Evaluation of Organic Acids and Ultrasound as Pretreatment in Convective Drying Kinetics and Quality Parameters of Pumpkin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Ultrasound and Organic Acids Pretreatment
2.3. Drying
2.4. Quality Evaluation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Pretreatment
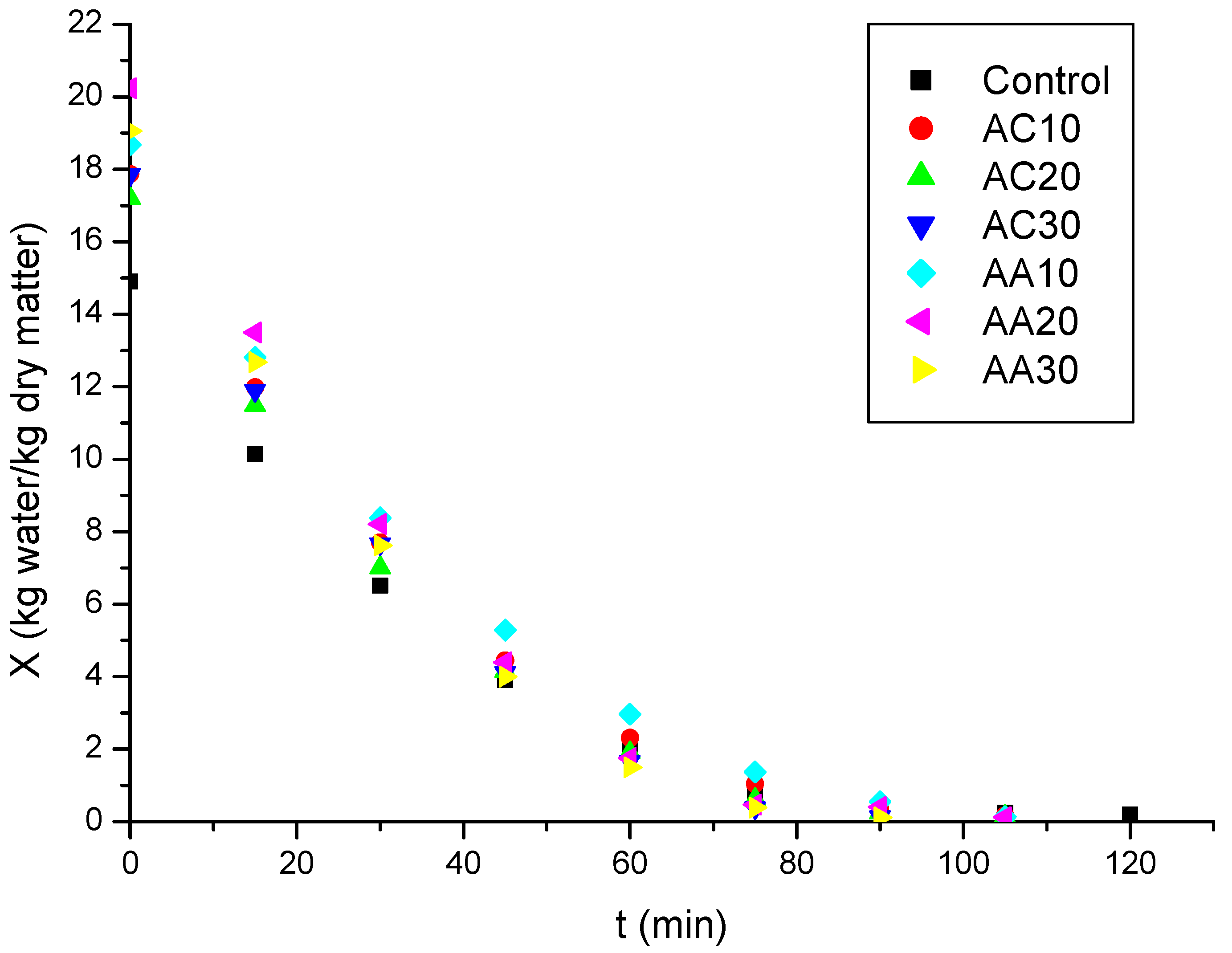
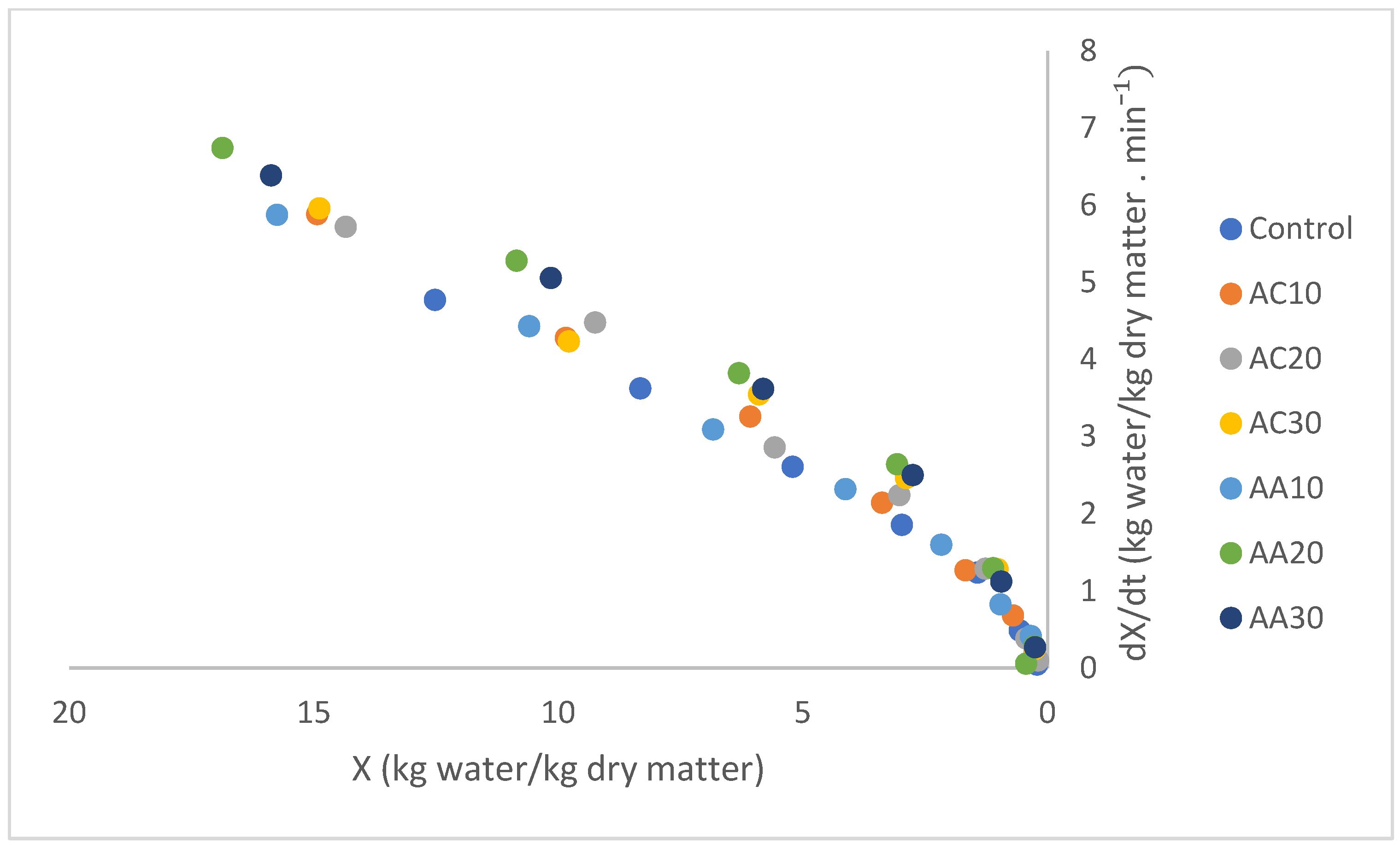
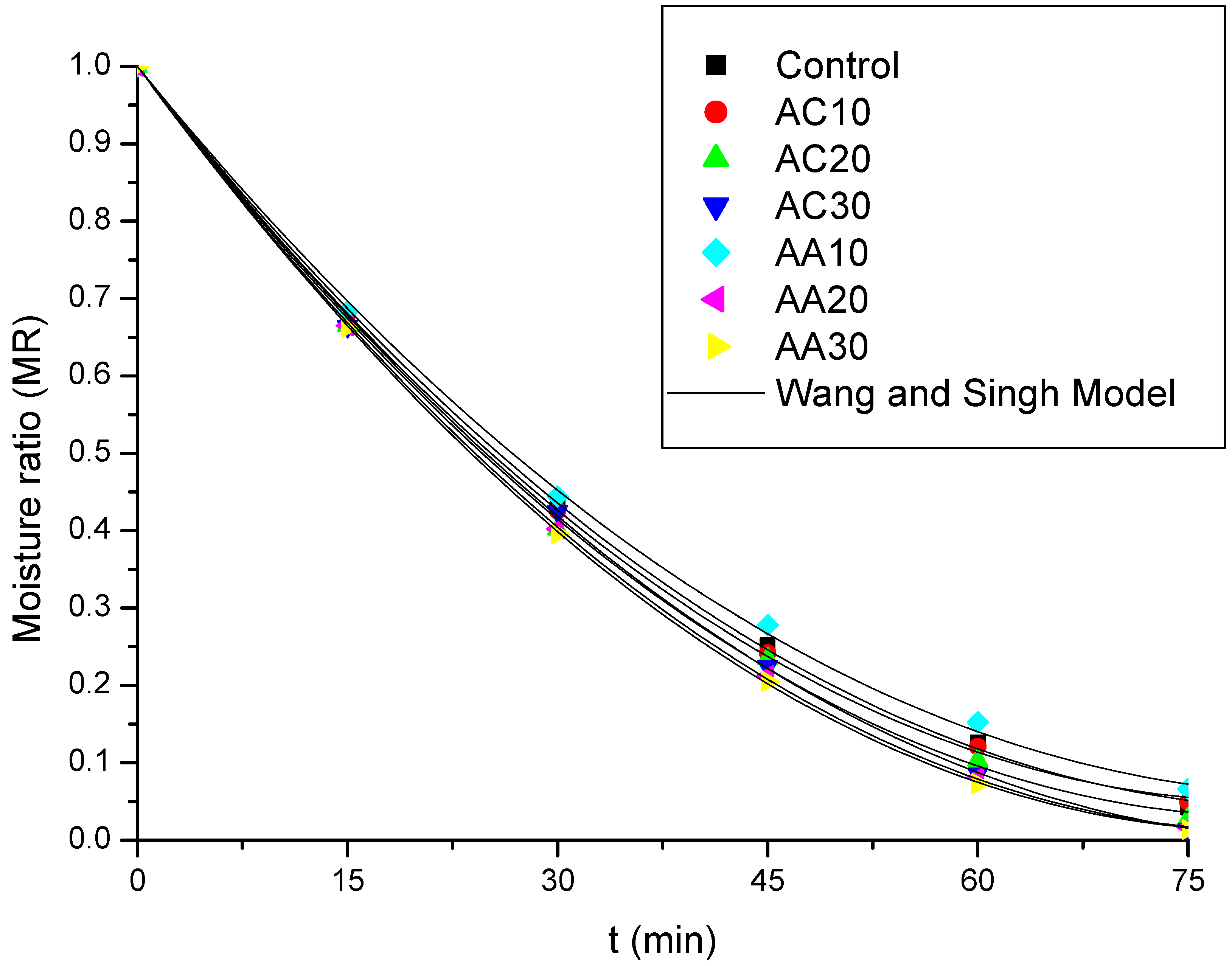
3.2. Drying
3.3. Quality Analysis
3.3.1. Water Activity (aw)
3.3.2. Total Phenolics, Total Carotenoids, and Ascorbic Acid Contents
3.3.3. Color
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ropelewska, E.; Popinska, W.; Sabanci, K.; Aslan, M.F. Flesh of pumpkin from ecological farming as part of fruit suitable for non-destructive cultivar classification using computer vision. Eur. Food Res. Technol. 2022, 248, 893–898. [Google Scholar] [CrossRef]
- Kulczynski, B.; Gramza-Michałowska, A. The profile of secondary metabolites and other bioactive compounds in Cucurbita pepo L. and Cucurbita moschata pumpkin cultivars. Molecules 2019, 24, 2945. [Google Scholar] [CrossRef] [PubMed]
- Chikpah, S.K.; Korese, J.K.; Sturm, B.; Hensel, O. Colour change kinetics of pumpkin (Cucurbita moschata) slices during convective air drying and bioactive compounds of the dried products. J. Agric. Food Res. 2022, 10, 100409. [Google Scholar] [CrossRef]
- Zubernik, J.; Dadan, M.; Cichowska, J.; Witrowa-Rajchert, D. The impact of the pre-treatment in ethanol solution on the drying kinetics and selected properties of convective dried apples. Int. J. Food Eng. 2020, 16, 20180338. [Google Scholar] [CrossRef]
- Huang, D.; Men, K.; Li, D.; Wen, T.; Gong, Z.; Sundenzan, B.; Wu, Z. Application of ultrasound technology in the drying of food products. Ultrason. Sonochem. 2020, 63, 104950. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Wiktor, A.; Śledź, M.; Jurek, N.; Witrowa-Rajchert, D. Drying of ultrasound pretreated apple and its selected physical properties. J. Food Eng. 2012, 113, 427–433. [Google Scholar] [CrossRef]
- Miano, A.C.; Rojas, M.L.; Augusto, P.E.D. Other mass transfer unit operations enhanced by ultrasound. In Ultrasound: Advances in Food Processing and Preservation; Elsevier: Amsterdam, The Netherlands, 2017; p. 556. [Google Scholar]
- Braga, A.M.P.; Pedroso, M.P.; Augusto, F.; Silva, M.A. Volatiles identification in pineapple submitted to drying in an ethanolic atmosphere. Dry. Technol. 2009, 27, 248–257. [Google Scholar] [CrossRef]
- Braga, A.M.P.; Silva, M.A. Effect of ethanol on the drying kinetics and on the quality of pineapple slices. In Proceedings of the 17th International Drying Symposium, Magdeburg, Germany, 3–6 October 2010; pp. 1492–1497. [Google Scholar]
- Rojas, M.L.; Silveira, I.; Augusto, P.E.D. Ultrasound and ethanol pre-treatments to improve convective drying: Drying, rehydration and carotenoid content of pumpkin. Food Bioprod. Process. 2020, 119, 20–30. [Google Scholar] [CrossRef]
- Rojas, M.L.; Augusto, P.E.D. Ethanol and ultrasound pre-treatment to improve infrared drying of potato slices. Innov. Food Sci. Emerg. Technol. 2018, 49, 65–75. [Google Scholar] [CrossRef]
