A Sustainable Approach for Degradation of Alternariol by Peroxidase Extracted from Soybean Hulls: Performance, Pathway, and Toxicity Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extraction of SHP from Soybean Hulls and Tested for Its Ability to Degrade AOH
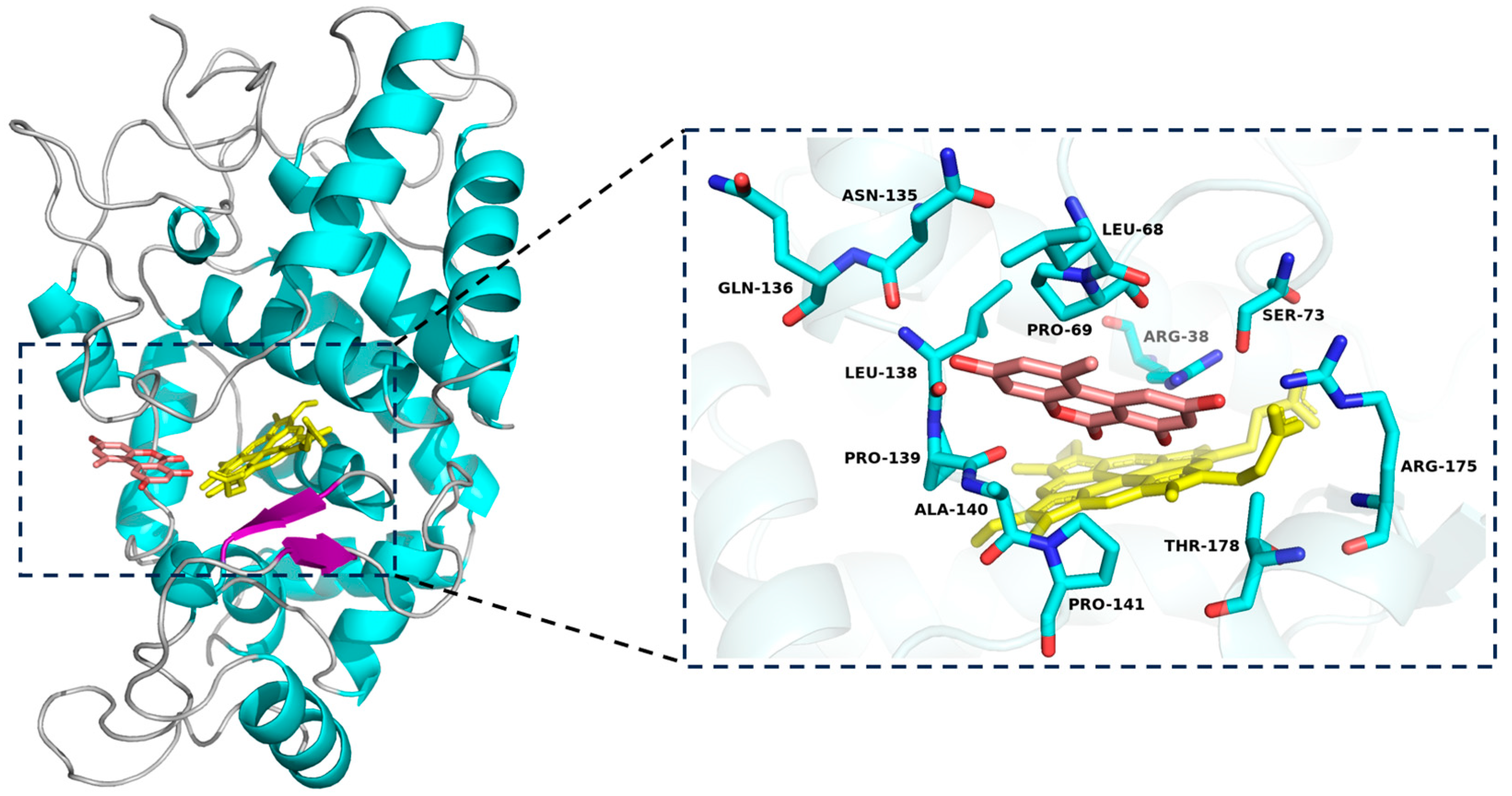
2.3. Molecular Docking Study
2.4. Enzymatic Characterization of AOH Degradation by Crude SHP
2.5. Determination of AOH by HPLC
2.6. Identification of AOH Degradation Products by UHPLC-HRMS
2.7. Cytotoxicity Assessment of AOH Degradation Products
2.8. Application of Crude SHP to Degrade AOH in Food Matrices
2.9. Statistical Analysis
3. Results and Discussion
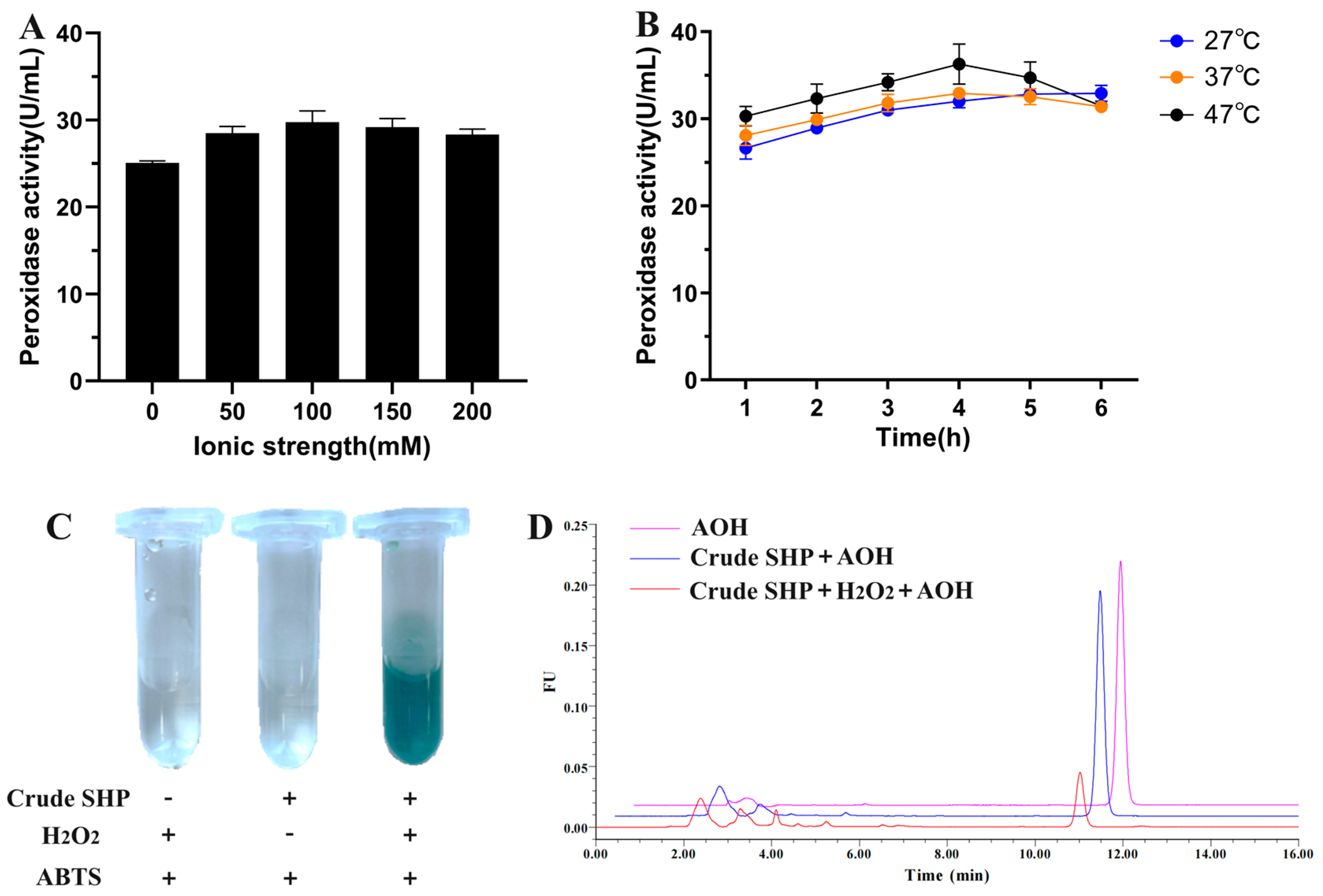
3.1. Extraction of SHP from Soybean Hulls and Tested for Its Ability to Degrade AOH
3.2. In Silico Analysis of SHP Interaction with AOH
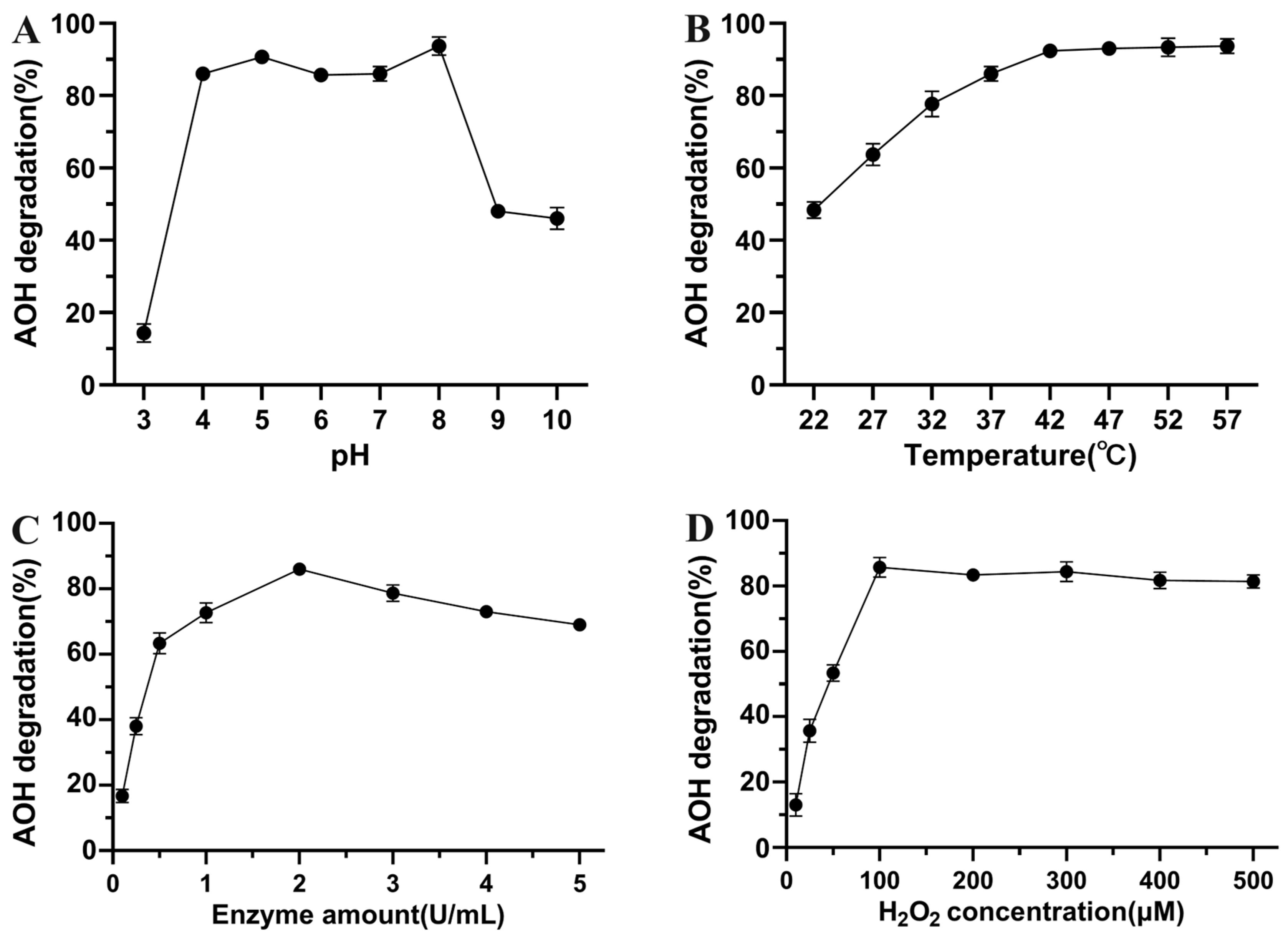
3.3. Enzymatic Characterization of AOH Degradation by Crude SHP
3.4. Identification of AOH Degradation Products
3.5. Cytotoxicity Evaluation of AOH Degradation Products
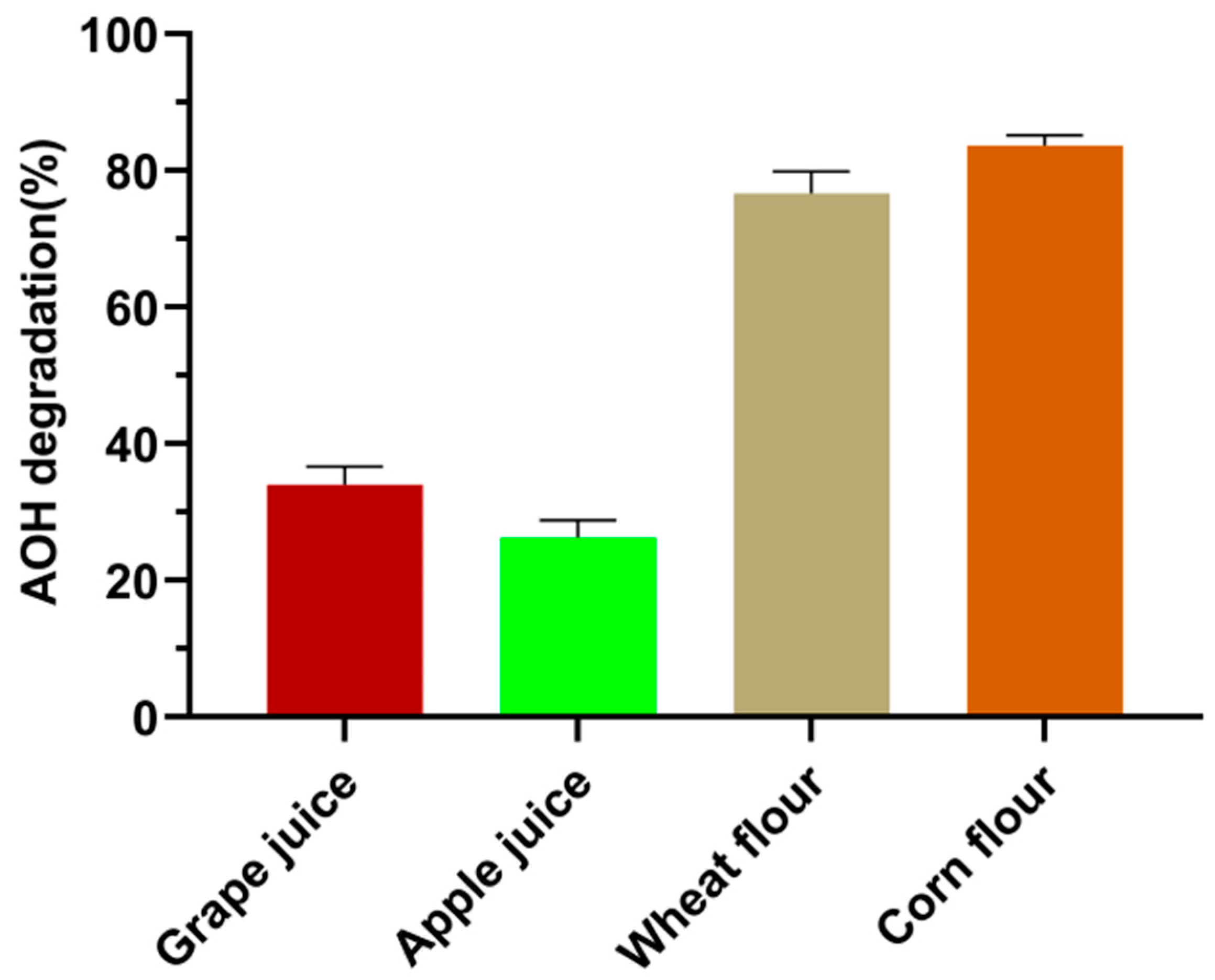
3.6. Performance of Crude SHP to Degrade AOH in Food Matrices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sá, S.V.M.; Monteiro, C.; Fernandes, J.O.; Pinto, E.; Faria, M.A.; Cunha, S.C. Emerging mycotoxins in infant and children foods: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Nagda, A.; Meena, M. Alternaria mycotoxins in food and feed: Occurrence, biosynthesis, toxicity, analytical methods, control and detoxification strategies. Food Control 2024, 158, 110211. