A Comparative Study of Microbial Communities, Biogenic Amines, and Volatile Profiles in the Brewing Process of Rice Wines with Hongqu and Xiaoqu as Fermentation Starters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Physicochemical Parameter Determination
2.3. Higher Alcohol Determination
2.4. Biogenic Amine Determination
2.5. Volatile Flavor Compound Determination
2.6. Microbial Metagenomic Sequencing
2.7. Statistical Analysis and Visualization
3. Results and Discussion
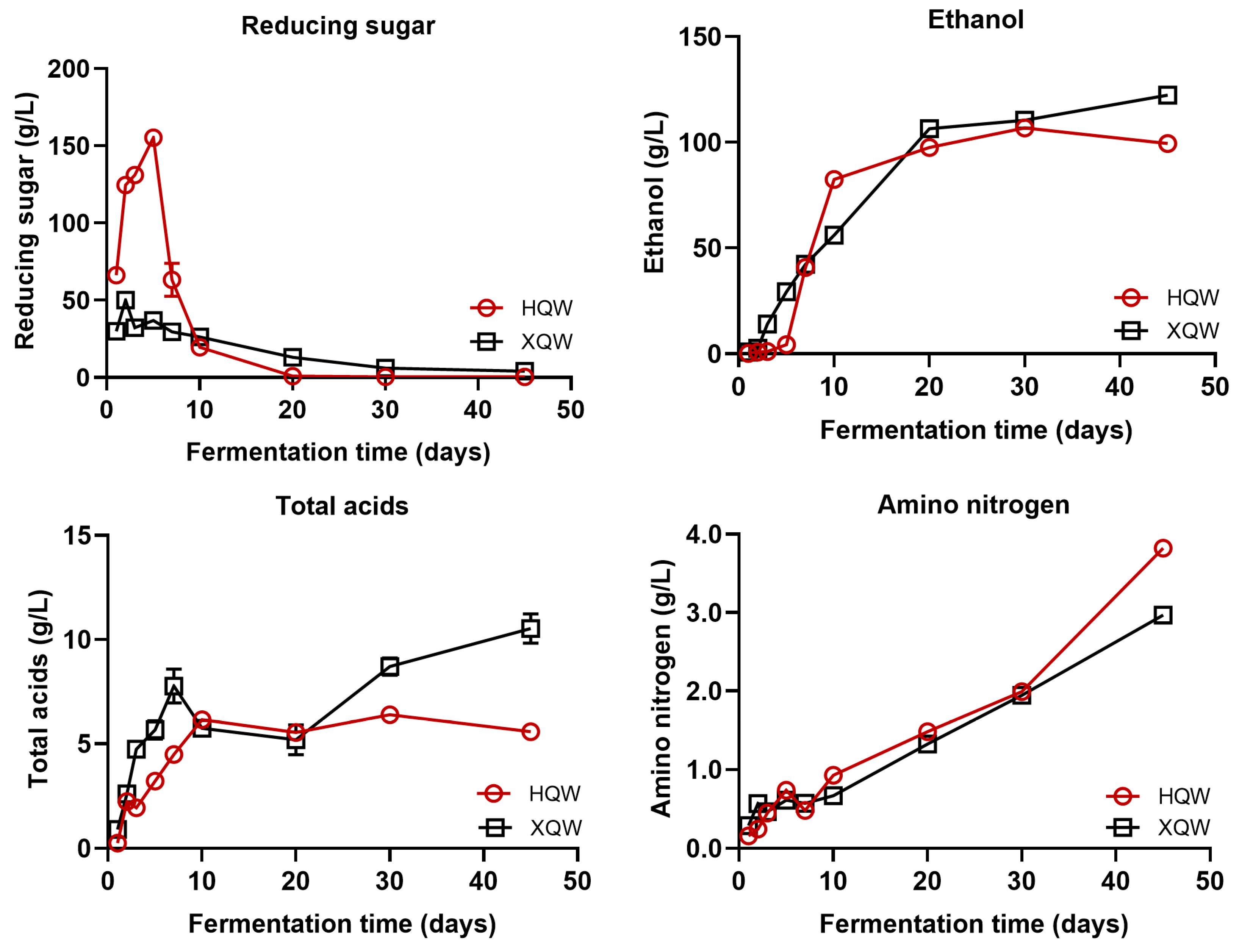
3.1. Dynamics of Physicochemical Parameters during HQW and XQW Brewing Processes
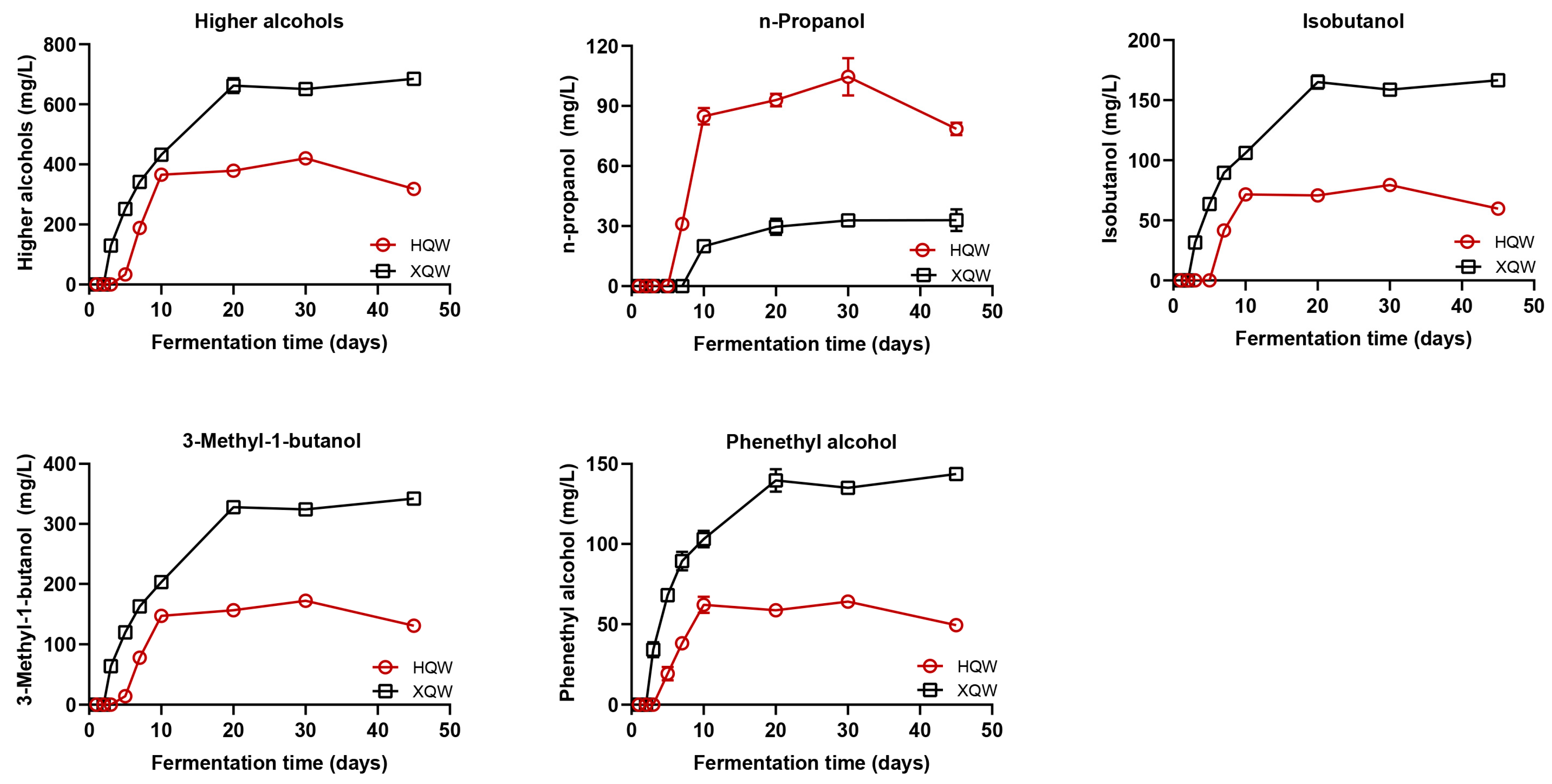
3.2. Dynamics of Higher Alcohols during HQW and XQW Brewing Processes
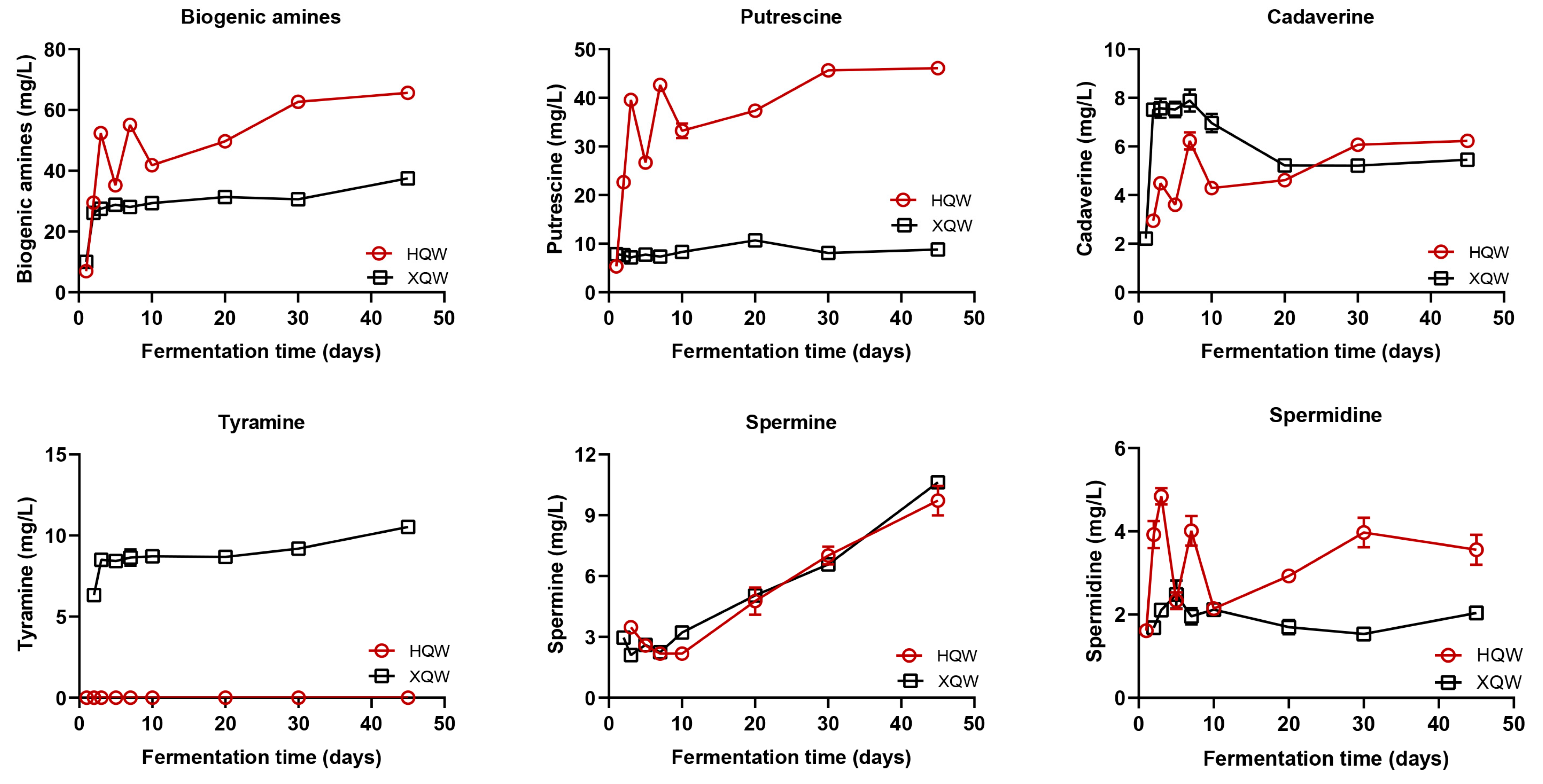
3.3. Dynamics of Biogenic Amines during HQW and XQW Brewing Processes
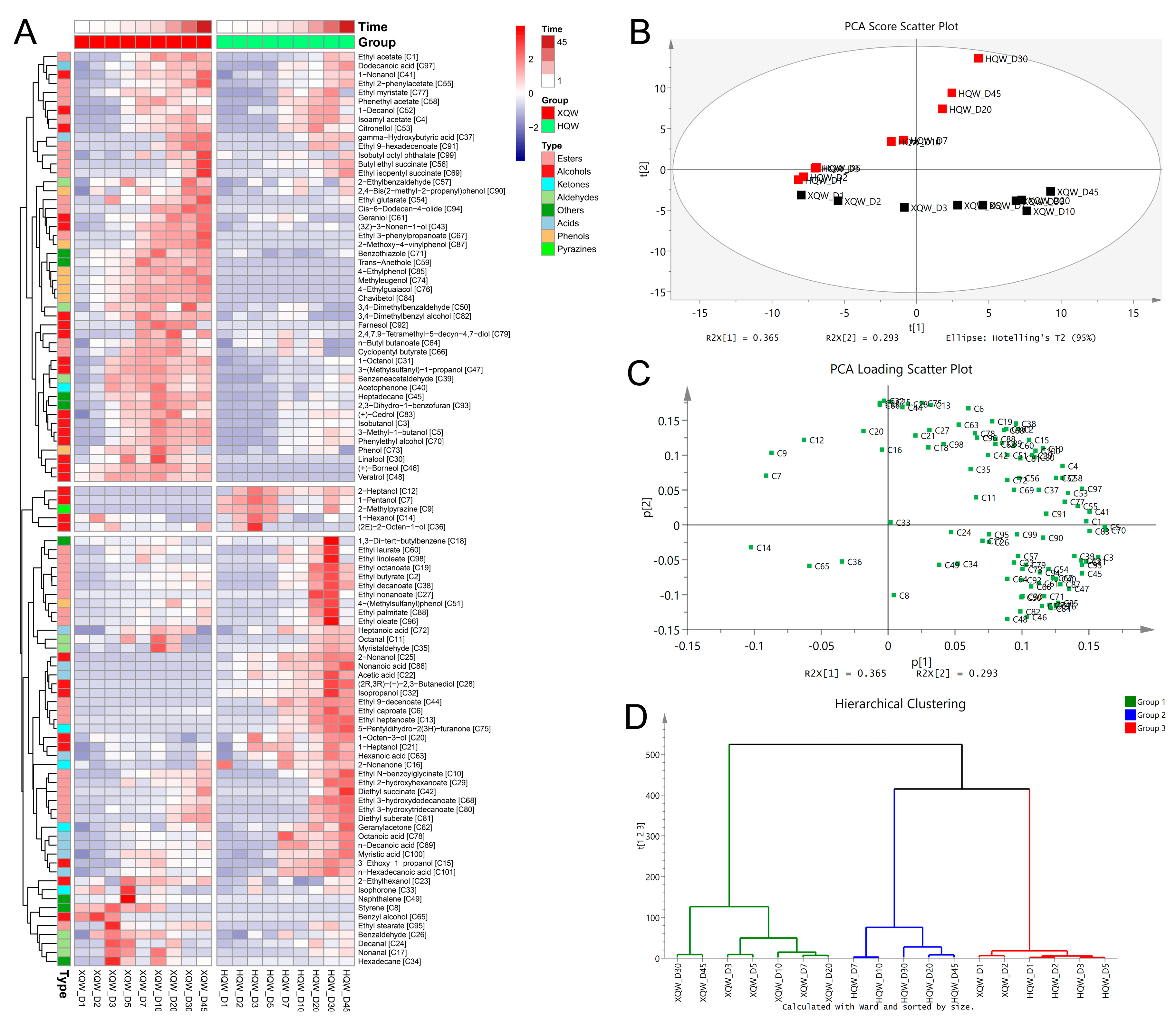
3.4. Dynamics of Volatile Organic Components during HQW and XQW Brewing
| Compound | CAS | Concentration (ug/L) | p-Value | Threshold (mg/L) # | OAV | ||
|---|---|---|---|---|---|---|---|
| XQW-D45 | HQW-D45 | XQW-D45 | HQW-D45 | ||||
| Acids | |||||||
| Acetic acid [C22] | 64-19-7 | 0.00 | 59.35 | 0.00180 | 60 | 0.00 | 0.00 |
| γ-Hydroxybutyric acid [C37] | 591-81-1 | 11.66 | 6.78 | 0.00869 | - | - | - |
| Hexanoic acid [C63] | 142-62-1 | 41,157.38 | 59,967.30 | 0.00104 | 5 | 8.23 | 11.99 |
| Heptanoic acid [C72] | 111-14-8 | 0.00 | 2415.11 | 0.00002 | 2.3 | 0.00 | 1.05 |
| Octanoic acid [C78] | 124-07-2 | 138.67 | 349.39 | 0.00267 | 0.91 | 0.15 | 0.38 |
| Nonanoic acid [C86] | 112-05-0 | 0.00 | 1865.22 | 0.00261 | 3 | 0.00 | 0.62 |
| n-Decanoic acid [C89] | 334-48-5 | 6481.45 | 11,408.41 | 0.10549 | 10 | 0.65 | 1.14 |
| Myristic acid [C100] | 544-63-8 | 4.19 | 8.71 | 0.01127 | 10 | 0.00 | 0.00 |
| n-Hexadecanoic acid [C101] | 57-10-3 | 17.51 | 27.97 | 0.03168 | - | - | - |
| Alcohols | |||||||
| Isobutanol [C3] | 78-83-1 | 56,372.81 | 21,780.91 | 0.02243 | 100 | 0.56 | 0.22 |
| 3-Methyl-1-butanol [C5] | 123-51-3 | 166,518.03 | 96,710.48 | 0.00073 | 50 | 3.33 | 1.93 |
| 1-Pentanol [C7] | 71-41-0 | 0.00 | 258.04 | 0.00095 | 50 | 0.00 | 0.01 |
| 2-Heptanol [C12] | 543-49-7 | 0.00 | 54.04 | 0.00012 | 0.25 | 0.00 | 0.22 |
| 1-Hexanol [C14] | 111-27-3 | 770.90 | 881.43 | 0.06357 | 4 | 0.19 | 0.22 |
| 3-Ethoxy-1-propanol [C15] | 111-35-3 | 2.39 | 6.21 | 0.00019 | 10 | 0.00 | 0.00 |
| 1-Octen-3-ol [C20] | 3391-86-4 | 19.35 | 36.38 | 0.00125 | 0.2 | 0.10 | 0.18 |
| 1-Heptanol [C21] | 111-70-6 | 66.05 | 94.95 | 0.00797 | 1 | 0.07 | 0.09 |
| 2-Ethylhexanol [C23] | 104-76-7 | 68.55 | 60.72 | 0.06074 | 0.83 | 0.08 | 0.07 |
| 2-Nonanol [C25] | 628-99-9 | 3.73 | 49.45 | 0.00001 | 0.075 | 0.05 | 0.66 |
| (2R,3R)-(−)-2,3-Butanediol [C28] | 24347-58-8 | 0.00 | 34.40 | 0.02356 | 400 | 0.00 | 0.00 |
| Linalool [C30] | 78-70-6 | 5.94 | 0.00 | 0.00000 | 0.08 | 0.07 | 0.00 |
| 1-Octanol [C31] | 111-87-5 | 115.13 | 61.49 | 0.00004 | 0.9 | 0.13 | 0.07 |
| Isopropanol [C32] | 67-63-0 | 0.00 | 17.77 | 0.01262 | 100 | 0.00 | 0.00 |
| (2E)-2-Octen-1-ol [C36] | 18409-17-1 | 6.94 | 0.00 | 0.00198 | 0.04 | 0.17 | 0.00 |
| 1-Nonanol [C41] | 143-08-8 | 85.12 | 55.00 | 0.00048 | 0.08 | 1.06 | 0.69 |
| (3Z)-3-Nonen-1-ol [C43] | 10340-23-5 | 15.84 | 3.08 | 0.00000 | - | - | - |
| (+)-Borneol [C46] | 507-70-0 | 6.65 | 0.00 | 0.00000 | - | - | - |
| 3-(Methylsulfanyl)-1-propanol [C47] | 505-10-2 | 3957.65 | 1152.95 | 0.00001 | 0.036 | 109.93 | 32.03 |
| 1-Decanol [C52] | 112-30-1 | 17.37 | 8.67 | 0.00041 | 0.18 | 0.10 | 0.05 |
| Geraniol [C61] | 106-24-1 | 13.28 | 0.00 | 0.01464 | 0.036 | 0.37 | 0.00 |
| Phenylethyl alcohol [C70] | 60-12-8 | 45,312.47 | 27,918.40 | 0.00003 | 5 | 9.06 | 5.58 |
