Hydrogen Gas-Grilling in Meat: Impact on Odor Profile and Contents of Polycyclic Aromatic Hydrocarbons and Volatile Organic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Meat Sample
2.1.2. Reagents and Standards
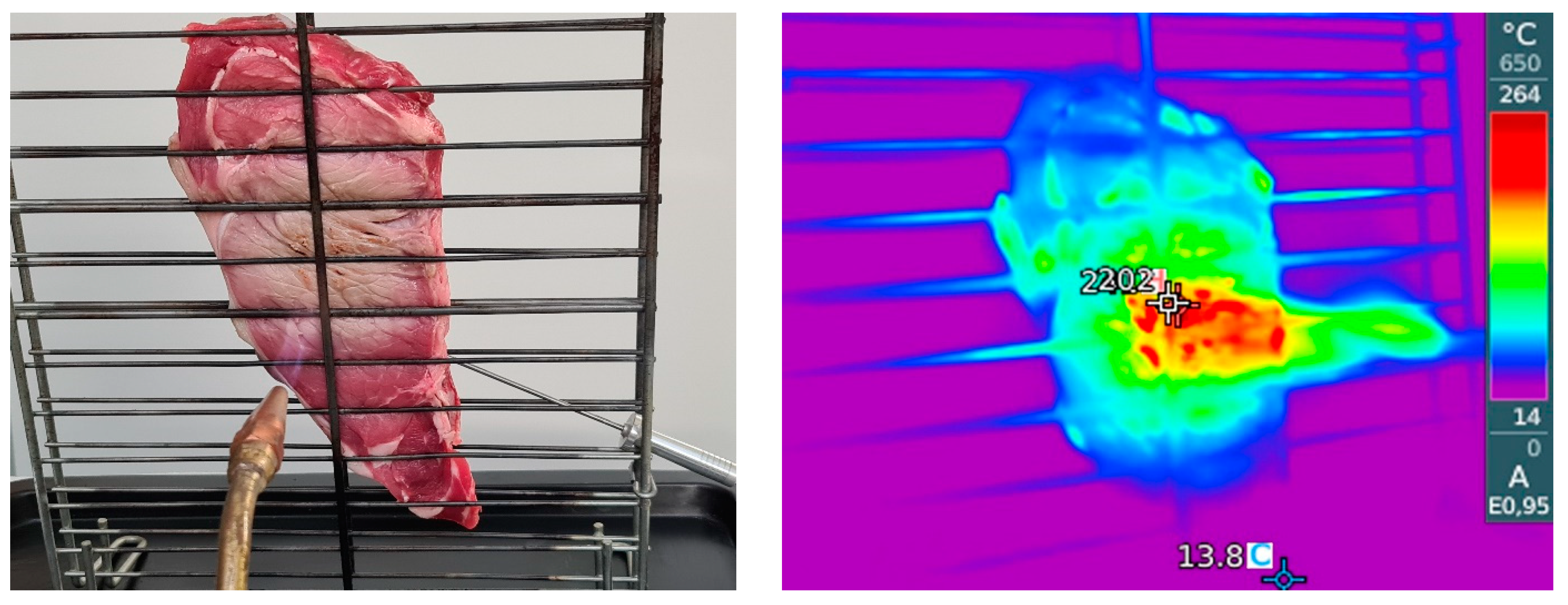
2.2. Sample Preparation and Gas-Grilling Method
2.3. Polycyclic Aromatic Hydrocarbon Analysis
2.3.1. Extraction by QuEChERS Method
2.3.2. GC-MS Analysis
2.3.3. Calibration, Quantification, and Method of Validation
2.4. Volatile Organic Compounds by GC-MS Analysis
2.5. Odor Profile by e-Nose
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effects of Gas-Grilling on PAHs
3.2. Effects of Gas-Grilling on VOCs
3.3. Effects of Gas-Grilling on Odor Profile by e-Nose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bleicher, J.; Ebner, E.E.; Bak, K.H. Formation and analysis of volatile and odor compounds in meat—A review. Molecules 2022, 27, 6703. [Google Scholar] [CrossRef] [PubMed]
- Sumer, G.; Oz, F. The effect of direct and indirect barbecue cooking on polycyclic aromatic hydrocarbon formation and beef quality. Foods 2023, 12, 1374. [Google Scholar] [CrossRef] [PubMed]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2022, 371, 131139. [Google Scholar] [CrossRef] [PubMed]
- WHO. Evaluation of Certain Food Contaminants: Sixty-Fourth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2006; p. 109. [Google Scholar]
- Jägerstad, M.; Skog, K. Genotoxicity of heat-processed foods. Mutat. Res. 2005, 574, 156–172. [Google Scholar] [CrossRef] [PubMed]
- EU. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance); EU: Maastricht, The Netherlands, 2023; Volume 119. [Google Scholar]
- Edna Hee, P.-T.; Liang, Z.; Zhang, P.; Fang, Z. Formation mechanisms, detection methods and mitigation strategies of acrylamide, polycyclic aromatic hydrocarbons and heterocyclic amines in food products. Food Control 2024, 158, 110236. [Google Scholar] [CrossRef]
- Onopiuk, A.; Kołodziejczak, K.; Szpicer, A.; Wojtasik-Kalinowska, I.; Wierzbicka, A.; Półtorak, A. Analysis of factors that influence the PAH profile and amount in meat products subjected to thermal processing. Trends Food Sci. Technol. 2021, 115, 366–379. [Google Scholar] [CrossRef]
