edna Regina Amante, Márlia Barbosa Pires, and Orquídea Vasconcelo dos Santos. 2024. "Valorization of Taioba Products and By-Products: Focusing on Starch" Foods 13, no. 15: 2415. https://doi.org/10.3390/foods13152415