| 2,4,7,9-Tetramethyl-5-decyn-4,7-diol [C79] | 126-86-3 | 60.09 | 3.56 | 0.00035 | - | - | - |
| 3,4-Dimethylbenzyl alcohol [C82] | 6966-10-5 | 6.73 | 0.00 | 0.00005 | - | - | - |
| (+)-Cedrol [C83] | 77-53-2 | 15.91 | 13.02 | 0.40790 | - | - | - |
| Aldehydes | |||||||
| Octanal [C11] | 124-13-0 | 0.00 | 28.02 | 0.09063 | 0.04 | 0.00 | 0.70 |
| Nonanal [C17] | 124-19-6 | 72.02 | 358.61 | 0.17653 | 0.015 | 4.80 | 23.91 |
| Decanal [C24] | 112-31-2 | 174.76 | 277.04 | 0.16765 | 0.005 | 34.95 | 55.41 |
| Benzaldehyde [C26] | 100-52-7 | 15.40 | 23.56 | 0.00021 | 1.5 | 0.01 | 0.02 |
| Myristaldehyde [C35] | 124-25-4 | 0.00 | 8.92 | 0.00599 | 100 | 0.00 | 0.00 |
| Benzeneacetaldehyde [C39] | 122-78-1 | 1324.39 | 1054.84 | 0.56011 | 0.1 | 13.24 | 10.55 |
| 3,4-Dimethylbenzaldehyde5973-71-7 [C50] | 5973-71-7 | 1.96 | 0.00 | 0.00007 | - | - | - |
| 2-Ethylbenzaldehyde [C57] | 22927-13-5 | 517.06 | 421.59 | 0.21568 | - | - | - |
| Esters | |||||||
| Ethyl acetate [C1] | 141-78-6 | 115,127.14 | 88,800.06 | 0.05713 | 30 | 3.84 | 2.96 |
| Ethyl butyrate [C2] | 105-54-4 | 142.71 | 237.50 | 0.40513 | 0.4 | 0.36 | 0.59 |
| Isoamyl acetate [C4] | 123-92-2 | 781.52 | 948.14 | 0.04757 | 0.68 | 1.15 | 1.39 |
| Ethyl caproate [C6] | 123-66-0 | 1322.60 | 4895.86 | 0.00000 | 0.21 | 6.30 | 23.31 |
| Ethyl N-benzoylglycinate [C10] | 1499-53-2 | 10.96 | 20.81 | 0.00038 | - | - | - |
| Ethyl heptanoate [C13] | 106-30-9 | 0.00 | 434.26 | 0.00014 | 0.4 | 0.00 | 1.09 |
| Ethyl octanoate [C19] | 106-32-1 | 1478.22 | 3056.34 | 0.00035 | 0.005 | 295.64 | 611.27 |
| Ethyl nonanoate [C27] | 123-29-5 | 0.00 | 99.90 | 0.00002 | 1.2 | 0.00 | 0.08 |
| Ethyl 2-hydroxyhexanoate [C29] | 6946-90-3 | 33.83 | 53.59 | 0.00045 | - | - | - |
| Ethyl decanoate [C38] | 110-38-3 | 3315.39 | 5265.61 | 0.04238 | 1.5 | 2.21 | 3.51 |
| Diethyl succinate [C42] | 123-25-1 | 982.12 | 1525.91 | 0.00011 | 75 | 0.01 | 0.02 |
| Ethyl 9-decenoate [C44] | 67233-91-4 | 0.00 | 9.46 | 0.00031 | - | - | - |
| Ethyl glutarate [C54] | 818-38-2 | 16.15 | 4.75 | 0.00044 | - | - | - |
| Ethyl 2-phenylacetate [C55] | 101-97-3 | 230.17 | 139.08 | 0.00011 | 0.15555 | 1.48 | 0.89 |
| Butyl ethyl succinate [C56] | 1000324-85-1 | 28.45 | 16.08 | 0.00017 | - | - | - |
| Phenethyl acetate [C58] | 103-45-7 | 377.34 | 560.37 | 0.00030 | 2 | 0.19 | 0.28 |
| Ethyl laurate [C60] | 106-33-2 | 15.06 | 13.21 | 0.40599 | 3.5 | 0.00 | 0.00 |
| n-Butyl butanoate [C64] | 109-21-7 | 133.20 | 33.95 | 0.00001 | 0.087 | 1.53 | 0.39 |
| Cyclopentyl butyrate [C66] | 6290-13-7 | 5.78 | 1.99 | 0.00030 | - | - | - |
| Ethyl 3-phenylpropanoate [C67] | 2021-28-5 | 6.07 | 0.00 | 0.00000 | 0.0016 | 3.79 | 0.00 |
| Ethyl 3-hydroxydodecanoate [C68] | 183613-15-2 | 31.76 | 66.73 | 0.00070 | - | - | - |
| Ethyl isopentyl succinate [C69] | 28024-16-0 | 47.86 | 29.84 | 0.00617 | - | - | - |
| Ethyl myristate [C77] | 124-06-1 | 23.57 | 3.55 | 0.00098 | 0.18 | 0.13 | 0.02 |
| Ethyl 3-hydroxytridecanoate [C80] | 107141-15-1 | 23.44 | 34.10 | 0.14882 | - | - | - |
| Diethyl suberate [C81] | 2050-23-9 | 9.55 | 11.91 | 0.24807 | - | - | - |
| Ethyl palmitate [C88] | 628-97-7 | 17,223.92 | 5502.63 | 0.00292 | 1.5 | 11.48 | 3.67 |
| Ethyl 9-hexadecenoate [C91] | 54546-22-4 | 16.09 | 0.00 | 0.00000 | - | - | - |
| Cis-6-Dodecen-4-olide [C94] | 18679-18-0 | 15.34 | 0.00 | 0.00000 | 0.0001 | 153.37 | 0.00 |
| Ethyl stearate [C95] | 111-61-5 | 142.08 | 29.85 | 0.00027 | 15000 | 0.00 | 0.00 |
| Ethyl oleate [C96] | 111-62-6 | 1346.04 | 465.10 | 0.00970 | 3.5 | 0.38 | 0.13 |
| Dodecanoic acid [C97] | 143-07-7 | 12.19 | 13.67 | 0.69195 | 0.5 | 0.02 | 0.03 |
| Isobutyl octyl phthalate [C99] | 1000309-04-5 | 43.27 | 24.98 | 0.02660 | - | - | - |