- Lee, J.-G.; Kim, S.-Y.; Moon, J.-S.; Kim, S.-H.; Kang, D.-H.; Yoon, H.-J. Effects of grilling procedures on levels of polycyclic aromatic hydrocarbons in grilled meats. Food Chem. 2016, 199, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Saint-Aubert, B.; Cooper, J.F.; Astre, C.; Spiliotis, J.; Joyeux, H. Evaluation of the induction of polycyclic aromatic hydrocarbons (PAH) by cooking on two geometrically different types of barbecue. J. Food Compos. Anal. 1992, 5, 257–263. [Google Scholar] [CrossRef]
- Kerth, C.R.; Miller, R.K. Beef flavor: A review from chemistry to consumer. J. Sci. Food Agric. 2015, 95, 2783–2798. [Google Scholar] [CrossRef]
- Anwar, H.; Anwar, T.; Murtaza, S. Review on food quality assessment using machine learning and electronic nose system. Biosens. Bioelectron. X 2023, 14, 100365. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Finardi, S.; de Souza, C.K.; Meinert, C.; Pateiro, M.; Hoffmann, T.G.; Domínguez, R.; Bertoli, S.L.; Kumar, M.; Lorenzo, J.M. Applications of electronic nose, electronic eye and electronic tongue in quality, safety and shelf life of meat and meat products: A review. Sensors 2023, 23, 672. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Kearley, M.E.; Marini, A.M.; Pressman, P.; Hayes, A.W. A review of horses sent to slaughter for human consumption: Impact of horsemeat consumption, residual banned drugs, and public health risks. Am. J. Vet. Res. 2023, 84. [Google Scholar] [CrossRef] [PubMed]
- Aaslyng, M.D.; Duedahl-Olesen, L.; Jensen, K.; Meinert, L. Content of heterocyclic amines and polycyclic aromatic hydrocarbons in pork, beef and chicken barbecued at home by Danish consumers. Meat Sci. 2013, 93, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 2014, 97, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Influence of thermal treatment on formation of volatile compounds, cooking loss and lipid oxidation in foal meat. LWT Food Sci. Technol. 2014, 58, 439–445. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Domínguez, R. Cooking losses, lipid oxidation and formation of volatile compounds in foal meat as affected by cooking procedure. Flavour Fragr. J. 2014, 29, 240–248. [Google Scholar] [CrossRef]
- Maggiolino, A.; Lorenzo, J.M.; Marino, R.; della Malva, A.; Centoducati, P.; De Palo, P. Foal meat volatile compounds: Effect of vacuum ageing on semimembranosus muscle. J. Sci. Food Agric. 2019, 99, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, F.S.; Yaqin, A.; Krasnikov, D.V.; Kondrashov, V.A.; Ovchinnikov, G.; Kostyukevich, Y.; Osipenko, S.; Nasibulin, A.G. Detecting cooking state of grilled chicken by electronic nose and computer vision techniques. Food Chem. 2021, 345, 128747. [Google Scholar] [CrossRef]
