Role of Quinoa (Chenopodium quinoa Willd) and Chickpea (Cicer arietinum L.) Ratio in Physicochemical Stability and Microbiological Quality of Fermented Plant-Based Beverages during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant-Based Beverages Preparation
2.2. Chemical Analysis during Storage
Determination of pH, Titratable Acidity, Water-Soluble Solids, and Organic Acids
2.3. Physical Analysis during Storage
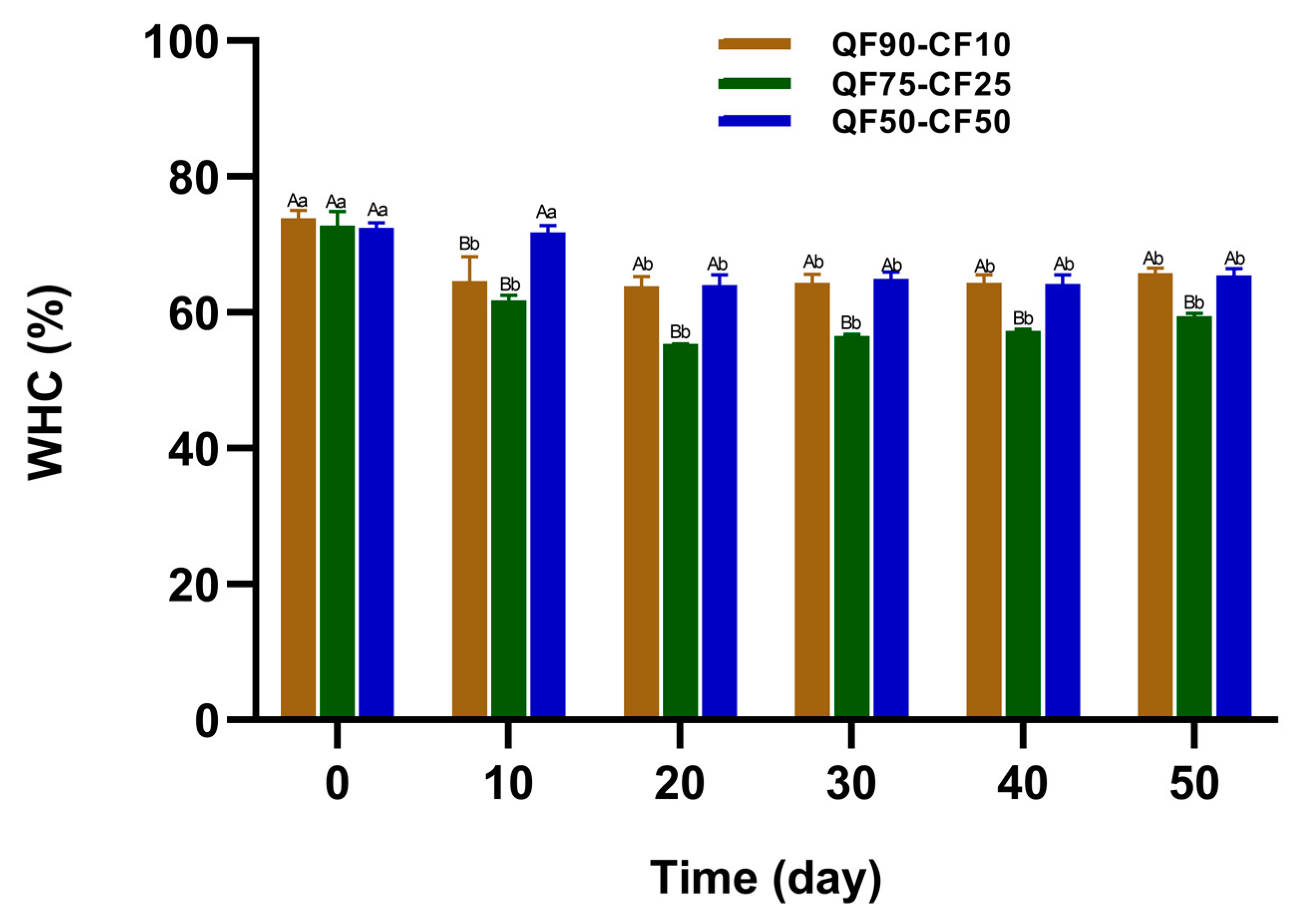
Determination of the Water-Holding Capacity
2.4. Rheological Measurements during Storage
2.5. Microbiological Quality
2.6. Probiotic Viability
