Comparative Evaluation of Chemical Composition and Nutritional Characteristics in Various Quinoa Sprout Varieties: The Superiority of 24-Hour Germination
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. The Growth Index Measurement
2.3. The Soluble Sugar, Soluble Protein, and Free Amino Acid Content Measurement
2.4. The Amino Acid and GABA Content Measurement
2.5. The Vitamin Content Measurement
2.6. The Total Phenol Content (TPC) and Total Flavonoid Content (TFC) Measurement
2.7. The Saponin Content Measurement
2.8. Antioxidant Activities Measurement
2.9. Statistical Analysis
3. Results
3.1. Morphological Indicators of Quinoa Sprouts
3.2. Soluble Protein, Soluble Sugar and Free Amino Acid
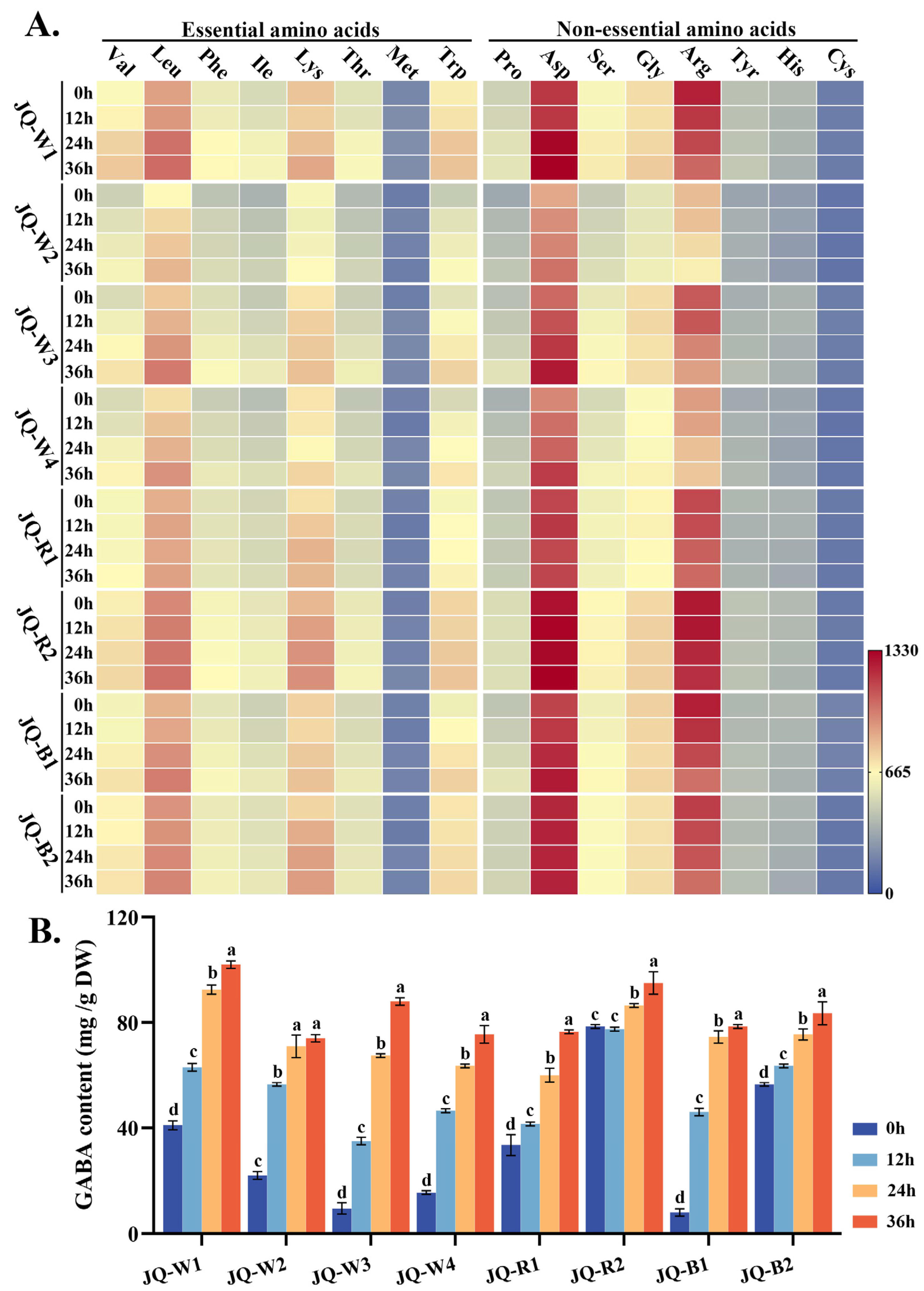
3.3. Hydrolyzed Amino Acid and Gamma-Aminobutyric Acid (GABA)
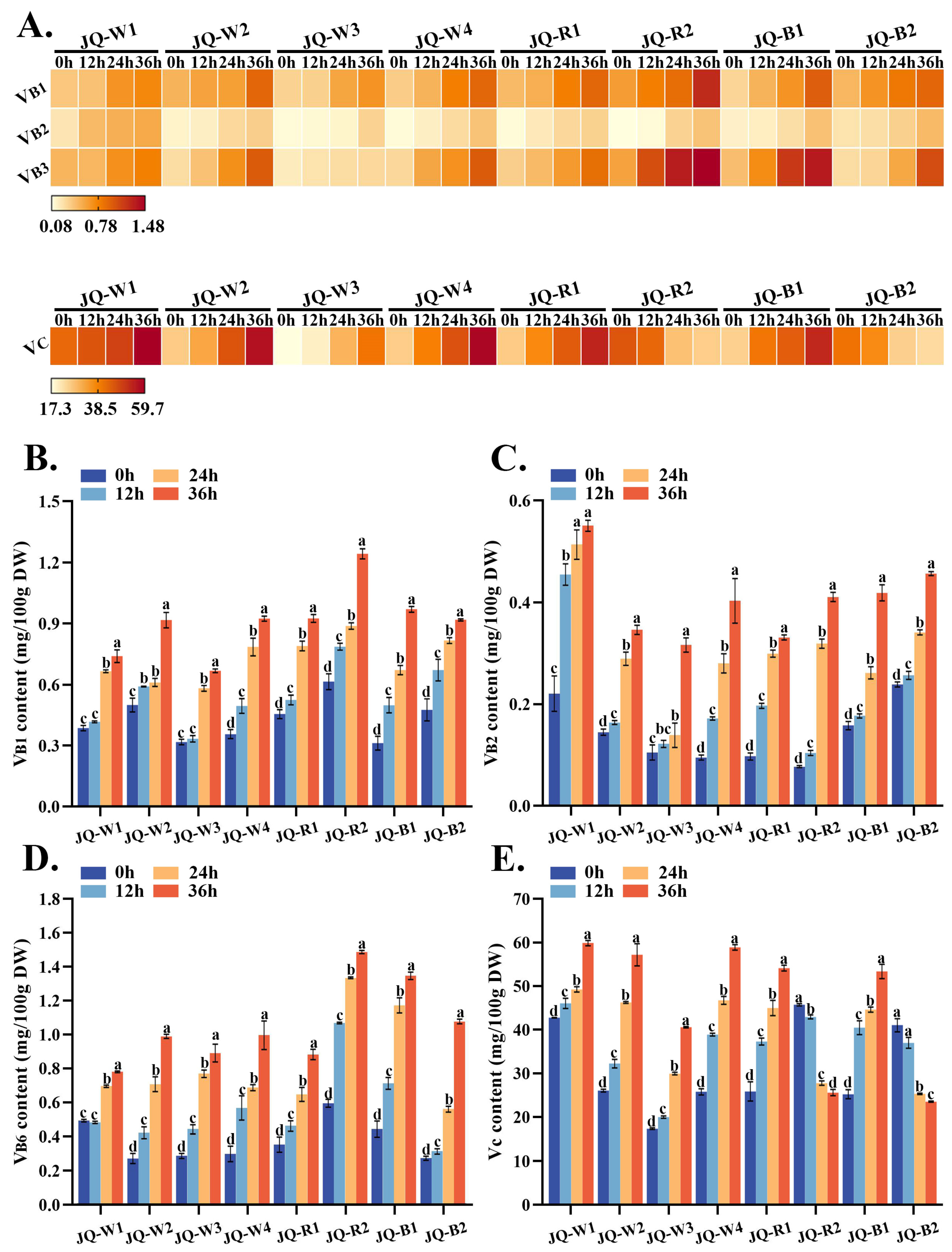
3.4. Vitamin Analysis
3.5. Total Phenol (TPC) and Total Flavonids (TFC)
3.6. Saponins Analysis
3.7. Antioxidant Activities
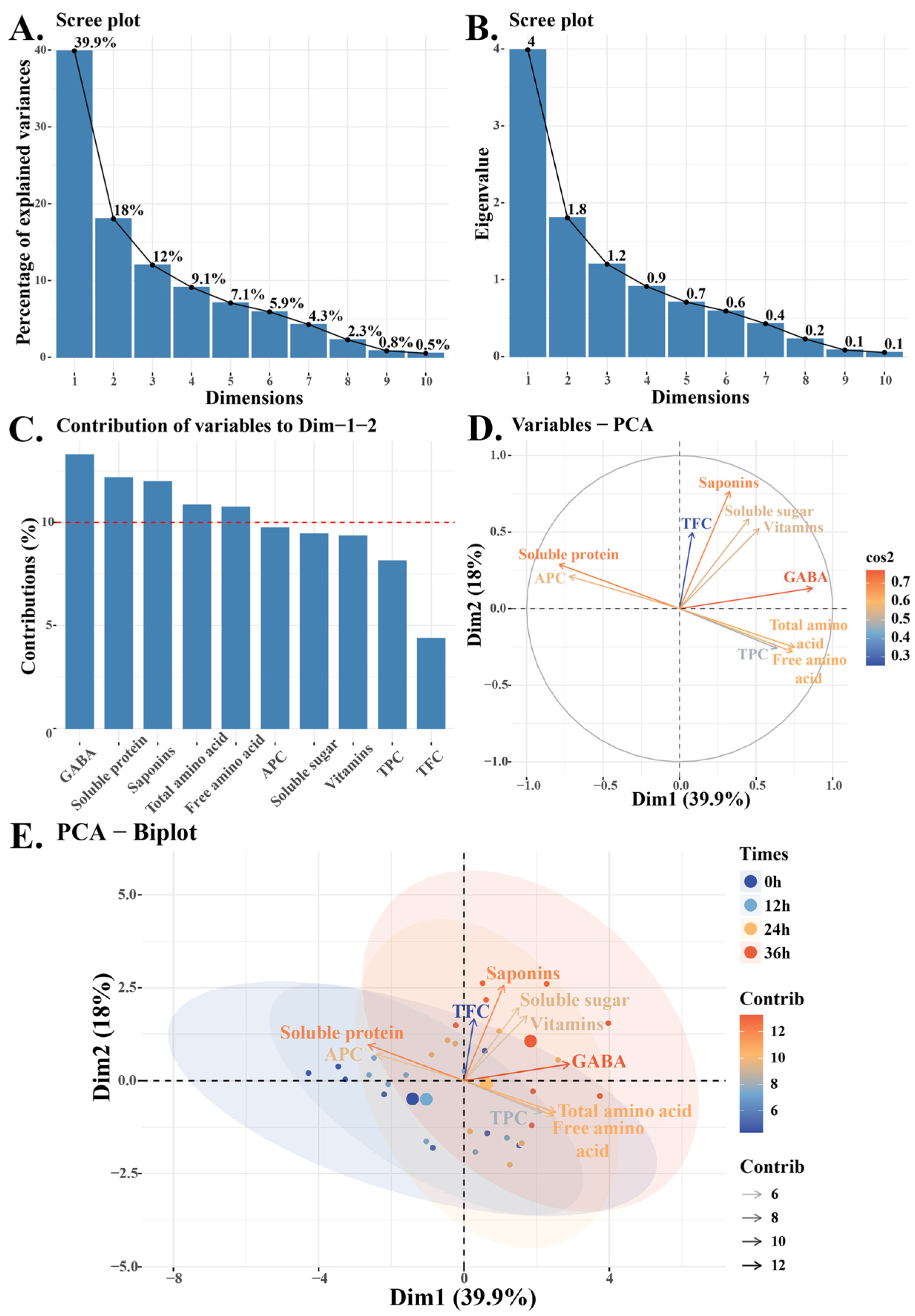
3.8. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhinder, S.; Kumari, S.; Singh, B.; Kaur, A.; Singh, N. Impact of germination on phenolic composition, antioxidant properties, antinutritional factors, mineral content and Maillard reaction products of malted quinoa flour. Food Chem. 2021, 346, 128915. [Google Scholar] [CrossRef]
- Gordillo-Bastidas, E.; Da, D. Quinoa (Chenopodium quinoa Willd), from nutritional value to potential health benefits: An integrative review. J. Nutr. Food Sci. 2016, 6, 3. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and other phenolic compounds in andean indigenous grains: Quinoa (Chenopodium quinoa), kaniwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Iafelice, G.; Lavini, A.; Pulvento, C.; Caboni, M.F.; Marconi, E. Phenolic compounds and saponins in quinoa samples (Chenopodium quinoa willd.) Grown under different saline and nonsaline irrigation regimens. J. Agric. Food. Chem. 2012, 60, 4620–4627. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Dimitrov, K.; Galván, D.; Alessandro, L. Effect of malting conditions on phenolic content, Maillard reaction products formation, and antioxidant activity of quinoa seeds. J. Food Sci. Technol. 2016, 53, 3978. [Google Scholar] [CrossRef]
