Conjugated Linoleic Acid Production in Pine Nut Oil: A Lactiplantibacillus plantarum Lp-01 Fermentation Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. Identification of Linoleic Acid Isomerase (LAI) Gene of L. plantarum Lp-01
2.3. Microorganism Culture and Substrate
2.4. Optimization of Fermentation Conditions for CLA Production
2.5. CLA Extraction and Determination
2.6. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.7. Bioinformatics Analysis
2.8. Statistical Analysis
3. Results and Discussion
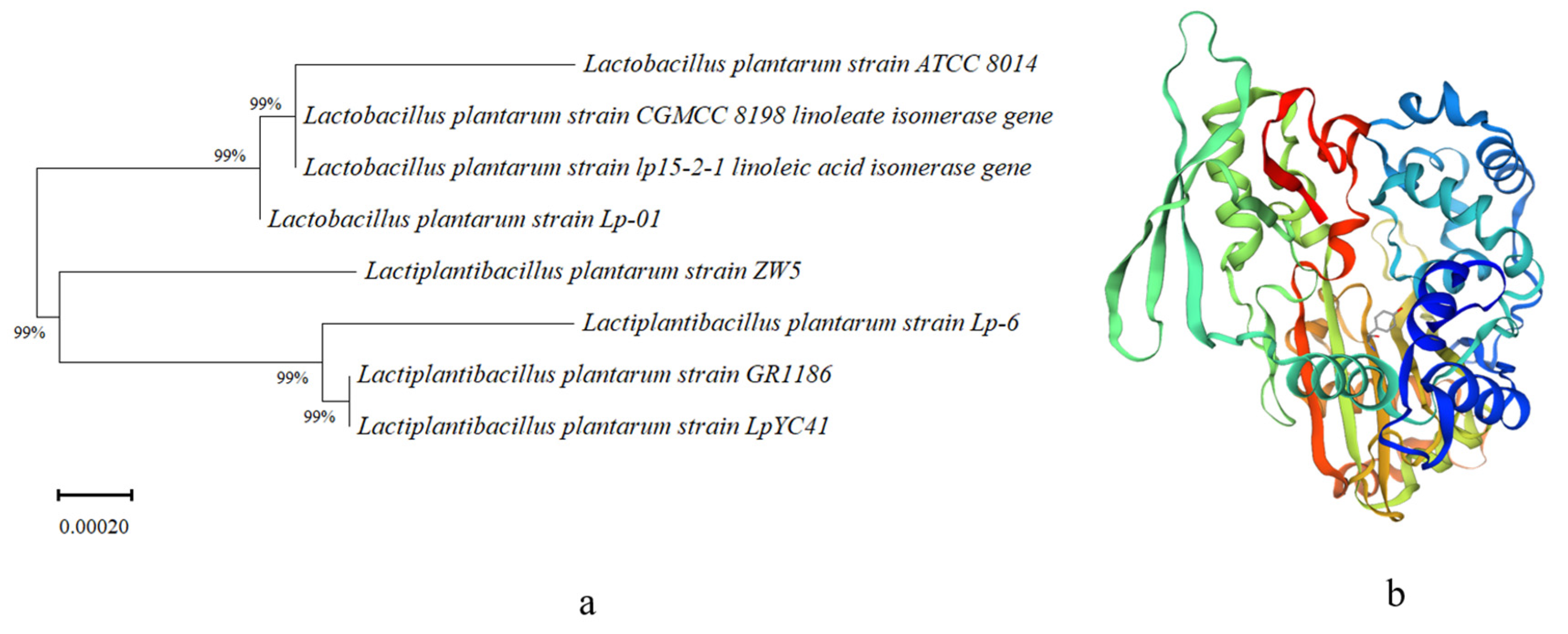
3.1. Identification, Analysis, and Phylogenetic Analysis of LAI in L. plantarum Lp-01
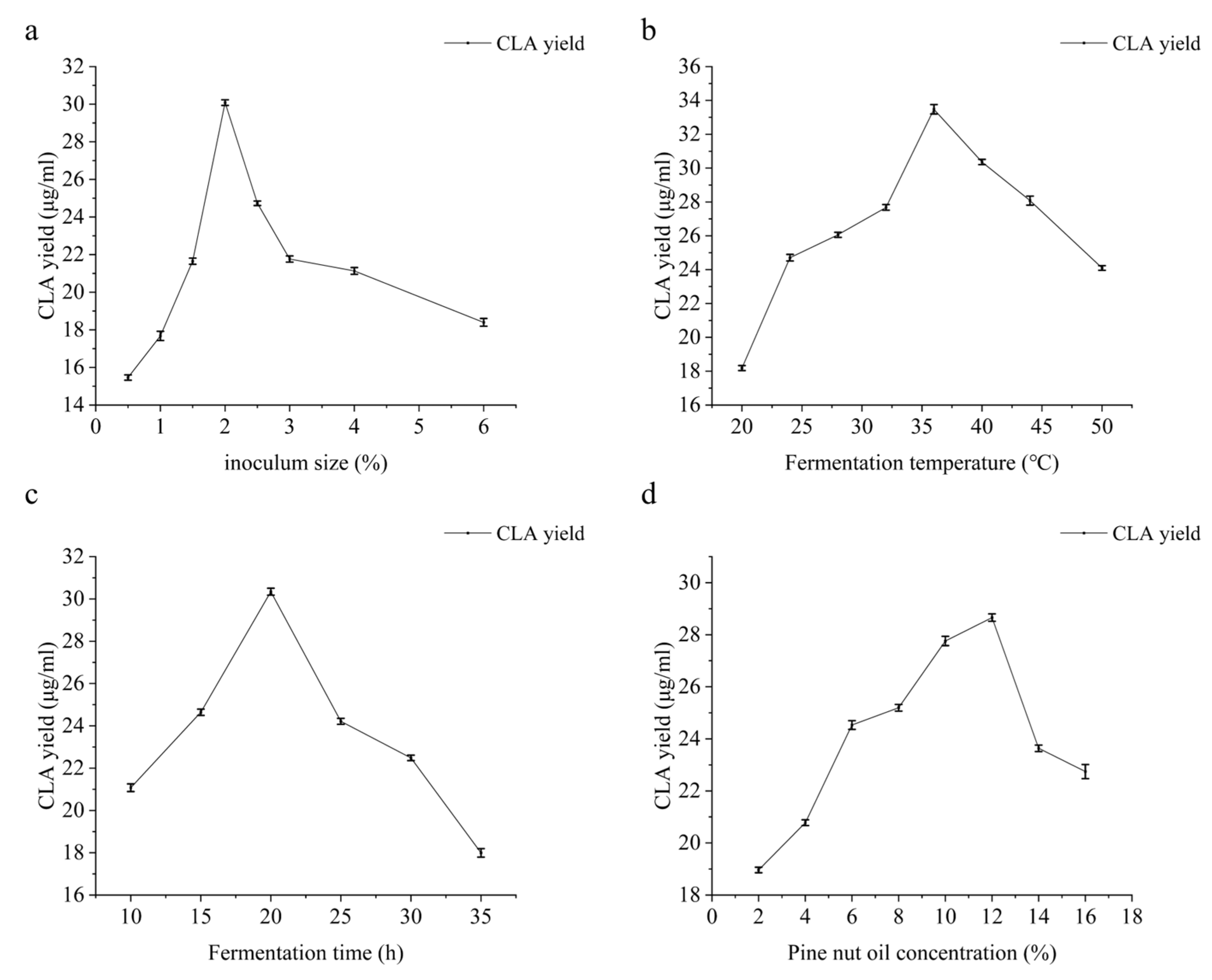
3.2. Effect of Inoculum Size on CLA Yield
3.3. Effect of Fermentation Temperature on CLA Yield
3.4. Effect of Fermentation Time on CLA Yield
3.5. Effect of Pine Nut Oil Concentration on CLA Yield
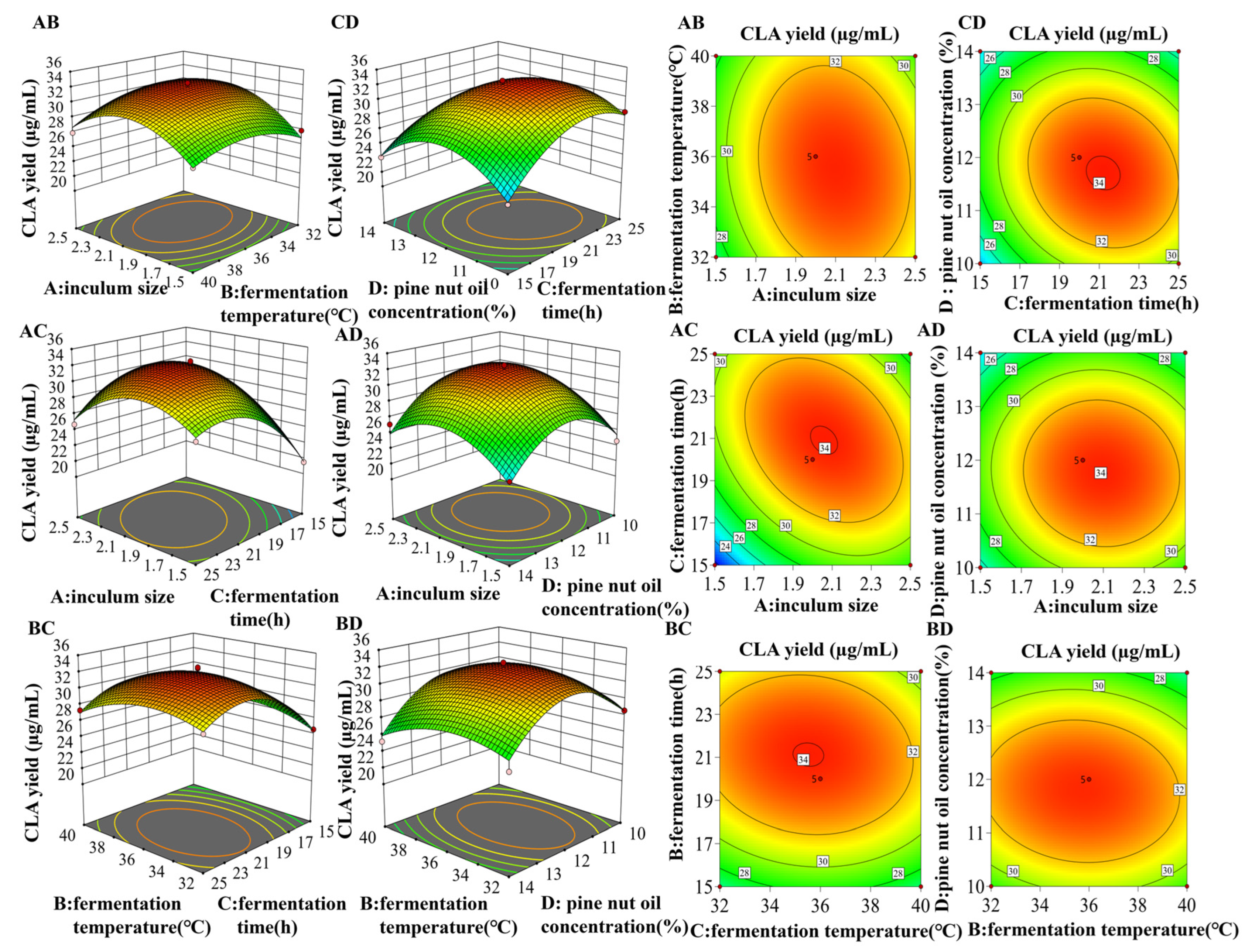
3.6. Response Surface Method to Optimize the Fermentation Process of L. plantarum Lp-01
3.6.1. Predicted Model and Analysis of Variance
3.6.2. Optimization and Validation for Fermentation Conditions
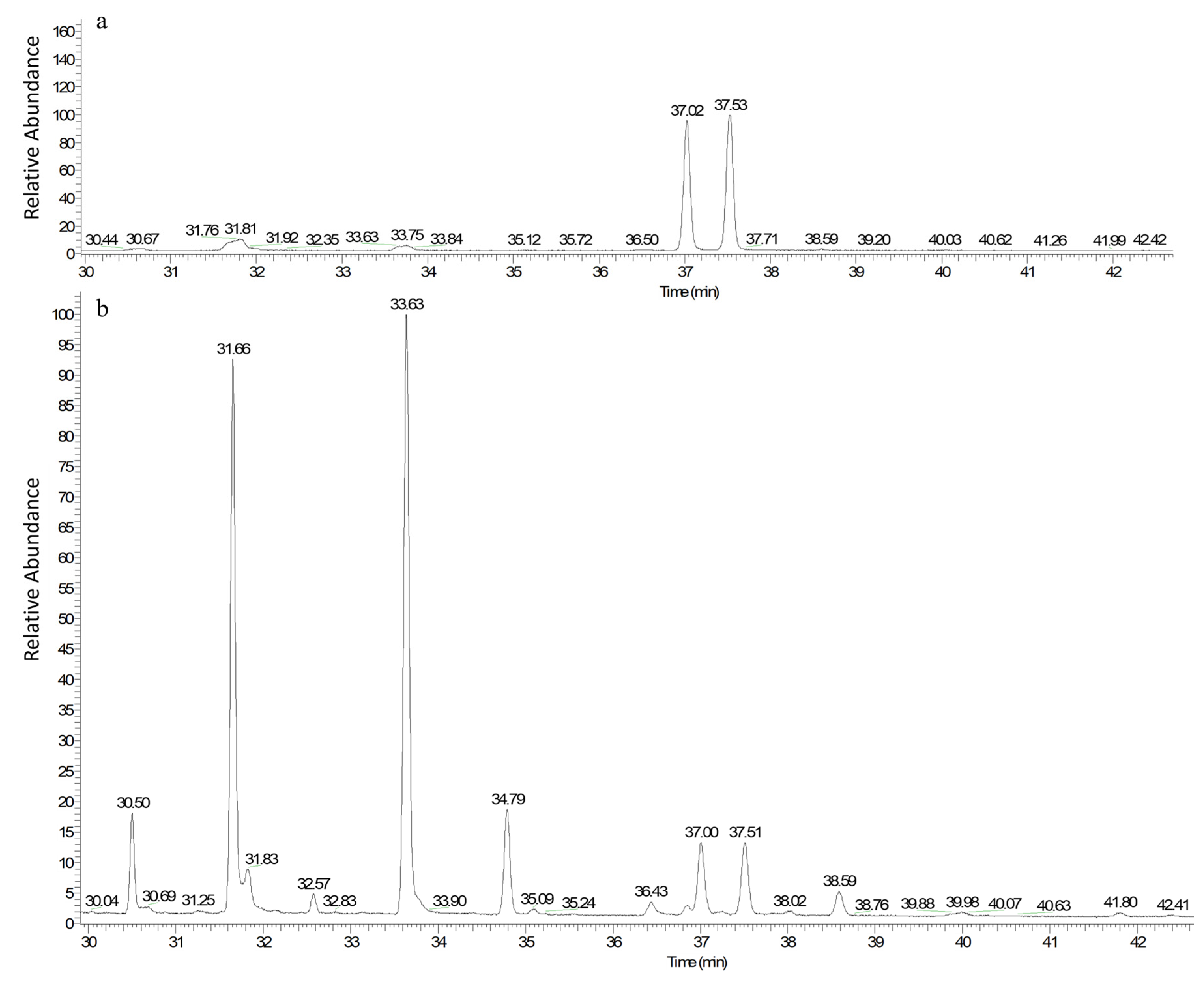
3.7. The Fermentation Products’ Composition Analysis
3.7.1. Results of the Extraction Liquid of Fermentation Product Scanned by Ultraviolet Wavelength
3.7.2. Gas Chromatography (GC) Figure of Fatty Acid Methyl Ester from Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iorizzo, M.; Di Martino, C.; Letizia, F.; Crawford, T.W.; Paventi, G. Production of Conjugated Linoleic Acid (CLA) by Lactiplantibacillus plantarum: A Review with Emphasis on Fermented Foods. Foods 2024, 13, 975. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Gong, M.; Wu, G.; Jin, J.; Wang, X.; Jin, Q. Conjugated Linolenic Acid (CLnA) vs. Conjugated Linoleic Acid (CLA): A Comprehensive Review of Potential Advantages in Molecular Characteristics, Health Benefits, and Production Techniques. J. Agric. Food. Chem. 2024, 72, 5503–5525. [Google Scholar] [CrossRef] [PubMed]
- Jamka, M.; Czochralska-Duszyńska, A.; Mądry, E.; Lisowska, A.; Jończyk-Potoczna, K.; Cielecka-Piontek, J.; Bogdański, P.; Walkowiak, J. The Effect of Conjugated Linoleic Acid Supplementation on Densitometric Parameters in Overweight and Obese Women—A Randomised Controlled Trial. Medicina 2023, 59, 1690. [Google Scholar] [CrossRef] [PubMed]
- Fuke, G.; Nornberg, J.L. Systematic evaluation on the effectiveness of conjugated linoleic acid in human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.C.; Cannelli, G.; Carta, G.; Cordeddu, L.; Melis, M.P.; Murru, E.; Stanton, C.; Banni, S. Metabolism of c9,t11-conjugated linoleic acid (CLA) in humans. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Hu, Y.; Wei, W.; Jin, Q.; Wang, X. Production of conjugated fatty acids: A review of recent advances. Biotechnol. Adv. 2019, 37, 107454. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, L.; Qing, J.; Wu, X.; Li, H.; Chen, H.; Liu, X. Multiple biological activities and biosynthesis mechanisms of specific conjugated linoleic acid isomers and analytical methods for prospective application. Food Chem. 2023, 409, 135257. [Google Scholar] [CrossRef]
