Effects of Different Drying Methods on the Structural Characteristics and Multiple Bioactivities of Rosa roxburghii Tratt Fruit Polysaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Drying Experiments
2.3. Preparation of RRT Polysaccharides
2.4. Structural Characterization of RRTPs
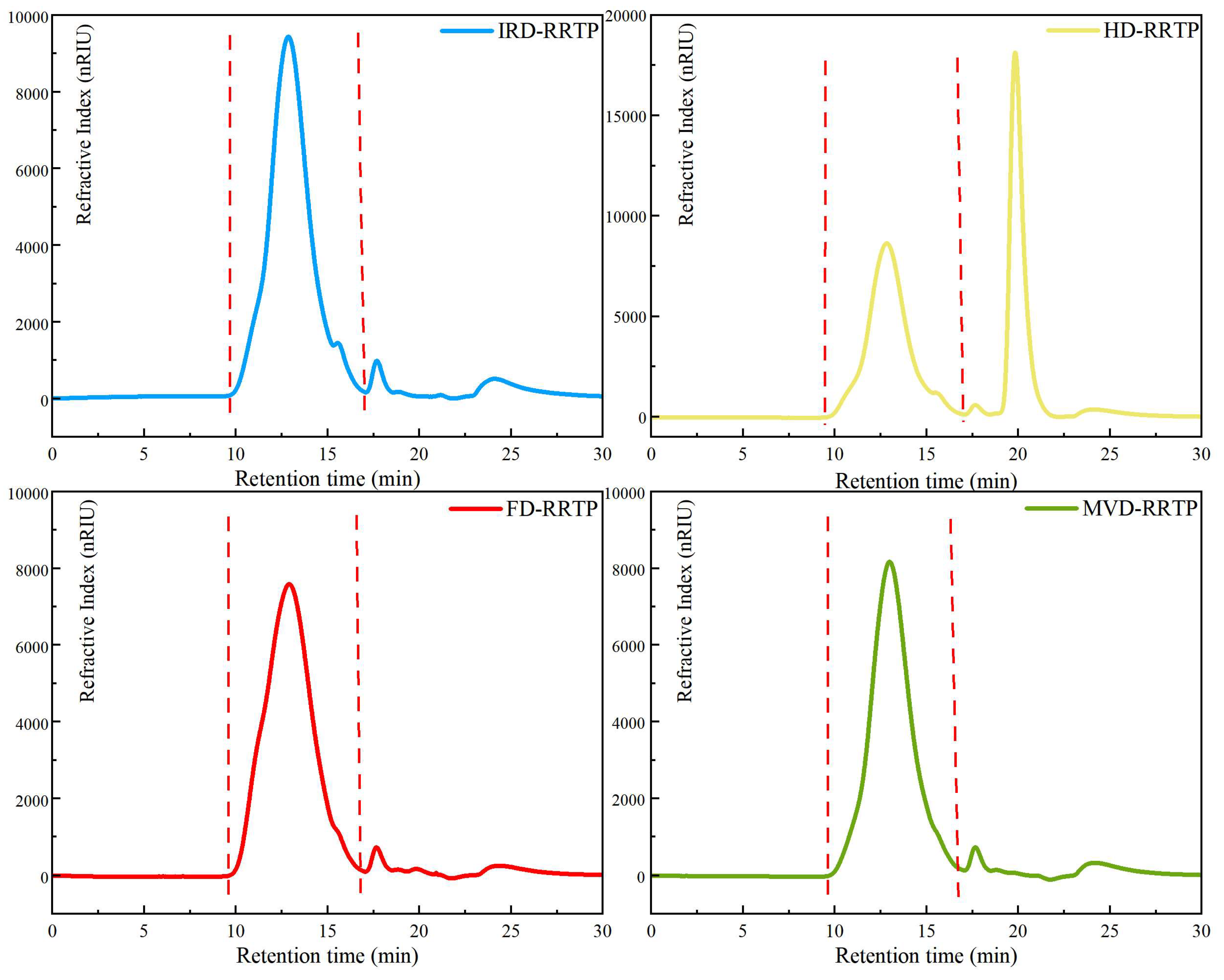
2.4.1. Chemical Composition and Molecular Weight Determination
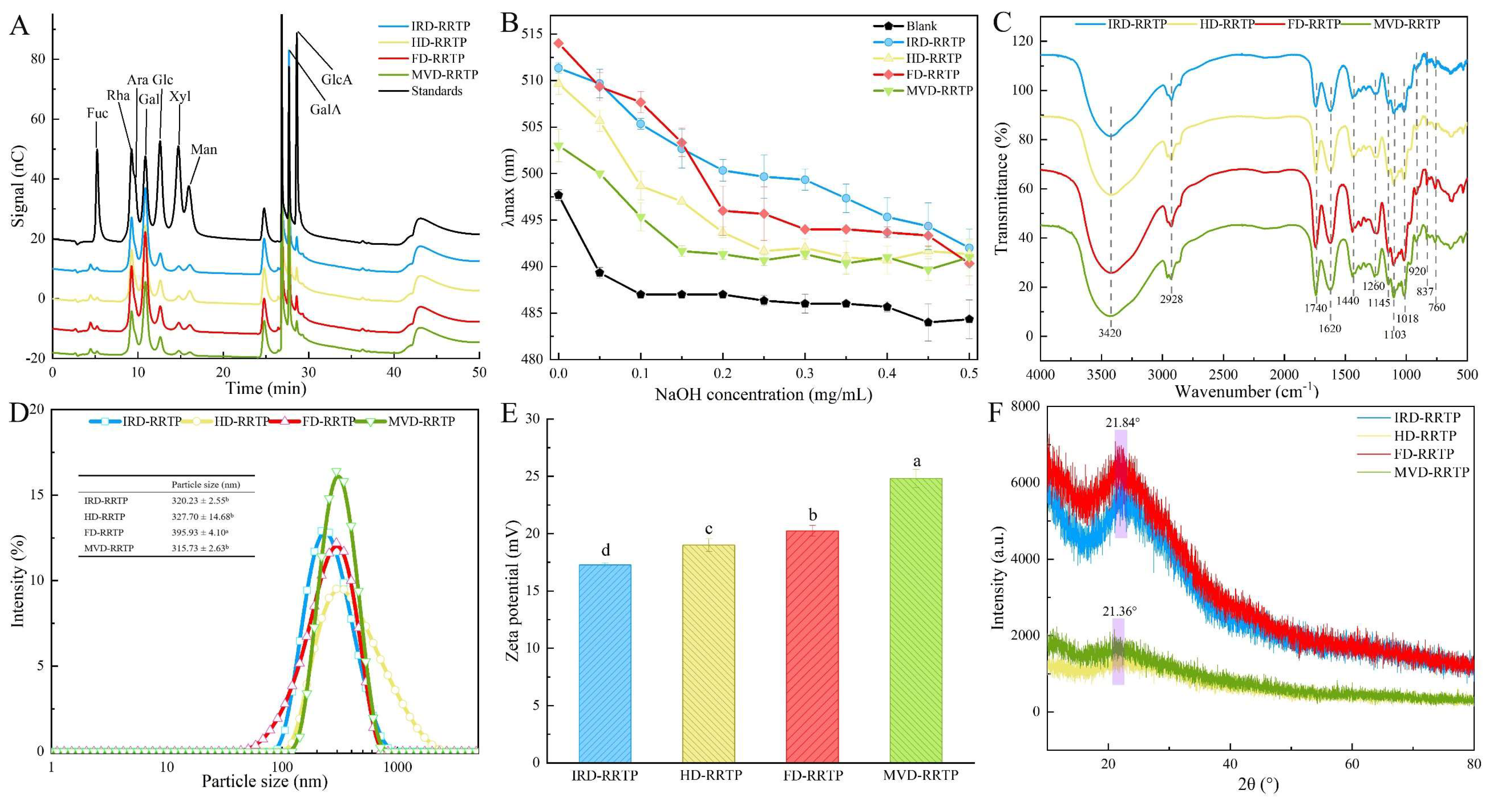
2.4.2. Monosaccharide Composition
2.4.3. Triple-Helical Structure and Fourier Transform Infrared (FTIR) Spectroscopy
2.4.4. Scanning Electron Microscopy (SEM) and X-ray Diffraction (XRD)
2.4.5. Particle Size and Potential
2.4.6. Differential Scanning Calorimetry (DSC)
2.5. Rheological Measurement
2.5.1. Steady Flow Behavior
2.5.2. Frequency Sweep
2.6. Antioxidant Activity in a Linoleic Acid System
2.7. In Vitro α-Glucosidase Activity Assay
2.7.1. Inhibitory Effect on α-Glucosidase
2.7.2. Inhibitory Kinetics of α-Glucosidase
2.7.3. Fluorescence Quenching
2.8. In Vitro Anti-Glycation Assay
2.8.1. Determination of Fructosamine Concentration
2.8.2. Determination of α-Dicarbonyl Compounds
2.8.3. Determination of Fluorescent AGEs
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different Drying Methods on the Physicochemical Characteristics of RRTPs
3.1.1. The Yield and Chemical Compositions of RRTPs
3.1.2. Molecular Weights and Constituent Monosaccharides of RRTPs
3.1.3. Triple-Helical Structure and FT-IR Analysis of RRTPs
3.1.4. Particle Size and Zeta-Potential Analysis of RRTPs
3.1.5. XRD and SEM Examination of RRTPs
3.1.6. DSC Analysis of RRTPs
3.2. Rheological Properties of RRTPs
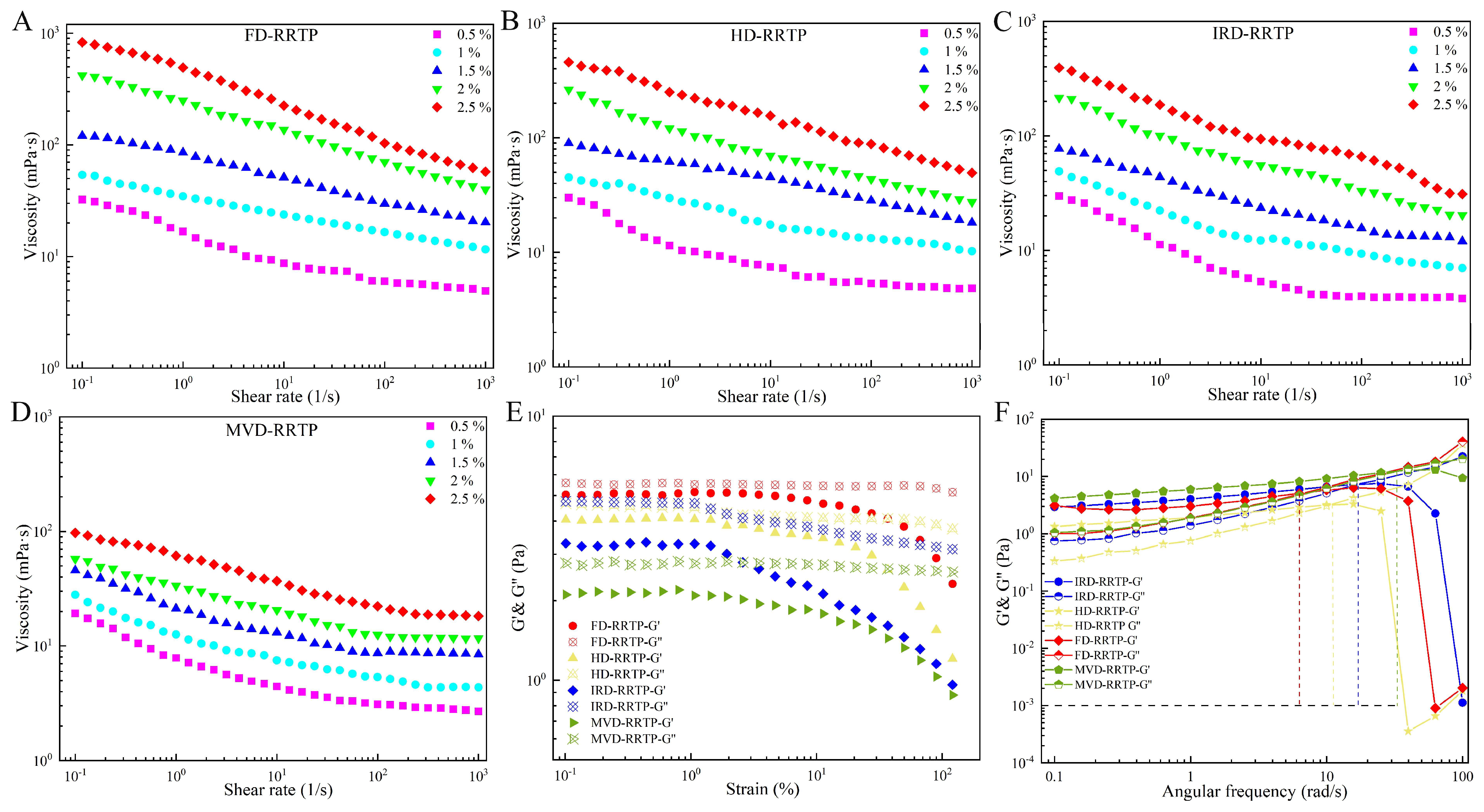
3.2.1. Apparent Viscosity
3.2.2. Oscillatory Measurements
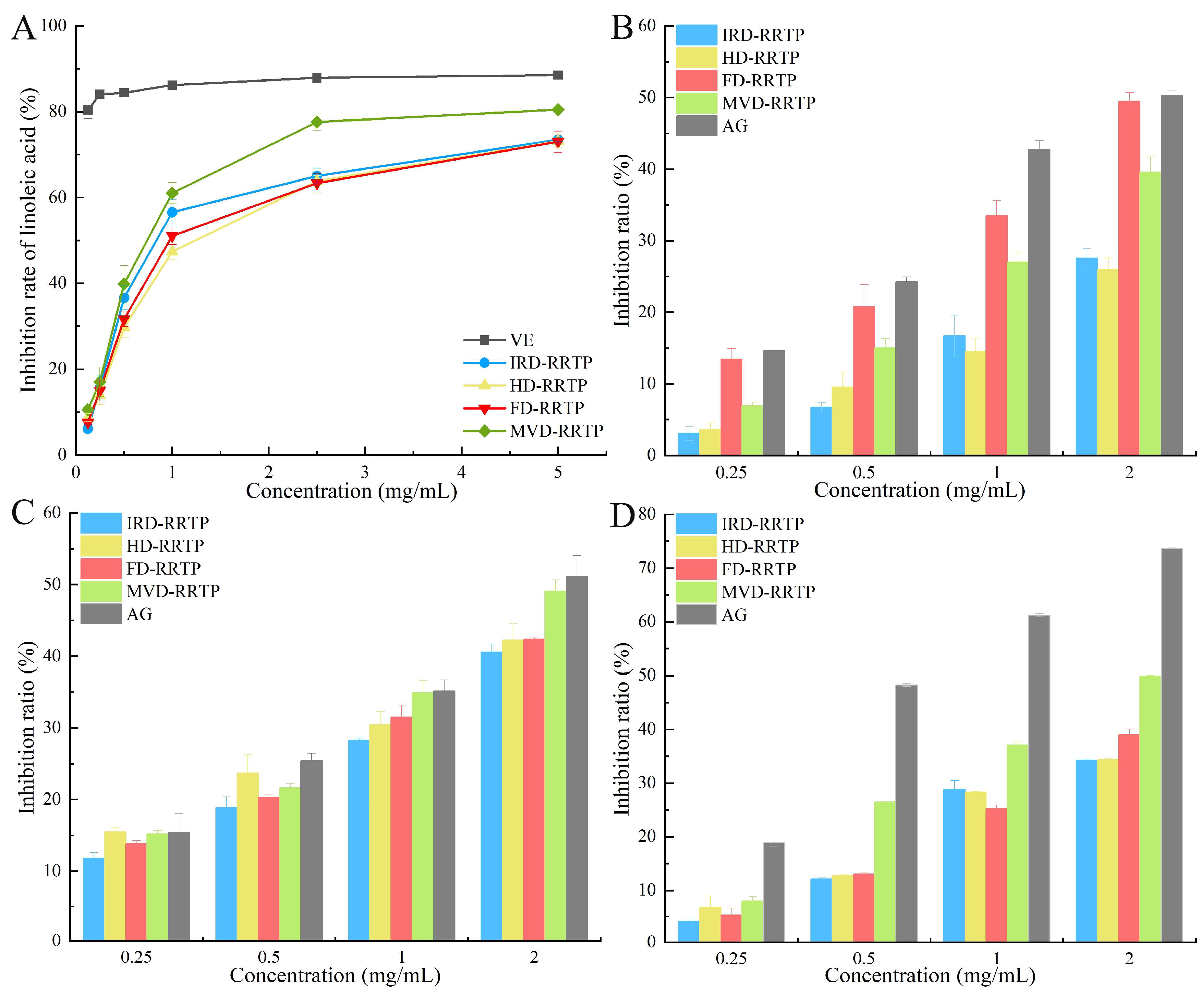
3.3. Antioxidant Activity in a Linoleic Acid System
3.4. Anti-Glycation Assay Analysis
3.5. In Vitro Hypoglycemic Activity
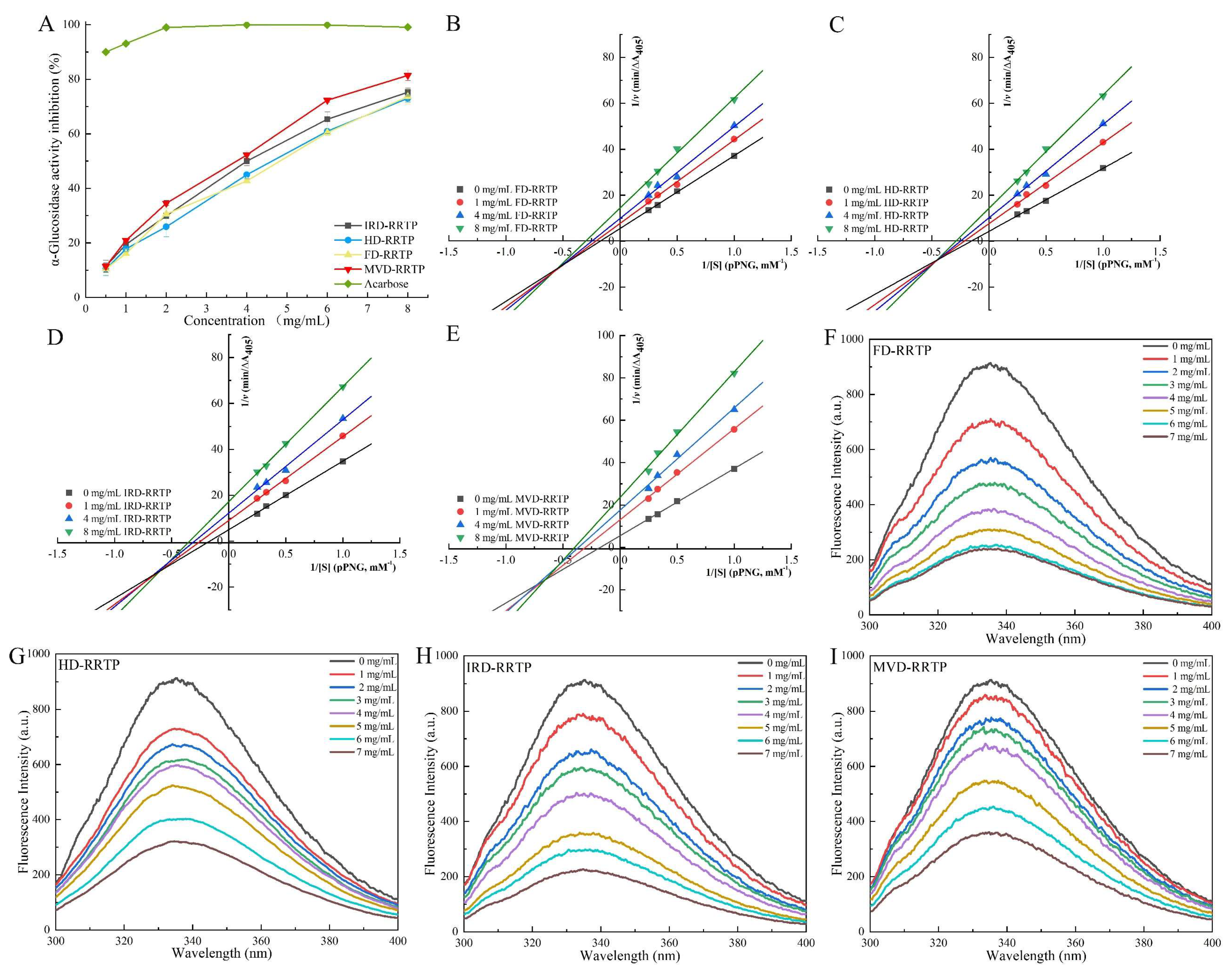
3.5.1. Inhibitory Activity on α-Glucosidase
3.5.2. Inhibitory Kinetics Analysis
3.5.3. Fluorescence Quenching
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.-T.; Lv, M.-J.; An, J.-Y.; Fan, X.-H.; Dong, M.-Z.; Zhang, S.-D.; Wang, J.-D.; Wang, Y.-Q.; Cai, Z.-H.; Fu, Y.-J. Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, Y.; Zhang, H.; Li, W.; Chen, W.; Kuang, M.; Song, Y.; Zhong, Z. Exploring the active components and potential mechanisms of Rosa roxburghii Tratt in treating type 2 diabetes mellitus based on UPLC-Q-exactive Orbitrap/MS and network pharmacology. Chin. Med. 2023, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Z.-J.; Liu, J.; Liu, L.-K.; Zhang, E.-S.; Li, W.-L. Inhibition of Metastasis and Invasion of Ovarian Cancer Cells by Crude Polysaccharides from Rosa Roxburghii Tratt In Vitro. Asian Pac. J. Cancer Prev. 2015, 15, 10351–10354. [Google Scholar] [CrossRef]
- Wu, H.; Li, M.; Yang, X.; Wei, Q.; Sun, L.; Zhao, J.; Shang, H. Extraction optimization, physicochemical properties and antioxidant and hypoglycemic activities of polysaccharides from roxburgh rose (Rosa roxburghii Tratt.) leaves. Int. J. Biol. Macromol. 2020, 165, 517–529. [Google Scholar] [CrossRef] [PubMed]