- Rojas, M.L.; Augusto, P.E.D.; Cárcel, J.A. Ethanol pre-treatment to ultrasound-assisted convective drying of apple. Innov. Food Sci. Emerg. Technol. 2020, 61, 102328. [Google Scholar] [CrossRef]
- Cunha, R.M.C.; Brandão, S.C.R.; Medeiros, R.A.B.; Silva Júnior, E.V.; Silva, J.H.F.; Azoubel, P.M. Effect of ethanol pretreatment on melon convective drying. Food Chem. 2020, 333, 127502. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.D.C.; Brandão, S.C.R.; da Silva, J.H.F.; da Rocha, O.R.S.; Azoubel, P.M. Effect of ethanol and ultrasound pretreatments on pineapple convective drying. Food Technol. Biotech. 2021, 59, 209–215. [Google Scholar] [CrossRef]
- Silva, M.A.; Braga, A.M.P.; Santos, P.H.S. Enhancement of fruit drying: The ethanol effect. In Proceedings of the 18th International Drying Symposium (IDS 2012), Xiamen, China, 11–15 November 2012. [Google Scholar]
- Gallmann, P. Food Additives: Second edition, revised and expanded. A.L. Branen, P.M. Davidson, S. Salminen, J.H. Thorngate; Marcel Dekker, New York, Basel, 2002. Int. Dairy J. 2002, 12, 863. [Google Scholar] [CrossRef]
- Hiranvarachat, B.; Devahastin, S.; Chiewchan, N. Effects of acid pretreatments on some physicochemical properties of carrot undergoing hot air drying. Food Bioprod. Process. 2011, 89, 116–127. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; Alvira, P.; Ballesteros, M.; Negro, M.J. Chapter 7—Pretreatment Technologies for Lignocellulose-to-Bioethanol Conversion; Larroche, C., Ricke, S.C., Dussap, C.-G., Gnansounou, E., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; pp. 149–176. [Google Scholar]
- Gorgüç, A.; Gençdağ, E.; Okuroğlu, F.; Yılmaz, F.M.; Halil Bıyık, H.; Kose Köse, S.Ö.; Ersus, S. Single and combined decontamination effects of power-ultrasound, peroxyacetic acid and sodium chloride sanitizing treatments on Escherichia coli, Bacillus cereus and Penicillium expansum inoculated dried figs. LWT 2021, 140, 110844. [Google Scholar] [CrossRef]
- Lyu, Y.; Bi, J.; Chen, Q.; Li, X.; Wu, X.; Gou, M. Effects of ultrasound, heat, ascorbic acid and CaCl2 treatments on color enhancement and flavor changes of freeze-dried carrots during the storage period. Food Chem. 2022, 373, 131526. [Google Scholar] [CrossRef]
- Mkhize, X.; Nkosi, N.; Zondi, L.; Tumba, K. Convective drying of pumpkin: Brief literature review and new data for organically produced indigenous pumpkin (Cucurbita pepo L.) over an expanded temperature range. J. Agric. Food Res. 2023, 14, 100800. [Google Scholar] [CrossRef]
- Chiewchan, N.; Praphraiphetch, C.; Devahastin, S. Effect of pretreatment on surface topographical features of vegetables during drying. J. Food Eng. 2010, 101, 41–48. [Google Scholar] [CrossRef]
- Zhao, W.; Shehzad, H.; Yan, S.; Li, J.; Wang, Q. Acetic acid pretreatment improves the hardness of cooked potato slices. Food Chem. 2017, 228, 204–210. [Google Scholar] [CrossRef]
- Xu, B.; Tiliwa, E.S.; Yan, W.; Roknul Azam, S.M.; Wei, B.; Zhou, C.; Ma, H.; Bhandari, B. Recent development in high quality drying of fruits and vegetables assisted by ultrasound: A review. Food Res. Int. 2022, 152, 110744. [Google Scholar] [CrossRef]