- Kuhl, G.C.; De Dea Lindner, J. Biohydrogenation of Linoleic Acid by Lactic Acid Bacteria for the Production of Functional Cultured Dairy Products: A Review. Foods 2016, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, A.; Mollaei Tavani, S.; Arjeh, E.; Jafari, S.M. Production of conjugated linoleic acid by lactic acid bacteria; important factors and optimum conditions. Food Chem. X 2023, 20, 100942. [Google Scholar] [CrossRef] [PubMed]
- Abedin, M.M.; Chourasia, R.; Phukon, L.C.; Sarkar, P.; Ray, R.C.; Singh, S.P.; Rai, A.K. Lactic acid bacteria in the functional food industry: Biotechnological properties and potential applications. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef]
- Yang, B.; Gao, H.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, Y.Q.; Chen, H.; Chen, W. Bacterial conjugated linoleic acid production and their applications. Prog. Lipid Res. 2017, 68, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-J.; Yan, L.-H.; Li, Q.; Zhang, C.-J.; Si, C.-L.; Li, Z.-Y.; Song, Y.-J.; Zhou, H.; Zhang, T.-C.; Luo, X.-G. Bioconversion of conjugated linoleic acid by Lactobacillus plantarum CGMCC8198 supplemented with Acer truncatum bunge seeds oil. Food Sci. Biotechnol. 2017, 26, 1595–1611. [Google Scholar] [CrossRef]
- Kouchak Yazdi, Z.; Alemzadeh, I.; Vossoughi, M. Comparison and optimization of conjugated linoleic acid production by Lactobacillus plantarum and Lactobacillus plantarum subsp. plantarum. Sci. Iran. 2017, 24, 1272–1280. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Kermanshahi, R.K.; Hosseinkhani, S.; Shojaosadati, S.A.; Nazari, M. Conjugated linoleic acid production from various substrates by probiotic Lactobacillus plantarum. Ann. Microbiol. 2015, 65, 27–32. [Google Scholar] [CrossRef]