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- Fan, K.; Guo, W.; Huang, Q.; Meng, J.; Yao, Q.; Nie, D.; Han, Z.; Zhao, Z. Assessment of Human Exposure to Five Alternaria Mycotoxins in China by Biomonitoring Approach. Toxins 2021, 13, 762. [Google Scholar] [CrossRef]
- Brugger, E.-M.; Wagner, J.; Schumacher, D.M.; Koch, K.; Podlech, J.; Metzler, M.; Lehmann, L. Mutagenicity of the mycotoxin alternariol in cultured mammalian cells. Toxicol. Lett. 2006, 164, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Fleck, S.C.; Burkhardt, B.; Pfeiffer, E.; Metzler, M. Alternaria toxins: Altertoxin II is a much stronger mutagen and DNA strand breaking mycotoxin than alternariol and its methyl ether in cultured mammalian cells. Toxicol. Lett. 2012, 214, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M. Recent advances on Alternaria mycotoxins. Curr. Opin. Food Sci. 2017, 17, 57–61. [Google Scholar] [CrossRef]
- Frizzell, C.; Ndossi, D.; Kalayou, S.; Eriksen, G.S.; Verhaegen, S.; Sørlie, M.; Elliott, C.T.; Ropstad, E.; Connolly, L. An in vitro investigation of endocrine disrupting effects of the mycotoxin alternariol. Toxicol. Appl. Pharmacol. 2013, 271, 64–71. [Google Scholar] [CrossRef]
- Kozieł, M.J.; Habrowska-Górczyńska, D.E.; Urbanek, K.A.; Domińska, K.; Piastowska-Ciesielska, A.W.; Kowalska, K. Estrogen receptor α mediates alternariol-induced apoptosis and modulation of the invasiveness of ovarian cancer cells. Toxicol. Lett. 2023, 386, 9–19. [Google Scholar] [CrossRef]
- Authority, E.F.S.; Arcella, D.; Eskola, M.; Gómez Ruiz, J.A. Dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016, 14, e04654. [Google Scholar]