- Pasko, P.; Barton, H.; Zagrodzki, P.; Gorinstein, S.; Folta, M.; Zachwieja, Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Pitzschke, A.; Fraundorfer, A.; Guggemos, M.; Fuchs, N. Antioxidative responses during germination in quinoa grown in vitamin B-rich medium. Food Sci. Nutr. 2015, 3, 242–251. [Google Scholar] [CrossRef]
- Landi, N.; Roucco, M.R.; Ragucci, S.; Aliotta, F.; Maro, A.D. Quinoa as source of type 1 ribosome inactivating proteins: A novel knowledge for a revision of its consumption. Food Chem. 2020, 342, 128337. [Google Scholar] [CrossRef]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmckel, S.M.; Li, B.; Borm, T.J.A.; Ohyanagi, H.; Mineta, K.; Michell, C.T.; Saber, N. Corrigendum: The genome of Chenopodium quinoa. Nature 2017, 545, 510. [Google Scholar] [CrossRef]
- Zou, C.; Chen, A.; Xiao, L.; Muller, H.M.; Ache, P.; Haberer, G.; Zhang, M.; Jia, W.; Deng, P.; Huang, R. A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value. Cell Res. 2017, 27, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hou, R.; Yang, N.; Li, L.; Deng, J.; Qin, G.; Ding, D. Physiological and tmt-labeled proteomic analyses reveal important roles of sugar and secondary metabolism in Citrus junos under cold stress. J. Proteom. 2021, 237, 104145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wei, X.; Liu, Z.; Wu, X.; Bao, C.; Sun, Y.; Su, N.; Cui, J. Transcriptome analysis reveals the molecular mechanism of gaba accumulation during quinoa (Chenopodium quinoa willd.) Germination. J. Agric. Food. Chem. 2021, 69, 12171–12186. [Google Scholar] [CrossRef] [PubMed]
- Reguera, M.; Conesa, C.M.; Gil-Gómez, A.; Haros, C.M.; Pérez-Casas, M.Á.; Briones-Labarca, V. The impact of different agroecological conditions on the nutritional composition of quinoa seeds. PeerJ 2018, 6, e4442. [Google Scholar] [CrossRef] [PubMed]