2.7. Statistical Analysis
3. Results and Discussion
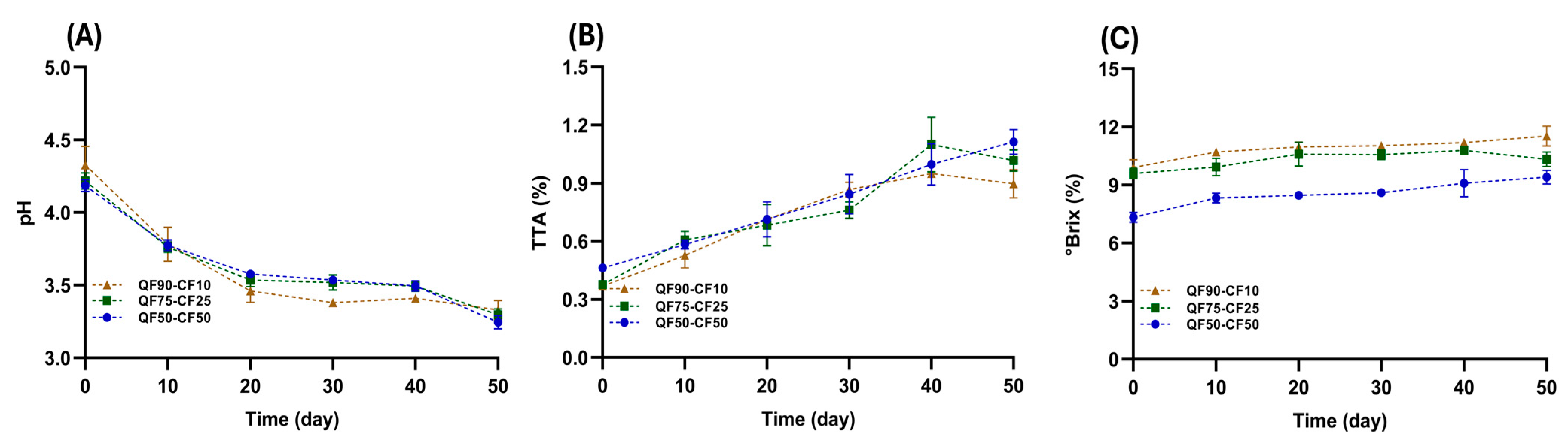
3.1. Chemical Properties of Fermented Plant-Based Beverages during Storage
3.2. Physical Stability of Fermented Plant-Based Beverages during Storage
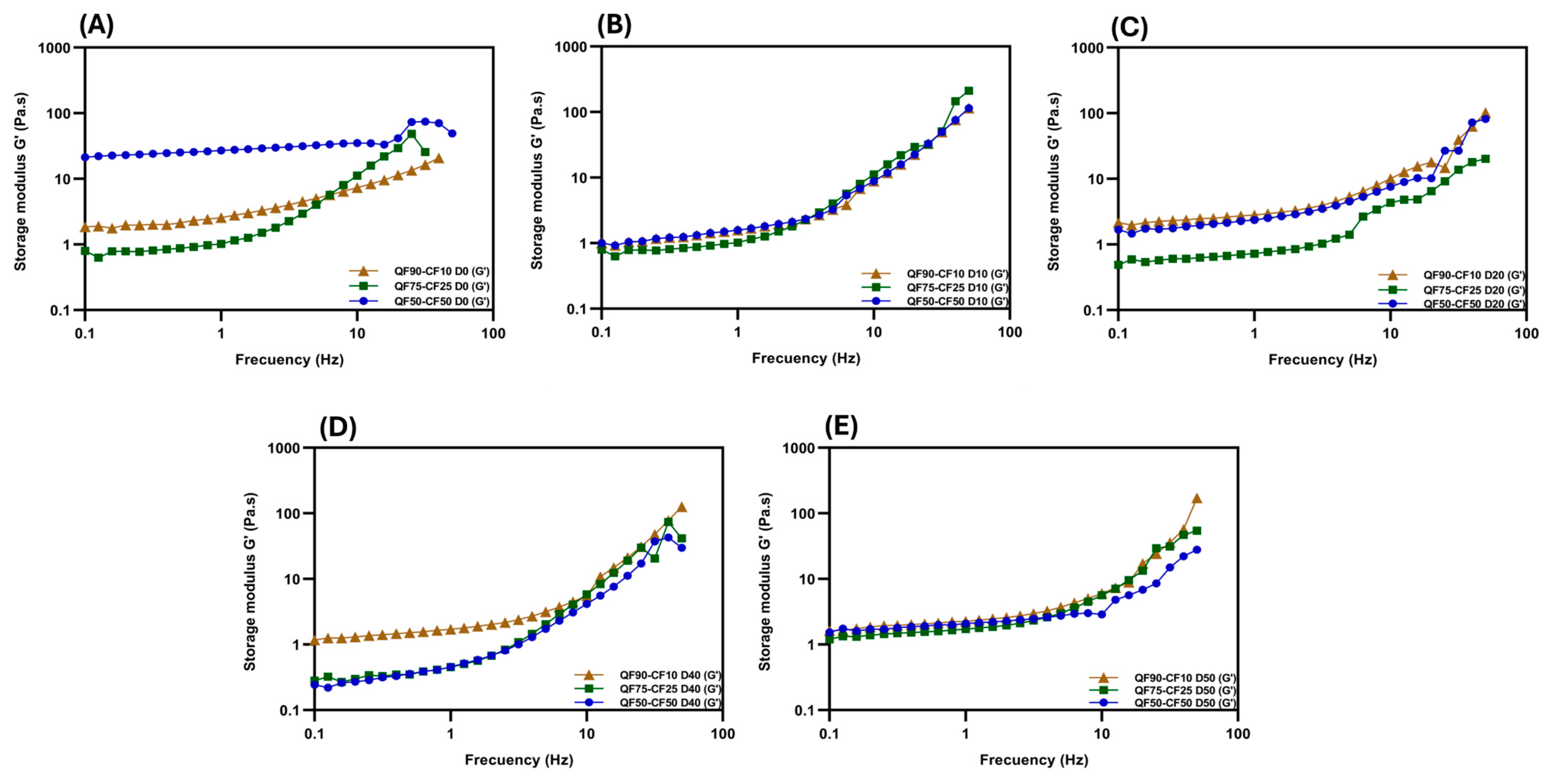
3.3. Changes in the Rheological Properties of Fermented Plant-Based Beverages during Storage
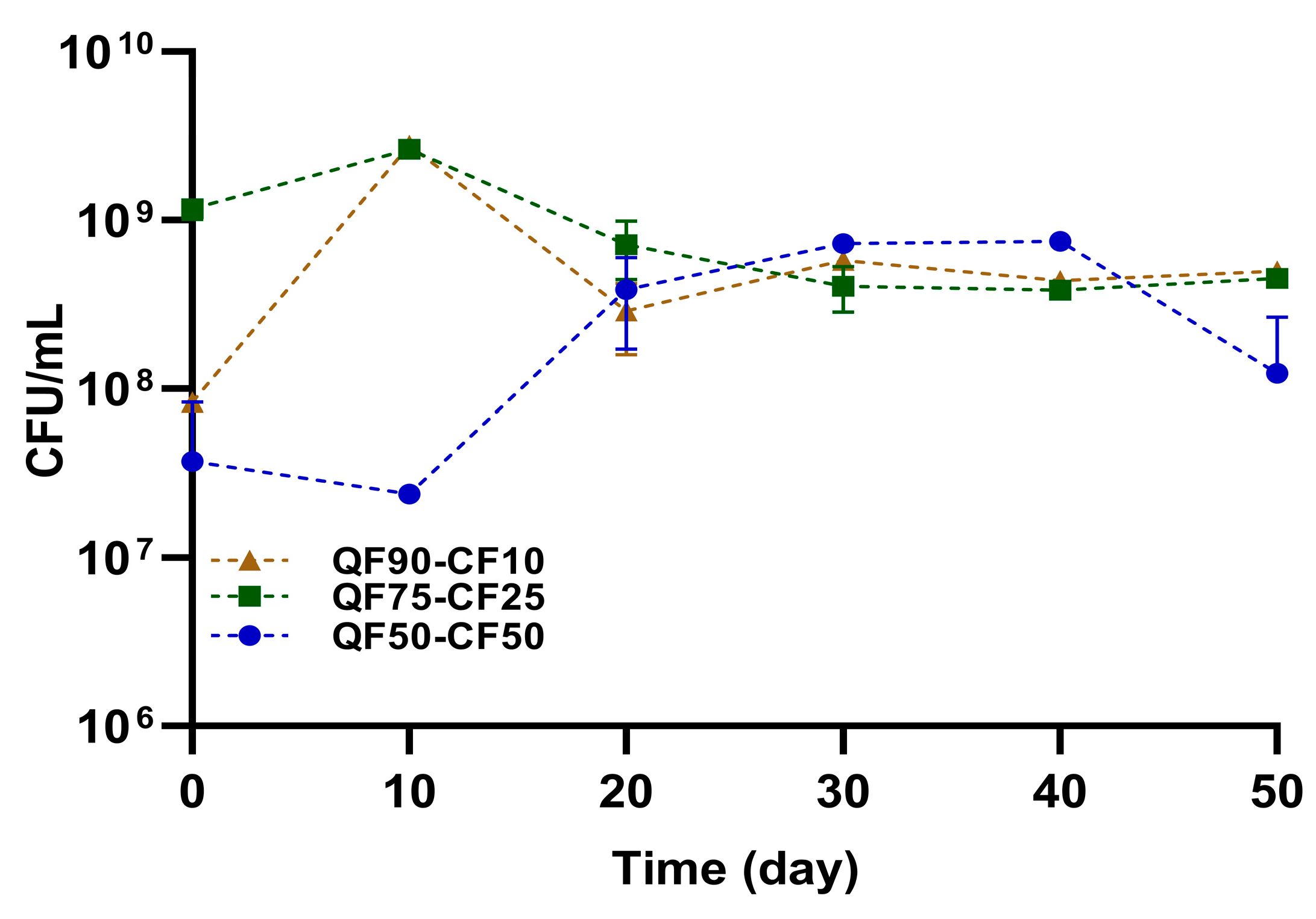
3.4. Microbiological Quality during Storage
3.5. Probiotic Viability under Storage Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medici, E.; Craig, W.J.; Rowland, I. A Comprehensive