- Estiarte, N.; Crespo-Sempere, A.; Marín, S.; Ramos, A.J.; Worobo, R.W. Stability of alternariol and alternariol monomethyl ether during food processing of tomato products. Food Chem. 2018, 245, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Elhariry, H.; Khiralla, G.; Gherbawy, Y.A.; Elrahman, H.A. Natural occurrence of Alternaria toxins in pomegranate fruit and the influence of some technological processing on their levels in juice. Acta Aliment. 2016, 45, 380–389. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Yan, Y.; Wang, W.; Zhang, L.; Zong, W. The degradation of Alternaria mycotoxins by dielectric barrier discharge cold plasma. Food Control 2020, 117, 107333. [Google Scholar] [CrossRef]
- Qi, L.; Zhong, X.; Gao, L.; Cai, R.; Yue, T.; Yuan, Y.; Wang, Z.; Zhao, X. Degradation of mycotoxins by pulsed light and evaluation of the safety of degradation products. J. Food Eng. 2024, 376, 112085. [Google Scholar] [CrossRef]
- Wen, S.; Guan, M.; Zhou, T.; Cao, H.; Xie, C.; Liu, D.; Yao, D. Cloning, expression, purification and characterization of an aflatoxin-converting enzyme from Armillaria tabescens. Acta Microbiol. Sin. 2011, 51, 1212–1221. [Google Scholar]
- Garnham, C.P.; Butler, S.G.; Telmer, P.G.; Black, F.E.; Renaud, J.B.; Sumarah, M.W. Identification and characterization of an Aspergillus niger amine oxidase that detoxifies intact fumonisins. J. Agric. Food Chem. 2020, 68, 13779–13790. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Ando, N.; Kimura, M.; Kakeya, H.; Osada, H.; Yamaguchi, I. A novel lactonohydrolase responsible for the detoxification of zearalenone: Enzyme purification and gene cloning. Biochem. J. 2002, 365, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dobritzsch, D.; Wang, H.; Schneider, G.; Yu, S. Structural and functional characterization of ochratoxinase, a novel mycotoxin-degrading enzyme. Biochem. J. 2014, 462, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, J.; Liu, Y.; Guo, Y.; Tang, Y.; Zhang, Q.; Ma, Q.; Ji, C.; Zhao, L. A quinoprotein dehydrogenase from Pelagibacterium halotolerans ANSP101 oxidizes deoxynivalenol to 3-keto-deoxynivalenol. Food Control 2022, 136, 108834. [Google Scholar] [CrossRef]
- Guo, Y.; Qin, X.; Tang, Y.; Ma, Q.; Zhang, J.; Zhao, L. CotA laccase, a novel aflatoxin oxidase from Bacillus licheniformis, transforms aflatoxin B1 to aflatoxin Q1 and epi-aflatoxin Q1. Food Chem. 2020, 325, 126877. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Liu, Y.; Ma, Q.; Ji, C.; Zhao, L. Detoxification of the mycoestrogen zearalenone by Bacillus licheniformis spore CotA laccase and application of immobilized laccase in contaminated corn meal. LWT-Food Sci. Technol. 2022, 163, 113548. [Google Scholar] [CrossRef]
- Sun, F.; Yu, D.; Zhou, H.; Lin, H.; Yan, Z.; Wu, A. CotA laccase from Bacillus licheniformis ZOM-1 effectively degrades zearalenone, aflatoxin B1 and alternariol. Food Control 2023, 145, 109472. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Hao, Z.; Luo, H.; Yao, B.; Su, X. Degradation of four major mycotoxins by eight manganese peroxidases in presence of a dicarboxylic acid. Toxins 2019, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tang, Y.; Zhang, L.; Liu, Y.; Ma, Q.; Zhao, L. Enzymatic characterization and application of soybean hull peroxidase as an efficient and renewable biocatalyst for degradation of zearalenone. Int. J. Biol. Macromol. 2024, 260, 129664. [Google Scholar] [CrossRef]
- de Oliveira, F.K.; Santos, L.O.; Buffon, J.G. Mechanism of action, sources, and application of peroxidases. Food Res. Int. 2021, 143, 110266. [Google Scholar] [CrossRef]
- Wang, Q.; Li, G.; Zheng, K.; Zhu, X.; Ma, J.; Wang, D.; Tang, K.; Feng, X.; Leng, J.; Yu, H.; et al. The soybean laccase gene family: Evolution and possible roles in plant defense and stem strength selection. Genes 2019, 10, 701. [Google Scholar] [CrossRef]
- de Oliveira Garcia, S.; Sibaja, K.V.M.; Nogueira, W.V.; Feltrin, A.C.P.; Pinheiro, D.F.A.; Cerqueira, M.B.R.; Badiale Furlong, E.; Garda-Buffon, J. Peroxidase as a simultaneous degradation agent of ochratoxin A and zearalenone applied to model solution and beer. Food Res. Int. 2020, 131, 109039. [Google Scholar] [CrossRef] [PubMed]
- Feltrin, A.C.P.; Garcia, S.D.O.; Caldas, S.S.; Primel, E.G.; Badiale-Furlong, E.; Garda-Buffon, J. Characterization and application of the enzyme peroxidase to the degradation of the mycotoxin DON. J. Environ. Sci. Health B 2017, 52, 777–783. [Google Scholar] [CrossRef]
- You, Y.; Qiu, Y.; Xu, H.; He, R.; Zhang, L.; Wang, Z.; Xia, Y. Effective and food-grade detoxification of multiple mycotoxins using yeast expressed manganese peroxidases. Food Biosci. 2024, 59, 103886. [Google Scholar] [CrossRef]