- Abderrahim, F.; Estrella, S.; Susín, C.; Arribas, S.M.; González, M.C.; Condezo-Hoyos, L. The antioxidant activity and thermal stability of lemon verbena (Aloysia triphylla) infusion. J. Med. Food 2011, 14, 517. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food. Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, D.E.; Oakenfull, D. Saponin content of food plants and some prepared foods. J. Sci. Food. Agric. 1983, 34, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kataria, A.; Singh, B. Effect of thermal processing on the bioactive compounds, antioxidative, antinutritional and functional characteristics of quinoa (Chenopodium quinoa). Lwt 2022, 160, 113256. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Alves, R.E.; Brito, E.S.D.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from brazil. Food Chem. 2011, 121, 996–1002. [Google Scholar] [CrossRef]
- Barakat, H.; Al-Qabba, M.M.; Algonaiman, R.; Radhi, K.S.; Almutairi, A.S.; Al Zhrani, M.M.; Mohamed, A. Impact of sprouting process on the protein quality of yellow and red quinoa (Chenopodium quinoa). Molecules 2024, 29, 404. [Google Scholar] [CrossRef]
- Le, L.; Gong, X.; An, Q.; Xiang, D.; Wan, Y. Quinoa sprouts as potential vegetable source: Nutrient composition and functional contents of different quinoa sprout varieties. Food Chem. 2021, 357, 129752. [Google Scholar] [CrossRef]
- Junior, A.Q.; Ida, E.I. Profile of the contents of different forms of soybean isoflavones and the effect of germination time on these compounds and the physical parameters in soybean sprouts. Food Chem. 2015, 166, 173–178. [Google Scholar] [CrossRef]
- Sacchi, G.A.; Zocchi, G.; Cocucci, S. Changes in form of elongation factor 1 during germination of wheat seeds. Febs J. 1984, 139, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nidhi; Prasad, N.; Sinha, S.K. Nutritional and antinutritional attributes of faba bean (Vicia faba L.) Germplasms growing in Bihar, India. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2015, 21, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Sangsukiam, T.; Duangmal, K. A comparative study of physico-chemical properties and antioxidant activity of freeze-dried mung bean (Vigna radiata) and adzuki bean (Vigna angularis) sprout hydrolysate powders. Int. J. Food Sci. Technol. 2017, 52, 1971–1982. [Google Scholar] [CrossRef]
