Effects of Different Pretreatments on Hot Air Drying Characteristics, Nutrition, and Antioxidant Capacity of Tartary Buckwheat Sprouts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemical Reagents
2.2. Sprout Sample Preparation
2.3. Sprouts Pretreatment
2.4. Hot Air Drying
2.5. Drying Curve
2.6. Rehydration Ratio
2.7. Determination of Color Profile
2.8. Physical Characteristics
2.9. Nutritional Properties
2.10. DPPH Radical Scavenging Ability
2.11. ABTS Radical Scavenging Ability
2.12. FRAP Assay
2.13. Data Processing
3. Result and Analysis
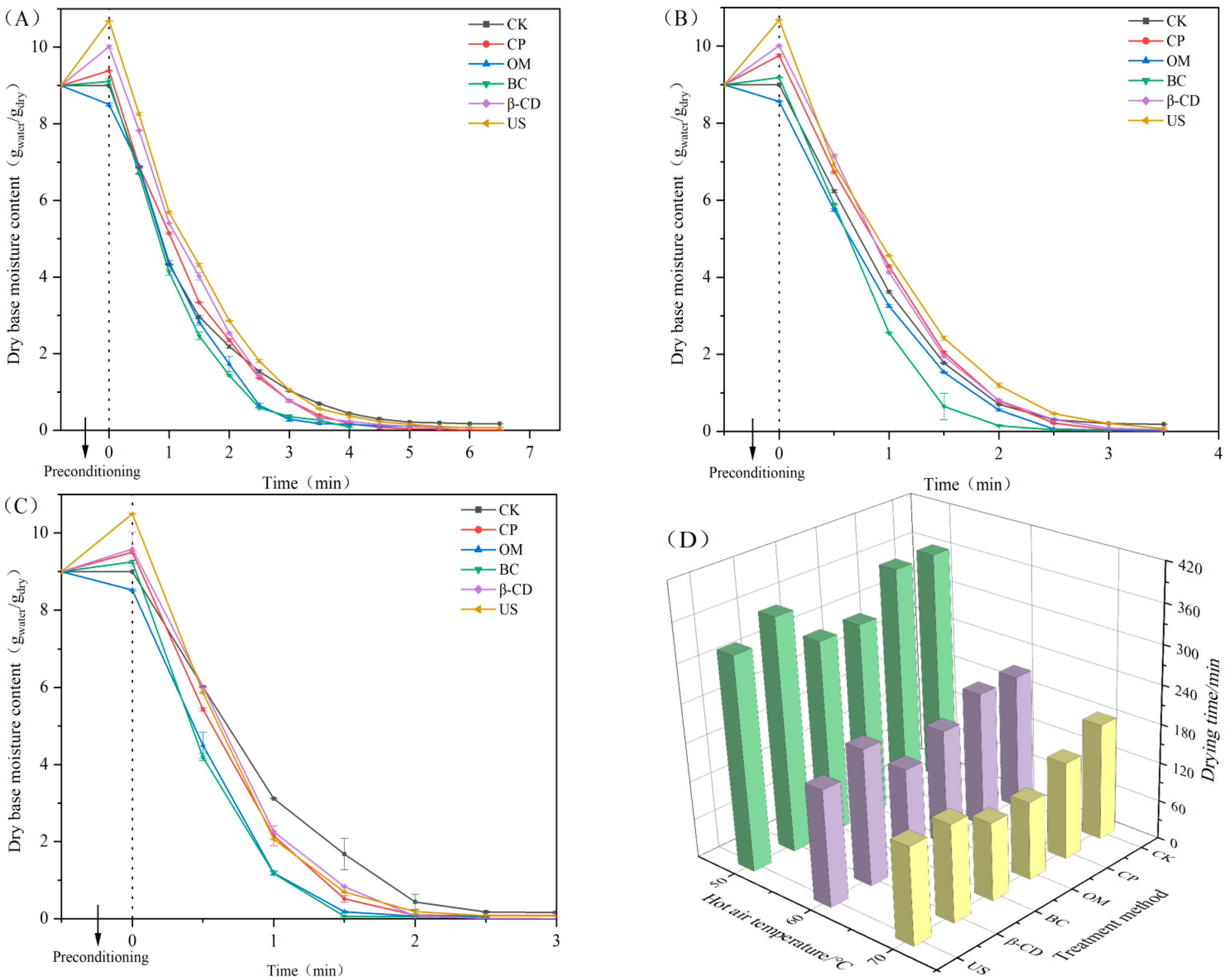
3.1. Effect of Different Pretreatment on Moisture Curve of Tartary Buckwheat Sprouts Dried by Hot Air
3.2. Effects of Ultrasound Combined with ZnO NPs Treatment on Colors of Fresh-Cut Lettuce
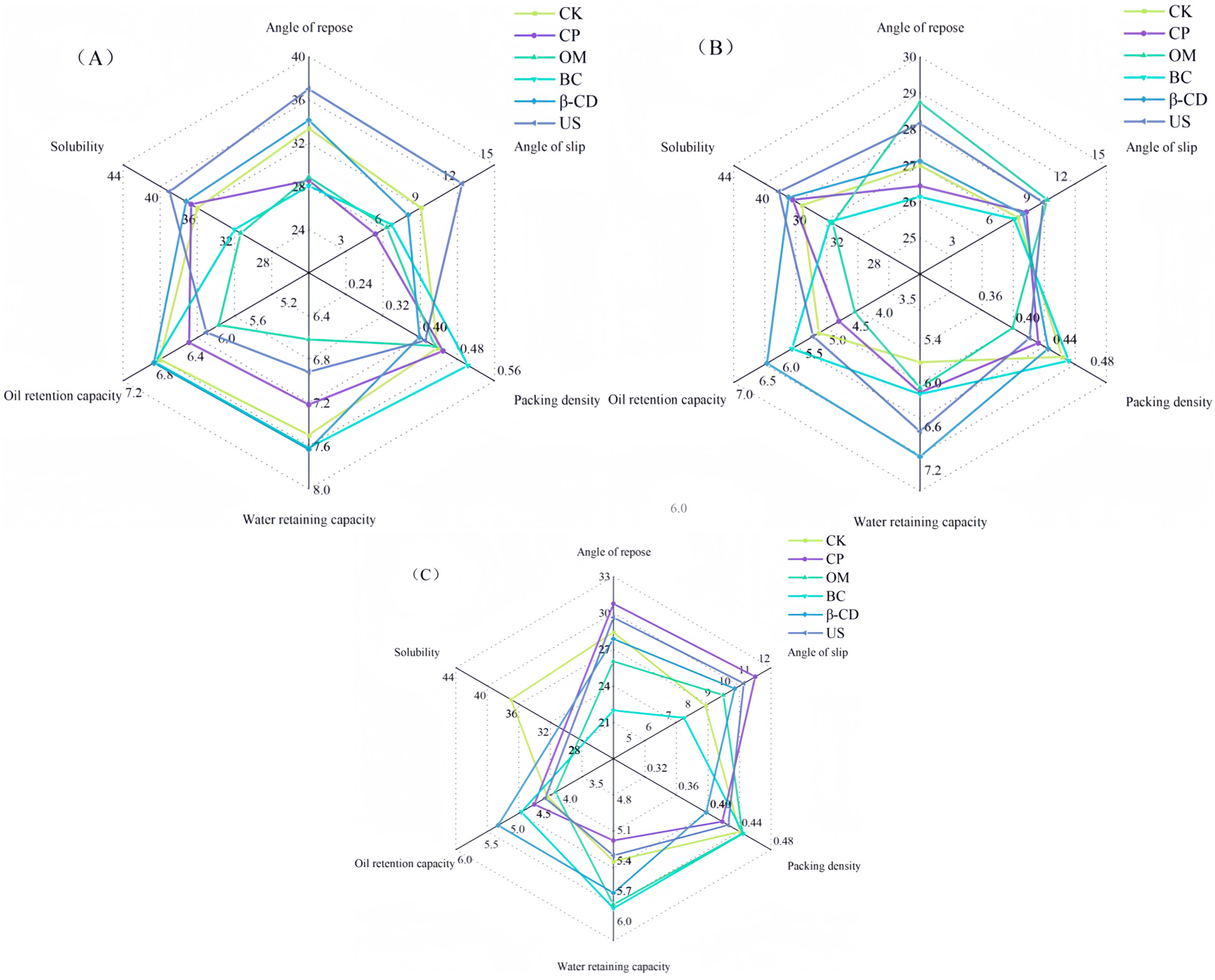
3.3. Effects of Hot Air Drying on Physical Characteristics of Tartary Buckwheat Sprouts under Different Pretreatment Conditions
3.4. Effects of Hot Air Drying on Nutrient Composition of Tartary Buckwheat Sprouts under Different Pretreatment Conditions