Analysis of the Nutritional Composition of Plant-Based Drinks and Yogurt Alternatives in Europe. Nutrients 2023, 15, 3415. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Fuentes, B.; de Jesús-José, E.; de Cabrera-Hidalgo, A.J.; Sandoval-Castilla, O.; Espinosa-Solares, T.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Liceaga, A.M.; Aguilar-Toalá, J.E. Plant-Based Fermented Beverages: Nutritional Composition, Sensory Properties, and Health Benefits. Foods 2024, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Mehany, T.; Siddiqui, S.A.; Olawoye, B.; Olabisi Popoola, O.; Hassoun, A.; Manzoor, M.F.; Punia Bangar, S. Recent Innovations and Emerging Technological Advances Used to Improve Quality and Process of Plant-Based Milk Analogs. Crit. Rev. Food Sci. Nutr. 2024, 64, 7237–7267. [Google Scholar] [CrossRef] [PubMed]
- Gungor, G.; Akpinar, A.; Yerlikaya, O. Production of Plant-Based Fermented Beverages Using Probiotic Starter Cultures and Propionibacterium spp. Food Biosci. 2024, 59, 103840. [Google Scholar] [CrossRef]
- Dhankhar, J.; Kundu, P. Stability Aspects of Non-Dairy Milk Alternatives; London. 2021. Available online: www.intechopen.com (accessed on 11 June 2024).