- Azoubel, P.M.; Baima, M.A.M.; Amorim, M.R.; Oliveira, S.S.B. Effect of ultrasound on banana cv Pacovan drying kinetics. J. Food Eng. 2010, 97, 194–198. [Google Scholar] [CrossRef]
- Medeiros, R.A.B.; Barros, Z.M.P.; Carvalho, C.B.O.; Neta, E.G.F.; Maciel, M.I.S.; Azoubel, P.M. Influence of dual-stage sugar substitution pretreatment on drying kinetics and quality parameters of mango. LWT 2016, 67, 167–173. [Google Scholar] [CrossRef]
- da Silva, E.S.; Brandão, S.C.R.; da Silva, A.L.; da Silva, J.H.F.; Coêlho, A.C.D.; Azoubel, P.M. Ultrasound-assisted vacuum drying of nectarine. J. Food Eng. 2019, 246, 119–124. [Google Scholar] [CrossRef]
- Lomauro, C.J.; Bakshi, A.S.; Labuza, T.P. Evaluation of food moisture sorption isotherm equations. Part I: Fruit, vegetable and meat products. LWT 1985, 18, 112–122. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; AOAC: Washington, DC, USA, 2002; 1115p. [Google Scholar]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; International Life Science Institute Press: Washington, DC, USA, 1999; 64p. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteau reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Garcia-Noguera, J.; Oliveira, F.I.P.; Gallão, M.I.; Weller, C.; Rodrigues, S.; Fernandes, F.A.N. Effect of ultrasonic and osmotic dehydration pre-treatments on the colour of freeze dried stawberries. J. Food Sci. Technol. 2010, 51, 2222–2227. [Google Scholar] [CrossRef]
- Silva, J.H.F.; Galvão, C.C.; Silva, E.S.; Cavalcanti, D.E.S.; Rocha, O.R.S.; Azoubel, P.M.; Benachour, M. Secagem convectiva de melão (Cucumis melo L.) com e sem pré-tratamento ultrassônico. In Proceedings of the XXII Congresso Brasileiro de Engenharia Química, São Paulo, Brazil, 23–26 September 2018; Volume 1, pp. 2845–2848. [Google Scholar]
- Wang, J.; Wang, J.; Ye, J.; Kranthi, S.; Raghavan, V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control 2019, 96, 128–136. [Google Scholar] [CrossRef]
- Padilha, C.E.A.; Nogueira, C.C.; Oliveira Filho, M.A.; Souza, D.F.S.; Oliveira, J.A.; Santos, E.S. Valorization of cashew apple bagasse using acetic acid pretreatment: Production of cellulosic ethanol and lignin for their use as sunscreen ingredients. Process Biochem. 2020, 91, 23–33. [Google Scholar] [CrossRef]
- TACO. Tabela Brasileira de Composição de Alimentos; NEPA-UNICAMP: Campinas, Brazil, 2011; 161p. [Google Scholar]
- Gomes, E.S.; Marins, A.R.; Gomes, R.G. Avaliação das características químicas e físicas da farinha de abóbora (Cucurbita maxima): Polpa e sementes. Res. Soc. Dev. 2022, 11, 36211931811. [Google Scholar] [CrossRef]
- Villamiel, M.; Garcia-Perez, J.V.; Montilla, A.; Cárcel, J.A.; Benedito, J. Ultrasound in Food Processing: Recent Advances; John Wiley & Sons: Hoboken, NJ, USA, 2017; 544p. [Google Scholar]
- Ozdemir, Y.; Ozturk, A.; Tüfekçi, S. Effect of two dipping pretreatment on drying kinetics of golden berry (Physalis peruviana L.). Afr. J. Agric. Res. 2016, 11, 40–47. [Google Scholar]
- Osidacz, R.C.; Anbrosio-Ugri, M.C.B. Physicochemical quality of eggplant dehydrated with varied pretreatments. Acta Sci. Technol. 2013, 35, 175–179. [Google Scholar] [CrossRef]