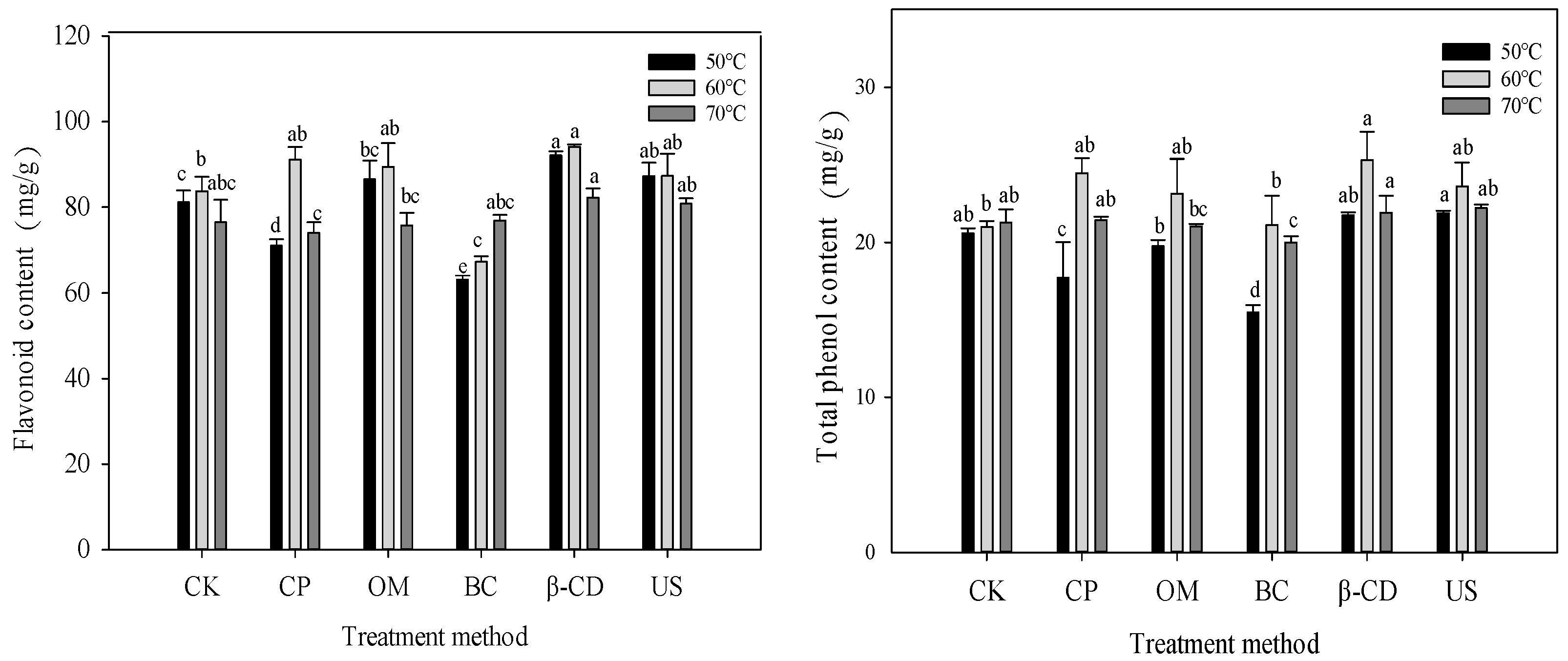
3.5. Effect of Different Pretreatment on Active Ingredients of Tartary Buckwheat Sprouts Dried by Hot Air
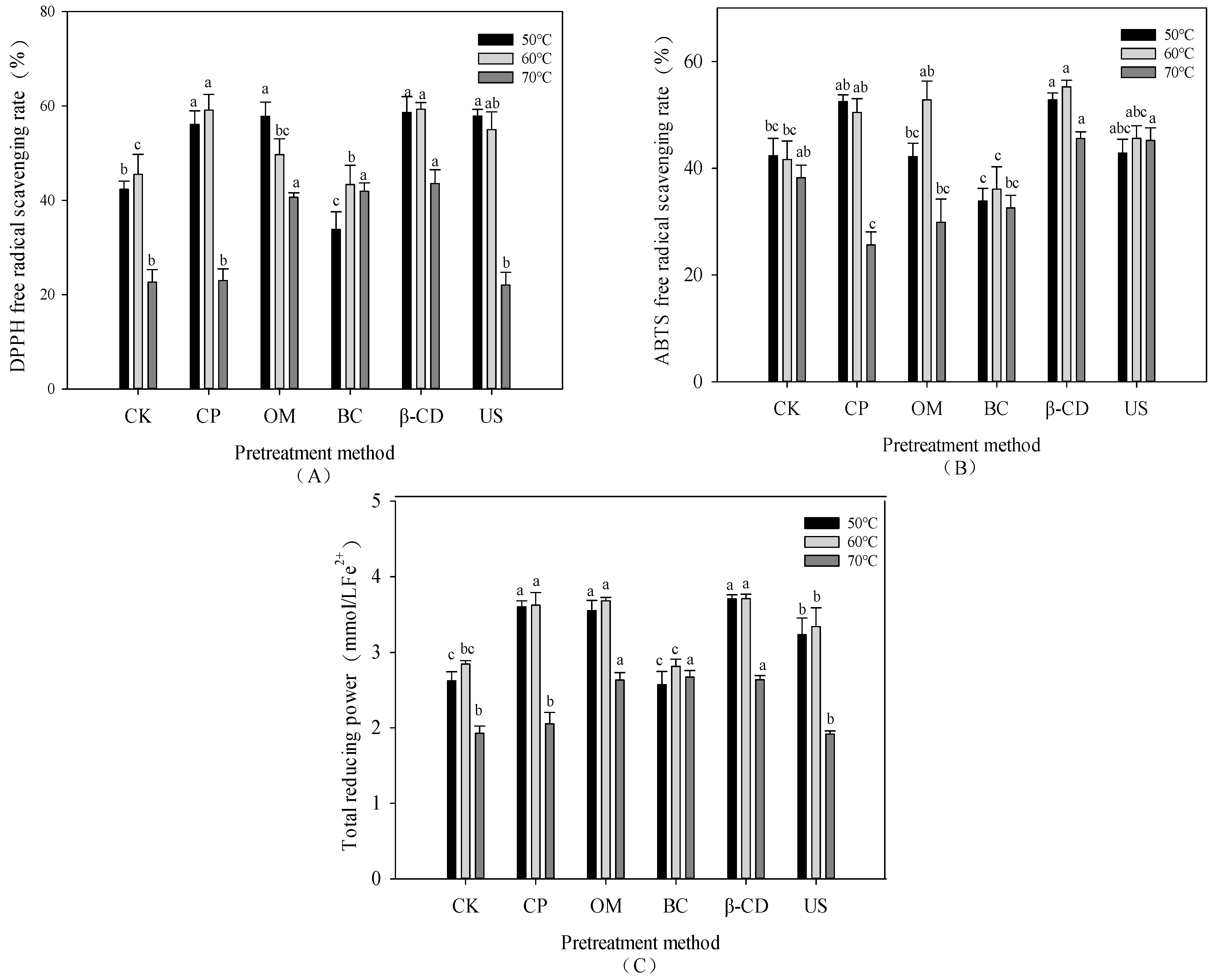
3.6. Effect of Hot Air Drying on Antioxidant Capacity of Tartary Buckwheat Sprouts under Different Pretreatment Conditions
3.7. Clustering Heat Map Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2023. Available online: http://ec.europa.eu/eurostat (accessed on 9 August 2023).