| Ketones | |||||||
| 2-Nonanone [C16] | 821-55-6 | 5.70 | 5.96 | 0.13240 | 0.2 | 0.03 | 0.03 |
| Isophorone [C33] | 78-59-1 | 2.55 | 7.08 | 0.00075 | 0.1 | 0.03 | 0.07 |
| Acetophenone [C40] | 98-86-2 | 15.81 | 8.81 | 0.00122 | 3 | 0.01 | 0.00 |
| Geranylacetone [C62] | 3796-70-1 | 90.00 | 139.19 | 0.01801 | 0.06 | 1.50 | 2.32 |
| 5-Pentyldihydro-2(3H)-furanone [C75] | 104-61-0 | 0.00 | 195.85 | 0.00001 | 0.0097 | 0.00 | 20.19 |
| Others | |||||||
| Hexadecane [C34] | 544-76-3 | 9.05 | 6.92 | 0.28655 | 300 | 0.00 | 0.00 |
| Heptadecane [C45] | 629-78-7 | 10.66 | 4.28 | 0.00596 | - | - | - |
| Naphthalene [C49] | 91-20-3 | 5.08 | 2.11 | 0.00385 | 0.36 | 0.01 | 0.01 |
| Trans-Anethole [C59] | 4180-23-8 | 21.55 | 0.00 | 0.00001 | 0.015 | 1.44 | 0.00 |
| Benzothiazole [C71] | 95-16-9 | 80.62 | 41.48 | 0.00164 | 0.08 | 1.01 | 0.52 |
| 2,3-Dihydro-1-benzofuran [C93] | 496-16-2 | 19.32 | 7.69 | 0.00344 | - | - | - |
| Phenols | |||||||
| 4-(Methylsulfanyl)phenol [C51] | 1073-72-9 | 11.46 | 3.98 | 0.00441 | 0.8 | 0.01 | 0.00 |
| Methyleugenol [C74] | 93-15-2 | 23.44 | 0.00 | 0.00001 | 1.25 | 0.02 | 0.00 |
| 4-Ethylguaiacol [C76] | 2785-89-9 | 227.57 | 0.00 | 0.00003 | 0.07 | 3.25 | 0.00 |
| Chavibetol [C84] | 501-19-9 | 231.37 | 0.00 | 0.00006 | - | - | - |
| 4-Ethylphenol [C85] | 123-07-9 | 186.66 | 0.00 | 0.00008 | 0.3 | 0.62 | 0.00 |
| 2-Methoxy-4-vinylphenol [C87] | 7786-61-0 | 1551.22 | 12.67 | 0.00003 | 0.003 | 517.07 | 4.22 |
| 2,4-Bis(2-methyl-2-propanyl)phenol [C90] | 96-76-4 | 452.19 | 362.51 | 0.44719 | 0.5 | 0.90 | 0.73 |
| Pyrazines | |||||||
| 2-Methylpyrazine [C9] | 109-08-0 | 0.00 | 191.91 | 0.00596 | 100 | 0.00 | 0.00 |
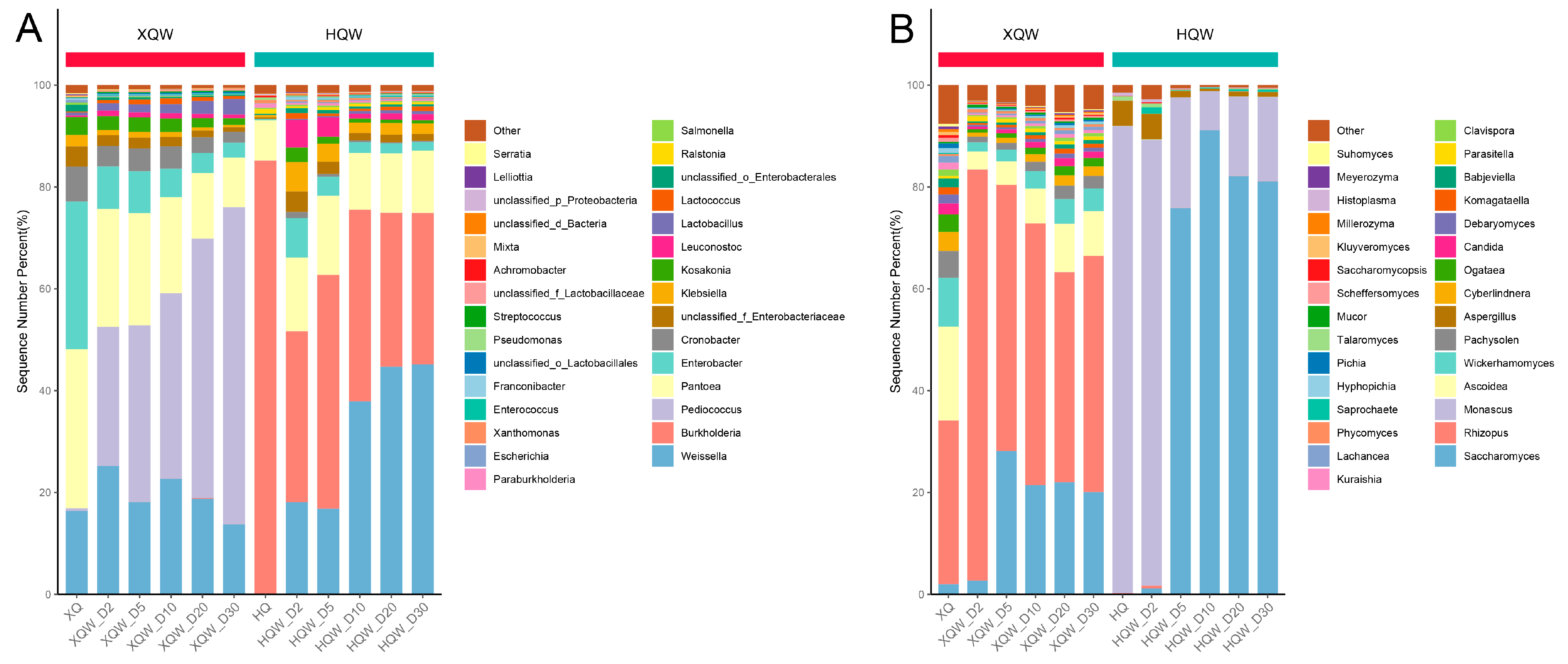
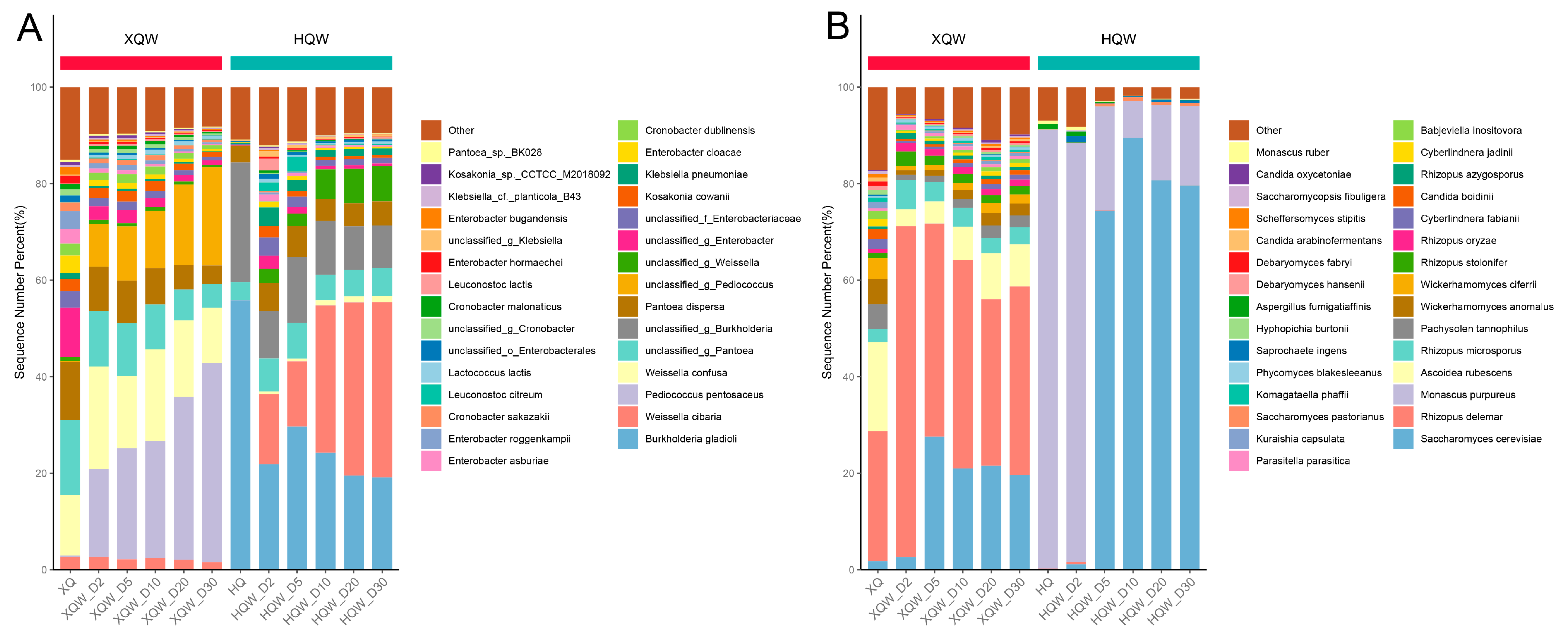
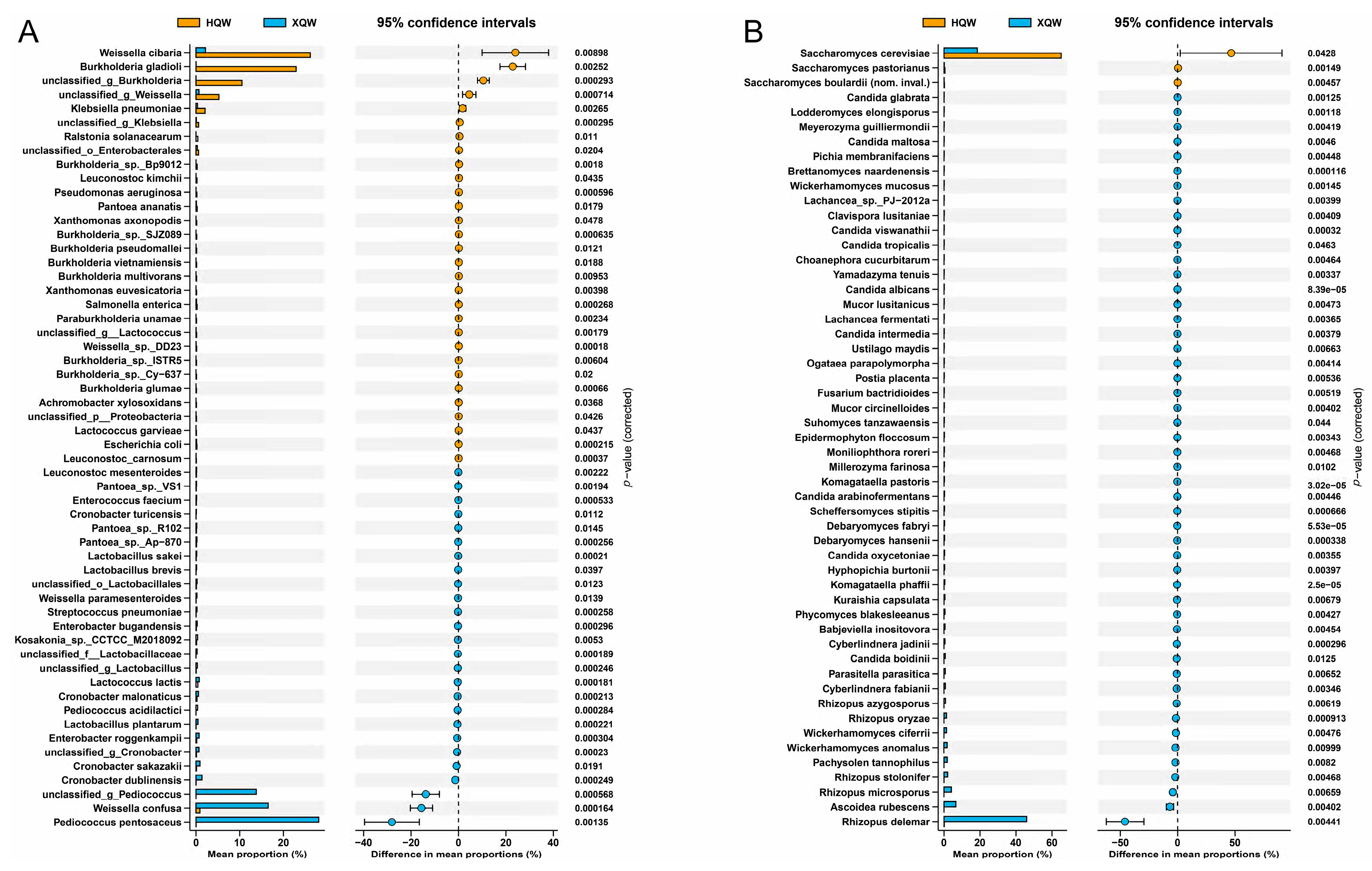
3.5. Dynamics of Microbial Community during HQW and XQW Brewing Processes
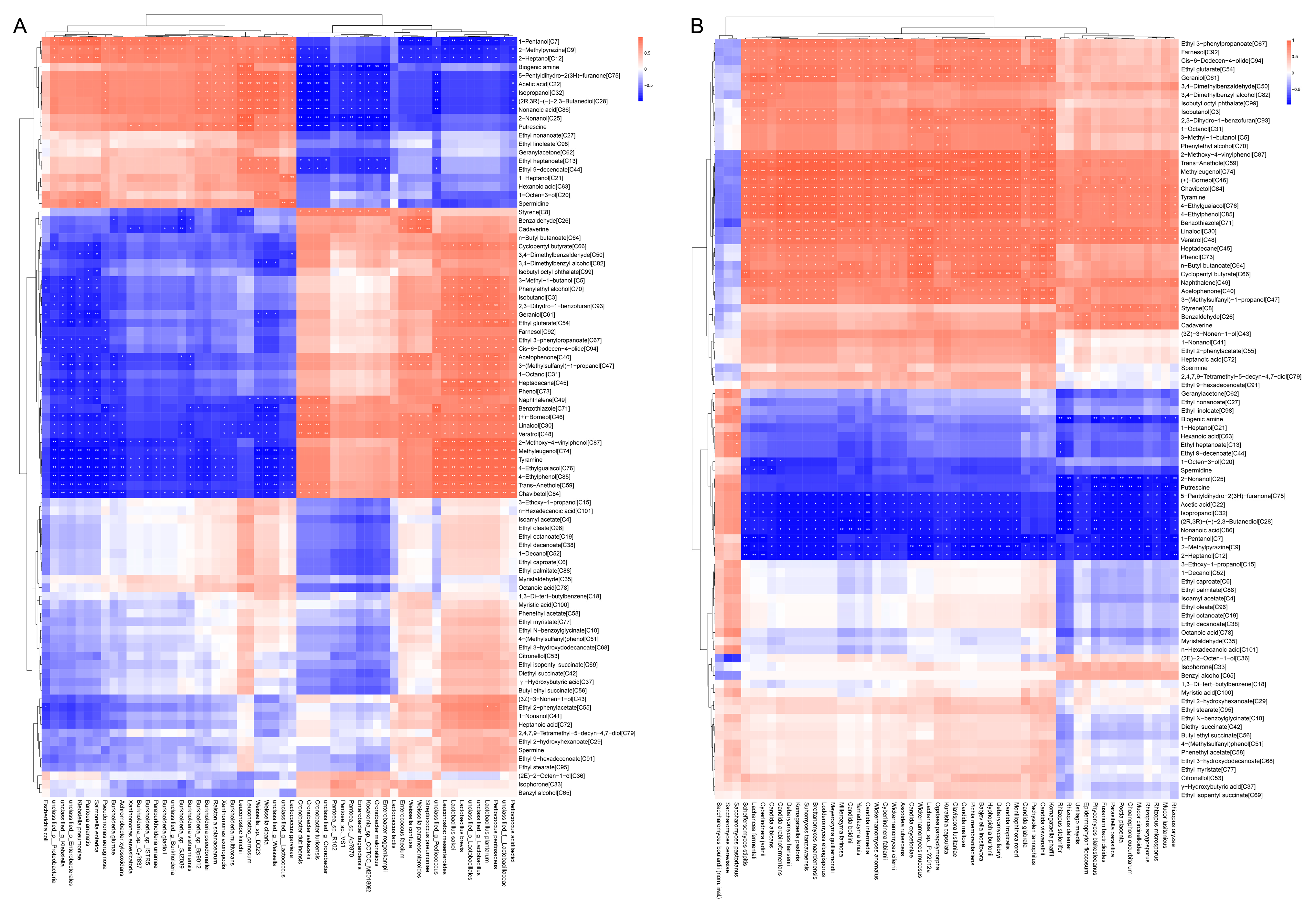
3.6. The Correlations between Microbial Communities and Metabolites
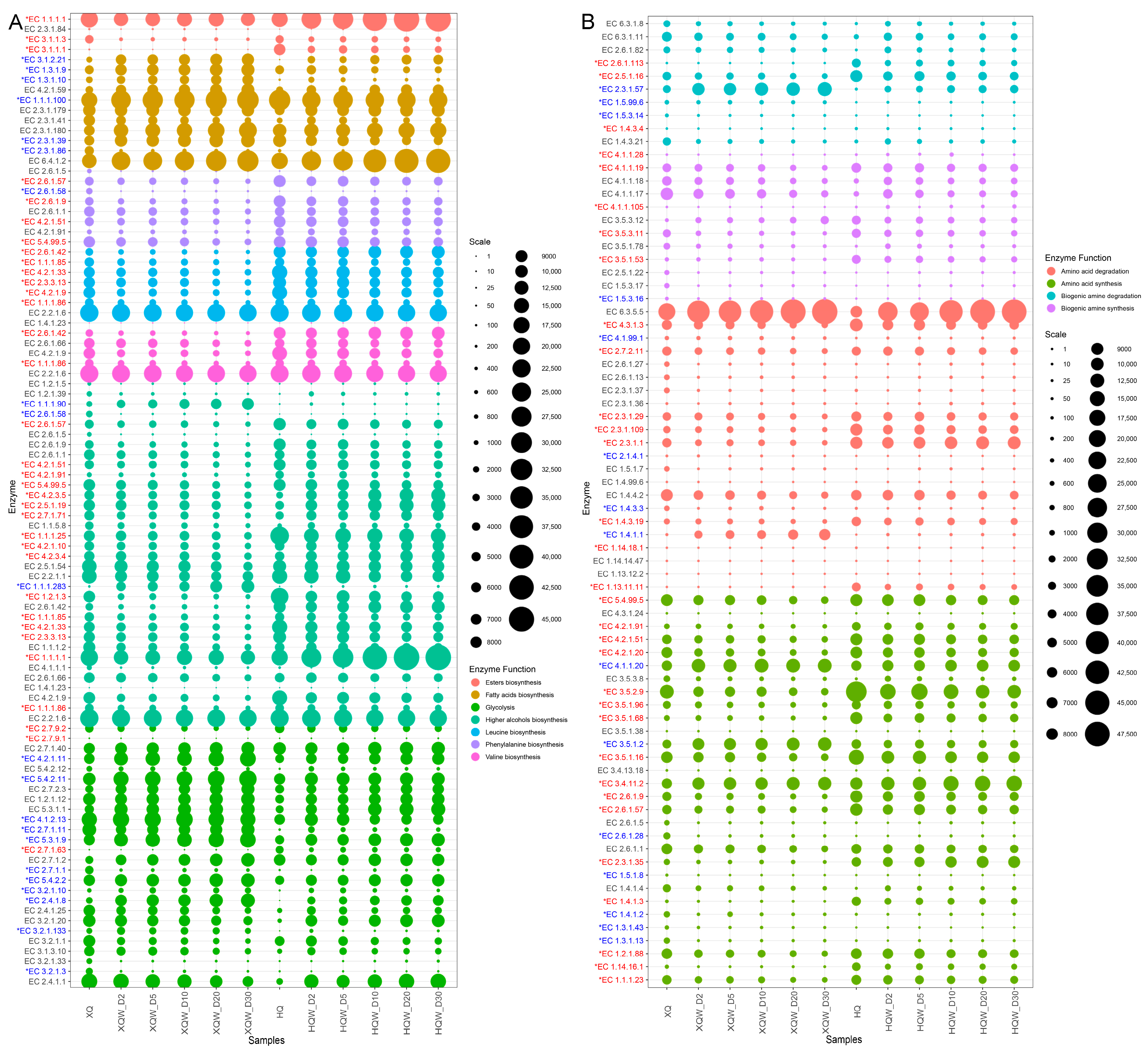
3.7. Functional Genes and Microorganisms for Flavor and Biogenic Amine Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, Y.; Cai, W.; Guo, Z.; Shan, C.; Wang, Y. Bacterial community structure of rice wine from China’s Xiaogan and Dazhou regions: Correlation with taste characteristics. LWT 2024, 192, 115733. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, J.; Zhao, W.; Ma, X.; Sun, H. Antifatigue and antiaging effects of Chinese rice wine in mice. Food Sci. Nutr. 2018, 6, 2386–2394. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, L.; Wu, H.; Wang, Y.; Wang, Z.; Zheng, F.; Lu, X.; Xu, J. The changes of microbial community and flavor compound in the fermentation process of Chinese rice wine using Fagopyrum tataricum grain as feedstock. Sci. Rep. 2019, 9, 3365. [Google Scholar] [CrossRef]
- Zhao, W.; Qian, M.; Dong, H.; Liu, X.; Bai, W.; Liu, G.; Lv, X.-c. Effect of Hong Qu on the flavor and quality of Hakka yellow rice wine (Huangjiu) produced in Southern China. LWT 2022, 160, 113264. [Google Scholar] [CrossRef]
- Cao, Y.; Xia, Q.; Chen, J.; Jin, Z.; Aniya. Understanding of microbial diversity in three representative Qu in China and characterization of the volatile compounds in the corresponding Chinese rice wine. LWT 2022, 164, 113680. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Q.; Wu, W.; Yang, J.; Zou, W. Wheat Qu and Its Production Technology, Microbiota, Flavor, and Metabolites. J. Food Sci. 2019, 84, 2373–2386. [Google Scholar] [CrossRef]
- Liang, Z.; He, B.; Lin, X.; Su, H.; He, Z.; Chen, J.; Li, W.; Zheng, Y. Effect of ADH7 gene loss on fusel oil metabolism of Saccharomyces cerevisiae for Huangjiu fermentation. LWT 2023, 175, 114444. [Google Scholar] [CrossRef]