- Doymaz, I. The kinetics of forced convective air-drying of pumpkin slices. J. Food Eng. 2007, 79, 243–248. [Google Scholar] [CrossRef]
- Koç, G.Ç.; Dirim, N. Determination of the freeze drying kinetics of pumpkin (Cucurbita moschata) Puree. In Proceedings of the 19th International Drying Symposium (IDS2014), Lyon, France, 24–27 August 2014. [Google Scholar]
- Oliveira, D.E.C.; Guimarães, J.M.; Bueno, J.M.G.S.; Costa Júnior, J.R.; Alves, E.M.; Silva, B.M.C. Drying kinetics of pumpkin seeds. Comun. Sci. 2021, 12, e3391. [Google Scholar]
- Mahapatra, A.; Tripathy, P.P. Modeling and simulation of moisture transfer during solar drying of carrot slices. J. Food Process Eng. 2018, 41, e12909. [Google Scholar] [CrossRef]
- Smaniotto, T.; Resende, O.; Sousa, K.; Oliveira, D.; Campos, R. Drying kinetics of sunflower grains. Rev. Bras. Eng. Agric. Ambient. 2017, 21, 203–208. [Google Scholar] [CrossRef]
- Khawas, P.; Dash, K.; Das, A.; Deka, S. Drying characteristics and assessment of physicochemical and microstructural properties of dried culinary banana slices. Int. J. Food Eng. 2015, 11, 667–678. [Google Scholar] [CrossRef]
- Skoog, D.; West, D.; Holler, J.; Crouch, S. Fundamentos de Química Analítica; Editora Thomson: São Paulo, Brazil, 2006. [Google Scholar]
- Dufera, L.T.; Hofacker, W.; Esper, A.; Hensel, O. Effect of different predrying treatments on physicochemical quality and drying kinetics of twin layer solar tunnel dried tomato (Lycopersicon esculentum L.) slices. J. Food Qual. 2022, 2022, 9095922. [Google Scholar]
- Reger, D.; Goode, S.; Mercer, E. Química: Princípios e Aplicações; Fundação Calouste Gulbenkian: Lisbon, Portugal, 2010; 1226p. [Google Scholar]
- Jha, A.K.; Sit, N. Drying characteristics and kinetics of colour change and degradation of phytocomponents and antioxidant activity during convective drying of deseeded Terminalia chebula fruit. J. Food Meas. Charact. 2020, 14, 2067–2077. [Google Scholar] [CrossRef]
- Onwude, D.; Hashim, N.; Janius, R.; Nawi, N.; Abdan, K. Modelling effective moisture diffusivity of pumpkin (Cucurbita moschata) slices under convective hot air drying condition. Int. J. Food Eng. 2013, 12, 481–489. [Google Scholar] [CrossRef]
- BRASIL. Ministério da Saúde. Resolução—RDC n° 272, de 22 de Setembro de 2005. Regulamento Técnico Para Produtos de Vegetais, Produtos de Frutas e Cogumelos Comestíveis; Diário Oficial da União: Brasília, Brazil, 2005.
- Szadzińska, J.; Mierzwa, D. The influence of hybrid drying (microwave-convective) on drying kinetics and quality of white mushrooms. Chem. Eng. Process. 2021, 167, 108532. [Google Scholar] [CrossRef]
- Köprüalan, Ö.; Altay, Ö.; Bodruk, A.; Kaymak-Ertekin, F. Effect of hybrid drying method on physical, textural and antioxidant properties of pumpkin chips. J. Food Meas. Charact. 2021, 15, 2995–3004. [Google Scholar] [CrossRef]
- Islam, M.Z.; Das, S.; Monalisa, K.; Sayem, A.S.M. Influence of osmotic dehydration on mass transfer kinetics and quality retention of ripe papaya (Carica papaya L.) during Drying. AgriEngineering 2019, 1, 220–234. [Google Scholar] [CrossRef]