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.R.; Gil Lee, S.; Lee, B.-H.; Han, S.G.; Choi, S.-W.; Song, W.-J.; Nam, T.G. Phenolic compounds in common buckwheat sprouts: Composition, isolation, analysis and bioactivities. Food Sci. Biotechnol. 2022, 31, 935–956. [Google Scholar] [CrossRef]
- Wang, N.; Dong, Y.; Wang, S.; Wang, J.; Wu, N. Changes in nutritional and antioxidant properties, structure, and flavor compounds of tartary buckwheat (Fagopyrum tataricums) sprouts during domestic cooking. Int. J. Gastron. Food Sci. 2024, 36, 100914. [Google Scholar] [CrossRef]
- Korese, J.K.; Achaglinkame, M.A.; Chikpah, S.K. Effect of hot air temperature on drying kinetics of palmyra (Borassus aethiopum Mart.) seed-sprout fleshy scale slices and quality attributes of its flour. J. Agric. Food Res. 2021, 6, 100249. [Google Scholar] [CrossRef]
- Yin, M.; Matsuoka, R.; Yanagisawa, T.; Xi, Y.; Zhang, L.; Wang, X. Effect of different drying methods on free amino acid and flavor nucleotides of scallop (Patinopecten yessoensis) adductor muscle. Food Chem. 2022, 396, 133620. [Google Scholar] [CrossRef]
- Bi, J.; Yang, Z.; Li, Y.; Li, B.; Gao, Y.; Ping, C.; Chen, Z.; Li, C. Effects of different cooking methods on volatile flavor compounds in garlic. Int. J. Gastron. Food Sci. 2023, 31, 100642. [Google Scholar] [CrossRef]
- Nudar, J.; Roy, M.; Ahmed, S. Combined osmotic pretreatment and hot air drying: Evaluation of drying kinetics and quality parameters of adajamir (Citrus assamensis). Heliyon 2023, 9, e19545. [Google Scholar] [CrossRef]
- Guo, X.; Hao, Q.; Qiao, X.; Li, M.; Qiu, Z.; Zheng, Z.; Zhang, B. An evaluation of different pretreatment methods of hot-air drying of garlic: Drying characteristics, energy consumption and quality properties. LWT 2023, 180, 114685. [Google Scholar] [CrossRef]
- Qiu, X.L.; Feng, L.; Nie, M.M.; Liu, C.Q.; Li, D.J.; Zhang, Z.Y.; Li, J.L.; Qiu, X.F.; Tang, D.M. Effects of ultrasonic pre-treatment on the microwave drying quality of shepherd’s purse (Capsella bursa-pastoris). Trans. Chin. Soc. Agric. Eng. 2024, 40, 155–167. [Google Scholar] [CrossRef]
- Lin, Y.W.; Li, A.Q.; Tian, X.Y.; Xie, Y.K.; Li, J.R.; Li, X.P. Effect of Medium and Short-Wave Infrared Drying Temperature on Drying Characteristics, Astaxanthin Content and Microstructure of Penaeus vannamei. J. Chin. Inst. Food Sci. Technol. 2023, 23, 129–139. [Google Scholar] [CrossRef]
- Suo, K.; Yang, Z.F.; Feng, Y.B.; Zhang, Y.; Zhou, C.S.; Shi, L.Y.; Chen, W. Effects of puncture combined with ethanol pretreatment on the hot air drying characteristics of carrot slices. Trans. Chin. Soc. Agric. Eng. 2024, 40, 124–133. [Google Scholar] [CrossRef]
- Karam, M.C.; Petit, J.; Zimmer, D.; Djantou, E.B.; Scher, J. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Xu, Y.; Wu, J.; Yu, Y.; Peng, J.; An, K.; Zou, B.; Yang, W. Effect of ultrasound-assisted osmotic dehydration pretreatment on the drying characteristics and quality properties of Sanhua plum (Prunus salicina L.). LWT 2021, 138, 110653. [Google Scholar] [CrossRef]
- Daniel, I.O.; Kamran, I.; Donato, R.; Seraina, S.; Jörg, S.; Alex, M.; Thijs, D. How much do process parameters affect the residual quality attributes of dried fruits and vegetables for convective drying? Food Bioprod. Process. 2021, 131, 179–190. [Google Scholar] [CrossRef]