- Kumar, G.N.M.; Knowles, N.R. Age of potato seed-tubers influences protein synthesis during sprouting. Physiol. Plant. 1993, 89, 262–270. [Google Scholar] [CrossRef]
- Fouad, A.A.; Rehab, F.M.A. Effect of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris medik.) Sprouts. Acta Sci. Pol. Technol. Aliment. 2015, 14, 233. [Google Scholar] [CrossRef]
- Angeli, V.; Miguel Silva, P.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa willd.): An overview of the potentials of the “golden grain” and socio-economic and environmental aspects of its cultivation and marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Kuppusamy, S.; Cho, K.M.; Kim, P.J.; Kwack, Y.B.; Lee, Y.B. Influence of cold stress on contents of soluble sugars, vitamin c and free amino acids including gamma-aminobutyric acid (gaba) in spinach (Spinacia oleracea). Food Chem. 2017, 132, 1959–1965. [Google Scholar] [CrossRef]
- Hussain, M.I.; Muscolo, A.; Ahmed, M.; Asghar, M.A.; Al-Dakheel, A.J. Agro-morphological, yield and quality traits and interrelationship with yield stability in quinoa (Chenopodium quinoa willd.) Genotypes under saline marginal environment. Plants 2020, 9, 1763. [Google Scholar] [CrossRef]
- Gahlowt, P.; Tripathi, D.K.; Singh, S.P.; Gupta, R.; Singh, V.P. Gaba in plants: Developmental and stress resilience perspective. Physiol. Plant. 2024, 176, e14116. [Google Scholar] [CrossRef]
- Jiao, C.; Duan, Y.; Lin, Q. MAPK mediates NO/cGMP-induced GABA accumulation in soybean sprouts. Lwt 2019, 100, 253–262. [Google Scholar] [CrossRef]
- Frank, L.; Anke, H.; Hillel, F.; Linda, B.; Nicolas, B.; Markus, G. Mutants of GABA transaminase (POP2) suppress the severe phenotype of succinic semialdehyde dehydrogenase (ssadh) mutants in arabidopsis. PLoS ONE 2008, 3, e3383. [Google Scholar] [CrossRef]