- He, J.-Y.; Zhang, Y.-H.; Ma, N.; Zhang, X.-L.; Liu, M.-H.; Fu, W.-M. Comparative analysis of multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities. J. Funct. Foods 2016, 27, 29–41. [Google Scholar] [CrossRef]
- Liu, M.-H.; Zhang, Q.; Zhang, Y.-H.; Lu, X.-Y.; Fu, W.-M.; He, J.-Y. Chemical Analysis of Dietary Constituents in Rosa roxburghii and Rosa sterilis Fruits. Molecules 2016, 21, 1204. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Huang, Q.; Fu, X.; Liu, R.H. In vitro digestibility and prebiotic potential of a novel polysaccharide from Rosa roxburghii Tratt fruit. J. Funct. Foods 2019, 52, 408–417. [Google Scholar] [CrossRef]
- Chen, G.; Kan, J. Ultrasound-assisted extraction, characterization, and antioxidant activity in vitro and in vivo of polysaccharides from Chestnut rose (Rosa roxburghii tratt) fruit. J. Food Sci. Technol. 2018, 55, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Vidyarthi, S.K.; Bai, W.; Pan, Z. Nutritional constituents, health benefits and processing of Rosa roxburghii: A review. J. Funct. Foods 2019, 60, 103456. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, M.; Mujumdar, A.S.; Yu, D. New drying technologies for animal/plant origin polysaccharide-based future food processing: Research progress, application prospects and challenges. Food Biosci. 2023, 56, 103315. [Google Scholar] [CrossRef]
- Ma, Q.; Santhanam, R.K.; Xue, Z.; Guo, Q.; Gao, X.; Chen, H. Effect of different drying methods on the physicochemical properties and antioxidant activities of mulberry leaves polysaccharides. Int. J. Biol. Macromol. 2018, 119, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Feng, K.-L.; Yang, J.-C.; He, Y.-S.; Guo, H.; Wang, S.-P.; Gan, R.-Y.; Wu, D.-T. Polysaccharides from dandelion (Taraxacum mongolicum) leaves: Insights into innovative drying techniques on their structural characteristics and biological activities. Int. J. Biol. Macromol. 2021, 167, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, D.-T.; Li, F.; Gan, R.-Y.; Hu, Y.-C.; Zou, L. Structural and Biological Properties of Water Soluble Polysaccharides from Lotus Leaves: Effects of Drying Techniques. Molecules 2021, 26, 4395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Y.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Effects of drying on the structural characteristics and antioxidant activities of polysaccharides from Stropharia rugosoannulata. J. Food Sci. Technol. 2021, 58, 3622–3631. [Google Scholar] [CrossRef]
- Zor, T.; Seliger, Z. Linearization of the bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkr, N.; Asboehan, G. New method for quantitative-determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Li, F.; Zhao, J.; Wei, Y.; Zhang, L.; Wang, H.; Yu, W.; Li, Q. Structural diversity and physicochemical properties of polysaccharides isolated from pumpkin (Cucurbita moschata) by different methods. Food Res. Int. 2023, 163, 112157. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zeng, J.; Wang, B.; Cheng, Z.; Xu, J.; Gao, W.; Chen, K. Structural characterization and antioxidant activities of Bletilla striata polysaccharide extracted by different methods. Carbohydr. Polym. 