- Lavelli, V.; Zanoni, B.; Zaniboni, A. Effect of water activity on carotenoid degradation in dehydrated carrots. Food Chem. 2007, 104, 1705–1711. [Google Scholar] [CrossRef]
- Corrêa, J.; Rasia, M.; Mulet, A.; Cárcel, J. Influence of ultrasound application on both the osmotic pretreatment and subsequent convective drying of pineapple (Ananas comosus). Innov. Food Sci. Emerg. Technol. 2017, 41, 284–291. [Google Scholar] [CrossRef]
- Doymaz, I. Effect of citric acid and blanching pre-treatments on drying and rehydration of Amasya red apples. Food Bioprod. Process. 2010, 88, 124–132. [Google Scholar] [CrossRef]
- Khatun, P.; Karmakar, A.; Chakraborty, I. Microwave-vacuum drying: Modeling validation of drying and rehydration kinetics, moisture diffusivity and physicochemical properties of dried dragon fruit slices. Food Humanit. 2024, 2, 100292. [Google Scholar] [CrossRef]
- Czarnowska-Kujawska, M.; Draszanowska, A.; Starowicz, M. Effect of different cooking methods on the folate content, organoleptic and functional properties of broccoli and spinach. LWT 2022, 167, 113825. [Google Scholar] [CrossRef]
- Sun, X.; Zou, J.; Wu, Y.; Li, Y. Investigation of effects of repose angle and critical sliding angle of snow on curved roofs. J. Wind. Eng. Ind. Aerodyn. 2022, 227, 105071. [Google Scholar] [CrossRef]
- Knop, Y.; Peled, A. Packing density modeling of blended cement with limestone having different particle sizes. Constr. Build. Mater. 2016, 102, 44–50. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, X.; Islam, N.; Zheng, H.; Li, J.; Liu, F.; Cao, Y.; Dai, Y. Comparison of hydrability, antioxidants, microstructure, and sensory quality of barley grass powder using ultra-micro-crushing combined with hot air and freeze drying. Food Sci. Nutr. 2021, 9, 1870–1880. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, H.; Zhang, X.; Ao, Q. Effect of pressure grinding technology on the physicochemical and antioxidant properties of Tremella aurantialba powder. J. Food Process. Preserv. 2018, 42, e13833. [Google Scholar] [CrossRef]
- Alves, E.S.; Ferreira, C.S.R.; Souza, P.R.; Bruni, A.R.S.; Castro, M.C.; Saqueti, B.H.F.; Santos, O.O.; Madrona, G.S.; Visentainer, J.V. Freeze-dried human milk microcapsules using gum arabic and maltodextrin: An approach to improving solubility. Int. J. Biol. Macromol. 2023, 238, 124100. [Google Scholar] [CrossRef]
- Xue, L.; Yang, L. Deriving leaf chlorophyll content of green-leafy vegetables from hyperspectral reflectance. ISPRS J. Photogramm. Remote Sens. 2009, 64, 97–106. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Zhang, Z.; Wang, C.; Li, Y.; Lu, H.; Ma, K.; Gao, Z.; Yin, X.; Chen, F.; et al. Foliar application of selenium promotes starch content accumulation and quality enhancement in foxtail millet grains. Field Crop. Res. 2024, 310, 109352. [Google Scholar] [CrossRef]
- Ma, H.; Xu, X.; Wang, S.; Wang, J.; Peng, W. Effects of microwave irradiation on the expression of key flavonoid biosynthetic enzyme genes and the accumulation of flavonoid products in Fagopyrum tataricum sprouts. J. Cereal Sci. 2021, 101, 103275. [Google Scholar] [CrossRef]
- Diamante, M.S.; Borges, C.V.; Minatel, I.O.; Jacomino, A.P.; Basílio, L.S.P.; Monteiro, G.C.; Corrêa, C.R.; de Oliveira, R.A.; Lima, G.P.P. Domestic cooking practices influence the carotenoid and tocopherol content in colored cauliflower. Food Chem. 2021, 340, 127901. [Google Scholar] [CrossRef]
