Changes in Lipid Metabolites and Enzyme Activities of Wheat Flour during Maturation
Abstract
1. Introduction
2. Materials and Methods
2.1. Wheat Preparation and Maturation
2.2. Measurement of Free Fatty Acids (FFA)
2.3. Conjugated Triene Value (K268 nm)
2.4. p-Anisidine Value (P-AV)
2.5. Total Oxidation Value (TOTOX)
2.6. Lipase Activity Assay
2.7. Lipoxygenase Activity Assay (LOX)
2.8. Catalase Activity Measurement (CAT)
2.9. Statistical Analysis
3. Results and Discussion
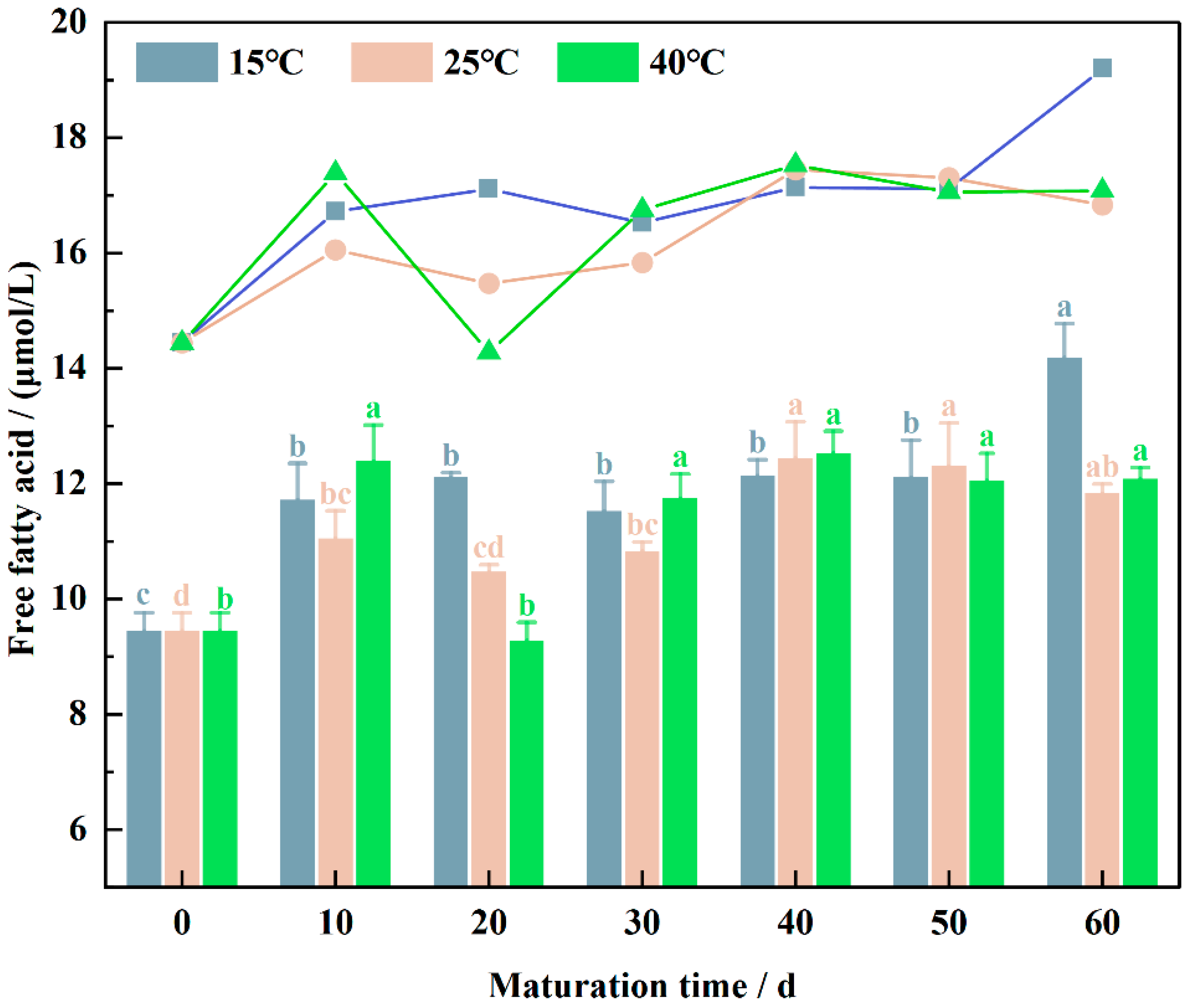
3.1. Free Fatty Acid Variation during Wheat Flour Maturation
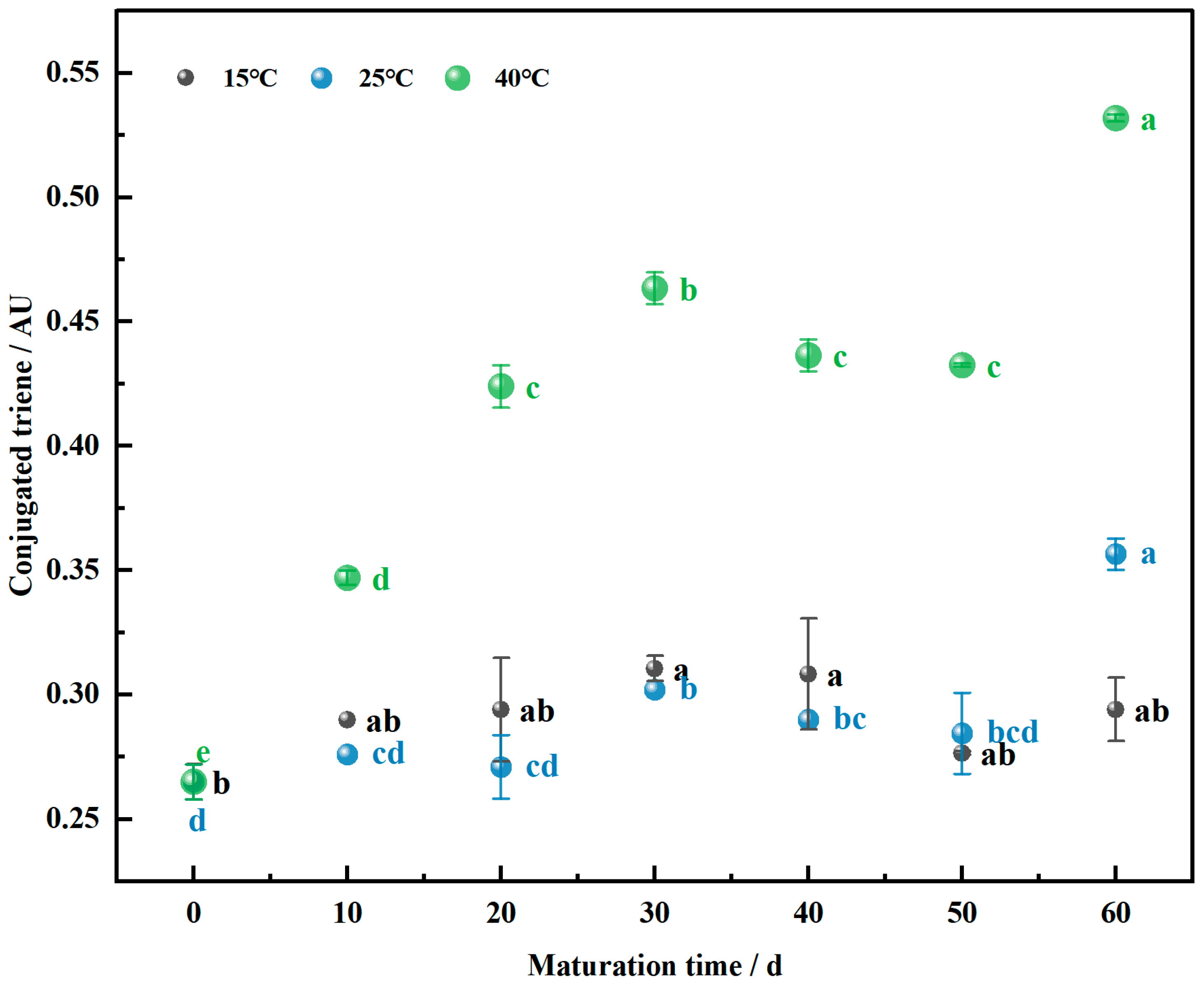
3.2. Conjugated Triene Levels in the Maturation of Wheat Flour