2021, 266, 118149. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Gao, X.; Xue, Z.; Wang, Y.; Lu, Y.; Zhang, M.; Panichayupakaranant, P.; Chen, H. Characterization, antioxidant activities, and inhibition on α-glucosidase activity of corn silk polysaccharides obtained by different extraction methods. Int. J. Biol. Macromol. 2020, 163, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Waterhouse, G.I.N.; Cui, T.; Sun-Waterhouse, D.; Wu, P. Pectin fractions extracted sequentially from Cerasus humilis: Their compositions, structures, functional properties and antioxidant activities. Food Sci. Hum. Wellness 2023, 12, 564–574. [Google Scholar] [CrossRef]
- Gong, P.; Pei, S.; Long, H.; Yang, W.; Yao, W.; Li, N.; Wang, J.; Zhao, Y.; Chen, F.; Xie, J.; et al. Potential inhibitory effect of Auricularia auricula polysaccharide on advanced glycation end-products (AGEs). Int. J. Biol. Macromol. 2024, 262, 129856. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, M.A.; Hafsa, J.; Rihouey, C.; Le Cerf, D.; Majdoub, H. Depolymerization of polysaccharides from Opuntia ficus indica: Antioxidant and antiglycated activities. Int. J. Biol. Macromol. 2015, 79, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, C.; Zhang, B.; Huang, Q.; Fu, X.; Li, C. Structural characterization of a novel acidic polysaccharide from Rosa roxburghii Tratt fruit and its alpha-glucosidase inhibitory activity. Food Funct. 2018, 9, 3974–3985. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, X.; Zhang, L.; Hu, Y.-M.; Wang, H.; Tu, Z.-C. Inhibition mechanism of α-glucosidase inhibitors screened from Artemisia selengensis Turcz root. Ind. Crops Prod. 2020, 143, 111941. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Chen, C.-R.; Wu, W.-H.; Wen, C.-L.; Chang, C.-I.; Hou, W.-C. Anti-α-glucosidase and Anti-dipeptidyl Peptidase-IV Activities of Extracts and Purified Compounds from Vitis thunbergii var. taiwaniana. J. Agric. Food Chem. 2015, 63, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Yin, X.; Wu, D.; Chen, J.; Ye, X. Natural polysaccharides from Glycyrrhiza uralensis residues with typical glucan structure showing inhibition on α-glucosidase activities. Int. J. Biol. Macromol. 2023, 224, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Qiu, C.; Guo, Y.; Zhang, C.; Li, D.; Gao, K.; Ma, Y.; Ma, H. Comparative Evaluation of Physicochemical Properties, Microstructure, and Antioxidant Activity of Jujube Polysaccharides Subjected to Hot Air, Infrared, Radio Frequency, and Freeze Drying. Agriculture 2022, 12, 1606. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Li, J.; Zhang, Z.; He, Y.; Yang, H.; Zhou, P. Inhibition on α-Glucosidase Activity and Non-Enzymatic Glycation by an Anti-Oxidative Proteoglycan from Ganoderma lucidum. Molecules 2022, 27, 1457. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.-M.; Chen, C.; Huang, Q.; Fu, X. Comparative study on the effect of extraction solvent on the physicochemical properties and bioactivity of blackberry fruit polysaccharides. Int. J. Biol. Macromol. 