- Ma, H.; Xu, X.; Wang, S.; Wang, J.; Wang, S. Effects of microwave irradiation of Fagopyrum tataricum seeds on the physicochemical and functional attributes of sprouts. LWT 2022, 165, 113738. [Google Scholar] [CrossRef]
- Fong-In, S.; Khwanchai, P.; Prommajak, T.; Boonsom, S. Physicochemical, nutritional, phytochemical properties and antioxidant activity of edible Astraeus odoratus mushrooms: Effects of different cooking methods. Int. J. Gastron. Food Sci. 2023, 33, 100743. [Google Scholar] [CrossRef]
- Peng, W.; Dong, Y.; Wang, J.; Wang, S.; Wang, N. Effects of exogenous solution treatment on germination, antioxidation and flavonoid biosynthesis of Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.). Food Biosci. 2023, 56, 103367. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Lv, W.-Q.; Li, D.; Wang, L.-J. Freeze-thaw and ultrasound pretreatment before microwave combined drying affects drying kinetics, cell structure and quality parameters of Platycodon grandiflorum. Ind. Crop. Prod. 2021, 164, 113391. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, M.; Shi, W. Evaluation of ultrasound pretreatment and drying methods on selected quality attributes of bitter melon (Momordica charantia L.). Dry. Technol. 2019, 37, 387–396. [Google Scholar] [CrossRef]
- An, N.-N.; Lv, W.-Q.; Li, D.; Wang, L.-J.; Wang, Y. Effects of hot-air microwave rolling blanching pretreatment on the drying of turmeric (Curcuma longa L.): Physiochemical properties and microstructure evaluation. Food Chem. 2023, 398, 133925. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.-Z.; Pan, Z.; Mujumdar, A.; Zhao, J.-H.; Zheng, Z.-A.; Gao, Z.-J.; Xiao, H.-W. High-humidity hot air impingement blanching (HHAIB) enhances drying quality of apricots by inactivating the enzymes, reducing drying time and altering cellular structure. Food Control. 2019, 96, 104–111. [Google Scholar] [CrossRef]
- Bai, J.-W.; Zhang, L.; Aheto, J.H.; Cai, J.-R.; Wang, Y.-C.; Sun, L.; Tian, X.-Y. Effects of different pretreatment methods on drying kinetics, three-dimensional deformation, quality characteristics and microstructure of dried apple slices. Innov. Food Sci. Emerg. Technol. 2023, 83, 103216. [Google Scholar] [CrossRef]
- Hong, S.; Das, P.R.; Eun, J. Effects of superfine grinding using ball-milling on the physical properties, chemical composition, and antioxidant properties of Quercus salicina (Blume) leaf powders. J. Sci. Food Agric. 2021, 101, 3123–3131. [Google Scholar] [CrossRef]
- Brzezowska, J.; Hendrysiak, A.; Wojdyło, A.; Michalska-Ciechanowska, A. Extraction-depended and thermally-modulated physical and chemical properties of powders produced from cranberry pomace extracts. Curr. Res. Food Sci. 2024, 8, 100664. [Google Scholar] [CrossRef]
- Xu, F.; Zheng, Y.; Yang, Z.; Cao, S.; Shao, X.; Wang, H. Domestic cooking methods affect the nutritional quality of red cabbage. Food Chem. 2014, 161, 162–167. [Google Scholar] [CrossRef]
- Badwaik, L.S.; Gautam, G.; Deka, S.C. Influence of blanching on antioxidant, nutritional and physical properties of bamboo shoot. J. Agric. Sci. 2015, 10, 140–150. [Google Scholar] [CrossRef]
- Mehmood, A.; Zeb, A. Effects of different cooking techniques on bioactive contents of leafy vegetables. Int. J. Gastron. Food Sci. 2020, 22, 100246. [Google Scholar] [CrossRef]