- Liang, Z.; Lin, X.; He, Z.; Li, W.; Ren, X.; Lin, X. Dynamic changes of total acid and bacterial communities during the traditional fermentation of Hong Qu glutinous rice wine. Electron. J. Biotechnol. 2020, 43, 23–31. [Google Scholar] [CrossRef]
- Huang, Z.-R.; Guo, W.-L.; Zhou, W.-B.; Li, L.; Xu, J.-X.; Hong, J.-L.; Liu, H.-P.; Zeng, F.; Bai, W.-D.; Liu, B.; et al. Microbial communities and volatile metabolites in different traditional fermentation starters used for Hong Qu glutinous rice wine. Food Res. Int. 2019, 121, 593–603. [Google Scholar] [CrossRef]
- Xiao, R.; Chen, S.; Wang, X.; Chen, K.; Hu, J.; Wei, K.; Ning, Y.; Xiong, T.; Lu, F. Microbial community starters affect the profiles of volatile compounds in traditional Chinese Xiaoqu rice wine: Assement via high-throughput sequencing and gas chromatography-ion mobility spectrometry. LWT 2022, 170, 114000. [Google Scholar] [CrossRef]
- Chen, G.-M.; Li, W.-L.; Tong, S.-G.; Qiu, Y.-T.; Han, J.-Z.; Lv, X.-C.; Ai, L.-Z.; Sun, J.-Y.; Sun, B.-G.; Ni, L. Effects of the microbial community on the formation of volatile compounds and biogenic amines during the traditional brewing of Hongqu rice wine. Curr. Res. Food Sci. 2022, 5, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; He, F.; Pan, Z.; Du, Y.; Chen, Z.; He, Y.; Sun, Y.; Li, M. Effect of microbial communities on flavor profile of Hakka rice wine throughout production. Food Chem. X 2024, 21, 101121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Z.; Sun, J.; Ni, L. The dynamics of volatile compounds and their correlation with the microbial succession during the traditional solid-state fermentation of Gutian Hong Qu glutinous rice wine. Food Microbiol. 2020, 86, 103347. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Ge, J.; Song, Y.; Feng, P.; Lin, L.; Guo, L.; Zhang, C. Regulating the ratio of higher alcohols to esters by simultaneously overexpressing ATF1 and deleting BAT2 in brewer’s yeast Saccharomyces pastorianus. Food Biosci. 2021, 43, 101231. [Google Scholar] [CrossRef]
- Chen, G.-M.; Li, W.-L.; Yang, Z.-Y.; Liang, Z.-H.; Chen, S.-Y.; Qiu, Y.-J.; Lv, X.-C.; Ai, L.-Z.; Ni, L. Insights into microbial communities and metabolic profiles in the traditional production of the two representative Hongqu rice wines fermented with Gutian Qu and Wuyi Qu based on single-molecule real-time sequencing. Food Res. Int. 2023, 173, 113488. [Google Scholar] [CrossRef] [PubMed]
- Margaux, C.; Georgia, L.; Sophie, T.; Jean-Christophe, B. Olfactory Impact of Higher Alcohols on Red Wine Fruity Ester Aroma Expression in Model Solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar]
- Sun, H.; Liu, S.; Mao, J.; Yu, Z.; Lin, Z.; Mao, J. New insights into the impacts of huangjiu compontents on intoxication. Food Chem. 2020, 317, 126420. [Google Scholar] [CrossRef] [PubMed]
- Cui-Ying, Z.; Ya-Nan, Q.; Hong-Xia, M.; Wei, L.; Long-Hai, D.; Dong-Guang, X. Decreased production of higher alcohols by Saccharomyces cerevisiae for Chinese rice wine fermentation by deletion of Bat aminotransferases. J. Ind. Microbiol. Biotechnol. 2015, 42, 617–625. [Google Scholar]
- Lachenmeier, D.W.; Haupt, S.; Schulz, K. Defining maximum levels of higher alcohols in alcoholic beverages and surrogate alcohol products. Regul. Toxicol. Pharmacol. 2007, 50, 313–321. [Google Scholar] [CrossRef]