- Behr, A.; Witte, H.; Bayrak, Z. Homogeneous metal complex catalyzed conjugation of methyl linoleate. Eur. J. Lipid Sci. Technol. 2013, 115, 721–728. [Google Scholar] [CrossRef]
- Kishino, S.; Ogawa, J.; Ando, A.; Omura, Y.; Shimizu, S. Ricinoleic Acid and Castor Oil as Substrates for Conjugated Linoleic Acid Production by Washed Cells of Lactobacillus plantarum. Biosci. Biotechnol. Biochem. 2002, 66, 2283–2286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, H.; Shi, H.; Wang, F.; Li, X. Green Synthesis of Conjugated Linoleic Acids from Plant Oils Using a Novel Synergistic Catalytic System. J. Agric. Food. Chem. 2017, 65, 5322–5329. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Lee, S.C.; Huh, C.K. Efficient conversion of conjugated linoleic acid c9,t11 by Lactobacillus fermentation from vegetable oil to generate fermented milk with high CLA content. Korean J. Food Preserv. 2018, 25, 482–489. [Google Scholar] [CrossRef]
- Fontes, A.L.; Pimentel, L.L.; Soares, A.M.S.; Domingues, M.d.R.; Rodríguez-Alcalá, L.M.; Gomes, A.M. Study of the viability of using lipase-hydrolyzed commercial vegetable oils to produce microbially conjugated linolenic acid-enriched milk. Food Chem. 2023, 413, 135665. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Miles, E.A.; Calder, P.C. A review of the potential health benefits of pine nut oil and itscharacteristic fatty acid pinolenic acid. J. Funct. Foods 2016, 23, 464–473. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Calder, P.C. A review of the functional effects of pine nut oil, pinolenic acid and its derivative eicosatrienoic acid and their potential health benefits. Prog. Lipid Res. 2021, 82, 101097. [Google Scholar] [CrossRef] [PubMed]
- Azemi, N.A.; Azemi, A.K.; Abu-Bakar, L.; Sevakumaran, V.; Muhammad, T.S.; Ismail, N. Effect of Linoleic Acid on Cholesterol Levels in a High-Fat Diet-Induced Hypercholesterolemia Rat Model. Metabolites 2023, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Burr, S.D.; Chen, Y.; Hartley, C.P.; Zhao, X.; Liu, J. Replacement of saturated fatty acids with linoleic acid in western diet attenuates atherosclerosis in a mouse model with inducible ablation of hepatic LDL receptor. Sci. Rep. 2023, 13, 16832. [Google Scholar] [CrossRef]
- Uslu, N.; Özcan, M.M. Fatty Acid Profiles of Some Nut Oils Harvested at The Different Harvest Periods. Erwerbs-Obstbau 2020, 62, 459–462. [Google Scholar] [CrossRef]
- Chen, C.; Tong, F.; Sun, R.; Zhang, Y.; Pang, Z.; Liu, X. Screening and Identification of High-Yielding Strains of Conjugated Linoleic Acid and Optimization of Conditions for the Conversion of CLA. Food 2024, 13, 1830. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Kim, C.S.; Cho, S.K.; Choi, H.J.; Ji, G.E.; Oh, D.