- Molet-Rodríguez, A.; Salvia-Trujillo, L.; Martín-Belloso, O. Beverage Emulsions: Key Aspects of Their Formulation and Physicochemical Stability. Beverages 2018, 4, 70. [Google Scholar] [CrossRef]
- Canaviri Paz, P.; Janny, R.J.; Håkansson, Å. Safeguarding of Quinoa Beverage Production by Fermentation with Lactobacillus plantarum DSM 9843. Int. J. Food Microbiol. 2020, 324, 108630. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liu, Y.; Zhang, Y.; Cao, H.; Luo, D.-K.; Yi, C.; Guan, X. Formulation of Plant-Based Yoghurt from Soybean and Quinoa and Evaluation of Physicochemical, Rheological, Sensory and Functional Properties. Food Biosci. 2022, 49, 101831. [Google Scholar] [CrossRef]
- Allahdad, Z.; Manus, J.; Aguilar-Uscanga, B.R.; Salmieri, S.; Millette, M.; Lacroix, M. Physico-Chemical Properties and Sensorial Appreciation of a New Fermented Probiotic Beverage Enriched with Pea and Rice Proteins. Plant Foods Human. Nutr. 2022, 77, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Pierrepont, C.; Duarte, C.M.; Filipe, A.; Medronho, B.; Sousa, I. Legume Beverages from Chickpea and Lupin, as New Milk Alternatives. Foods 2020, 9, 1458. [Google Scholar] [CrossRef]
- Itagi, H.B.N.; Singh, V. Preparation, Nutritional Composition, Functional Properties and Antioxidant Activities of Multigrain Composite Mixes. J. Food Sci. Technol. 2012, 49, 74–81. [Google Scholar] [CrossRef]
- Kyrylenko, A.; Eijlander, R.T.; Alliney, G.; van de Bos, E.L.; Wells-Bennik, M.H.J. Levels and Types of Microbial Contaminants in Different Plant-Based Ingredients Used in Dairy Alternatives. Int. J. Food Microbiol. 2023, 407, 110392. [Google Scholar] [CrossRef] [PubMed]
- Dridi, C.; Millette, M.; Aguilar, B.; Salmieri, S.; Lacroix, M. Storage Stability of a Fermented Probiotic Beverage Enriched with Cricket Protein Hydrolysates. Food Bioproc Tech. 2022, 15, 2587–2600. [Google Scholar] [CrossRef]
- Roland, I.S.; Le, T.T.; Chen, T.; Aguilera-Toro, M.; Nielsen, S.D.H.; Larsen, L.B.; Poulsen, N.A. Storage Stability of Plant-Based Drinks Related to Proteolysis and Generation of Free Amino Acids. Foods 2024, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Chasquibol, N.; Sotelo, A.; Alarcón, R. Development of Powdered Beverage with Cushuro (Nostoc commune) Concentrated Protein and Quinoa (Chenopodium quinoa). Biol. Life Sci. Forum 2023, 25, 2. [Google Scholar] [CrossRef]
- Ng, C.Y.; Wang, M. The Functional Ingredients of Quinoa (Chenopodium quinoa) and Physiological Effects of Consuming Quinoa: A Review. Food Front. 2021, 2, 329–356. [Google Scholar] [CrossRef]
- Urquizo, F.E.L.; Torres, S.M.G.; Tolonen, T.; Jaakkola, M.; Pena-Niebuhr, M.G.; von Wright, A.; Repo-Carrasco-Valencia, R.; Korhonen, H.; Plumed-Ferrer, C. Development of a Fermented Quinoa-Based Beverage. Food Sci. Nutr. 2017, 5, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Contardo, I.; Guzmán, F.; Enrione, J. Conformational and Structural Changes in Chickpea Proteins Caused by Simulated Salivary Alterations in the Elderly. Foods 2023, 12, 3668. [Google Scholar] [CrossRef] [PubMed]
- Mefleh, M.; Faccia, M.; Natrella, G.; De Angelis, D.; Pasqualone, A.; Caponio, F.; Summo, C. Development and Chemical-Sensory Characterization of Chickpeas-Based Beverages Fermented with Selected Starters. Foods 2022, 11, 3578. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Raymundo, V.G.; Vélez-Ruiz, J.F. Yoghurt-Type Beverage with Partial Substitution of Milk by a Chickpea Extract: Effect on Physicochemical and Flow Properties. Int. J. Dairy. Technol. 2019, 72, 266–274. [Google Scholar] [CrossRef]
- Masiá, C.; Geppel, A.; Jensen, P.E.; Buldo, P.; Smith, C.J.; Lluna, A.G. Effect of Lactobacillus Rhamnosus on Physicochemical Properties of Fermented Plant-Based Raw Materials. Foods 2021, 9, 573. [Google Scholar] [CrossRef]
- Sidhu, M.K.; Lyu, F.; Sharkie, T.P.; Ajlouni, S.; Ranadheera, C.S. Probiotic Yogurt Fortified with Chickpea Flour: Physico-Chemical Properties and Probiotic Survival during Storage and Simulated Gastrointestinal Transit. Foods 2020, 9, 1144. [Google Scholar] [CrossRef] [PubMed]