- Shen, C.; Cai, Y.; Ding, M.; Wu, X.; Cai, G.; Wang, B.; Gai, S.; Liu, D. Predicting VOCs content and roasting methods of lamb shashliks using deep learning combined with chemometrics and sensory evaluation. Food Chem. X 2023, 19, 100755. [Google Scholar] [CrossRef]
- Grigioni, G.; Paschettas, F.; Soteras, T.; Messina, V. Odour profile of beef using an electronic nose based on MOS-sensor. Sens. Transducers J. 2013, 149, 199–204. [Google Scholar]
- Cittadini, A.; Domínguez, R.; Pateiro, M.; Sarriés, M.V.; Lorenzo, J.M. Fatty acid composition and volatile profile of longissimus thoracis et lumborum muscle from Burguete and Jaca Navarra foals fattened with different finishing diets. Foods 2021, 10, 2914. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.; Sadowska-Rociek, A.; Cieślik, E. The application of d-SPE in the QuEChERS method for the determination of PAHs in food of animal origin with GC–MS detection. Eur. Food Res. Technol. 2014, 238, 1029–1036. [Google Scholar] [CrossRef]
- Duedahl-Olesen, L.; Aaslyng, M.; Meinert, L.; Christensen, T.; Jensen, A.H.; Binderup, M.-L. Polycyclic aromatic hydrocarbons (PAH) in Danish barbecued meat. Food Control 2015, 57, 169–176. [Google Scholar] [CrossRef]
- Gorraiz, C.; Beriain, M.j.; Chasco, J.; Insausti, K. Effect of aging time on volatile compounds, odor, and flavor of cooked beef from Pirenaica and Friesian bulls and heifers. J. Food Sci. 2002, 67, 916–922. [Google Scholar] [CrossRef]
- Castello, G.; Moretti, P.; Vezzani, S. Retention models for programmed gas chromatography. J. Chromatogr. A 2009, 1216, 1607–1623. [Google Scholar] [CrossRef] [PubMed]
- Doleman, B.J.; Severin, E.J.; Lewis, N.S. Trends in odor intensity for human and electronic noses: Relative roles of odorant vapor pressure vs. molecularly specific odorant binding. Proc. Natl. Acad. Sci. USA 1998, 95, 5442–5447. [Google Scholar] [CrossRef] [PubMed]
- Devore, J.L. Probability and Statistics for Engineering and the Sciences (International Metric Edition), 9th ed.; Cengage Learning (EMEA) Ltd.: Hampshire, UK, 2016; ISBN 978-1-337-41938-3. [Google Scholar]