- Latorre-Moratalla, M.L.; Bover-Cid, S.; Talon, R.; Garriga, M.; Zanardi, E.; Ianieri, A.; Fraqueza, M.J.; Elias, M.; Drosinos, E.H.; Vidal-Carou, M.C. Strategies to reduce biogenic amine accumulation in traditional sausage manufacturing. LWT-Food Sci. Technol. 2010, 43, 20–25. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Liu, Y.; Li, X.; Wang, F.; Huang, Y.; Shi, P.; Brennan, C.S.; Wang, M. Mechanisms and factors influencing the ability of lactic acid bacteria on reducing biogenic amines in fermented food: A mini review. LWT 2024, 197, 115890. [Google Scholar] [CrossRef]
- Curiel, J.A.; Ruiz-Capillas, C.; Rivas, B.D.L.; Carrascosa, A.V.; Jiménez-Colmenero, F.; Muñoz, R. Production of biogenic amines by lactic acid bacteria and enterobacteria isolated from fresh pork sausages packaged in different atmospheres and kept under refrigeration. Meat Sci. 2011, 88, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Luo, Y.; Zhang, Q.; Huang, Y.; Zhang, B. Mixed Starter Culture Regulates Biogenic Amines Formation via Decarboxylation and Transamination during Chinese Rice Wine Fermentation. J. Agric. Food Chem. 2018, 66, 6348–6356. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Shi, R.; Gong, P.; Liu, Y.; Chen, W.; Wang, C. Biogenic amines in Huangjiu (Chinese rice wine): Formation, hazard, detection, and reduction. LWT 2022, 168, 113952. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Custódio, F.B.; Gloria, M.B.A. Health concerns associated with biogenic amines in food and interaction with amine oxidase drugs. Curr. Opin. Food Sci. 2023, 54, 101090. [Google Scholar] [CrossRef]
- Kalač, P.; Krausová, P. A review of dietary polyamines: Formation, implications for growth and health and occurrence in foods. Food Chem. 2005, 90, 219–230. [Google Scholar] [CrossRef]
- Li, B.; Lu, S. The Importance of Amine-degrading Enzymes on the Biogenic Amine Degradation in Fermented Foods: A review. Process Biochem. 2020, 99, 331–339. [Google Scholar] [CrossRef]
- Teixeira, G.G.; Santos, P.M. Simple and cost-effective approaches for quantification of reducing sugar exploiting digital image analysis. J. Food Compos. Anal. 2022, 113, 104719. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Z.; Liang, Z.; Wu, Q.; Yan, Y.; Chen, S.; Li, X.; Ai, L.; Ni, L.; Lv, X. The regulatory effects of microbial community on the formation of higher alcohols and volatile flavor components in Hongqu rice wine brewing. Food Biosci. 2023, 56, 103142. [Google Scholar] [CrossRef]
- Pineda, A.; Carrasco, J.; Pena-Farfal, C.; Henriquez-Aedo, K.; Aranda, M. Preliminary evaluation of biogenic amines content in Chilean young varietal wines by HPLC. Food Control 2012, 23, 251–257. [Google Scholar] [CrossRef]
- Liu, S.P.; Mao, J.; Liu, Y.Y.; Meng, X.Y.; Ji, Z.W.; Zhou, Z.L.; Ai-lati, A. Bacterial succession and the dynamics of volatile compounds during the fermentation of Chinese rice wine from Shaoxing region. World J. Microbiol. Biotechnol. 2015, 31, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Bruns, M.A.; Tiedje, J.M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 1996, 62, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-H.; Lu, Z.-M.; Zhang, X.-J.; Wang, Z.-M.; Yu, Y.-J.; Shi, J.-S.; Xu, Z.-H. Metagenomics reveals flavour metabolic network of cereal vinegar microbiota. Food Microbiol. 2017, 62, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Y.; Zhao, Q.-S.; Su, C.; Yang, J.-G. Analysis of the Microbial Community Structure during Brewing of Sichuan Xiaoqu Baijiu. J. Am. Soc. Brew. Chem. 2019, 77, 210–219. [Google Scholar] [CrossRef]
- Toshiaki, N.; Tsuyoshi, H.; Hideaki, T.; Yasubumi, S. MetaVelvet: An extension of Velvet assembler to de novo metagenome assembly from short sequence reads. Nucleic Acids Res. 2012, 40, e155. [Google Scholar]