- Márquez, O.; Waliszewski, K.N.; Oliart, R.M.; Pardio, V.T. Purification and characterization of cell wall-bound peroxidase from vanilla bean. LWT-Food Sci. Technol. 2008, 41, 1372–1379. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.; Bhushan Jha, A.; Misra, A.N.; Sharma, P. Amorphophallus paeoniifolius corm: A potential source of peroxidase for wide applications. Int. J. Food Prop. 2017, 20, 2658–2664. [Google Scholar] [CrossRef]
- Marimón Sibaja, K.V.; de Oliveira Garcia, S.; Feltrin, A.C.P.; Diaz Remedi, R.; Cerqueira, M.B.R.; Badiale-Furlong, E.; Garda-Buffon, J. Aflatoxin biotransformation by commercial peroxidase and its application in contaminated food. J. Chem. Technol. Biotechnol. 2019, 94, 1187–1194. [Google Scholar] [CrossRef]
- Fernandes, M.; Souza, D.H.; Henriques, R.O.; Alves, M.V.; Skoronski, E.; Junior, A.F. Obtaining soybean peroxidase from soybean hulls and its application for detoxification of 2,4-dichlorophenol contaminated water. J. Environ. Chem. Eng. 2020, 8, 103786. [Google Scholar] [CrossRef]
- Hu, X.; Yang, T.; Li, C.; Zhang, L.; Li, M.; Huang, W.; Zhou, P. Human fetal hepatocyte line, L-02, exhibits good liver function in vitro and in an acute liver failure model. Transplant. Proc. 2013, 45, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, A.; Eriksen, G.S.; Holme, J.A. Mechanisms of action and toxicity of the mycotoxin alternariol: A review. Basic Clin. Pharmacol. Toxicol. 2016, 119, 533–539. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Tang, Y.; Ma, Q.; Ji, C.; Zhao, L. Combined in silico investigation and in vitro characterization of the zearalenone detoxificaiton potential of dye-decolorizing peroxidase from Bacillus subtilis 168. Food Control 2023, 146, 109549. [Google Scholar] [CrossRef]