- Demarinis, C.; Verni, M.; Pinto, L.; Rizzello, C.G.; Baruzzi, F. Use of Selected Lactic Acid Bacteria for the Fermentation of Legume-Based Water Extracts. Foods 2022, 11, 3346. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, S.M.; Zahedani, M.R.; Rahmdel, S.; Hemmati, F.; Mazloomi, S.M. Development of Lactobacillus acidophilus-Fermented Milk Fortified with Date Extract. LWT 2018, 98, 577–582. [Google Scholar] [CrossRef]
- Iskakova, J.; Smanalieva, J.; Methner, F.J. Investigation of Changes in Rheological Properties during Processing of Fermented Cereal Beverages. J. Food Sci. Technol. 2019, 56, 3980–3987. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; McClements, D.J.; Luo, S.; Ye, J.; Liu, C. A Review of the Effects of Fermentation on the Structure, Properties, and Application of Cereal Starch in Foods. Crit. Rev. Food Sci. Nutr. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Bontsidis, C.; Mallouchos, A.; Terpou, A.; Nikolaou, A.; Batra, G.; Mantzourani, I.; Alexopoulos, A.; Plessas, S. Microbiological and Chemical Properties of Chokeberry Juice Fermented by Novel Lactic Acid Bacteria with Potential Probiotic Properties during Fermentation at 4 °C for 4 Weeks. Foods 2021, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Ani, E.; Amove, J.; Igbabul, B. Physicochemical, Microbiological, Sensory Properties and Storage Stability of Plant-Based Yoghurt Produced from Bambaranut, Soybean and Moringa oleifera Seed Milks. Am. J. Food Nutr. 2018, 6, 115–125. [Google Scholar] [CrossRef]
- Udayakumar, S.; Rasika, D.M.D.; Priyashantha, H.; Vidanarachchi, J.K.; Ranadheera, C.S. Probiotics and Beneficial Microorganisms in Biopreservation of Plant-Based Foods and Beverages. Appl. Sci. 2022, 12, 11737. [Google Scholar] [CrossRef]
- Tabasco, R.; García-Cayuela, T.; Peláez, C.; Requena, T. Lactobacillus acidophilus La-5 Increases Lactacin B Production When It Senses Live Target Bacteria. Int. J. Food Microbiol. 2009, 132, 109–116. [Google Scholar] [CrossRef]
- Cabello-Olmo, M.; Oneca, M.; Torre, P.; Díaz, J.V.; Encio, I.J.; Barajas, M.; Araña, M. Influence of Storage Temperature and Packaging on Bacteria and Yeast Viability in a Plant-Based Fermented Food. Foods 2020, 9, 302. [Google Scholar] [CrossRef]
- Xu, X.; Cui, H.; Yuan, Z.; Xu, J.; Li, J.; Liu, J.; Liu, H.; Zhu, D. Effects of Different Combinations of Probiotics on Rheology, Microstructure, and Moisture Distribution of Soy Materials-Based Yogurt. J. Food Sci. 2022, 87, 2820–2830. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Iturra, N.; Contardo, I.; Millao, S.; Morales, E.; Rubilar, M. Food-Grade Bigels with Potential to Replace Saturated and Trans Fats in Cookies. Gels 2022, 8, 445. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, S.; Li, Y.; Sun, F.; Huang, D.; Chen, X. Alternations in the Multilevel Structures of Chickpea Protein during Fermentation and Their Relationship with Digestibility. Food Res. Int. 2023, 165, 112453. [Google Scholar] [CrossRef] [PubMed]
- Rincon, L.; Braz Assunção Botelho, R.; de Alencar, E.R. Development of Novel Plant-Based Milk Based on Chickpea and Coconut. LWT 2020, 128, 109479. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.J.; Frutos, M.J.; Pérez-Llamas, F. Probiotic Red Quinoa Drinks for Celiacs and Lactose Intolerant People: Study of Functional, Physicochemical and Probiotic Properties during Fermentation and Gastrointestinal Digestion. Int. J. Food Sci. Nutr. 2022, 73, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Mauro, C.S.I.; Garcia, S. Coconut Milk Beverage Fermented by Lactobacillus reuteri: Optimization Process and Stability during Refrigerated Storage. J. Food Sci. Technol. 2019, 56, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, G.; Kaushal, K. Extraction, Characterization, Physicochemical and Rheological Properties of Two Different Varieties of Chickpea Starch. Legume Sci. 2020, 2, e17. [Google Scholar] [CrossRef]