2021, 183, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Feng, K.-L.; Wei, S.-Y.; Xiang, X.-R.; Ding, Y.; Li, H.-Y.; Zhao, L.; Qin, W.; Gan, R.-Y.; Wu, D.-T. Comparison of structural characteristics and bioactivities of polysaccharides from loquat leaves prepared by different drying techniques. Int. J. Biol. Macromol. 2020, 145, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, H.-Y.; Li, H.; Wu, D.-T.; Zhong, L.L.D.; Gan, R.-Y.; Gao, H. Recent advances in the influences of drying technologies on physicochemical properties and biological activities of plant polysaccharides. Crit. Rev. Food Sci. Nutr. 2023; online ahead of print. [Google Scholar] [CrossRef]
- An, K.; Wu, J.; Xiao, H.; Hu, T.; Yu, Y.; Yang, W.; Xiao, G.; Xu, Y. Effect of various drying methods on the physicochemical characterizations, antioxidant activities and hypoglycemic activities of lychee (Litchi chinensis Sonn.) pulp polysaccharides. Int. J. Biol. Macromol. 2022, 220, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Meneguin, A.B.; Silvestre, A.L.P.; Sposito, L.; de Souza, M.P.C.; Sabio, R.M.; Araujo, V.H.S.; Cury, B.S.F.; Chorilli, M. The role of polysaccharides from natural resources to design oral insulin micro- and nanoparticles intended for the treatment of Diabetes mellitus: A review. Carbohydr. Polym. 2021, 256, 117504. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, C.; Wang, S.; Mei, X.; Zhang, H.; Kan, J. Characterization of physicochemical properties and antioxidant activity of polysaccharides from shoot residues of bamboo (Chimonobambusa quadrangularis): Effect of drying procedures. Food Chem. 2019, 292, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yu, X.; Ma, H.; Liu, S.; Qin, X.; Yagoub, A.E.-G.A.; Owusu, J. Examining of athermal effects in microwave-induced glucose/glycine reaction and degradation of polysaccharide from Porphyra yezoensis. Carbohydr. Polym. 2013, 97, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Dai, T.; Liang, L.; Deng, L.; Liu, C.; Li, Q.; Liang, R.; Chen, J. Physicochemical properties of pectin extracted from navel orange peel dried by vacuum microwave. LWT-Food Sci. Technol. 2021, 151, 112100. [Google Scholar] [CrossRef]
- Li, L.; Wang, B.; Wang, Y.; Liu, J. Effect of drying methods on the characterisation of pectin extracted from dried hawthorn fruit. J. Food Meas. Charact. 2022, 16, 3670–3681. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wu, L.-X.; Qiao, Z.-R.; Cai, W.-D.; Ma, H. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chem. 2019, 271, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, C.; Fu, X.; Huang, Q.; Chen, Q. Physicochemical, functional and biological properties of soluble dietary fibers obtained from Rosa roxburghii Tratt pomace using different extraction methods. Process Biochem. 2023, 128, 40–48. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Ren, Z.; Cong, Z.; Chen, M.; Shi, L.; Han, X.; Pei, J. Optimization of the microwave-assisted enzymatic extraction of Rosa roxburghii Tratt. polysaccharides using response surface methodology and its antioxidant and α-d-glucosidase inhibitory activity. Int. J. Biol. Macromol. 2018, 112, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Tian, S.; Li, H.; Zhu, L.; Zhao, Z.; Zheng, G.; Wen, Q.; Tian, H.; Yang, D. Extraction, characterization, and antioxidant properties of cell wall polysaccharides from the pericarp of Citrus reticulata cv. Chachiensis. Food Hydrocoll. 2023, 136, 108237. [Google Scholar] [CrossRef]