- Shao, L.; Zhao, Y.; Zou, B.; Li, X.; Dai, R. Ohmic heating in fruit and vegetable processing: Quality characteristics, enzyme inactivation, challenges and prospective. Trends Food Sci. Technol. 2021, 118, 601–616. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Y.; Zhang, F.; Wang, I.; Yi, J.; Liao, X. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food Agric. 2011, 91, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Movagharnejad, K.; Sadeghi, E. Infrared drying effects on the quality of eggplant slices and process optimization using response surface methodology. Food Chem. 2020, 333, 127423. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.H. Effect of Pretreatments and Solar Tunnel Dryer Zones on Drying Characteristics and Quality of Pumpkin (Cucurbita maxima) Pulp Slice and Powder. Master’s Thesis, Jimma University, Jimma, Etiopia, 2022. [Google Scholar]
- Liu, Y.; Miao, S.; Wu, J.; Liu, J.; Yu, H.; Duan, X. Drying characteristics and modeling of vacuum far-infrared radiation drying of Flos lonicerae. J. Food Process. Preserv. 2015, 39, 338–348. [Google Scholar] [CrossRef]
- Djendoubi, N.M.; Boudhrioua, N.; Kechaou, N.; Courtis, F.; Bonazzi, C. Influence of air drying temperature on kinetics, physicochemical properties, total phenolic content and ascorbic acid of pears. Food Bioprod. Process. 2012, 90, 433–441. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Costa, M.G.M.; Jesus, A.L.T.; Miranda, M.R.A.; Fernandes, F.A.N.; Rodrigues, S. Power ultrasound processing of cantaloupe melon juice: Effects on quality parameters. Food Res. Int. 2012, 48, 41–48. [Google Scholar] [CrossRef]
- Dyab, A.; El-El-Sherif, G.; Gab-Allah, R. Evaluate the effect of pretreatments and drying techniques on the sweet potato slices. Food Technol. Res. J. 2023, 1, 9–19. [Google Scholar] [CrossRef]
- Ramos-Parra, P.A.; García-Salinas, C.; Rodríguez-López, C.E.; García, N.; García-Rivas, G.; Hernández-Brenes, C.; Díaz de la Garza, R.I. High hydrostatic pressure treatments trigger de novo carotenoid biosynthesis in papaya fruit (Carica papaya cv. Maradol). Food Chem. 2019, 277, 362–372. [Google Scholar] [CrossRef]
- Kreck, M.; Kuerbel, P.; Ludwig, M.; Paschold, P.; Dietrich, H. Identification and quantification of carotenoids in pumpkin cultivars (Cucurbita maxima L.) and their juices by liquid chromatography with ultraviolet-diode array detection. J. Appl. Bot. Food Qual. 2006, 80, 93–99. [Google Scholar]
- Rodriguez-Amaya, D.B. Latin American food sources of carotenoids. Arch. Latinoam. Nutr. 1999, 49, 74S–84S. [Google Scholar] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Skibsted, L.H. Kinetics and mechanism of the primary steps of degradation of carotenoids by acid in homogeneous solution. J. Agric. Food Chem. 2000, 48, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Veda, S.; Platel, K.; Srinivasan, K. Influence of food acidulants and antioxidant spices on the bioaccessibility of β-carotene from selected vegetables. J. Agric. Food Chem. 2008, 56, 8714–8719. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Cao, S.; Huang, Y. Phenolics and ascorbic acid in pumpkin (Cucurbita maxima) slices: Effects of hot air drying and degradation kinetics. Food Meas. 2021, 15, 247–255. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Pinheiro, J.; Abreu, M.; Brandão, T.R.S.; Silva, C.L.M. Kinetics of quality changes of pumpkin (Curcurbita maxima L.) stored under isothermal and non-isothermal frozen conditions. J. Food Eng. 2011, 106, 40–47. [Google Scholar] [CrossRef]