-K. Bioconversion of linoleic acid into conjugated linoleic acid during fermentation and by washed cells of Lactobacillus reuteri. Biotechnol. Lett. 2003, 25, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Sieber, R.; Collomb, M.; Aeschlimann, A.; Jelen, P.; Eyer, H. Impact of microbial cultures on conjugated linoleic acid in dairy products—A review. Int. Dairy J. 2004, 14, 1–15. [Google Scholar] [CrossRef]
- Razmjooei, M.; Shad, E.; Nejadmansouri, M.; Safdarianghomsheh, R.; Delvigne, F.; Khalesi, M. Effect of metal support and different carbon sources on CLA production using Lactobacillus plantarum. Biochem. Eng. J. 2020, 162, 107715. [Google Scholar] [CrossRef]
- Khosravi, A.; Safari, M.; Khodaiyan, F.; Gharibzahedi, S.M.T. Bioconversion enhancement of conjugated linoleic acid by Lactobacillus plantarum using the culture media manipulation and numerical optimization. J. Food Sci. Technol. 2015, 52, 5781–5789. [Google Scholar] [CrossRef]
- Özer, C.O.; Kılıç, B. Optimization of pH, time, temperature, variety and concentration of the added fatty acid and the initial count of added lactic acid Bacteria strains to improve microbial conjugated linoleic acid production in fermented ground beef. Meat Sci. 2021, 171, 108303. [Google Scholar] [CrossRef] [PubMed]

| ID | Primer Sequence | |
|---|---|---|
| Forward | Reverse | |
| 1 | AATAAGAATCATCCGATGGCTAA | GTTCCGTGAAGATCATCTGGTAT |
| 2 | AATAAGAATCATCCGATGGCTAA | GTTCCGTGAAGATCATCTGGTAT |
| 3 | GAATAAGAATCATCCGATGGCTA | GTTCCGTGAAGATCATCTGGTAT |
| Level | Factors | |||
|---|---|---|---|---|
| A (%) | B (°C) | C (h) | D (%) | |
| −1 | 1.5 | 32 | 15 | 10 |
| 0 | 2 | 36 | 20 | 12 |
| 1 | 2.5 | 40 | 25 | 14 |
| Run Numbers | A: Inoculum Size/% | B: Temperature/ °C | C: Time/ h | D: Pine Nut Oil Concentration/% | CLA Yield/ μg/mL |