- Chen, S.; Luan, L.; Zhang, Y.; Liu, F.; Ye, X.; Hou, Z. A comparison study on polysaccharides extracted from Rosa sterilis S.D.Shi using different methods: Structural and in vitro fermentation characterizations. Food Chem. X 2023, 17, 100533. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, B.; Zheng, T.; Zhao, C.; Gao, Y.; Wu, W.; Fan, Y.; Wang, X.; Qiu, M.; Fan, J. Structural Characteristics, Rheological Properties, and Antioxidant and Anti-Glycosylation Activities of Pectin Polysaccharides from Arabica Coffee Husks. Foods 2023, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Pan, Y.; Chen, W.; Chen, W.; Yun, Y.; Zhong, Q.; Zhang, W.; Chen, H. Effects of cultivar and growth region on the structural, emulsifying and rheological characteristic of mango peel pectin. Food Hydrocoll. 2020, 103, 105707. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Xiong, F.; Li, X.; Zheng, L.; Hu, N.; Cui, M.; Li, H. Characterization and antioxidant activities of polysaccharides from Passiflora edulis Sims peel under different degradation methods. Carbohydr. Polym. 2019, 218, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Kan, J. Characterization of a novel polysaccharide isolated from Rosa roxburghii Tratt fruit and assessment of its antioxidant in vitro and in vivo. Int. J. Biol. Macromol. 2018, 107, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; He, Y.; Xiang, P.-Y.; Huang, Y.-J.; Cao, Z.-W.; Shen, S.-W.; Zhao, L.; Zhang, Q.; Qin, W.; Wu, D.-T. Influences of different drying methods on the structural characteristics and multiple bioactivities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2020, 147, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dou, Z.; Duan, Q.; Chen, C.; Liu, R.; Jiang, Y.; Yang, B.; Fu, X. A comparison study on structure-function relationship of polysaccharides obtained from sea buckthorn berries using different methods: Antioxidant and bile acid-binding capacity. Food Sci. Hum. Wellness 2024, 13, 494–505. [Google Scholar] [CrossRef]
- Iranshahi, K.; Rubinetti, D.; Onwude, D.I.; Psarianos, M.; Schlüter, O.K.; Defraeye, T. Electrohydrodynamic drying versus conventional drying methods: A comparison of key performance indicators. Energy Convers. Manag. 2023, 279, 116661. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Zhao, H.; Liu, M.; Lin, C.; Li, L.; Ma, B. Pectin polysaccharide from Flos Magnoliae (Xin Yi, Magnolia biondii Pamp. flower buds): Hot-compressed water extraction, purification and partial structural characterization. Food Hydrocoll. 2022, 122, 107061. [Google Scholar] [CrossRef]