- Yuan, D.; Wu, X.; Gong, B.; Huo, R.; Zhao, L.; Li, J.; Lü, G.; Gao, H. Gaba metabolism, transport and their roles and mechanisms in the regulation of abiotic stress (hypoxia, salt, drought) resistance in plants. Metabolites 2023, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Avezum, L.; Madode, Y.E.; Mestres, C.; Achir, N.; Delpech, C.; Chapron, M.; Gibert, O.; Rajjou, L.; Rondet, E. New insights into the rapid germination process of lentil and cowpea seeds: High thiamine and folate, and low α-galactoside content. Food Chem. 2024, 439, 138027. [Google Scholar] [CrossRef]
- Coello, K.E.; Frias, J.; Martínez-Villaluenga, C.; Cartea, M.E.; Abilleira, R.; Peas, E. Potential of germination in selected conditions to improve the nutritional and bioactive properties of moringa (Moringa oleifera L.). Foods 2020, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Vega-Galvez, A.; Quispe-Fuentes, I.; Rodriguez, M.J.; Martinez, E.A. Nutritional aspects of six quinoa (Chenopodium quinoa willd.) Ecotypes from three geographical areas of chile. Chil. J. Agric. Res. 2012, 72, 175–181. [Google Scholar] [CrossRef]
- Jiadkong, K.; Fauzia, A.N.; Yamaguchi, N.; Ueda, A. Exogenous riboflavin (vitamin B2) application enhances salinity tolerance through the activation of its biosynthesis in rice seedlings under salinity stress. Plant Sci. 2024, 339, 111929. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-García, N.; Martínez-Villaluenga, C.; Frias, J.; Peas, E. Sprouted oat as a potential gluten-free ingredient with enhanced nutritional and bioactive properties. Food Chem. 2020, 338, 127972. [Google Scholar] [CrossRef]
- Mizutani, Y.M.H. Pyridoxine (vb6) restores the down-regulation of serine palmitoyltransferase mrna expression in keratinocytes cultured in highly oxidative conditions through enhancement of the intracellular antioxidant system. Exp. Dermatol. 2019, 28, 43–49. [Google Scholar] [CrossRef]
- Francis, H.; Debs, E.; Koubaa, M.; Alrayess, Z.; Maroun, R.G.; Louka, N. Sprouts use as functional foods. Optimization of germination of wheat (Triticum aestivum L.), Alfalfa (Medicago sativa L.), And radish (Raphanus sativus L.) Seeds based on their nutritional content evolution. Foods 2022, 11, 1460. [Google Scholar] [CrossRef]