- Cittadini, A.; Sarriés, M.V.; Domínguez, R.; Pateiro, M.; Lorenzo, J.M. Effect of breed and finishing diet on chemical composition and quality parameters of meat from Burguete and Jaca Navarra foals. Animals 2022, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- López-Pedrouso, M.; Lorenzo, J.M.; Cittadini, A.; Sarries, M.V.; Gagaoua, M.; Franco, D. A proteomic approach to identify biomarkers of foal meat quality: A focus on tenderness, color and intramuscular fat traits. Food Chem. 2023, 405, 134805. [Google Scholar] [CrossRef] [PubMed]
- Sarriés, M.V.; Beriain, M.J. Carcass characteristics and meat quality of male and female foals. Meat Sci. 2005, 70, 141–152. [Google Scholar] [CrossRef]
- Ahmad Kamal, N.H.; Selamat, J.; Sanny, M. Simultaneous formation of polycyclic aromatic hydrocarbons (PAHs) and heterocyclic aromatic amines (HCAs) in gas-grilled beef satay at different temperatures. Food Addit. Contam. Part A 2018, 35, 848–869. [Google Scholar] [CrossRef]
- Das, A.K.; Bhattacharya, D.; Das, A.; Nath, S.; Bandyopadhyay, S.; Nanda, P.K.; Gagaoua, M. Current innovative approaches in reducing polycyclic aromatic hydrocarbons (PAHs) in processed meat and meat products. Chem. Biol. Technol. Agric. 2023, 10, 109. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Zhou, H.; Cai, K.; Xu, B. A review of hazards in meat products: Multiple pathways, hazards and mitigation of polycyclic aromatic hydrocarbons. Food Chem. 2024, 445, 138718. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef] [PubMed]
- Beldarrain, L.R.; Morán, L.; Sentandreu, M.Á.; Barron, L.J.R.; Aldai, N. Effect of ageing time on the volatile compounds from cooked horse meat. Meat Sci. 2022, 184, 108692. [Google Scholar] [CrossRef] [PubMed]
- Le Quéré, J.-L.; Schoumacker, R. Dynamic instrumental and sensory methods used to link aroma release and aroma perception: A review. Molecules 2023, 28, 6308. [Google Scholar] [CrossRef]
- Górska-Horczyczak, E.; Guzek, D.; Molęda, Z.; Wojtasik-Kalinowska, I.; Brodowska, M.; Wierzbicka, A. Applications of electronic noses in meat analysis. Food Sci. Technol. 2016, 36, 389–395. [Google Scholar] [CrossRef]
 : group centroid.
: group centroid.
 : group centroid.
: group centroid.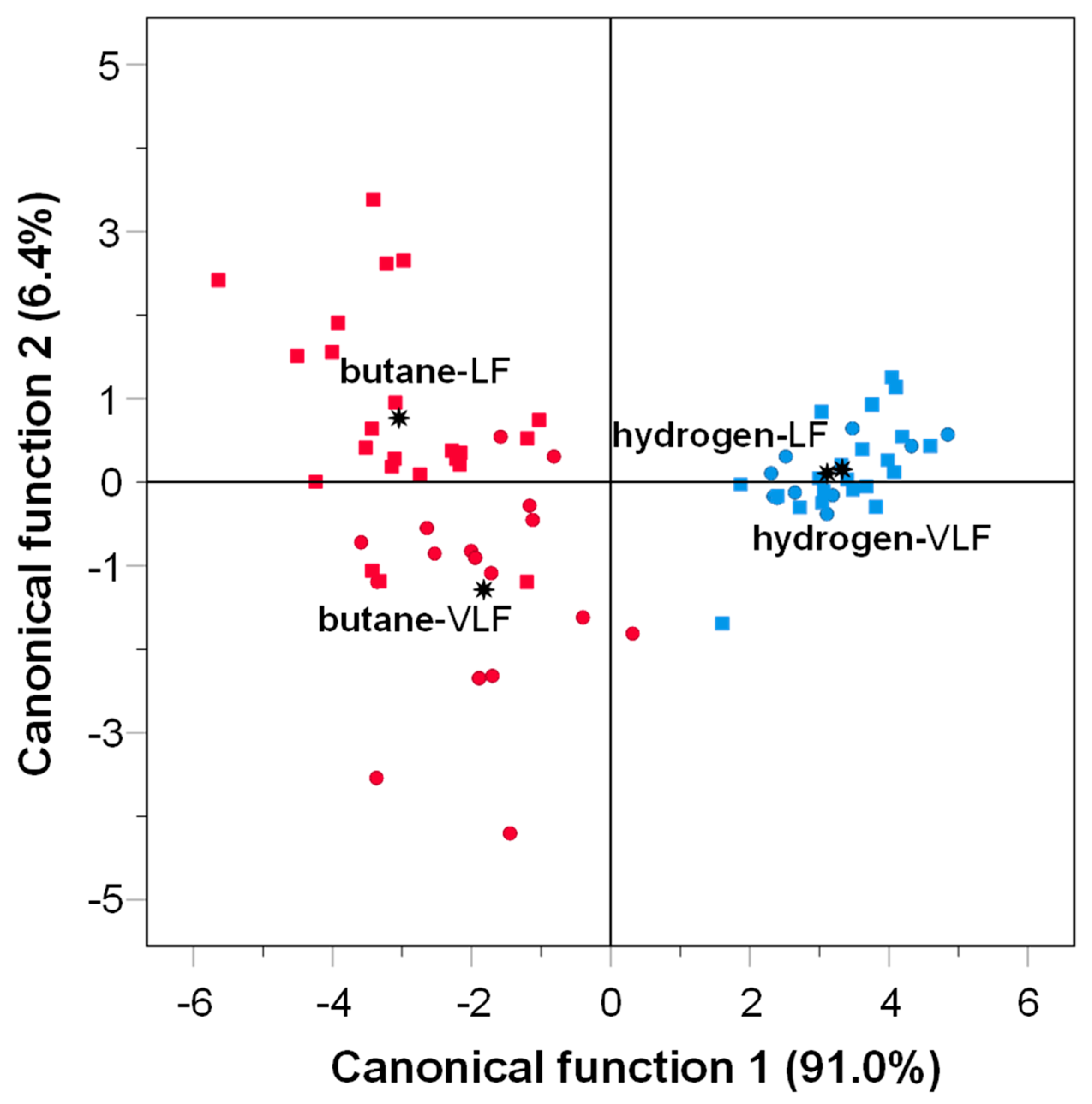
 : group centroid.
: group centroid.
 : group centroid.
: group centroid.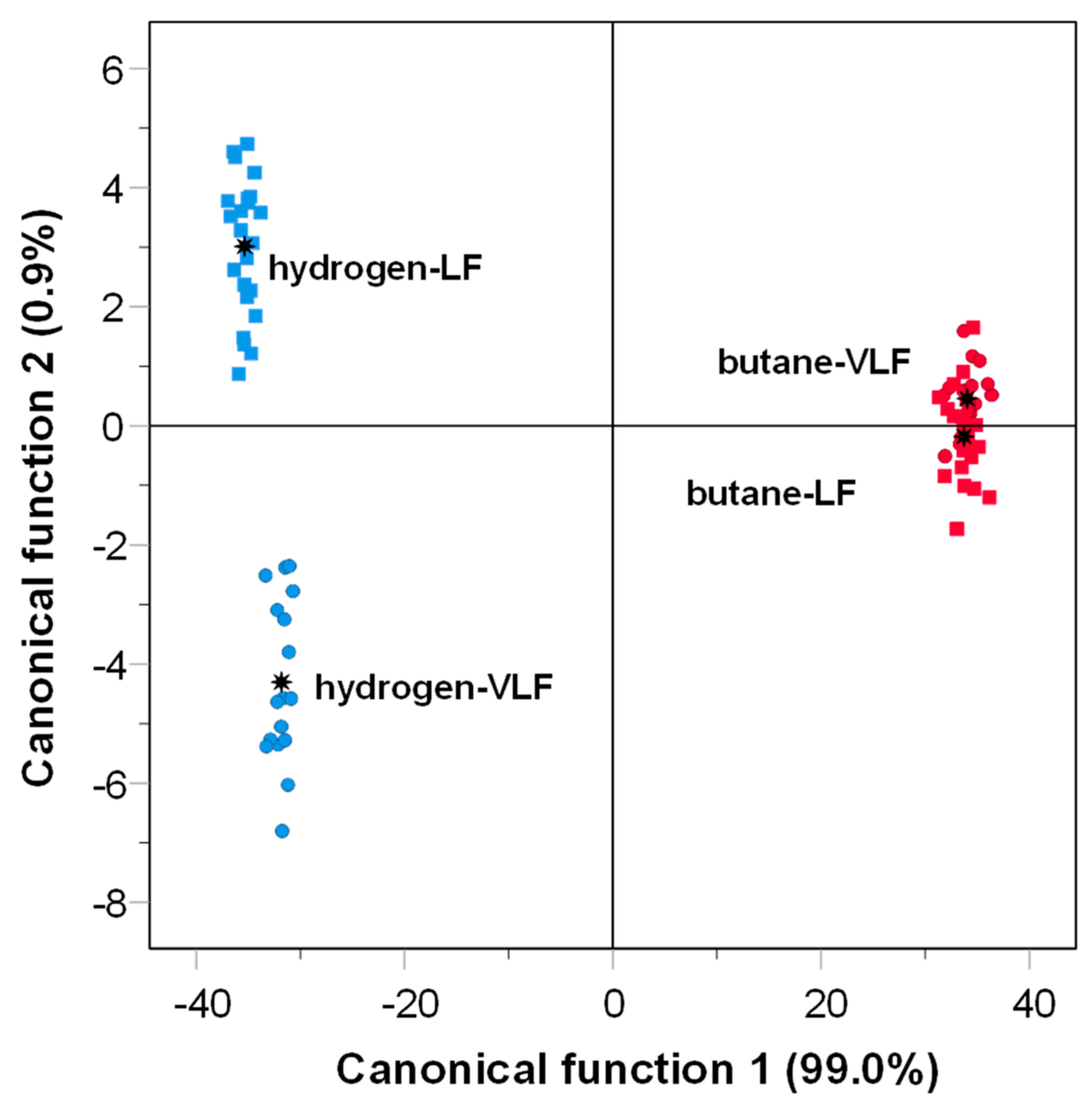
 : group centroid.
