Experimental Investigation of Bacterial Inactivation of Beef Using Indirect Cold Plasma in Cold Chain and at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Beef Preparation
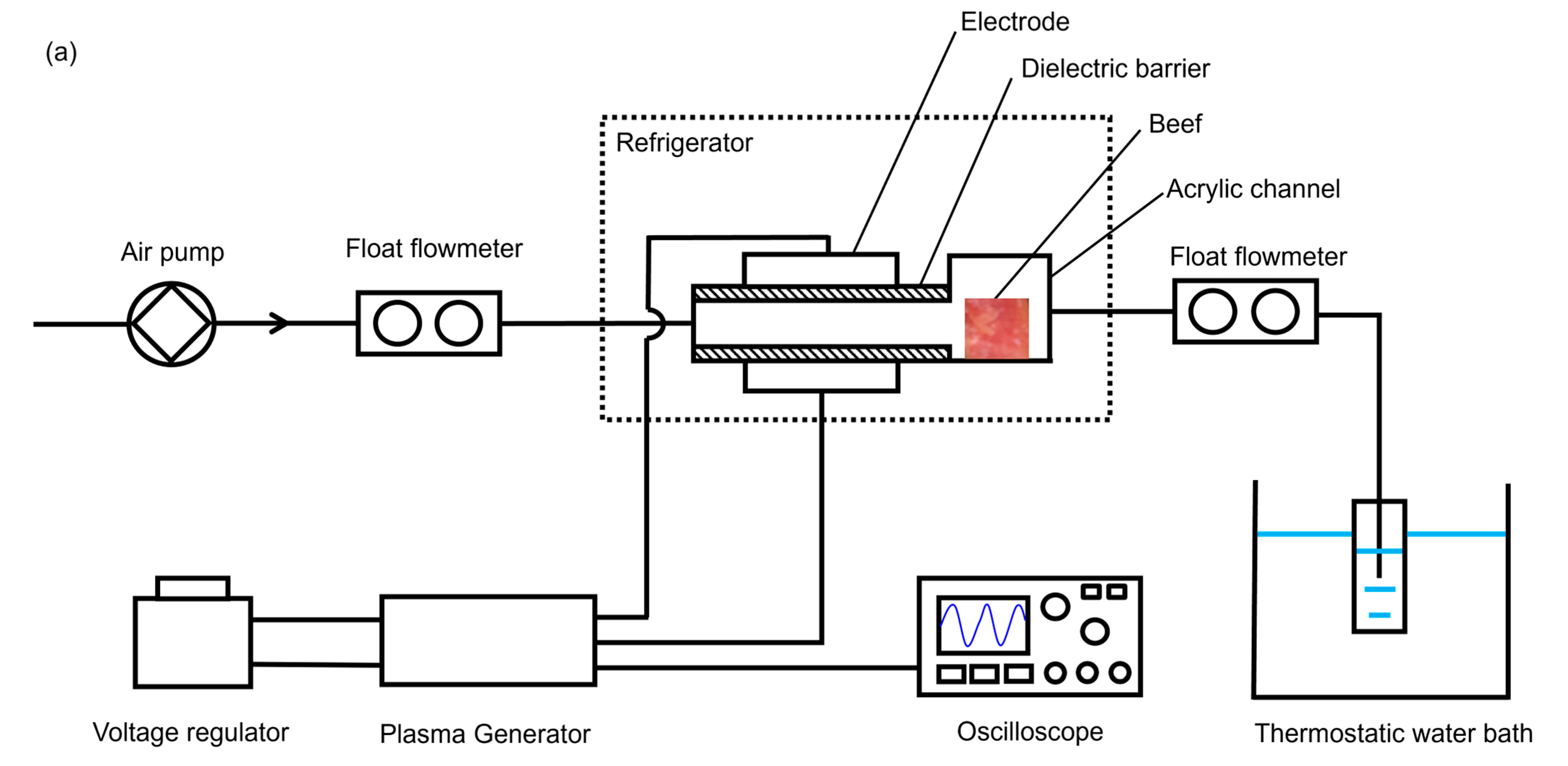
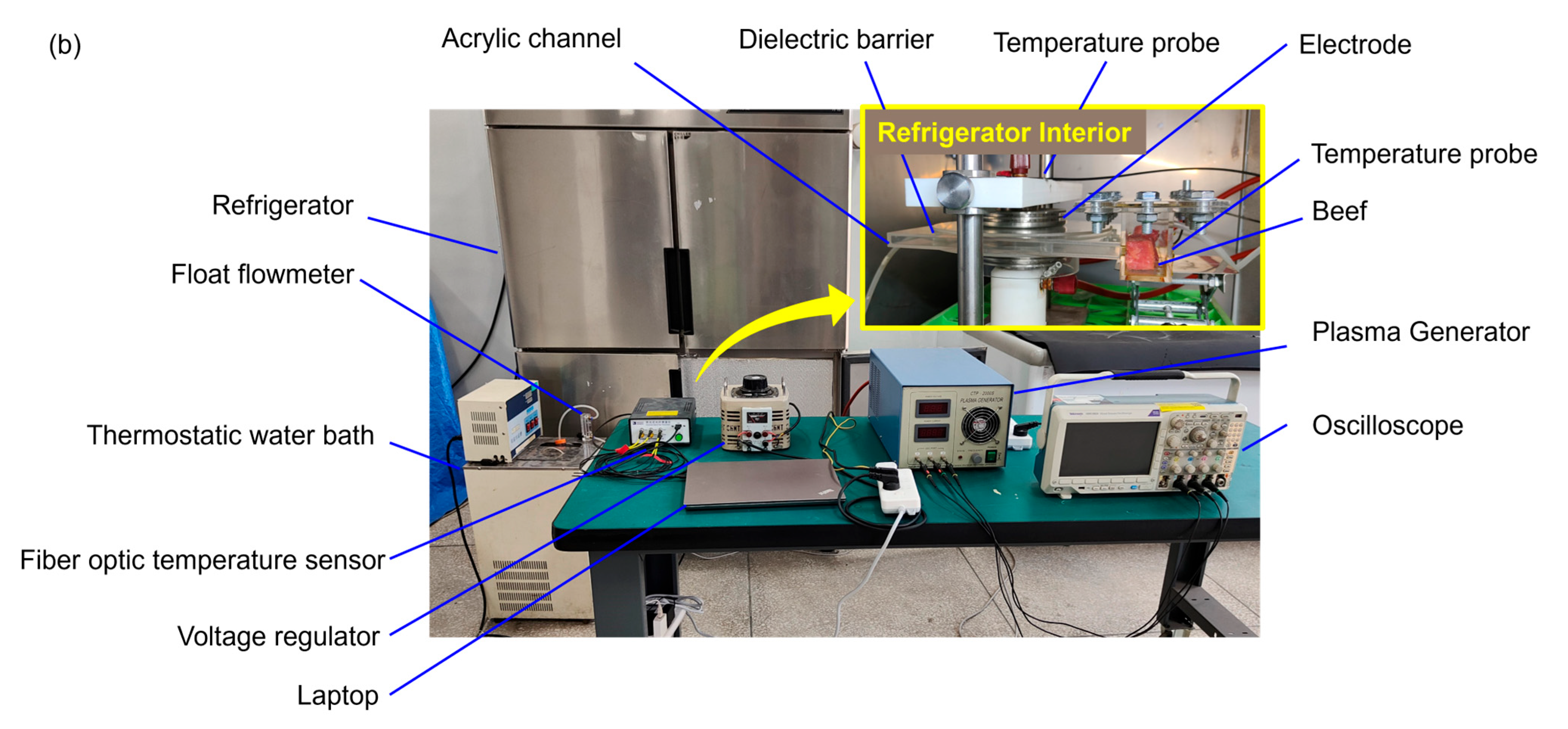
2.2. Experimental Apparatus
2.3. Detection of Reactive Species in CP
2.4. Bacteria Preparation
2.5. Measurement of Quality Indexes
2.6. Statistical Analysis
3. Results and Discussion
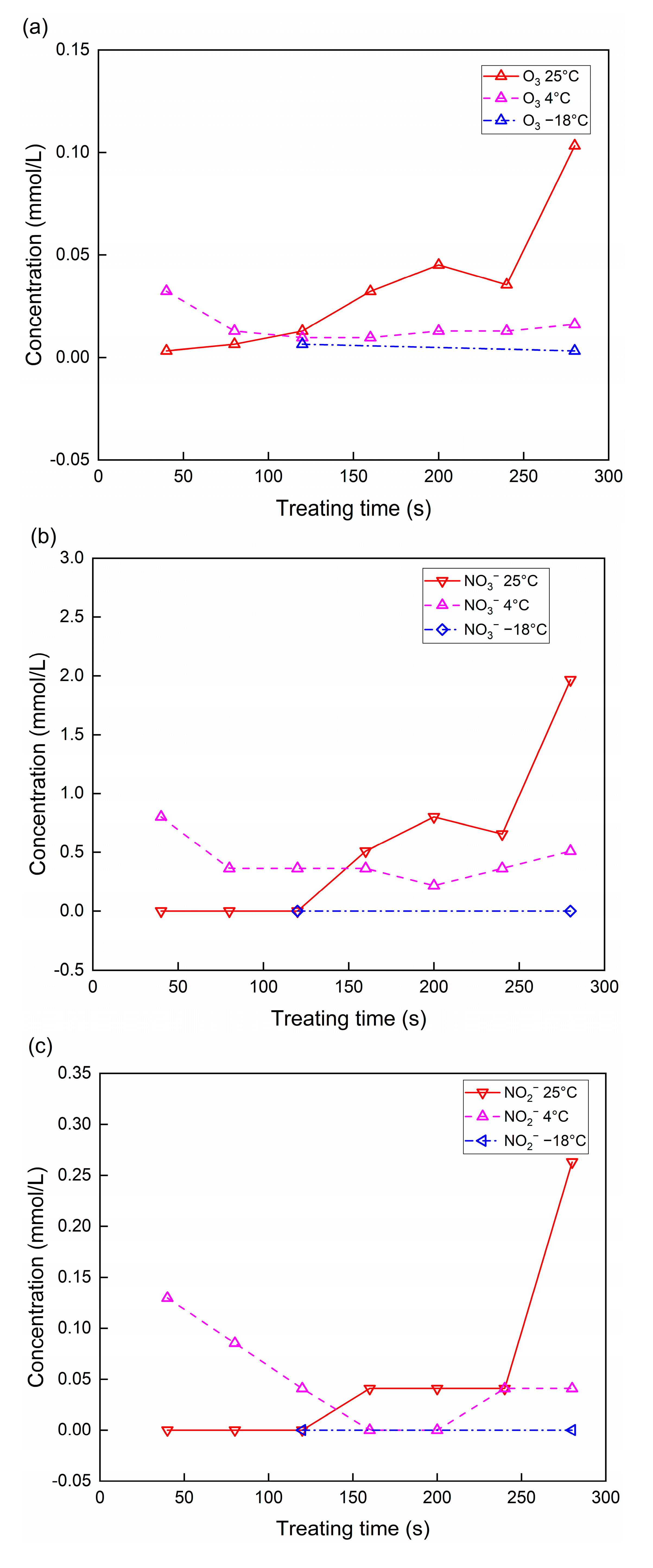
3.1. Concentrations of Substances
3.2. Bacterial Inactivation
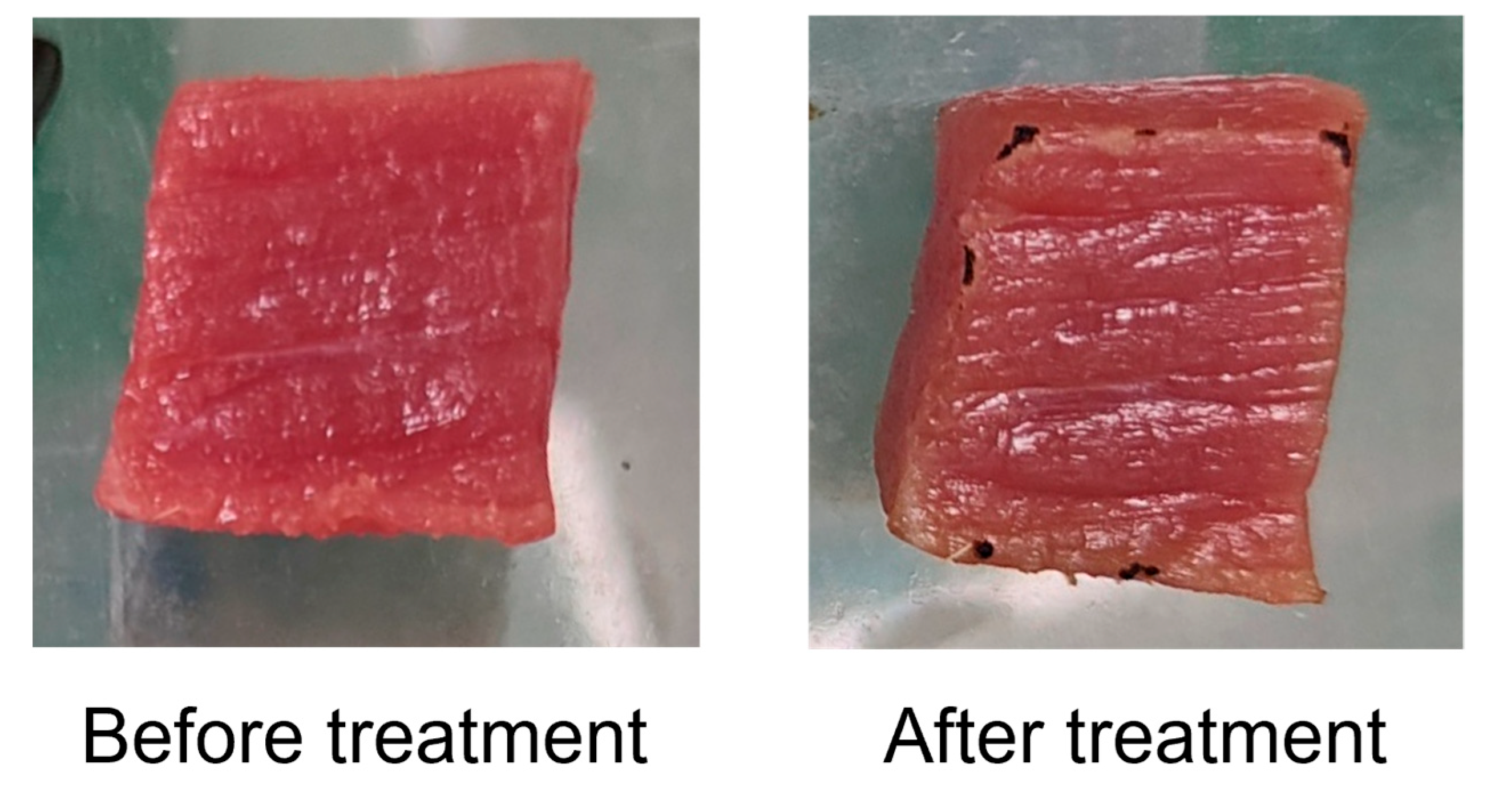
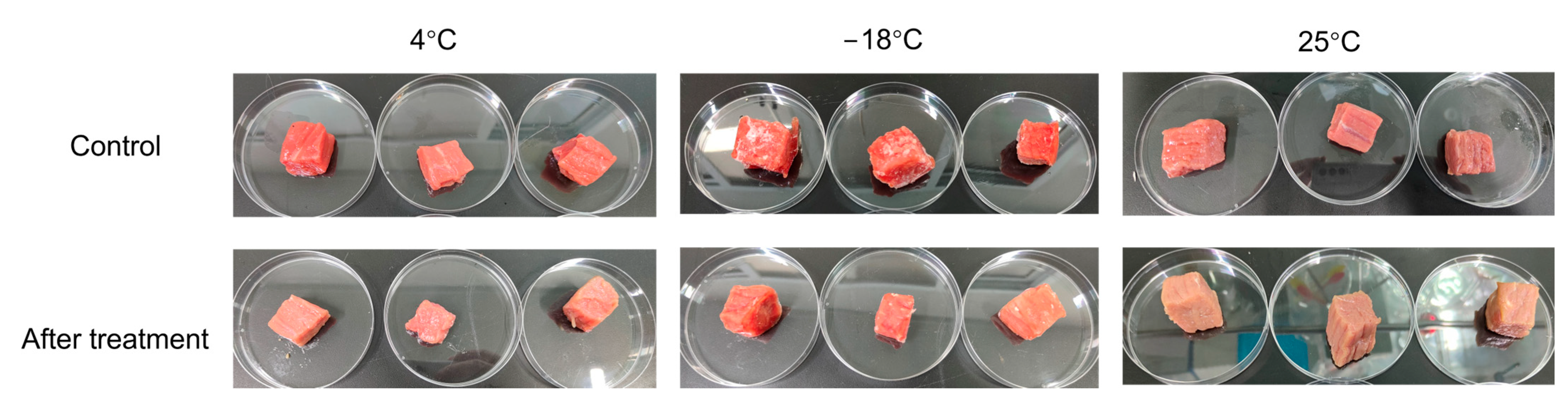
3.3. Quality Index
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warmate, D.; Onarinde, B.A. Food Safety Incidents in the Red Meat Industry: A Review of Foodborne Disease Outbreaks Linked to the Consumption of Red Meat and Its Products, 1991 to 2021. Int. J. Food Microbiol. 2023, 398, 110240. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhu, Y.; Yu, B.; Du, M.; Feng, J.; Zhuang, J.; Ma, R.; Jiao, Z. Comparative Analysis of Helium and Air Surface Micro-Discharge Plasma Treatment on the Microbial Reduction and Quality Attributes of Beef Slices. Meat Sci. 2023, 204, 109259. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Wang, L. The Microbial Safety of Fish and Fish Products: Recent Advances in Understanding Its Significance, Contamination Sources, and Control Strategies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 738–786. [Google Scholar] [CrossRef]