- Mohammed, J.K.; Mahdi, A.A.; Ahmed, M.I.; Ma, M.; Wang, H. Preparation, deproteinization, characterization, and antioxidant activity of polysaccharide from Medemia argun fruit. Int. J. Biol. Macromol. 2020, 155, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Mannai, F.; Elhleli, H.; Yilmaz, M.; Khiari, R.; Belgacem, M.N.; Moussaoui, Y. Precipitation solvents effect on the extraction of mucilaginous polysaccharides from Opuntia ficus-indica (Cactaceae): Structural, functional and rheological properties. Ind. Crops Prod. 2023, 202, 117072. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, H.-M.; Cheng, X.-C.; Wang, X.-D. Effect of drying pretreatment methods on structure and properties of pectins extracted from Chinese quince fruit. Int. J. Biol. Macromol. 2019, 137, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, T.; Hu, X.; Ren, G.; Zhang, H.; Wang, Z.; Teng, Z.; Wu, R.; Wu, J. Structural, rheological properties and antioxidant activities of polysaccharides from mulberry fruits (Murus alba L.) based on different extraction techniques with superfine grinding pretreatment. Int. J. Biol. Macromol. 2021, 183, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cui, N.; Liu, J.; Ma, Q.; Zhao, T.; Yang, Z.; Zhao, H.; Zhang, B.; Liang, L. Application of metabolomics to explore the automatic oxidation process of hazelnut oil. Food Res. Int. 2022, 162, 111888. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; He, Q.; Qiu, Y.; Liu, H.; Wu, J.; Liu, G.; Brennan, C.; Brennan, M.A.; Zhu, L. Food matrixes play a key role in the distribution of contaminants of lipid origin: A case study of malondialdehyde formation in vegetable oils during deep-frying. Food Chem. 2021, 347, 129080. [Google Scholar] [CrossRef] [PubMed]
- Bertolin, J.R.; Joy, M.; Blanco, M. Malondialdehyde determination in raw and processed meat products by UPLC-DAD and UPLC-FLD. Food Chem. 2019, 298, 125009. [Google Scholar] [CrossRef]
- Sun, L.; Su, X.; Zhuang, Y. Preparation, characterization and antiglycation activities of the novel polysaccharides from Boletus snicus. Int. J. Biol. Macromol. 2016, 92, 607–614. [Google Scholar] [CrossRef]
- Zha, X.Q.; Li, X.L.; Zhang, H.L.; Cui, S.H.; Liu, J.; Wang, J.H.; Pan, L.H.; Luo, J.P. Pectinase hydrolysis of Dendrobium huoshanense polysaccharide and its effect on protein nonenzymatic glycation. Int. J. Biol. Macromol. 2013, 61, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhang, X.; Wang, Y.; Zhang, L.; Zhao, J.; Chen, G.; Fan, J.; Jia, Y.; Yan, F.; Ning, C. Characterization of polysaccharide fractions from fruit of Actinidia arguta and assessment of their antioxidant and antiglycated activities. Carbohydr. Polym. 2019, 210, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Ding, H.; Hu, X.; Zhang, G.; Gong, D. Galangin inhibits α-glucosidase activity and formation of non-enzymatic glycation products. Food Chem. 2019, 271, 70–79. [Google Scholar] [CrossRef]