- Laus, M.N.; Cataldi, M.P.; Robbe, C.; D’Ambrosio, T.; Amodio, M.L.; Colelli, G.; De Santis, G.; Flagella, Z.; Pastore, D. Antioxidant capacity, phenolic and vitamin c contents of quinoa (Chenopodium quinoa willd.) as affected by sprouting and storage conditions. Ital. J. Agron. 2017, 12, 63–68. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Petrucci, N.S.; Lopez, M.L. Interpretation of possible processing modalities in charred remains of the genus chenopodium recovered from the site of Soria 2, Catamarca, Argentina. Lat. Am. Antiq. 2020, 31, 733–746. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa willd. Genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Chen, X.; Zhou, W.; Guo, Y.; Yuan, X.; Cui, J.; Shen, Z.; Su, N. Multi-omics analysis provides insights into lysine accumulation in quinoa (Chenopodium quinoa Willd.) Sprouts. Food Res. Int. 2023, 171, 113026. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Walia, S.; Kumar, R. Functional composition, physiological effect and agronomy of future food quinoa (Chenopodium quinoa willd.): A review. J. Food Compos. Anal. 2023, 118, 105192. [Google Scholar] [CrossRef]
- Lazo-Vélez, M.A.; Guajardo-Flores, D.; Mata-Ramírez, D.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. Characterization and quantitation of triterpenoid saponins in raw and sprouted Chenopodium berlandieri spp. (Huauzontle) grains subjected to germination with or without selenium stress conditions. J. Food Sci. 2016, 81, C19–C26. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Cao, B.; Wei, X.; Shen, Z.; Su, N. Assessment and comparison of nutritional qualities of thirty quinoa (Chenopodium quinoa Willd.) Seed varieties. Food Chem. X 2023, 19, 100808. [Google Scholar] [CrossRef]
- Pako, P.; Sajewicz, M.; Gorinstein, S.; Zachwieja, A.Z. Analysis of selected phenolic acids and flavonoids in amaranthus cruentus and Chenopodium quinoa seeds and sprouts by hplc. Acta Chromatogr. 2008, 20420411, 661–672. [Google Scholar] [CrossRef]


| Number | Seed Coat Color | Origin | Common Name |
|---|---|---|---|
| JQ-W1 | white | Bolivia | DB-2 |