- Yao, J.; Zhou, B.; Wang, R.; Wang, T.; Hu, X.; Liao, X.; Zhang, Y. Inactivation of Staphylococcus Aureus by High Hydrostatic Pressure in Saline Solution and Meat Slurry with Different Initial Inoculum Levels. Food Bioprod. Process. 2015, 94, 592–600. [Google Scholar] [CrossRef]
- Food Safety and Inspection Service. Connoisseur’s Kitchen Recalls Imported Frozen Chicken Products Due to Possible Listeria Contamination. 2022. Available online: http://www.fsis.usda.gov/recalls-alerts/connoisseurs-kitchen-recalls-imported-frozen-chicken-products-due-possible-listeria (accessed on 23 August 2024).
- Food Safety and Inspection Service. Morasch Meats Inc. Recalls Raw Frozen Diced Beef Products Due to Possible E. coli O157:H7 Contamination. 2022. Available online: https://www.fsis.usda.gov/recalls-alerts/morasch-meats-inc--recalls-raw-frozen-diced-beef-products-due-possible-e--coli (accessed on 23 August 2024).
- CDC Factors Affecting the Efficacy of Disinfection and Sterilization. Available online: https://www.cdc.gov/infection-control/hcp/disinfection-sterilization/efficacy-factors.html (accessed on 8 August 2024).
- Singh, H.; Bhardwaj, S.K.; Khatri, M.; Kim, K.-H.; Bhardwaj, N. UVC Radiation for Food Safety: An Emerging Technology for the Microbial Disinfection of Food Products. Chem. Eng. J. 2021, 417, 128084. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, Y.; Wang, L.; Lu, W.; Li, S.; Xu, D.; Fu, Y.V. Inactivation of Porcine Epidemic Diarrhea Virus with Electron Beam Irradiation under Cold Chain Conditions. Environ. Technol. Innov. 2022, 27, 102715. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dutta, H. Use of Nature-derived Antimicrobial Substances as Safe Disinfectants and Preservatives in Food Processing Industries: A Review. Food Process. Preserv. 2022, 46, e15999. [Google Scholar] [CrossRef]