: group centroid.
 : group centroid.
: group centroid.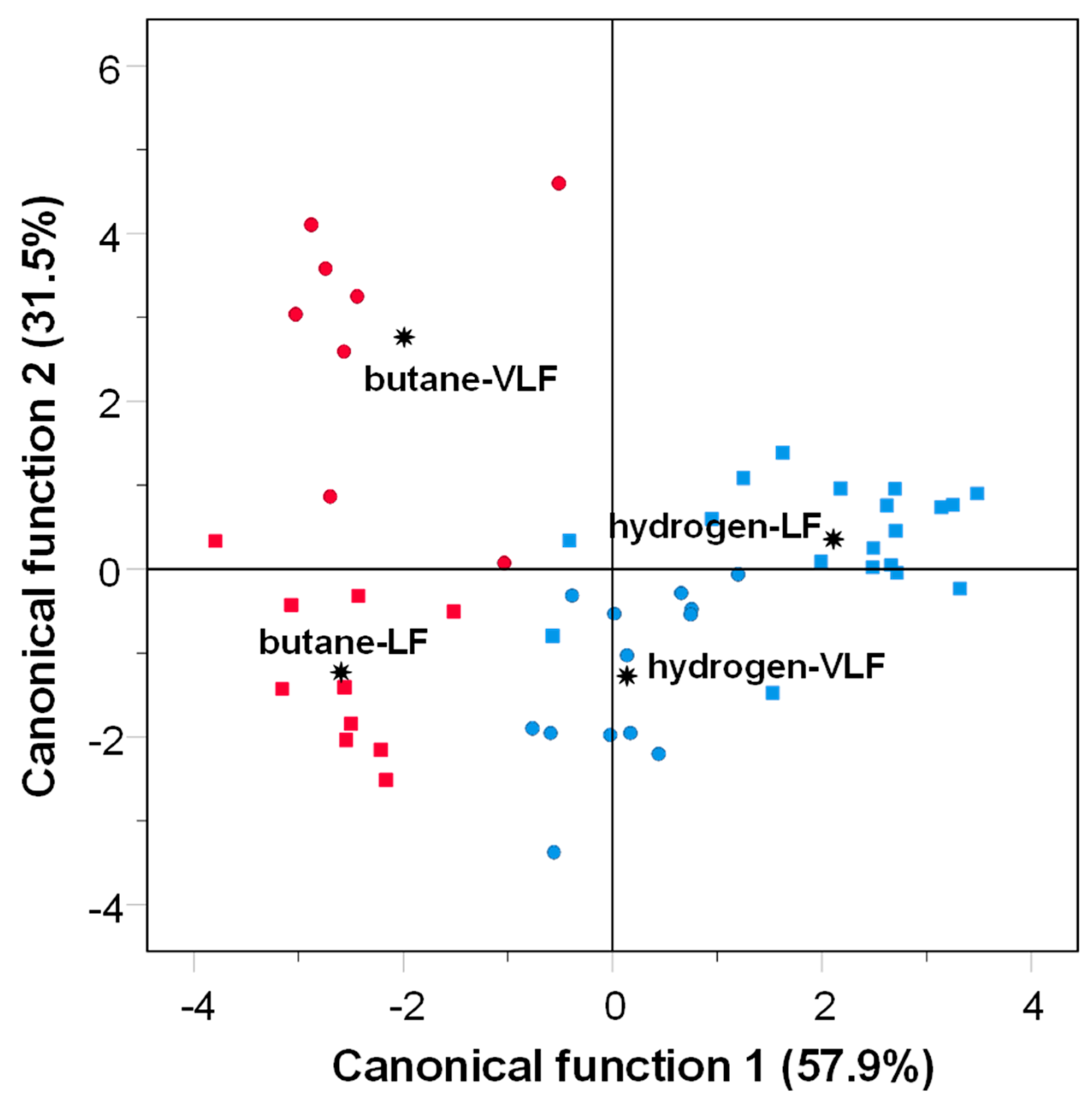
| Very Low-Fat Horse Meat | Low-Fat Horse Meat | |||
|---|---|---|---|---|
| Compounds | Butane Grill | Hydrogen Grill | Butane Grill | Hydrogen Grill |
| LMW PAHs | ||||
| acenaphthylene | 43.45 ± 21.02 b | 1.13 ± 1.06 a | 73.34 ± 32.74 c | 0.98 ± 0.89 a |
| naphthalene | 41.62 ± 12.86 b | 23.43 ± 3.00 a | 68.63 ± 25.36 c | 28.36 ± 10.73 b |
| pyrene | 34.56 ± 19.21 b | 0.55 ± 0.23 a | 51.13 ± 16.74 c | 0.62 ± 0.27 a |
| phenanthrene | 26.72 ± 12.82 b | 2.68 ± 0.53 a | 39.01 ± 12.86 c | 3.24 ± 0.65 a |
| fluoranthene | 17.82 ± 9.87 b | 0.53 ± 0.19 a | 27.00 ± 8.88 c | 0.59 ± 0.21 a |
| fluorene | 7.31 ± 3.39 b | 0.74 ± 0.33 a | 11.15 ± 4.38 c | 1.11 ± 0.33 a |
| acenaphthene | 5.57 ± 2.14 a | 7.22 ± 1.87 b | 5.35 ± 1.37 a | 6.06 ± 1.41 a |
| anthracene | 4.09 ± 1.76 b | 0.18 ± 0.06 a | 5.76 ± 2.46 c | 0.25 ± 0.08 a |
| chrysene | 1.67 ± 0.83 b | 0.17 ± 0.03 a | 2.34 ± 0.79 c | 0.13 ± 0.05 a |
| benzo[a]anthracene | 1.60 ± 0.81 b | 0.27 ± 0.09 a | 2.33 ± 0.75 c | 0.30 ± 0.07 a |
| Total LMW PAHs | 184.41 ± 77.73 b | 36.88 ± 5.56 a | 286.03 ± 95.66 c | 41.63 ± 11.47 a |
| HMW PAHs | ||||
| benzo[ghi]perylene | 9.61 ± 7.09 b | 0.60 ± 0.16 a | 13.97 ± 4.78 c | 0.41 ± 0.22 a |
| indeno[1,2,3-cd]pyrene | 3.38 ± 2.23 b | 0.28 ± 0.04 a | 4.81 ± 1.56 c | 0.24 ± 0.06 a |
| benzo[a]pyrene | 2.90 ± 1.62 b | 0.23 ± 0.12 a | 4.46 ± 1.76 c | 0.11 ± 0.07 a |
| benzo[b]fluoranthene | 1.96 ± 1.73 b | 0.13 ± 0.03 a | 3.13 ± 1.40 c | 0.19 ± 0.07 a |
| benzo[k]fluoranthene | 1.06 ± 0.81 b | 0.38 ± 0.08 a | 1.55 ± 0.67 b | 0.39 ± 0.13 a |
| dibenzo[a,h]anthracene | 0.69 ± 0.03 b | 0.38 ± 0.11 a | 0.73 ± 0.08 b | 0.31 ± 0.29 a |
| Total HMW PAHs | 19.59 ± 13.36 b | 1.83 ± 0.38 a | 28.65 ± 9.70 c | 1.61 ± 0.57 a |
| Very Low-Fat Horse Meat | Low-Fat Horse Meat | |||
|---|---|---|---|---|
| Compounds | Butane Grill | Hydrogen Grill | Butane Grill | Hydrogen Grill |
| Aldehydes | ||||
| hexanal | 20.03 ± 6.49 b | 0.84 ± 0.44 a | 21.42 ± 7.64 b | 0.37 ± 0.23 a |
| propanal | 19.75 ± 5.16 | 13.9 ± 3.73 | 18.69 ± 7.50 | 14.72 ± 9.86 |