- Tian, J.H.; Chen, J.C.; Ye, X.Q.; Chen, S.G. Health benefits of the potato affected by domestic cooking: A review. Food Chem. 2016, 202, 165–175. [Google Scholar] [CrossRef] [PubMed]

| Pretreatment Method | Operation Method | Abbreviation |
|---|---|---|
| Control check | Without any treatment. | CK |
| Color-protecting | Immerse in 0.3% citric acid solution; the ratio of solid to liquid is 1:20. Soak at room temperature for 5 min, then drain and wipe the surface water. | CP |
| Osmosis | Immerse in a beaker containing 3% NaCl solution for 30 min with a solid-liquid ratio of 1:20. Soak at room temperature for 30 min, then drain and wipe the surface moisture. | OM |
| Blanching | Immersed in a beaker containing distilled water. The ratio of material to liquid is 1:20. Blanch at 85 °C for 30 s, then drain and quickly cool with cold water; wipe the surface moisture. | BC |
| β-cyclodextrin | Immerse in 1.5% β-cyclodextrin solution with a ratio of material to liquid of 1:20, soak at room temperature for 30 min, then drain and wipe the surface moisture. | β-CD |
| Ultrasound | Immerse in a beaker filled with distilled water, the ratio of material to liquid is 1:20, and treat at 150 W, 40 Hz, at room temperature for 30 min, then drain and wipe the surface water. | US |
| Drying Temperature (°C) | Method | L* | a* | b* | C* | h° | Rehydration Property |
|---|---|---|---|---|---|---|---|
| 50 | CK | 64.67 ± 0.77 ab | 5.73 ± 0.60 b | 17.97 ± 0.26 b | 18.87 ± 0.42 a | 72.37 ± 1.52 c | 4.89 ± 0.31 bc |
| CP | 66.05 ± 0.55 a | 4.44 ± 0.60 c | 18.71 ± 0.84 a | 19.24 ± 0.77 a | 76.65 ± 2.03 ab | 6.36 ± 0.59 a | |
| OM | 63.64 ± 0.61 c | 4.51 ± 0.44 c | 17.77 ± 0.97 bc | 18.34 ± 0.83 b | 75.73 ± 2.03 b | 5.01 ± 0.57 bc | |
| BC | 58.03 ± 0.70 de | 9.35 ± 0.64 a | 11.23 ± 0.30 d | 14.62 ± 0.53 d | 50.26 ± 1.83 d | 4.33 ± 0.68 c | |
| β-CD | 66.00 ± 0.88 a | 3.92 ± 0.36 d | 17.48 ± 0.03 c | 17.92 ± 0.10 c | 77.39 ± 1.12 a | 6.16 ± 0.28 a | |
| US | 65.85 ± 0.70 a | 4.54 ± 0.50 c | 18.11 ± 0.69 a | 18.67 ± 0.62 b | 75.93 ± 1.74 b | 5.36 ± 0.45 ab | |
| 60 | CK | 62.53 ± 0.14 d | 6.43 ± 0.88 b | 17.64 ± 0.32 c | 18.79 ± 0.16 c | 70.00 ± 2.80 c | 4.15 ± 0.30 d |
| CP | 65.48 ± 0.88 a | 5.60 ± 0.57 b | 17.95 ± 0.38 c | 18.81 ± 0.19 c | 72.68 ± 2.00 ab | 5.19 ± 0.37 bc | |
| OM | 65.39 ± 0.81 a | 5.83 ± 0.47 b | 19.06 ± 0.08 a | 19.94 ± 0.09 a | 73.04 ± 1.33 a | 5.65 ± 0.59 b | |
| BC | 60.02 ± 2.31 de | 10.18 ± 1.33 a | 12.21 ± 0.85 d | 15.95 ± 0.23 d | 50.26 ± 5.59 d | 4.68 ± 0.21 cd | |
| β-CD | 65.78 ± 1.09 a | 5.63 ± 0.62 b | 18.30 ± 0.38 b | 19.15 ± 0.41 b | 72.95 ± 1.80 ab | 6.42 ± 0.43 a | |
| US | 64.04 ± 0.55 ab | 5.75 ± 0.60 b | 18.48 ± 0.31 b | 19.36 ± 0.26 b | 72.74 ± 1.85 ab | 6.50 ± 0.13 a | |
| 70 | CK | 59.61 ± 1.25 d | 10.44 ± 0.79 c | 17.06 ± 0.17 ab | 20.01 ± 0.36 a | 58.58 ± 2.06 b | 3.64 ± 0.44 c |
| CP | 62.23 ± 1.01 c | 8.09 ± 0.56 d | 17.81 ± 0.33 a | 19.56 ± 0.46 b | 65.61 ± 1.35 a | 5.42 ± 0.53 a | |
| OM | 59.78 ± 0.62 d | 11.69 ± 0.51 b | 16.65 ± 0.61 c | 20.35 ± 0.24 a | 54.94 ± 2.15 c | 5.14 ± 0.44 ab | |
| BC | 56.41 ± 1.11 f | 14.61 ± 0.61 a | 10.62 ± 0.75 e | 18.08 ± 0.26 c | 36.02 ± 2.95 d | 4.33 ± 0.37 bc | |
| β-CD | 61.52 ± 0.82 cd | 9.97 ± 0.84 c | 17.28 ± 0.49 a | 19.97 ± 0.32 ab | 60.04 ± 2.62 b | 5.07 ± 0.39 ab | |
| US | 59.77 ± 0.79 d | 11.42 ± 0.54 b | 15.62 ± 0.68 d | 19.36 ± 0.60 b | 53.83 ± 1.84 c | 3.71 ± 0.30 c |