- Oliinychenko, Y.K.; Ekonomou, S.I.; Tiwari, B.K.; Stratakos, A.C. Assessing the Effects of Cold Atmospheric Plasma on the Natural Microbiota and Quality of Pork during Storage. Foods 2024, 13, 1015. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C. Applications of Cold Plasma Technology for Microbiological Safety in Meat Industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Birania, S.; Attkan, A.K.; Kumar, S.; Kumar, N.; Singh, V.K. Cold Plasma in Food Processing and Preservation: A Review. J. Food Process Eng. 2022, 45, e14110. [Google Scholar] [CrossRef]
- D’Angola, A.; Colonna, G.; Kustova, E. Editorial: Thermal and Non-Thermal Plasmas at Atmospheric Pressure. Front. Phys. 2022, 10, 852905. [Google Scholar] [CrossRef]
- Farooq, S.; Dar, A.H.; Dash, K.K.; Srivastava, S.; Pandey, V.K.; Ayoub, W.S.; Pandiselvam, R.; Manzoor, S.; Kaur, M. Cold Plasma Treatment Advancements in Food Processing and Impact on the Physiochemical Characteristics of Food Products. Food Sci. Biotechnol. 2023, 32, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Laroque, D.A.; Seó, S.T.; Valencia, G.A.; Laurindo, J.B.; Carciofi, B.A.M. Cold Plasma in Food Processing: Design, Mechanisms, and Application. J. Food Eng. 2022, 312, 110748. [Google Scholar] [CrossRef]