- Arruda, G.M.P.; Brandão, S.C.R.; Silva Junior, E.V.; Silva, E.M.; Barros, Z.M.P.; Silva, E.S.; Shinohara, N.K.S.; Azoubel, P.M. Influence of ultrasound and ethanol as a pretreatment on papaya infrared and convective drying characteristics and quality parameters. J. Food Process Eng. 2023, 46, e14255. [Google Scholar] [CrossRef]
- Carvalho, G.R.; Rojas, M.L.; Silveira, I.; Augusto, P.E.D. Drying accelerators to enhance processing and properties: Ethanol, isopropanol, acetone and acetic acid as pre-treatments to convective drying of pumpkin. Food Bioprocess Technol. 2020, 13, 1984–1996. [Google Scholar] [CrossRef]
- Onwude, D.; Hashim, N.; Janius, R.; Nawi, N.; Abdan, E. Color change kinetics and total carotenoid content of pumpkin as affected by drying temperature. Ital. J. Food Sci. 2017, 29, 1–18. [Google Scholar]
- Lee, F.A. Browning reactions. In Basic Food Chemistry; The Avi Publishing Company: Westport, CT, USA, 1983. [Google Scholar]
- Rizzi, G.P. Chemical structure of colored Maillard reaction products. Food Rev. Int. 1997, 13, 1–28. [Google Scholar] [CrossRef]
- Marquez-Rios, E.; Ocaño-Higuera, V.M.; Maeda-Martínez, A.N.; Lugo-Sánchez, M.E.; Carvallo-Ruiz, M.G.; Pacheco-Aguilar, R. Citric acid as pretreatment in drying of Pacific Lion’s Paw Scallop (Nodipecten subnodosus) meats. Food Chem. 2009, 112, 599–603. [Google Scholar] [CrossRef]
- Doymaz, I. Impact of citric acid on the drying characteristics of kiwifruit slices. Acta Sci. Technol. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Sun, X.; Jin, X.; Fu, N.; Chen, X. Effects of different pretreatment methods on the drying characteristics and quality of potatoes. Food Sci. Nutr. 2020, 8, 5767–5775. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.; Titikshya, S.; Aradwad, P.; Kumar, V.; Naik, S.N. Study of the drying behaviour and color kinetics of convective drying of yam (Dioscorea hispida) slices. Ind. Crops Prod. 2022, 176, 114258. [Google Scholar] [CrossRef]
- Seerangurayar, T.; Al-Ismaili, A.M.; Jeewantha, L.J.; Al-Habsi, N.A. Effect of solar drying methods on color kinetics and texture of dates. Food Bioprod. Process. 2019, 116, 227–239. [Google Scholar]
- Xiao, H.W.; Yao, X.D.; Lin, H.; Yang, W.X.; Meng, J.S.; Gao, Z.J. Effect of SSB (superheated steam blanching) time and drying temperature on hot air impingement drying kinetics and quality attributes of yam slices. J. Food Process Eng. 2012, 35, 370–390. [Google Scholar] [CrossRef]
- Kumar, Y.; Sharanagat, V.S.; Singh, L.; Nema, P.K. Convective drying of spine gourd (Momordica dioica): Effect of ultrasound pre-treatment on drying characteristics, color, and texture attributes. J. Food Process. Preserv. 2020, 44, e14639. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Peressini, D. Effects of osmotic dehydration (with and without sonication) and pectin-based coating pretreatments on functional properties and color of hot-air dried apricot cubes. Food Chem. 2020, 311, 125978. [Google Scholar] [CrossRef]