| Chemical Name | Retention Time (min) | Formula | m/z | Fragment Ions (m/z) | Inferred Chemical Structure |
|---|---|---|---|---|---|
| AOH | 6.734 | C14H10O5 | 257.0459 | 215.0385, 147.0477 |  |
| M1 | 11.718 | C16H20O8 | 339.2319 | 163.1150 | 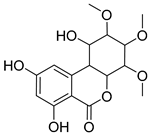 |
| M2 | 12.455 | C19H24O8 | 379.2289 | 333.2308, 275.1523 | 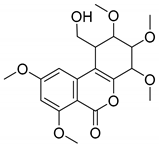 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zheng, H.; Lv, H.; Yin, J.; Li, Y.; Zhang, K.; Zhang, L.; Zhang, W.; Wang, Z.; Zhao, L.; et al. A Sustainable Approach for Degradation of Alternariol by Peroxidase Extracted from Soybean Hulls: Performance, Pathway, and Toxicity Evaluation. Foods 2024, 13, 2434. https://doi.org/10.3390/foods13152434
Zhang X, Zheng H, Lv H, Yin J, Li Y, Zhang K, Zhang L, Zhang W, Wang Z, Zhao L, et al. A Sustainable Approach for Degradation of Alternariol by Peroxidase Extracted from Soybean Hulls: Performance, Pathway, and Toxicity Evaluation. Foods. 2024; 13(15):2434. https://doi.org/10.3390/foods13152434
Chicago/Turabian StyleZhang, Xingke, Hao Zheng, Hao Lv, Jiyuan Yin, Yi Li, Kexin Zhang, Liangyu Zhang, Wei Zhang, Zhixiang Wang, Lihong Zhao, and et al. 2024. "A Sustainable Approach for Degradation of Alternariol by Peroxidase Extracted from Soybean Hulls: Performance, Pathway, and Toxicity Evaluation" Foods 13, no. 15: 2434. https://doi.org/10.3390/foods13152434