| ethanal | 11.74 ± 6.58 ab | 16.16 ± 17.18 b | 8.74 ± 3.49 a | 7.19 ± 2.09 a |
| pentanal | 2.75 ± 1.10 b | 0.08 ± 0.04 a | 3.21 ± 1.48 b | 0.02 ± 0.01 a |
| heptanal | 1.57 ± 0.65 b | 0.00 ± 0.00 a | 1.70 ± 1.06 b | 0.02 ± 0.01 a |
| 2-methylpropanal | 1.14 ± 0.44 | 1.56 ± 0.75 | 1.21 ± 0.43 | 1.18 ± 0.50 |
| octanal | 0.79 ± 0.36 bc | 0.75 ± 0.38 bc | 0.87 ± 0.42 c | 0.36 ± 0.25 a |
| butanal | 0.71 ± 0.24 a | 0.75 ± 0.34 a | 0.87 ± 0.22 a | 1.41 ± 0.41 b |
| 3-methylbutanal | 0.60 ± 0.38 a | 1.35 ± 0.79 b | 0.64 ± 0.29 a | 0.89 ± 0.39 a |
| 2-methyl-butanal | 0.32 ± 0.26 a | 0.83 ± 0.67 b | 0.28 ± 0.17 a | 0.51 ± 0.28 ab |
| 2-pentenal | 0.13 ± 0.11 b | 0.00 ± 0.00 a | 0.19 ± 0.14 b | 0.00± 0.00 a |
| nonanal | 0.11 ± 0.11 b | 0.00 ± 0.00 a | 0.20 ± 0.13 b | 0.00± 0.00 a |
| (E)-2-octenal | 0.07 ± 0.05 | 0.20 ± 0.17 | 0.10 ± 0.08 | 0.58 ± 0.38 |
| benzaldehyde | 0.07 ± 0.05 | 0.27 ± 0.15 | 0.07 ± 0.06 | 0.16 ± 0.07 |
| 2-methyl-2-pentenal | <0.01 a | 0.16 ± 0.13 b | <0.01 a | 0.16 ± 0.13 b |
| Total aldehydes | 59.76 ±7.92 c | 36.84 ± 16.24 b | 58.13 ± 9.04 c | 27.37 ± 9.86 a |
| Alcohols | ||||
| ethanol | 5.16 ± 1.45 b | 1.03 ± 0.85 a | 1.01 ± 0.39 a | 0.63 ± 0.28 a |
| 1-penten-3-ol | 3.64 ± 1.74 b | 0.04 ± 0.01 a | 4.28 ± 1.58 b | 0.00 ± 0.00 a |
| 1-pentanol | 0.86 ± 0.45 a | 2.00 ± 1.63 b | 0.80 ± 0.39 a | 1.46 ± 1.14 ab |
| 2-penten-1-ol | 0.19 ± 0.16 a | 0.33 ± 0.24 a | 0.22 ± 0.13 a | 1.12 ± 1.09 b |
| 1-hexanol | 0.04 ± 0.02 a | 0.20 ± 0.10 c | 0.01 ± 0.03 a | 0.09 ± 0.08 b |
| 1-octen-3-ol | 0.02 ± 0.01 a | 8.54 ± 4.22 c | 0.01 ± 0.01 a | 4.2 0± 2.35 b |
| 1-butanol | <0.01 | 0.63 ± 0.43 | <0.01 | 0.51 ± 0.28 |
| Total alcohols | 9.88 ± 3.37 ab | 12.66 ± 5.57 b | 6.34 ± 1.98 a | 7.69 ± 3.51 a |
| Ketones | ||||
| 2-butanone | 1.11 ± 0.46 | 1.06 ± 0.39 | 0.83 ± 0.33 | 0.87 ± 0.38 |
| 2.3-butanedione | 0.38 ± 0.26 b | 0.13 ± 0.06 a | 0.26 ± 0.13 b | 0.13 ± 0.11 a |
| 2-pentanone | 0.20 ± 0.10 c | 0.07 ± 0.06 ab | 0.15 ± 0.05 bc | 0.11 ± 0.10 b |
| 2-heptanone | 0.14 ± 0.08 b | 0.04 ± 0.01 a | 0.15 ± 0.12 b | 0.03 ± 0.01 a |
| 3-octanone | 0.13 ± 0.09 a | 0.61 ± 0.45 b | 0.15 ± 0.08 a | 1.04 ± 0.90 b |