| Model | Equation |
|---|---|
| Single exponential | |
| Henderson and Pabis | |
| Logarithmic | |
| Two-term exponential | |
| Wang and Singh |
| Sample | WL (%) | SG (%) |
|---|---|---|
| AC10 | −0.56 ± 0.03 a | −0.13 ± 0.02 a |
| AC20 | −1.19 ± 0.08 ᵇ | −0.27 ± 0.04 ᵇ |
| AC30 | −1.49 ± 0.07 c | −0.35 ± 0.03 c |
| AA10 | −1.12 ± 0.10 ᵇ | −0.25 ± 0.02 ᵇ |
| AA20 | −1.65 ± 0.14 c | −0.46 ± 0.01 ᵈ |
| AA30 | −2.03 ± 0.15 ᵈ | −0.52 ± 0.03 ᵉ |
| Sample | X (Wet Basis, kg Water/100 kg Sample) | X (Dry Basis, kg Water/kg Dry Matter) |
|---|---|---|
| AC10 | 94.25 ± 0.03 | 16.39 ± 0.08 |
| AC20 | 94.88 ± 0.08 | 18.53 ± 0.14 |
| AC30 | 95.18 ± 0.07 | 19.75 ± 0.16 |
| AA10 | 94.81 ± 0.10 | 18.27 ± 0.21 |
| AA20 | 95.34 ± 0.14 | 20.46 ± 0.30 |
| AA30 | 95.72 ± 0.15 | 22.36 ± 0.32 |
| Samples | Time to Reach Equilibrium Moisture Content (min) | Time Reduction Compared to Untreated Sample (min) | Final Moisture Content (kg Water/kg Sample) | Final Moisture Content (kg Water/kg Dry Matter) |
|---|---|---|---|---|
| Control | 120 | - | 16.03 ± 0.14 a | 0.19 ± 0.01 a |
| AC10 | 105 | 12.5 | 14.17 ± 0.13 ᵇ | 0.17 ± 0.01 ᵇ |
| AC20 | 105 | 12.5 | 12.43 ± 0.12 c | 0.14 ± 0.01 c |
| AC30 | 90 | 25.0 | 11.70 ± 0.11 ᵈ | 0.13 ± 0.01 ᵈ |
| AA10 | 105 | 12.5 | 11.80 ± 0.03 ᵈ | 0.13 ± 0.01 ᵈ |
| AA20 | 105 | 12.5 | 11.06 ± 0.07 ᵉ | 0.12 ± 0.01 ᵉ |
| AA30 | 90 | 25.0 | 10.54 ± 0.11 ᶠ | 0.12 ± 0.01 ᶠ |
| Models | Sample | Parameters | R2 | P (%) | |||
|---|---|---|---|---|---|---|---|
| Henderson and Pabis | a | k | |||||
| Control | 1.0232 | 0.0312 | 0.9901 | 28.0948 | |||
| AC10 | 1.0222 | 0.0317 | 0.9909 | 25.7271 | |||
| AC20 | 1.0246 | 0.0331 | 0.9888 | 44.0220 | |||
| AC30 | 1.0286 | 0.0335 | 0.9827 | 95.6486 | |||
| AA10 | 1.0194 | 0.0294 | 0.9932 | 16.0379 | |||
| AA20 | 1.0291 | 0.0343 | 0.9847 | 75.4536 | |||
| AA30 | 1.0300 | 0.0349 | 0.9839 | 89.3456 | |||
| Logarithmic | a | k | c | ||||
| Control | 1.2009 | 0.0216 | −0.1988 | 0.9999 | 1.9995 | ||
| AC10 | 1.1822 | 0.0225 | −0.1797 | 0.9998 | 3.7916 | ||
| AC20 | 1.1952 | 0.0230 | −0.1917 | 0.9997 | 5.0739 | ||
| AC30 | 1.2604 | 0.0210 | −0.2577 | 0.9993 | 17.1681 | ||
| AA10 | 1.1726 | 0.0213 | −0.1714 | 0.9999 | 0.8006 | ||
| AA20 | 1.1772 | 0.0212 | −0.1761 | 0.9999 | 0.8608 | ||
| AA30 | 1.2246 | 0.0230 | −0.2181 | 0.9989 | 21.0980 | ||
| Two-term exponential | a | k | b | w | |||
| Control | 0.5179 | 0.0312 | 0.5053 | 0.0312 | 0.9901 | 28.0950 | |
| AC10 | 0.5501 | 0.0317 | 0.4721 | 0.0317 | 0.9909 | 25.7276 | |
| AC20 | −42.9453 | 0.0283 | 43.9469 | 0.0284 | 0.9992 | 9.2724 | |
| AC30 | −73.2640 | 0.0281 | 74.2643 | 0.0283 | 0.9982 | 25.5953 | |
| AA10 | −95.6213 | 0.0248 | 96.6198 | 0.0250 | 0.9996 | 2.2760 | |
| AA20 | −61.0873 | 0.0297 | 62.0896 | 0.0230 | 0.9987 | 21.2206 | |
| AA30 | −84.5977 | 0.0304 | 85.6013 | 0.0303 | 0.9985 | 27.0797 | |
| Wang and Singh | a | b | |||||
| Control | −0.0229 | 0.000137 | 0.9994 | 5.4474 | |||