- Niveditha, A.; Pandiselvam, R.; Prasath, V.A.; Singh, S.K.; Gul, K.; Kothakota, A. Application of Cold Plasma and Ozone Technology for Decontamination of Escherichia Coli in Foods—A Review. Food Control 2021, 130, 108338. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Moraes, J.; Ferreira, M.V.S.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; et al. Cold Plasma Processing of Milk and Dairy Products. Trends Food Sci. Technol. 2018, 74, 56–68. [Google Scholar] [CrossRef]
- Waghmare, R. Cold Plasma Technology for Fruit Based Beverages: A Review. Trends Food Sci. Technol. 2021, 114, 60–69. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, J.; Liu, C.; Chen, G.; Sang, X.; Zhang, J. Effects of High Voltage Atmospheric Cold Plasma Treatment on the Number of Microorganisms and the Quality of Trachinotus Ovatus during Refrigerator Storage. Foods 2022, 11, 2706. [Google Scholar] [CrossRef]
- Dharini, M.; Jaspin, S.; Mahendran, R. Cold Plasma Bubbling: Impact on Safety, Physicochemical Properties, and Nutritional Quality of Sesame Milk. Food Bioprod. Process. 2023, 139, 109–120. [Google Scholar] [CrossRef]
- Han, J.-Y.; Song, W.-J.; Eom, S.; Kim, S.B.; Kang, D.-H. Antimicrobial Efficacy of Cold Plasma Treatment against Food-Borne Pathogens on Various Foods. J. Phys. D Appl. Phys. 2020, 53, 204003. [Google Scholar] [CrossRef]
- Moutiq, R.; Misra, N.N.; Mendonça, A.; Keener, K. In-Package Decontamination of Chicken Breast Using Cold Plasma Technology: Microbial, Quality and Storage Studies. Meat Sci. 2020, 159, 107942. [Google Scholar] [CrossRef]
- Nasiru, M.M.; Frimpong, E.B.; Muhammad, U.; Qian, J.; Mustapha, A.T.; Yan, W.; Zhuang, H.; Zhang, J. Dielectric Barrier Discharge Cold Atmospheric Plasma: Influence of Processing Parameters on Microbial Inactivation in Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2626–2659. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, J.-H.; Sun, D.-W. Enhancing Microorganism Inactivation Performance through Optimization of Plate-to-Plate Dielectric Barrier Discharge Cold Plasma Reactors. Food Control 2024, 157, 110164. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, J.-H. Assessing the Effect of Cold Plasma on the Softening of Postharvest Blueberries through Reactive Oxygen Species Metabolism Using Transcriptomic Analysis. Foods 2024, 13, 1132. [Google Scholar] [CrossRef]
- Gan, Z.; Feng, X.; Hou, Y.; Sun, A.; Wang, R. Cold Plasma Jet with Dielectric Barrier Configuration: Investigating Its Effect on the Cell Membrane of E. Coli and S. Cerevisiae and Its Impact on the Quality of Chokeberry Juice. LWT 2021, 136, 110223. [Google Scholar] [CrossRef]
- Georgescu, N.; Apostol, L.; Gherendi, F. Inactivation of Salmonella Enterica Serovar Typhimurium on Egg Surface, by Direct and Indirect Treatments with Cold Atmospheric Plasma. Food Control 2017, 76, 52–61. [Google Scholar] [CrossRef]
- Liu, M.; Li, P.; Xu, H.; Tian, C.; Zhang, H.; Kong, F.; Zou, H. Experimental Investigation of Integrated Systems of Precooling and Plasma-activated Water-based Bacterial Inactivation. J. Food Process. Eng. 2024, 47, e14547. [Google Scholar] [CrossRef]
- Ferre-Aracil, J.; Cardona, S.C.; Navarro-Laboulais, J. Determination and Validation of Henry’s Constant for Ozone in Phosphate Buffers Using Different Analytical Methodologies. Ozone Sci. Eng. 2015, 37, 106–118. [Google Scholar] [CrossRef]