- Bulut, M.; Tunçtürk, Y.; Alwazeer, D. Effect of Fortification of Set-Type Yoghurt with Different Plant Extracts on Its Physicochemical, Rheological, Textural and Sensory Properties during Storage. Int. J. Dairy. Technol. 2021, 74, 723–736. [Google Scholar] [CrossRef]
- Chekdid, A.A.; Kahn, C.J.F.; Prévot, E.; Ferrières, M.; Lemois, B.; Choquet, C.; Linder, M. Mixture Design Applied for Formulation and Characterization of Vegetal-Based Fermented Products. LWT 2021, 146, 111336. [Google Scholar] [CrossRef]
- Petersson, K.; Nordlund, E.; Tornberg, E.; Eliasson, A.C.; Buchert, J. Impact of Cell Wall-Degrading Enzymes on Water-Holding Capacity and Solubility of Dietary Fibre in Rye and Wheat Bran. J. Sci. Food Agric. 2013, 93, 882–889. [Google Scholar] [CrossRef]
- Ziarno, M.; Zaręba, D.; Ścibisz, I.; Kozłowska, M. Comprehensive Studies on the Stability of Yogurt-Type Fermented Soy Beverages during Refrigerated Storage Using Dairy Starter Cultures. Front. Microbiol. 2023, 14, 1230025. [Google Scholar] [CrossRef]
- Ismail, H.A.; Rayan, A.M. Preparation and Evaluation of Quinoa-Kishk as a Novel Functional Fermented Dairy Product. J. Food Sci. Technol. 2022, 59, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Manus, J.; Millette, M.; Uscanga, B.R.A.; Salmieri, S.; Maherani, B.; Lacroix, M. In Vitro Protein Digestibility and Physico-Chemical Properties of Lactic Acid Bacteria Fermented Beverages Enriched with Plant Proteins. J. Food Sci. 2021, 86, 4172–4182. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, D.; Torley, P.J.; Chandrapala, J.; Terefe, N.S. Microbial Fermentation for Improving the Sensory, Nutritional and Functional Attributes of Legumes. Fermentation 2023, 9, 635. [Google Scholar] [CrossRef]
- Uruc, K.; Tekin, A.; Sahingil, D.; Hayaloglu, A.A. An Alternative Plant-Based Fermented Milk with Kefir Culture Using Apricot (Prunus armeniaca L. ) Seed Extract: Changes in Texture, Volatiles, and Bioactivity during Storage. Innov. Food Sci. Emerg. Technol. 2022, 82, 103189. [Google Scholar] [CrossRef]
- Xie, X.; Zheng, M.; Bai, Y.; Zhang, Z.; Zhang, M.; Chen, Z.; Hu, X.; Li, J. Effect of Lactiplantibacillus plantarum and Saccharomyces cerevisiae Fermentation on the Multi-Scale Structure and Physicochemical Properties of Highland Barley Starch. Food Biosci. 2023, 52, 102419. [Google Scholar] [CrossRef]
- Codină, G.G.; Franciuc, S.G.; Mironeasa, S. Rheological Characteristics and Microstructure of Milk Yogurt as Influenced by Quinoa Flour Addition. J. Food Qual. 2016, 39, 559–566. [Google Scholar] [CrossRef]
- Lopez-Ochoa, J.D.; Cadena-Chamorro, E.; Ciro-Velasquez, H.; Rodríguez-Sandoval, E. Enzymatically Modified Cassava Starch as a Stabilizer for Fermented Dairy Beverages. Starch/Staerke 2022, 74, 2100242. [Google Scholar] [CrossRef]
- Chen, X.; Singh, M.; Bhargava, K.; Ramanathan, R. Yogurt Fortification with Chickpea (Cicer arietinum) Flour: Physicochemical and Sensory Effects. JAOCS 2018, 95, 1041–1048. [Google Scholar] [CrossRef]
- Pachekrepapol, U.; Kokhuenkhan, Y.; Ongsawat, J. Formulation of Yogurt-like Product from Coconut Milk and Evaluation of Physicochemical, Rheological, and Sensory Properties. Int. J. Gastron. Food Sci. 2021, 25, 100393. [Google Scholar] [CrossRef]
- Singh, N. Functional and Physicochemical Properties of Pulse Starch. In Pulse Foods: Processing, Quality and Nutraceutical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 87–112. [Google Scholar] [CrossRef]
- Bender, D.; Schönlechner, R. Recent Developments and Knowledge in Pseudocereals Including Technological Aspects. Acta Aliment. 2021, 50, 583–609. [Google Scholar] [CrossRef]
- Standard 1407; Microbiological criteria that foods and beverages intended for human consumption must satisfy title. Ministry of Health and Social Protection: Bogotá, Colombian Republic, 2022. Available online: https://www.icbf.gov.co/cargues/avance/compilacion/docs/resolucion_minsaludps_1407_2022.htm (accessed on 5 July 2024).