- Espinosa-Ramírez, J.; Pérez-Carrillo, E.; Serna-Saldívar, S.O. Maltose and glucose utilization during fermentation of barley and sorghum lager beers as affected by β-amylase or amyloglucosidase addition. J. Cereal Sci. 2014, 60, 602–609. [Google Scholar] [CrossRef]
- Zhao, C.; Su, W.; Mu, Y.; Jiang, L.; Mu, Y. Correlations between microbiota with physicochemical properties and volatile flavor components in black glutinous rice wine fermentation. Food Res. Int. 2020, 138, 109800. [Google Scholar] [CrossRef]
- Longo, R.; Carew, A.; Sawyer, S.; Kemp, B.; Kerslake, F. A review on the aroma composition of Vitis vinifera L. Pinot noir wines: Origins and influencing factors. Crit. Rev. Food Sci. Nutr. 2021, 61, 1589–1604. [Google Scholar] [CrossRef]
- Liang, Z.-C.; Lin, X.-Z.; He, Z.-G.; Su, H.; Li, W.-X.; Guo, Q.-Q. Comparison of microbial communities and amino acid metabolites in different traditional fermentation starters used during the fermentation of Hong Qu glutinous rice wine. Food Res. Int. 2020, 136, 109329. [Google Scholar] [CrossRef]
- Huang, D.; Zhong, Y.; Liu, Y.; Song, Y.; Zhao, X.; Qin, Y. Reducing higher alcohols by integrating indigenous Saccharomyces cerevisiae, nitrogen compensation, and chaptalization methods during fermentation of kiwifruit wine. LWT 2023, 184, 115059. [Google Scholar] [CrossRef]
- Guo, L.; Luo, Y.; Zhou, Y.; Bianba, C.; Guo, H.; Zhao, Y.; Fu, H. Exploring microbial dynamics associated with flavours production during highland barley wine fermentation. Food Res. Int. 2020, 130, 108971. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, C.; He, R.; Zhang, Y.; Wang, B.; Zhang, Z.-H.; Ho, C.-T. Research advances on biogenic amines in traditional fermented foods: Emphasis on formation mechanism, detection and control methods. Food Chem. 2023, 405, 134911. [Google Scholar] [CrossRef]
- Fong, F.L.Y.; El-Nezami, H.; Sze, E.T.P. Biogenic amines—Precursors of carcinogens in traditional Chinese fermented food. NFS J. 2021, 23, 52–57. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, X.; Chen, X.; Jiang, M.; Li, C.; Dong, M. A survey of biogenic amines in Chinese rice wines. Food Chem. 2007, 100, 1424–1428. [Google Scholar]
- Pardo, I. Biogenic amine synthesis in high quality Tempranillo wines. Relationship with lactic acid bacteria and vinification conditions. Ann. Microbiol. 2011, 61, 191–198. [Google Scholar]
- Jae-Hyung, M. Biogenic amine reduction by vasoactive amine-degrading Bacillus licheniformis CH7P22 in Enterococcus faecium-contaminated Cheonggukjang, Korean fermented soybean paste. Food Control 2023, 153, 109956. [Google Scholar]
- Wang, Z.; Wang, Y.; Zhu, T.; Wang, J.; Huang, M.; Wei, J.; Ye, H.; Wu, J.; Zhang, J.; Meng, N. Characterization of the key odorants and their content variation in Niulanshan Baijiu with different storage years using flavor sensory omics analysis. Food Chem. 2022, 376, 131851. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ai, L.; Mu, Z.; Liu, H.; Yan, X.; Ni, L.; Zhang, H.; Xia, Y. Flavor compounds with high odor activity values (OAV > 1) dominate the aroma of aged Chinese rice wine (Huangjiu) by molecular association. Food Chem. 2022, 383, 132370. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, Y.; Qian, M.C. Aroma characterization of chinese rice wine by gas chromatography-olfactometry, chemical quantitative analysis, and aroma reconstitution. J. Agric. Food Chem. 2013, 61, 11295–11302. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, F.; Sun, J.; Ni, L. Dynamic changes of volatile and phenolic components during the whole manufacturing process of Wuyi Rock tea (Rougui). Food Chem. 2022, 367, 130624. [Google Scholar] [CrossRef]