| AC10 | −0.0234 | 0.000143 | 0.9995 | 4.4087 | |||
| AC20 | −0.0239 | 0.000148 | 0.9992 | 7.5585 | |||
| AC30 | −0.0236 | 0.000140 | 0.9997 | 2.3111 | |||
| AA10 | −0.0222 | 0.000131 | 0.9991 | 4.5355 | |||
| AA20 | −0.0243 | 0.000150 | 0.9999 | 2.6132 | |||
| AA30 | −0.0247 | 0.000154 | 0.9999 | 1.3180 | |||
| Single exponential | k | ||||||
| Control | 0.0305 | 0.9891 | 29.3033 | ||||
| AC10 | 0.0311 | 0.9900 | 27.0462 | ||||
| AC20 | 0.0324 | 0.9878 | 45.7841 | ||||
| AC30 | 0.0326 | 0.9813 | 101.6866 | ||||
| AA10 | 0.0288 | 0.9925 | 16.7395 | ||||
| AA20 | 0.0334 | 0.9834 | 78.9527 | ||||
| AA30 | 0.0340 | 0.9825 | 93.5021 | ||||
| Sample | Def × 108 (m2∙s−1) | R2 |
|---|---|---|
| Control | 6.68 ± 0.15 ᵇ | 0.9658 |
| AC10 | 6.73 ± 0.15 ᶜ | 0.9655 |
| AC20 | 7.02 ± 0.04 ᵈ | 0.9646 |
| AC30 | 7.01 ± 0.13 ᵈ | 0.9549 |
| AA10 | 6.24 ± 0.05 ᵃ | 0.9655 |
| AA20 | 7.22 ± 0.10 ᵉ | 0.9615 |
| AA30 | 7.31 ± 0.06 ᶠ | 0.9570 |
| Methods | Water Activity (aw) | Total Phenolics Content (mg GAE∙100 g−1 DM) | Total Carotenoids Content (µg∙g−1 DM) | Ascorbic Acid Content (mg/∙100 g−1 DM) | Color | |||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | |||||
| Fresh | 0.99 ± 0.01 ᵃ | 0.64 ± 0.02 a | 91.93 ± 1.82 a | 128.71 ± 8.17 ᵃ | 77.70 ± 0.03 ᵇ | 17.12 ± 1.28 ᵃ | 44.07 ± 1.42 ᵇ | - |
| AC30 | 0.59 ± 0.02 ᵇ | 0.58 ± 0.01 ᵇ | 30.52 ± 0.23 ᵈ | 63.39 ± 1.54 ᵇ | 79.61 ± 1.16 ᵃ | 15.37 ± 2.35 ᵃᵇ | 63.93 ± 2.26 ᵃ | 18.17 ± 0.72 ᵃ |
| AA10 | 0.51 ± 0.01 ᶜ | 0.58 ± 0.01 ᵇ | 50.47 ± 0.29 ᵇ | 42.39 ± 0.91 c | 78.94 ± 0.23 ᵃ | 13.08 ± 0.34 ᵇ | 61.80 ± 1.25 ᵃ | 18.51 ± 0.56 ᵃ |
| AA20 | 0.51 ± 0.03 ᶜ | 0.52 ± 0.03 c | 51.10 ± 1.12 ᵇ | 31.48 ± 0.90 ᵈ | 79.29 ± 0.50 ᵃ | 14.11 ± 0.08 ᵇ | 63.79 ± 0.67 ᵃ | 18.90 ± 2.53 ᵃ |
| AA30 | 0.51 ± 0.03 ᶜ | 0.45 ± 0.01 ᵈ | 45.71 ± 0.69 ͨ | 20.93 ± 0.49 ᵉ | 80.10 ± 0.40 ᵃ | 13.99 ± 0.01 ᵇ | 62.53 ± 0.15 ᵃ | 17.00 ± 1.00 ᵃ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, J.R.R.d.O.; de Morais, B.R.S.; da Silva, J.H.F.; Alves, A.S.S.; Brandão, S.C.R.; Azoubel, P.M. Evaluation of Organic Acids and Ultrasound as Pretreatment in Convective Drying Kinetics and Quality Parameters of Pumpkin. Foods 2024, 13, 2502. https://doi.org/10.3390/foods13162502
Moura JRRdO, de Morais BRS, da Silva JHF, Alves ASS, Brandão SCR, Azoubel PM. Evaluation of Organic Acids and Ultrasound as Pretreatment in Convective Drying Kinetics and Quality Parameters of Pumpkin. Foods. 2024; 13(16):2502. https://doi.org/10.3390/foods13162502
Chicago/Turabian StyleMoura, José R. R. de O., Blenda R. S. de Morais, João H. F. da Silva, Amanda S. S. Alves, Shirley C. R. Brandão, and Patricia M. Azoubel. 2024. "Evaluation of Organic Acids and Ultrasound as Pretreatment in Convective Drying Kinetics and Quality Parameters of Pumpkin" Foods 13, no. 16: 2502. https://doi.org/10.3390/foods13162502
APA StyleMoura, J. R. R. d. O., de Morais, B. R. S., da Silva, J. H. F., Alves, A. S. S., Brandão, S. C. R., & Azoubel, P. M. (2024). Evaluation of Organic Acids and Ultrasound as Pretreatment in Convective Drying Kinetics and Quality Parameters of Pumpkin. Foods, 13(16), 2502. https://doi.org/10.3390/foods13162502