- Fu, X.; Yang, H.; Ma, C.; Li, X.; Li, D.; Yang, Y.; Xu, Y.; Wang, L. Characterization and inhibitory activities on alpha-amylase and alpha-glucosidase of the polysaccharide from blue honeysuckle berries. Int. J. Biol. Macromol. 2020, 163, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Jia, R.B.; Ou, Z.R.; Li, Z.R.; Zhao, M.; Luo, D.; Lin, L. Comparative study on the structural characterization and alpha-glucosidase inhibitory activity of polysaccharide fractions extracted from Sargassum fusiforme at different pH conditions. Int. J. Biol. Macromol. 2022, 194, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Bai, J.; Bu, X.; Yin, Y.; Wang, L.; Yang, Y.; Xu, Y. Characterization of selenized polysaccharides from Ribes nigrum L. and its inhibitory effects on α-amylase and α-glucosidase. Carbohydr. Polym. 2021, 259, 117729. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Guo, J.; Cao, T.; Zhang, T.; Liu, Y.; Yan, Y. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Wellness 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Niu, G.; You, G.; Zhou, X.; Fan, H.; Liu, X. Physicochemical properties and in vitro hypoglycemic activities of hsian-tsao polysaccharide fractions by gradient ethanol precipitation method. Int. J. Biol. Macromol. 2023, 231, 123274. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, Q.; Yang, S.; Zhang, S.; Chen, G. Comparative Study on the Impact of Different Extraction Technologies on Structural Characteristics, Physicochemical Properties, and Biological Activities of Polysaccharides from Seedless Chestnut Rose (Rosa sterilis) Fruit. Foods 2024, 13, 772. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shi, J.; Wang, L.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Inhibitory kinetics and mechanism of flavonoids from lotus (Nelumbo nucifera Gaertn.) leaf against pancreatic α-amylase. Int. J. Biol. Macromol. 2018, 120, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhou, X.; Huo, D.; Chen, J.; Weng, L.; Li, B.; Wu, Z.; Zhang, X.; Li, L. Optimization for the extraction of polysaccharides from Huidouba and their in vitro α-glucosidase inhibition mechanism. Food Biosci. 2022, 49, 101910. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory mechanism of morin on alpha-glucosidase and its anti-glycation properties. Food Funct. 2016, 7, 3953–3963. [Google Scholar] [CrossRef] [PubMed]


| IRD-RRTP | HD-RRTP | FD-RRTP | MVD-RRTP | |
|---|---|---|---|---|
| Yield (%) | 2.96 ± 0.71 d | 3.23 ± 0.55 c | 3.75 ± 0.34 b | 4.38 ± 0.86 a |
| Carbohydrate (%) | 58.93 ± 2.01 c | 57.47 ± 1.42 c | 63.90 ± 2.14 b | 68.56 ± 1.51 a |
| Uronic acid (%) | 15.78 ± 1.52 c | 15.46 ± 1.66 c | 18.09 ± 1.16 b | 22.16 ± 1.28 a |
| Protein (%) | 1.61 ± 0.22 c | 1.57 ± 0.20 c | 2.09 ± 0.33 b | 2.82 ± 0.15 a |
| Molecular weight distribution | ||||
| Mw (kDa) | 164.73 ± 1.22 c | 175.01 ± 2.07 b | 196.72 ± 1.64 a | 145.68 ± 2.54 d |
| Mn (kDa) | 18.04 ± 0.92 a | 18.72 ± 1.56 a | 20.26 ± 1.46 a | 18.44 ± 2.54 a |
| Mw/Mn | 9.15 ± 0.52 a | 9.38 ± 0.64 a | 9.73 ± 0.60 a | 7.95 ± 0.71 b |
| Monosaccharide composition (molar ratio, %) | ||||
| Fuc | 0.38 ± 0.29 b | 0.42 ± 0.19 b | 0.74 ± 0.10 a | 0.21 ± 0.11 b |
| Rha | 17.68 ± 0.48 a | 17.58 ± 0.48 a | 16.98 ± 0.83 ab | 15.83 ± 1.23 b |
| Ara | 6.45 ± 0.27 b | 6.51 ± 0.35 b | 6.35 ± 0.31 b | 8.99 ± 1.35 a |