- Chaturvedi, S.; Chakraborty, S. Evaluation of Quality Attributes and In Vitro Characteristics of Synbiotic Legume-Based Beverage during Storage. Food Biosci. 2023, 55, 103000. [Google Scholar] [CrossRef]
- Ahsan, S.; Khaliq, A.; Chughtai, M.F.J.; Nadeem, M.; Tahir, A.B.; Din, A.A.; Ntsefong, G.N.; Shariati, M.A.; Rebezov, M.; Yessimbekov, Z.; et al. Technofunctional Quality Assessment of Soymilk Fermented with Lactobacillus acidophilus and Lactobacillus casei. Biotechnol. Appl. Biochem. 2022, 69, 172–182. [Google Scholar] [CrossRef] [PubMed]

| Composition | QF90-CF10 | QF75-CF25 | QF50-CF50 | |
|---|---|---|---|---|
| Time (Day) | Organic Acid (g/L) | |||
| 0 | Lactic acid | 1.9 ± 0.25 b | 2.1 ± 0.17 b | 2.6 ± 0.15 a |
| Acetic acid | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | |
| Ascorbic acid | BDL | BDL | BDL | |
| 30 | Lactic acid | 5.1± 0.01 a | 4.6 ± 0.04 b | 5.6 ± 0.02 a |
| Acetic acid | 1.1 ± 0.2 a | 0.7 ± 0.4 b | 0.7 ± 0.4 b | |
| Ascorbic acid | BDL | BDL | BDL | |
| 50 | Lactic acid | 4.3 ± 0.03 b | 4.7 ± 0.05 b | 5.6 ± 0.01 a |
| Acetic acid | 1.1 ± 0.8 a | 0.6 ± 0.6 b | 0.7 ± 0.8 b | |
| Ascorbic acid | BDL | BDL | BDL |
| Composition | QF90-CF10 | QF75-CF25 | QF50-CF50 | |
|---|---|---|---|---|
| Time (Day) | Cell Count (CFU/mL) | |||
| 0 | AMC | 5.4 × 101 a | 2.87 × 101 a | 3.01 × 101 a |
| Fungi | ND | ND | ND | |
| Coliforms | 1 × 101 a | ND | 1 × 101 a | |
| 10 | AMC | 1.3 × 101 a | 1.1 × 101 a | 1 × 101 a |
| Fungi | ND | ND | ND | |
| Coliforms | ND | ND | ND | |
| 20 | AMC | ND | ND | ND |
| Fungi | ND | ND | ND | |
| Coliforms | ND | ND | ND | |
| 30 | AMC | ND | ND | ND |
| Fungi | ND | ND | ND | |
| Coliforms | ND | ND | ND | |
| 40 | AMC | ND | ND | ND |
| Fungi | ND | ND | ND | |
| Coliforms | ND | ND | ND | |
| 50 | AMC | ND | ND | ND |
| Fungi | ND | ND | ND | |
| Coliforms | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurtado-Murillo, J.; Franco, W.; Contardo, I. Role of Quinoa (Chenopodium quinoa Willd) and Chickpea (Cicer arietinum L.) Ratio in Physicochemical Stability and Microbiological Quality of Fermented Plant-Based Beverages during Storage. Foods 2024, 13, 2462. https://doi.org/10.3390/foods13152462
Hurtado-Murillo J, Franco W, Contardo I. Role of Quinoa (Chenopodium quinoa Willd) and Chickpea (Cicer arietinum L.) Ratio in Physicochemical Stability and Microbiological Quality of Fermented Plant-Based Beverages during Storage. Foods. 2024; 13(15):2462. https://doi.org/10.3390/foods13152462
Chicago/Turabian StyleHurtado-Murillo, John, Wendy Franco, and Ingrid Contardo. 2024. "Role of Quinoa (Chenopodium quinoa Willd) and Chickpea (Cicer arietinum L.) Ratio in Physicochemical Stability and Microbiological Quality of Fermented Plant-Based Beverages during Storage" Foods 13, no. 15: 2462. https://doi.org/10.3390/foods13152462