|---|---|---|---|---|---|
| 1 | 2.00 | 32.00 | 20.00 | 10.00 | 28.6 |
| 2 | 2.50 | 40.00 | 20.00 | 12.00 | 27.9 |
| 3 | 2.00 | 32.00 | 15.00 | 12.00 | 26.6 |
| 4 | 1.50 | 36.00 | 15.00 | 12.00 | 21.5 |
| 5 | 2.50 | 36.00 | 20.00 | 10.00 | 28.7 |
| 6 | 2.00 | 36.00 | 20.00 | 12.00 | 31.3 |
| 7 | 2.00 | 36.00 | 20.00 | 12.00 | 34.4 |
| 8 | 2.00 | 36.00 | 15.00 | 14.00 | 24.1 |
| 9 | 1.50 | 36.00 | 20.00 | 14.00 | 24.6 |
| 10 | 2.00 | 40.00 | 15.00 | 12.00 | 27.3 |
| 11 | 2.00 | 36.00 | 20.00 | 12.00 | 34.3 |
| 12 | 2.00 | 32.00 | 20.00 | 14.00 | 26.2 |
| 13 | 2.00 | 32.00 | 15.00 | 10.00 | 23.7 |
| 14 | 1.50 | 36.00 | 20.00 | 10.00 | 24.6 |
| 15 | 2.50 | 32.00 | 20.00 | 12.00 | 31.0 |
| 16 | 2.00 | 36.00 | 20.00 | 12.00 | 33.6 |
| 17 | 1.50 | 36.00 | 25.00 | 12.00 | 28.8 |
| 18 | 1.50 | 40.00 | 20.00 | 12.00 | 27.6 |
| 19 | 2.50 | 36.00 | 15.00 | 12.00 | 28.2 |
| 20 | 2.00 | 36.00 | 20.00 | 12.00 | 33.3 |
| 21 | 2.00 | 40.00 | 20.00 | 14.00 | 25.2 |
| 22 | 2.00 | 36.00 | 25.00 | 10.00 | 30.0 |
| 23 | 2.00 | 36.00 | 25.00 | 14.00 | 25.8 |
| 24 | 2.50 | 36.00 | 25.00 | 12.00 | 26.7 |
| 25 | 2.00 | 32.00 | 25.00 | 12.00 | 30.4 |
| 26 | 2.00 | 40.00 | 25.00 | 12.00 | 29.3 |
| 27 | 1.50 | 32.00 | 20.00 | 12.00 | 27.9 |
| 28 | 2.00 | 40.00 | 20.00 | 10.00 | 29.2 |
| 29 | 2.50 | 36.00 | 20.00 | 14.00 | 27.1 |
| Variables | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 303.96 | 14 | 21.71 | 34.85 | <0.0001 | significance |
| A | 17.76 | 1 | 17.76 | 28.51 | 0.0001 | |
| B | 1.47 | 1 | 1.47 | 2.36 | 0.1468 | |