| 1-penten-3-one | 0.11 ± 0.08 a | 2.52 ± 1.08 b | 0.10 ± 0.08 a | 4.46 ± 1.50 c |
| 2,3-octanediona | 0.06 ± 0.03 | 0.07 ± 0.02 | 0.05 ± 0.03 | 0.10 ± 0.05 |
| 3-heptanone | 0.03 ± 0.03 a | 0.25 ± 0.10 b | 0.03 ± 0.02 a | 0.13 ± 0.06 b |
| 3-hexanone | <0.01 a | 0.05 ± 0.05 a | <0.01 a | 0.18 ± 0.15 b |
| Total ketones | 2.13 ± 0.77 a | 4.80 ± 1.26 b | 1.70 ± 0.47 a | 7.05 ± 2.18 c |
| Aliphatic hydrocarbons | ||||
| pentane | 10.72 ± 7.43 | 11.69 ± 5.12 | 9.40 ± 5.24 | 11.33 ± 6.76 |
| 2.2.4.6.6-pentamethylheptane | 4.73 ± 2.59 b | 0.09 ± 0.01 a | 5.77 ± 2.95 b | 0.05 ± 0.01 a |
| heptane | 2.13 ± 1.30 a | 2.49 ± 1.22 a | 2.66 ± 1.23 a | 4.86 ± 1.94 b |
| hexane | 0.83 ± 0.43 a | 1.02 ± 0.38 a | 1.08 ± 0.37 ab | 1.48 ± 0.73 b |
| 2-pentene | 0.72 ± 0.68 | 0.53 ± 0.48 | 0.68 ± 0.63 | 0.51 ± 0.42 |
| 3-methylene heptane | 0.52 ± 0.33 b | 0.04 ± 0.03 a | 0.61 ± 0.37 b | 0.04 ± 0.01 a |
| tetramethyl octane | 0.34 ± 0.22 b | 0.06 ± 0.05 a | 0.41 ± 0.19 b | 0.05 ± 0.02 a |
| 2.4-octadiene | 0.17 ± 0.08 c | 0.02 ± 0.01 a | 0.10 ± 0.06 b | 0.02 ± 0.01 a |
| 1,3-trans-5-cis-octatriene | 0.16 ± 0.10 b | 0.04 ± 0.04 a | 0.22 ± 0.13 b | 0.04 ± 0.02 a |
| 2,6,7-trimethyldecane | 0.14 ± 0.11 b | 0.04 ± 0.02 a | 0.16 ± 0.11 b | 0.02 ± 0.02 a |
| 1,3-cyclopentadiene | 0.13 ± 0.10 b | 0.10 ± 0.05 ab | 0.14 ± 0.11 b | 0.04 ± 0.01 a |
| 3-ethyl-1.5-octadiene | 0.12 ± 0.09 b | 0.03 ± 0.02 a | 0.17 ± 0.08 bc | 0.12 ± 0.10 b |
| 3-ethyl heptane | 0.07 ± 0.06 a | 0.29 ± 0.19 b | 0.14 ± 0.09 ab | 0.09 ± 0.05 a |
| decane | 0.07 ± 0.05 | 0.26 ± 0.21 | 0.11 ± 0.09 | 0.13 ± 0.09 |
| butyl-cyclopentane | 0.06 ± 0.03 a | 0.24 ± 0.16 b | 0.07 ± 0.06 a | 0.15 ± 0.11 ab |
| (Z)-2-octene | 0.04 ± 0.01 a | 13.77 ± 5.80 b | 0.06 ± 0.03 a | 28.71 ± 9.74 b |
| 1-heptene | <0.01 a | 0.15 ± 0.02 b | 0.01 ± 0.00 a | 0.13 ± 0.01 b |
| Total aliphatic hydrocarbons | 20.81 ±9.66 a | 30.78 ±10.71 b | 27.77 ±8.14 a | 47.75 ±9.74 c |
| Aromatic hydrocarbons | ||||
| benzene | 1.44 ± 1.05 b | 0.09 ± 0.02 a | 2.08 ± 1.33 b | 0.09 ± 0.06 a |
| toluene | 1.35 ± 0.95 b | 0.29 ± 0.19 a | 1.29 ± 0.74 b | 0.04 ± 0.03 a |
| ethenylbenzene | 0.13 ± 0.11 a | 1.66 ± 0.76 b | 0.19 ± 0.09 a | 2.96 ± 1.74 c |