- Moreno, J.A.; Zea, L.; Moyano, L.; Medina, M. Aroma compounds as markers of the changes in sherry wines subjected to biological ageing. Food Control 2005, 16, 333–338. [Google Scholar] [CrossRef]
- Salo, P.; NykÄNen, L.; Suomalainen, H. Odor thresholds and relative intensities of volatile aroma components in an artificial beverage imitating whisky. J. Food Sci. 2006, 37, 394–398. [Google Scholar] [CrossRef]
- Hamilton, E.I. Compilation of Odour Threshold Values in Air and Water; van Gemert, L.J., Nettenbreijer, A.H., Eds.; Price: DFl. 22.00. Science of The Total Environment 1978; National Institute for Water Supply: Voorburg, The Netherlands, 1977; Volume 9, pp. 300–301. [Google Scholar]
- Wang, S.; Wu, Q.; Nie, Y.; Wu, J.; Xu, Y. Construction of Synthetic Microbiota for Reproducible Flavor Compound Metab-olism in Chinese Light-Aroma-Type Liquor Produced by Solid-State Fermentation. Appl. Environ. Microbiol. 2019, 85, e03090-18. [Google Scholar] [CrossRef] [PubMed]
- Monnin, L.; Nidelet, T.; Noble, J.; Galeote, V. Insights into intraspecific diversity of central carbon metabolites in Saccharomyces cerevisiae during wine fermentation. Food Microbiol. 2024, 121, 104513. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Peng, P.; Yu, L.; Wan, B.; Liang, X.; Liu, P.; Liao, W.; Miao, L. Metagenomic analysis reveals the correlations between microbial communities and flavor compounds during the brewing of traditional Fangxian huangjiu. Food Biosci. 2024, 58, 103816. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Yang, Q.; Han, X.; Zhou, Z.; Mao, J. Saccharomyces cerevisiae strains with low-yield higher alcohols and high-yield acetate esters improve the quality, drinking comfort and safety of huangjiu. Food Res. Int. 2022, 161, 111763. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Q.; Chen, D.; Fu, B.; Zhang, Y.; Zhang, Y.; Xia, X.; Peng, N.; Liang, Y.; Zhao, S. Study on microbial communities and higher alcohol formations in the fermentation of Chinese Xiaoqu Baijiu produced by traditional and new mechanical technologies. Food Res. Int. 2021, 140, 109876. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Du, B.; Zhang, C.; Zhu, L.; Yan, Y.; Li, W.; Leng, W.; Pang, Z.; Li, X.; Sun, B. Effects of Saccharomyces cerevisiae on microbial community and flavor metabolites in solid-state fermentation of strong-flavor Baijiu. Food Biosci. 2024, 59, 103925. [Google Scholar] [CrossRef]
- Ben Salah, R.; Ghamghui, H.; Miled, N.; Mejdoub, H.; Gargouri, Y. Production of butyl acetate ester by lipase from novel strain of Rhizopus oryzae. J. Biosci. Bioeng. 2007, 103, 368–372. [Google Scholar] [CrossRef]
- Zha, M.; Sun, B.; Wu, Y.; Yin, S.; Wang, C. Improving flavor metabolism of Saccharomyces cerevisiae by mixed culture with Wickerhamomyces anomalus for Chinese Baijiu making. J. Biosci. Bioeng. 2018, 126, 189–195. [Google Scholar] [CrossRef]
- Tian, M.; Lin, K.; Yang, L.; Jiang, B.; Zhang, B.; Zhu, X.; Ren, D.; Yu, H. Characterization of key aroma compounds in gray sufu fermented using Leuconostoc mesenteroides subsp. Mesenteroides F24 as a starter culture. Food Chem. X 2023, 20, 100881. [Google Scholar] [CrossRef]
- Zhao, J.; Niu, C.; Du, S.; Liu, C.; Zheng, F.; Wang, J.; Li, Q. Reduction of biogenic amines formation during soybean paste fermentation by using Staphylococcus carnosus M43 and Pediococcus acidilactici M28 as starter culture. LWT 2020, 133, 109917. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, T.-T.; Guo, R.-R.; Ye, Q.; Zhao, H.-L.; Huang, X.-H. The regulation of key flavor of traditional fermented food by microbial metabolism: A review. Food Chem. X 2023, 19, 100871. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Tan, Y.; Kan, J. Regulation of quality and biogenic amine production during sufu fermentation by pure Mucor strains. LWT 2020, 117, 108637. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, Y.; Fang, C.; Wijffels, R.H.; Xu, Y. Can we control microbiota in spontaneous food fermentation?—Chinese liquor as a case example. Trends Food Sci. Technol. 2021, 110, 321–331. [Google Scholar] [CrossRef]