| Gal | 35.69 ± 1.10 b | 35.44 ± 0.14 b | 39.23 ± 1.81 a | 34.58 ± 0.34 b |
| Glc | 4.10 ± 1.17 a | 4.87 ± 0.40 a | 4.26 ± 0.42 a | 3.54 ± 0.69 b |
| Xyl | 1.56 ± 1.01 a | 0.81 ± 0.32 b | 1.58 ± 0.43 a | 0.70 ± 0.11 b |
| Man | 3.15 ± 1.43 ab | 4.91 ± 1.21 a | 1.78 ± 1.52 b | 1.17 ± 0.15 b |
| GalA | 29.52 ± 2.70 b | 28.82 ± 0.99 bc | 27.47 ± 1.45 c | 33.79 ± 1.64 a |
| GlcA | 1.48 ± 0.06 a | 1.64 ± 0.24 a | 1.61 ± 0.31 a | 1.20 ± 0.19 a |
| HG (%) | 11.84 ± 1.07 b | 10.49 ± 1.67 b | 11.24 ± 1.16 b | 17.96 ± 1.87 a |
| RG-I (%) | 77.49 ± 2.10 ab | 79.53 ± 2.34 a | 76.12 ± 1.23 b | 75.23 ± 3.88 b |
| (Ara + Gal)/Rha | 2.38 ± 0.06 b | 2.69 ± 0.07 a | 2.33 ± 0.05 b | 2.75 ± 0.06 a |
| Rha/GalA | 0.60 ± 0.07 a | 0.62 ± 0.04 a | 0.61 ± 0.03 a | 0.48 ± 0.11 b |
| Linearity | 0.47 ± 0.05 a | 0.41 ± 0.03 b | 0.47 ± 0.02 a | 0.56 ± 0.12 a |
| DE (%) | 57.02 ± 0.97 b | 58.23 ± 3.42 a | 59.12 ± 2.35 a | 59.31 ± 0.10 a |
| IRD-RRTP | HD-RRTP | FD-RRTP | MVD-RRTP | |
|---|---|---|---|---|
| Tm (°C) | 121.33 ± 1.16 b | 123.58 ± 5.88 b | 148.72 ± 3.06 a | 136.49 ± 1.41 ab |
| ΔHm (J/g) | 141.85 ± 43.51 b | 112.46 ± 28.33 c | 183.89 ± 40.94 a | 137.58 ± 29.55 b |
| To (°C) | 237.49 ± 2.69 c | 241.57 ± 3.90 bc | 247.58 ± 5.04 ab | 251.65 ± 1.69 a |
| Tg (°C) | 257.80 ± 0.72 a | 258.91 ± 3.28 a | 256.25 ± 0.81 a | 258.37 ± 2.68 a |
| ΔHg (J/g) | 30.18 ± 14.61 a | 5.03 ± 2.39 b | 21.67 ± 6.72 a | 3.21 ± 1.80 b |
| Sample | Concentration (mg/mL) | Km (mM) | Vmax (∆A405/min) | Ki (mg/mL) | Kis (mg/mL) | Kq (M−1 s−1) | KSV (M−1) | Kα (M−1) | n |
|---|---|---|---|---|---|---|---|---|---|
| IRD-RRTP | 0 | 6.02 | 0.20 | 13.81 | 4.49 | 6.98 × 1012 | 6.98 × 104 | 1.01 × 107 | 1.53 |
| 1 | 4.07 | 0.11 | |||||||
| 4 | 3.35 | 0.08 | |||||||
| 8 | 2.93 | 0.06 | |||||||
| HD-RRTP | 0 | 6.48 | 0.24 | 12.09 | 4.64 | 4.11 × 1012 | 4.11 × 104 | 2.91 × 104 | 1.00 |
| 1 | 4.65 | 0.13 | |||||||
| 4 | 4.02 | 0.10 | |||||||
| 8 | 3.47 | 0.07 | |||||||
| FD-RRTP | 0 | 6.95 | 0.22 | 18.08 | 4.92 | 8.54 × 1012 | 8.54 × 104 | 9.88 × 105 | 1.25 |
| 1 | 4.73 | 0.13 | |||||||
| 4 | 4.06 | 0.10 | |||||||
| 8 | 3.33 | 0.07 | |||||||
| MVD-RRTP | 0 | 5.80 | 0.18 | 11.74 | 4.16 | 3.78 × 1012 | 3.78 × 104 | 1.50 × 107 | 1.63 |
| 1 | 3.30 | 0.08 | |||||||
| 4 | 2.75 | 0.06 | |||||||
| 8 | 2.25 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Wu, S.; Dai, Q.; Hu, P.; Chen, G. Effects of Different Drying Methods on the Structural Characteristics and Multiple Bioactivities of Rosa roxburghii Tratt Fruit Polysaccharides. Foods 2024, 13, 2417. https://doi.org/10.3390/foods13152417
Zhang Q, Wu S, Dai Q, Hu P, Chen G. Effects of Different Drying Methods on the Structural Characteristics and Multiple Bioactivities of Rosa roxburghii Tratt Fruit Polysaccharides. Foods. 2024; 13(15):2417. https://doi.org/10.3390/foods13152417
Chicago/Turabian StyleZhang, Qiuqiu, Sha Wu, Qinghua Dai, Peng Hu, and Guangjing Chen. 2024. "Effects of Different Drying Methods on the Structural Characteristics and Multiple Bioactivities of Rosa roxburghii Tratt Fruit Polysaccharides" Foods 13, no. 15: 2417. https://doi.org/10.3390/foods13152417