| JQ-W2 | white | Bolivia | WXY-9-4 |
| JQ-W3 | white | Chile | Faro |
| JQ-W4 | white | USA | Brightest Brilliant |
| JQ-R1 | red | Bolivia | WXY-1-Y2 |
| JQ-R2 | red | Bolivia | WXY-12-1 |
| JQ-B1 | black | Argentina | JQ-B-2 |
| JQ-B2 | black | Bolivia | WXY-13-4 |
| Time | Sample | DPPH (%) | FRAP (mmol Fe2+ Equivalents/100 g) | ABTS (mM TEAC/g DW) | APC | Rank |
|---|---|---|---|---|---|---|
| 0 h | JQ-W1 | 6.95 ± 1.27 c | 2.10 ± 0.22 | 7012.34 ± 239.45 f | 73.19 | 7 |
| JQ-W2 | 8.99 ± 0.45 b | 2.33 ± 0.21 | 8912.79 ± 79.35 c | 88.81 | 2 | |
| JQ-W3 | 6.39 ± 0.72 c | 2.02 ± 0.14 | 9832.53 ± 191.55 b | 79.47 | 4 | |
| JQ-W4 | 5.62 ± 0.55 c | 2.28 ± 0.13 | 10,266.53 ± 87.24 a | 82.31 | 3 | |
| JQ-R1 | 6.51 ± 0.98 c | 2.20 ± 0.26 | 8097.56 ± 92.43 d | 76.74 | 5 | |
| JQ-R2 | 6.39 ± 1.98 c | 2.15 ± 0.37 | 8135.69 ± 63.76 d | 75.95 | 6 | |
| JQ-B1 | 11.09 ± 0.33 a | 2.37 ± 0.37 | 7567.90 ± 106.35 e | 91.24 | 1 | |
| JQ-B2 | 5.62 ± 0.55 c | 2.22 ± 0.17 | 7478.09 ± 21.64 e | 72.48 | 8 | |
| 12 h | JQ-W1 | 7.39 ± 0.72 b | 2.47 ± 0.12 | 7095.52 ± 56.43 g | 76.52 | 7 |
| JQ-W2 | 9.52 ± 0.26 a | 2.43 ± 0.11 | 9435.75 ± 75.36 c ** | 89.91 | 2 | |
| JQ-W3 | 7.96 ± 0.91 b | 2.51 ± 0.16 * | 9962.70 ± 74.97 b | 87.77 | 4 | |
| JQ-W4 | 6.93 ± 1.06 b | 2.62 ± 0.16 * | 10,747.80 ± 59.34 a ** | 88.27 | 3 | |
| JQ-R1 | 8.14 ± 0.82 b | 2.52 ± 0.36 | 8126.70 ± 173.85 d | 82.76 | 5 | |
| JQ-R2 | 6.97 ± 0.46 b | 2.54 ± 0.21 | 8263.46 ± 38.64 d * | 79.73 | 6 | |
| JQ-B1 | 10.7 ± 0.57 a | 2.59 ± 0.22 | 7845.64 ± 83.47 e * | 90.62 | 1 | |
| JQ-B2 | 6.93 ± 1.06 b | 2.41 ± 0.25 | 7643.64 ± 95.37 f * | 75.97 | 8 | |
| 24 h | JQ-W1 | 7.39 ± 0.65 c | 2.89 ± 0.16 ** | 7265.76 ± 209.24 f | 70.98 | 7 |
| JQ-W2 | 10.00 ± 0.43 b * | 2.93 ± 0.18 * | 9523.64 ± 46.67 c *** | 83.94 | 2 | |
| JQ-W3 | 8.82 ± 0.83 b * | 2.68 ± 0.27 * | 10,678.58 ± 49.35 b ** | 81.55 | 4 | |
| JQ-W4 | 7.15 ± 0.12 c ** | 2.83 ± 0.27 * | 11,909.36 ± 135.45 a *** | 82.71 | 3 | |
| JQ-R1 | 9.66 ± 1.16 b * | 2.64 ± 0.21 | 8167.68 ± 306.23 e | 76.08 | 6 | |
| JQ-R2 | 10.14 ± 1.49 b | 2.70 ± 0.11 | 8533.63 ± 30.34 d ** | 78.94 | 5 | |