| ethylbenzene | 0.04 ± 0.03 ab | 0.06 ± 0.02 b | 0.06 ± 0.03 b | 0.03 ± 0.02 a |
| Total aromatic hydrocarbons | 2.96 ±1.93 ab | 2.01 ± 0.65 a | 3.63 ± 1.89 b | 3.13 ± 1.73 b |
| Sulfur-containing compounds | ||||
| metanethiol | 0.36 ± 0.18 | 0.27 ± 0.24 | 0.23 ± 0.12 | 0.16 ± 0.12 |
| thiobis-methane | 0.38 ± 0.28 | 0.31 ± 0.25 | 0.25 ± 0.18 | 0.25 ± 0.17 |
| Total sulfur compounds | 0.76 ± 0.33 | 0.55 ± 0.31 | 0.48 ± 0.26 | 0.41 ± 0.21 |
| Others | ||||
| ethoxyethane | 1.60 ± 1.46 ab | 1.92 ± 1.15 ab | 5.52 ± 4.75 b | 1.09 ± 0.77 a |
| 4-bromoheptane | 0.39 ± 0.17 | 0.00 ± 0.00 | 0.26 ± 0.19 | 0.00 ± 0.00 |
| 2-ethylfurane | 0.22 ± 0.10 a | 2.73 ± 1.39 b | 0.29 ± 0.24 a | 3.46 ± 1.42 b |
| trichloromethane | 0.18 ± 0.10 a | 0.22 ± 0.15 a | 0.49 ± 0.35 b | 0.27 ± 0.18 a |
| hexyl formate | <0.01 a | 0.13 ± 0.10 b | <0.01 a | 0.14 ± 0.10 b |
| Total VOCs | 98.48 ± 1.09 | 92.64 ± 14.90 | 97.53 ± 4.14 | 98.37 ± 3.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beriain, M.J.; Gómez, I.; García, S.; Urroz, J.C.; Diéguez, P.M.; Ibañez, F.C. Hydrogen Gas-Grilling in Meat: Impact on Odor Profile and Contents of Polycyclic Aromatic Hydrocarbons and Volatile Organic Compounds. Foods 2024, 13, 2443. https://doi.org/10.3390/foods13152443
Beriain MJ, Gómez I, García S, Urroz JC, Diéguez PM, Ibañez FC. Hydrogen Gas-Grilling in Meat: Impact on Odor Profile and Contents of Polycyclic Aromatic Hydrocarbons and Volatile Organic Compounds. Foods. 2024; 13(15):2443. https://doi.org/10.3390/foods13152443
Chicago/Turabian StyleBeriain, María José, Inmaculada Gómez, Susana García, José Carlos Urroz, Pedro María Diéguez, and Francisco C. Ibañez. 2024. "Hydrogen Gas-Grilling in Meat: Impact on Odor Profile and Contents of Polycyclic Aromatic Hydrocarbons and Volatile Organic Compounds" Foods 13, no. 15: 2443. https://doi.org/10.3390/foods13152443
APA StyleBeriain, M. J., Gómez, I., García, S., Urroz, J. C., Diéguez, P. M., & Ibañez, F. C. (2024). Hydrogen Gas-Grilling in Meat: Impact on Odor Profile and Contents of Polycyclic Aromatic Hydrocarbons and Volatile Organic Compounds. Foods, 13(15), 2443. https://doi.org/10.3390/foods13152443