| C | 32.01 | 1 | 32.01 | 51.39 | <0.0001 | |
| D | 11.60 | 1 | 11.60 | 18.62 | 0.0007 | |
| AB | 1.96 | 1 | 1.96 | 3.15 | 00979 | |
| AC | 19.36 | 1 | 19.36 | 31.08 | <0.0001 | |
| AD | 0.6400 | 1 | 0.6400 | 1.03 | 0.3280 | |
| BC | 0.8100 | 1 | 0.8100 | 1.30 | 0.2733 | |
| BD | 0.6400 | 1 | 0.6400 | 1.03 | 0.3280 | |
| CD | 5.29 | 1 | 5.29 | 8.49 | 0.0113 | |
| A2 | 78.10 | 1 | 78.10 | 125.37 | <0.0001 | |
| B2 | 23.29 | 1 | 23.29 | 37.39 | <0.0001 | |
| C2 | 90.97 | 1 | 90.97 | 146.02 | <0.0001 | |
| D2 | 121.05 | 1 | 121.05 | 194.31 | <0.0001 | |
| Residual | 8.72 | 14 | 0.6230 | |||
| Lack of fit | 7.79 | 10 | 0.7790 | 3.34 | 0.1280 | Not significance |
| Pure error | 0.9320 | 4 | 0.2330 | |||
| Cor.Total | 312.68 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, G.; Wu, G.; Sun, J.; Qi, Y.; Zhao, Q.; Xu, F.; Zhang, Z.; Peng, L. Conjugated Linoleic Acid Production in Pine Nut Oil: A Lactiplantibacillus plantarum Lp-01 Fermentation Approach. Foods 2024, 13, 2472. https://doi.org/10.3390/foods13162472
Wei G, Wu G, Sun J, Qi Y, Zhao Q, Xu F, Zhang Z, Peng L. Conjugated Linoleic Acid Production in Pine Nut Oil: A Lactiplantibacillus plantarum Lp-01 Fermentation Approach. Foods. 2024; 13(16):2472. https://doi.org/10.3390/foods13162472
Chicago/Turabian StyleWei, Gang, Ge Wu, Jiajia Sun, Yi Qi, Qi Zhao, Fengde Xu, Zhi Zhang, and Lanzhi Peng. 2024. "Conjugated Linoleic Acid Production in Pine Nut Oil: A Lactiplantibacillus plantarum Lp-01 Fermentation Approach" Foods 13, no. 16: 2472. https://doi.org/10.3390/foods13162472