| JQ-B1 | 13.91 ± 0.6 a ** | 2.76 ± 0.14 | 8054.74 ± 57.73 e ** | 87.39 | 1 | |
| JQ-B2 | 7.15 ± 0.12 c ** | 2.87 ± 0.37 * | 7535.26 ± 286.45 f | 70.93 | 8 | |
| 36 h | JQ-W1 | 8.29 ± 0.47 d | 2.99 ± 0.26 b * | 7534.76 ± 57.99 f * | 60.83 | 8 |
| JQ-W2 | 13.47 ± 1.15 c ** | 3.02 ± 0.27 b * | 9529.47 ± 27.35 c *** | 75.87 | 6 | |
| JQ-W3 | 16.89 ± 0.38 b *** | 3.22 ± 0.38 b ** | 11,098.87 ± 290.46 b ** | 87.94 | 1 | |
| JQ-W4 | 12.72 ± 0.09 c *** | 3.33 ± 0.40 b * | 11,989.46 ± 104.46 a *** | 83.94 | 2 | |
| JQ-R1 | 12.72 ± 0.15 c *** | 3.59 ± 0.13 ab ** | 8556.48 ± 83.46 d ** | 76.67 | 5 | |
| JQ-R2 | 13.95 ± 2.07 c ** | 3.13 ± 0.19 b * | 8593.25 ± 57.46 d ** | 75.00 | 7 | |
| JQ-B1 | 18.77 ± 1.62 a ** | 3.06 ± 0.17 b * | 8154.64 ± 140.34 e ** | 81.75 | 3 | |
| JQ-B2 | 12.72 ± 0.09 c *** | 3.95 ± 0.41 a ** | 7964.24 ± 46.80 e *** | 78.06 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, B.; Bao, C.; Zhu, Z.; Gong, Y.; Wei, J.; Shen, Z.; Su, N. Comparative Evaluation of Chemical Composition and Nutritional Characteristics in Various Quinoa Sprout Varieties: The Superiority of 24-Hour Germination. Foods 2024, 13, 2513. https://doi.org/10.3390/foods13162513
Cao B, Bao C, Zhu Z, Gong Y, Wei J, Shen Z, Su N. Comparative Evaluation of Chemical Composition and Nutritional Characteristics in Various Quinoa Sprout Varieties: The Superiority of 24-Hour Germination. Foods. 2024; 13(16):2513. https://doi.org/10.3390/foods13162513
Chicago/Turabian StyleCao, Beier, Changjian Bao, Zhiqiang Zhu, Yanning Gong, Junyu Wei, Zhenguo Shen, and Nana Su. 2024. "Comparative Evaluation of Chemical Composition and Nutritional Characteristics in Various Quinoa Sprout Varieties: The Superiority of 24-Hour Germination" Foods 13, no. 16: 2513. https://doi.org/10.3390/foods13162513
APA StyleCao, B., Bao, C., Zhu, Z., Gong, Y., Wei, J., Shen, Z., & Su, N. (2024). Comparative Evaluation of Chemical Composition and Nutritional Characteristics in Various Quinoa Sprout Varieties: The Superiority of 24-Hour Germination. Foods, 13(16), 2513. https://doi.org/10.3390/foods13162513