- Tachibana, K.; Nakamura, T. Examination of UV-Absorption Spectroscopy for Analysis of O3, NO2−, and HNO2 Compositions and Kinetics in Plasma-Activated Water. Jpn. J. Appl. Phys. 2020, 59, 056004. [Google Scholar] [CrossRef]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiology: An Introduction, Global Edition, 13th ed.; Pearson: London, UK, 2020. [Google Scholar]
- Chen, M.; Yan, J.; Feng, Y.; Liu, D.; Wang, Z.; Liu, L.; Huang, L.; Guo, L.; Zhang, J.; Liu, C.; et al. Sterilization of Salmonella Typhimurium and Staphylococcus Aureus in Different Cold-Chain Environments by Combining NOx Mode and O3 Mode Air Discharges. Plasma Sources Sci. Technol. 2022, 31, 125006. [Google Scholar] [CrossRef]
- Herianto, S.; Arcega, R.D.; Hou, C.-Y.; Chao, H.-R.; Lee, C.-C.; Lin, C.-M.; Mahmudiono, T.; Chen, H.-L. Chemical Decontamination of Foods Using Non-Thermal Plasma-Activated Water. Sci. Total Environ. 2023, 874, 162235. [Google Scholar] [CrossRef]
- Park, S.; Choe, W.; Jo, C. Interplay among Ozone and Nitrogen Oxides in Air Plasmas: Rapid Change in Plasma Chemistry. Chem. Eng. J. 2018, 352, 1014–1021. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, M.; Huang, L.; Guo, L.; Xu, S.; Zhang, J.; Xi, W.; Wang, Z.; Liu, D.; Kong, M.G.; et al. Using Cold Atmospheric Plasma Treated-Air for COVID-19 Disinfection in Cold-Chain Environment. J. Phys. D Appl. Phys. 2021, 54, 40LT01. [Google Scholar] [CrossRef]

| L* | a* | b* | |
|---|---|---|---|
| Control group | 41.01 ± 1.31 a | 13.72 ± 1.13 b | 7.07 ± 1.13 a |
| Direct CP treatment | 42.68 ± 1.00 ab | 8.81 ± 0.93 a | 8.41 ± 0.97 ab |
| Active Substance | Characteristic Wavelength/nm | Correlation between Absorbance A (Abs) and Concentration C (mmol/L) |