| Drying Temperature (°C) | Method | Soluble Protein (mg/g) | Reducing Sugar (mg/g) | Starch (mg/g) | Cellulose (mg/g) | Chlorophyll a (mg/g) | Chlorophyll b (mg/g) | Total Chlorophyll (mg/g) |
|---|---|---|---|---|---|---|---|---|
| 50 | CK | 13.68 ± 0.47 b | 37.04 ± 1.02 b | 33.65 ± 1.02 b | 24.72 ± 1.69 d | 0.53 ± 0.05 c | 0.10 ± 0.02 c | 0.63 ± 0.04 c |
| CP | 14.75 ± 0.85 a | 37.42 ± 2.40 ab | 21.63 ± 0.92 d | 24.68 ± 1.65 d | 0.72 ± 0.01 a | 0.26 ± 0.01 a | 0.98 ± 0.06 a | |
| OM | 14.32 ± 0.47 a | 39.03 ± 1.06 a | 22.10 ± 0.40 d | 30.67 ± 1.03 ab | 0.66 ± 0.02 b | 0.23 ± 0.03 a | 0.89 ± 0.01 ab | |
| BC | 5.57 ± 0.47 c | 29.73 ± 2.21 c | 27.35 ± 2.94 c | 28.93 ± 0.84 c | 0.53 ± 0.02 c | 0.10 ± 0.01 c | 0.63 ± 0.03 c | |
| β-CD | 15.16 ± 0.42 a | 38.56 ± 1.96 a | 30.73 ± 2.45 c | 15.73 ± 2.57 e | 0.73 ± 0.03 a | 0.16 ± 0.04 ab | 0.90 ± 0.08 ab | |
| US | 12.87 ± 0.76 b | 34.52 ± 0.58 b | 45.83 ± 1.93 a | 32.63 ± 1.27 a | 0.64 ± 0.02 b | 0.18 ± 0.04 ab | 0.82 ± 0.02 b | |
| 60 | CK | 11.67 ± 0.45 c | 32.51 ± 2.36 b | 26.88 ± 3.50 c | 17.98 ± 0.84 d | 0.58 ± 0.08 a | 0.18 ± 0.02 a | 0.76 ± 0.04 a |
| CP | 13.48 ± 11.94 b | 33.56 ± 0.59 b | 20.81 ± 1.06 d | 23.03 ± 1.68 bc | 0.58 ± 0.05 a | 0.15 ± 0.06 ab | 0.73 ± 0.02 a | |
| OM | 11.95 ± 0.68 c | 31.83 ± 2.58 b | 31.54 ± 1.45 b | 19.94 ± 0.49 cd | 0.56 ± 0.04 a | 0.17 ± 0.02 a | 0.73 ± 0.04 a | |
| BC | 5.20 ± 0.52 d | 29.26 ± 1.25 c | 31.90 ± 1.80 b | 21.68 ± 2.65 bc | 0.52 ± 0.04 a | 0.14 ± 0.01 b | 0.67 ± 0.03 c | |
| β-CD | 14.34 ± 0.50 a | 36.09 ± 1.48 a | 34.93 ± 1.40 b | 27.25 ± 0.84 a | 0.58 ± 0.03 a | 0.18 ± 0.02 a | 0.76 ± 0.23 a | |
| US | 13.91 ± 0.13 b | 35.64 ± 0.95 a | 39.37 ± 2.53 a | 23.31 ± 1.75 b | 0.57 ± 0.04 a | 0.16 ± 0.02 a | 0.74 ± 0.05 a | |
| 70 | CK | 8.76 ± 0.65 a | 35.97 ± 1.23 a | 18.71 ± 4.85 c | 31.74 ± 1.29 ab | 0.48 ± 0.01 b | 0.11 ± 0.01 a | 0.60 ± 0.03 c |
| CP | 7.09 ± 0.30 bc | 37.29 ± 1.10 a | 27.11 ± 0.53 ab | 30.34 ± 2.57 bc | 0.51 ± 0.04 a | 0.14 ± 0.04 a | 0.66 ± 0.03 a | |
| OM | 7.45 ± 0.37 b | 32.51 ± 0.89 a | 31.43 ± 1.05 a | 32.58 ± 2.57 ab | 0.53 ± 0.01 a | 0.13 ± 0.01 a | 0.66 ± 0.01 ab | |
| BC | 6.15 ± 0.68 c | 27.12 ± 2.54 c | 17.19 ± 0.53 c | 33.99 ± 0.84 a | 0.50 ± 0.02 a | 0.08 ± 0.01 a | 0.59 ± 0.01 c | |
| β-CD | 8.00 ± 0.67 a | 34.58 ± 1.52 b | 24.78 ± 0.92 b | 28.09 ± 1.69 c | 0.47 ± 0.02 b | 0.18 ± 0.02 a | 0.65 ± 0.01 ab | |
| US | 8.07 ± 0.74 a | 35.43 ± 2.44 a | 23.96 ± 2.63 b | 34.49 ± 0.89 a | 0.49 ± 0.01 b | 0.08 ± 0.01 a | 0.57 ± 0.02 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Wang, N.; Wang, S.; Wang, J.; Wu, N.; Xu, Y.; Xu, M. Effects of Different Pretreatments on Hot Air Drying Characteristics, Nutrition, and Antioxidant Capacity of Tartary Buckwheat Sprouts. Foods 2024, 13, 2473. https://doi.org/10.3390/foods13162473
Xu X, Wang N, Wang S, Wang J, Wu N, Xu Y, Xu M. Effects of Different Pretreatments on Hot Air Drying Characteristics, Nutrition, and Antioxidant Capacity of Tartary Buckwheat Sprouts. Foods. 2024; 13(16):2473. https://doi.org/10.3390/foods13162473
Chicago/Turabian StyleXu, Xianmeng, Nan Wang, Shunmin Wang, Junzhen Wang, Ningning Wu, Yudie Xu, and Min Xu. 2024. "Effects of Different Pretreatments on Hot Air Drying Characteristics, Nutrition, and Antioxidant Capacity of Tartary Buckwheat Sprouts" Foods 13, no. 16: 2473. https://doi.org/10.3390/foods13162473