- El-Dalatony, M.M.; Saha, S.; Govindwar, S.P.; Abou-Shanab, R.A.I.; Jeon, B.-H. Biological Conversion of Amino Acids to Higher Alcohols. Trends Biotechnol. 2019, 37, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Tezuka, H.; Ishii, J.; Matsuda, F.; Ogino, C.; Kondo, A. Genetic engineering to enhance the Ehrlich pathway and alter carbon flux for increased isobutanol production from glucose by Saccharomyces cerevisiae. J. Biotechnol. 2012, 159, 32–37. [Google Scholar] [CrossRef]
- Zhang, J.; Du, R.; Niu, J.; Ban, S.; Zhang, Y.; Xu, L.; Nie, H.; Wu, Q.; Xu, Y. Daqu and environmental microbiota regulate fatty acid biosynthesis via driving the core microbiota in soy sauce aroma type liquor fermentation. Int. J. Food Microbiol. 2024, 408, 110423. [Google Scholar] [CrossRef]
- Suárez, R.; Suárez-Lepe, J.A.; Morata, A.; Calderón, F. The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: A review. Food Chem. 2007, 102, 10–21. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, S.; Du, T.; Xu, Y.; Zhao, X.; Huang, G.; Guan, Q.; Xiong, T. Unraveling the core functional microbiota involved in metabolic network of characteristic flavor development during soy sauce fermentation. Food Biosci. 2024, 58, 103697. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Q.; Zou, H.; Yu, Y.; Zhou, Z.; Mao, J.; Zhang, S. A metagenomic analysis of the relationship between microorganisms and flavor development in Shaoxing mechanized huangjiu fermentation mashes. Int. J. Food Microbiol. 2019, 303, 9–18. [Google Scholar] [CrossRef]
- Xiong, S.; Xu, X.; Liu, Q.; Xu, Y.; Ren, H.; Xiong, T.; Xie, M. Integrated metatranscriptomics and metabolomics revealed the metabolic pathways of biogenic amines during Laotan Suancai fermentation. Food Biosci. 2024, 57, 103517. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Chen, Y.; Guo, J.; Li, C.; Zhang, J. Metatranscriptomic approach reveals the functional and enzyme dynamics of core microbes during noni fruit fermentation. Food Res. Int. 2021, 141, 109999. [Google Scholar] [CrossRef]
- Wang, D.; Chen, G.; Tang, Y.; Li, J.; Huang, R.; Ye, M.; Ming, J.; Wu, Y.; Xu, F.; Lai, X.; et al. Correlation between autochthonous microbial communities and flavor profiles during the fermentation of mustard green paocai (Brassica juncea Coss.), a typical industrial-scaled salted fermented vegetable. LWT 2022, 172, 114212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Liang, Z.; Huo, Y.; Wu, Q.; Ni, L.; Lv, X. A Comparative Study of Microbial Communities, Biogenic Amines, and Volatile Profiles in the Brewing Process of Rice Wines with Hongqu and Xiaoqu as Fermentation Starters. Foods 2024, 13, 2452. https://doi.org/10.3390/foods13152452
Yan Y, Liang Z, Huo Y, Wu Q, Ni L, Lv X. A Comparative Study of Microbial Communities, Biogenic Amines, and Volatile Profiles in the Brewing Process of Rice Wines with Hongqu and Xiaoqu as Fermentation Starters. Foods. 2024; 13(15):2452. https://doi.org/10.3390/foods13152452
Chicago/Turabian StyleYan, Yingyin, Zihua Liang, Yujia Huo, Qi Wu, Li Ni, and Xucong Lv. 2024. "A Comparative Study of Microbial Communities, Biogenic Amines, and Volatile Profiles in the Brewing Process of Rice Wines with Hongqu and Xiaoqu as Fermentation Starters" Foods 13, no. 15: 2452. https://doi.org/10.3390/foods13152452