|---|---|---|
| O3 | 260.0 | A = 0.31·C [31] |
| NO3− | 302.1 | A = 0.00686·C + 0.00451 |
| L* | a* | b* | pH | Shear Force (g) | Cooking Loss (%) | |
|---|---|---|---|---|---|---|
| 25 °C control | 43.67 ± 0.36 ab | 16.04 ± 0.73 bc | 9.17 ± 0.42 ab | 6 ± 0 a | 4115 ± 179 b | 41.7 ± 0.3 a |
| 25 °C CP | 44.86 ± 0.93 b | 9.90 ± 0.57 a | 9.38 ± 0.58 b | 6 ± 0 a | 4128 ± 189 b | 42.0 ± 0.2 a |
| 4 °C control | 50.46 ± 1.61 c | 21.43 ± 0.89 e | 15.37 ± 0.45 d | 6 ± 0 a | 3151 ± 219 a | 39.2 ± 2.0 a |
| 4 °C CP | 48.36 ± 0.93 c | 17.97 ± 0.82 cd | 12.65 ± 0.45 c | 6 ± 0 a | 3100 ± 355 a | 35.8 ± 4.7 a |
| −18 °C control | 44.08 ± 1.00 ab | 19.66 ± 0.70 de | 12.61 ± 0.44 c | 6 ± 0 a | 3994 ± 127 b | 49.4 ± 0.3 b |
| −18 °C CP | 43.18 ± 1.01 ab | 18.33 ± 1.15 cd | 11.48 ± 0.83 c | 6 ± 0 a | 4486 ± 225 b | 48.5 ± 0.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhang, H.; Tian, C.; Zou, H. Experimental Investigation of Bacterial Inactivation of Beef Using Indirect Cold Plasma in Cold Chain and at Room Temperature. Foods 2024, 13, 2846. https://doi.org/10.3390/foods13172846
Li P, Zhang H, Tian C, Zou H. Experimental Investigation of Bacterial Inactivation of Beef Using Indirect Cold Plasma in Cold Chain and at Room Temperature. Foods. 2024; 13(17):2846. https://doi.org/10.3390/foods13172846
Chicago/Turabian StyleLi, Peiru, Hainan Zhang, Changqing Tian, and Huiming Zou. 2024. "Experimental Investigation of Bacterial Inactivation of Beef Using Indirect Cold Plasma in Cold Chain and at Room Temperature" Foods 13, no. 17: 2846. https://doi.org/10.3390/foods13172846