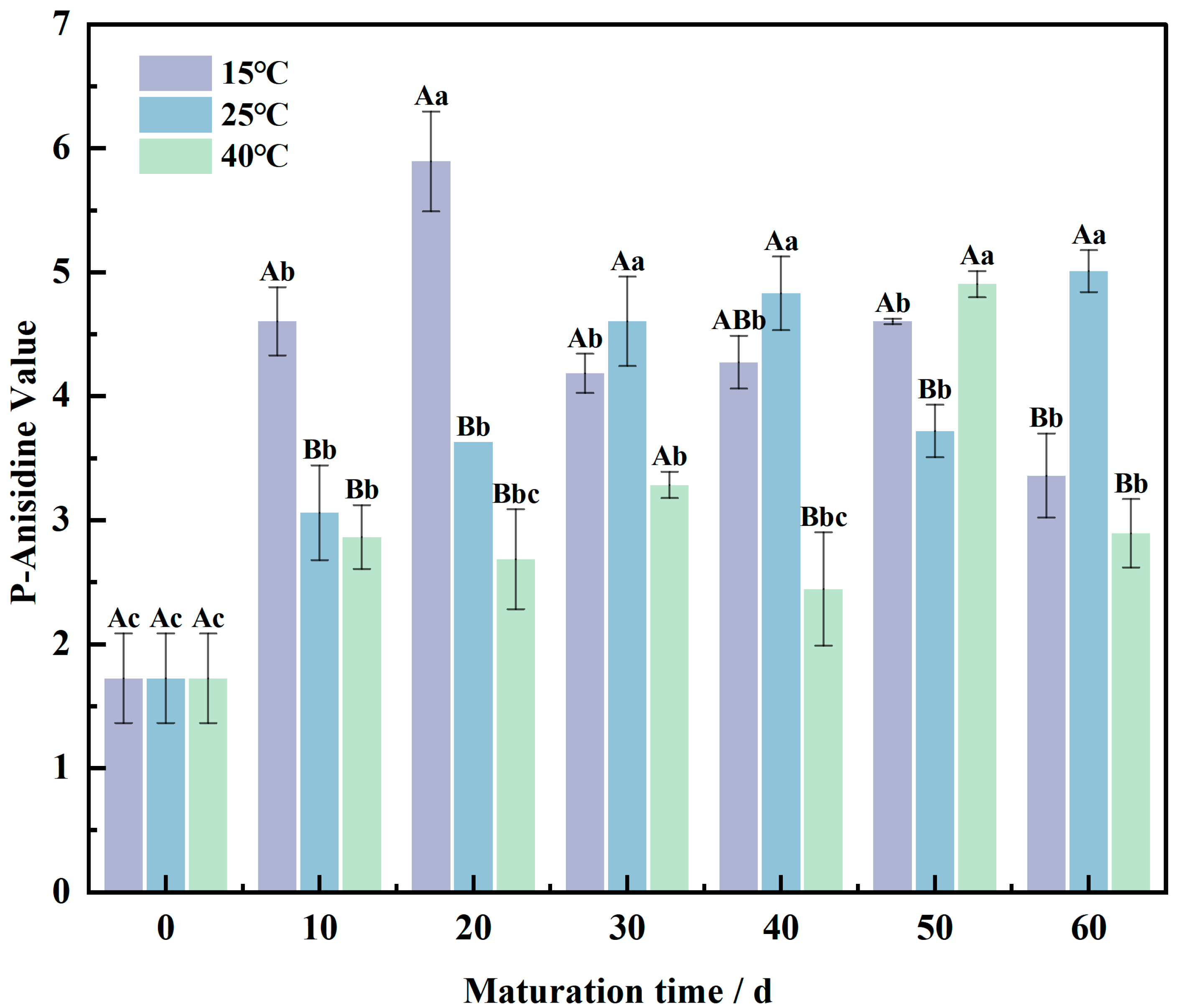
3.3. p-Anisidine Values Shift in the Wheat Flour Maturation Process
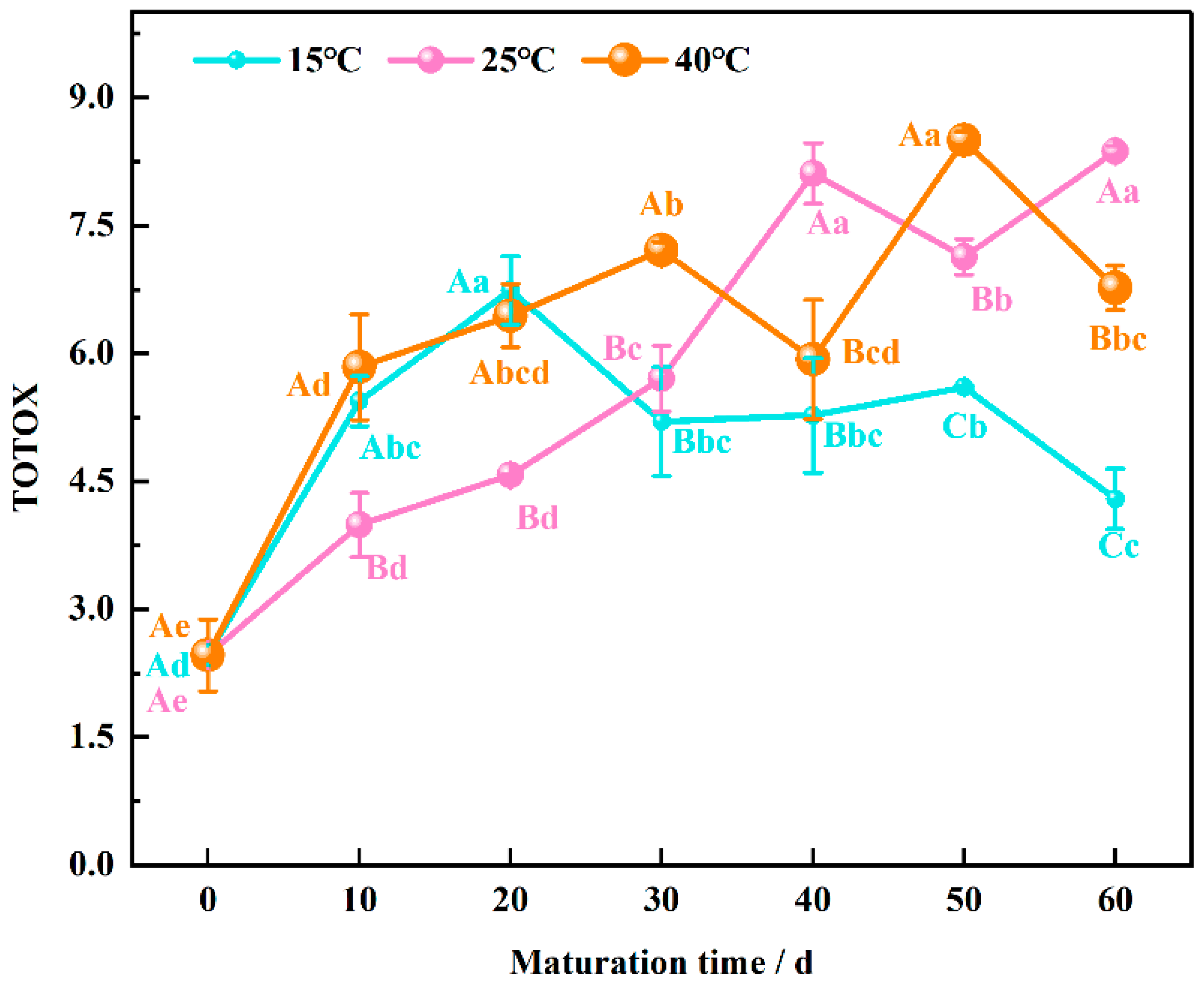
3.4. Total Oxidizing Value Evolution during Wheat Flour Maturation
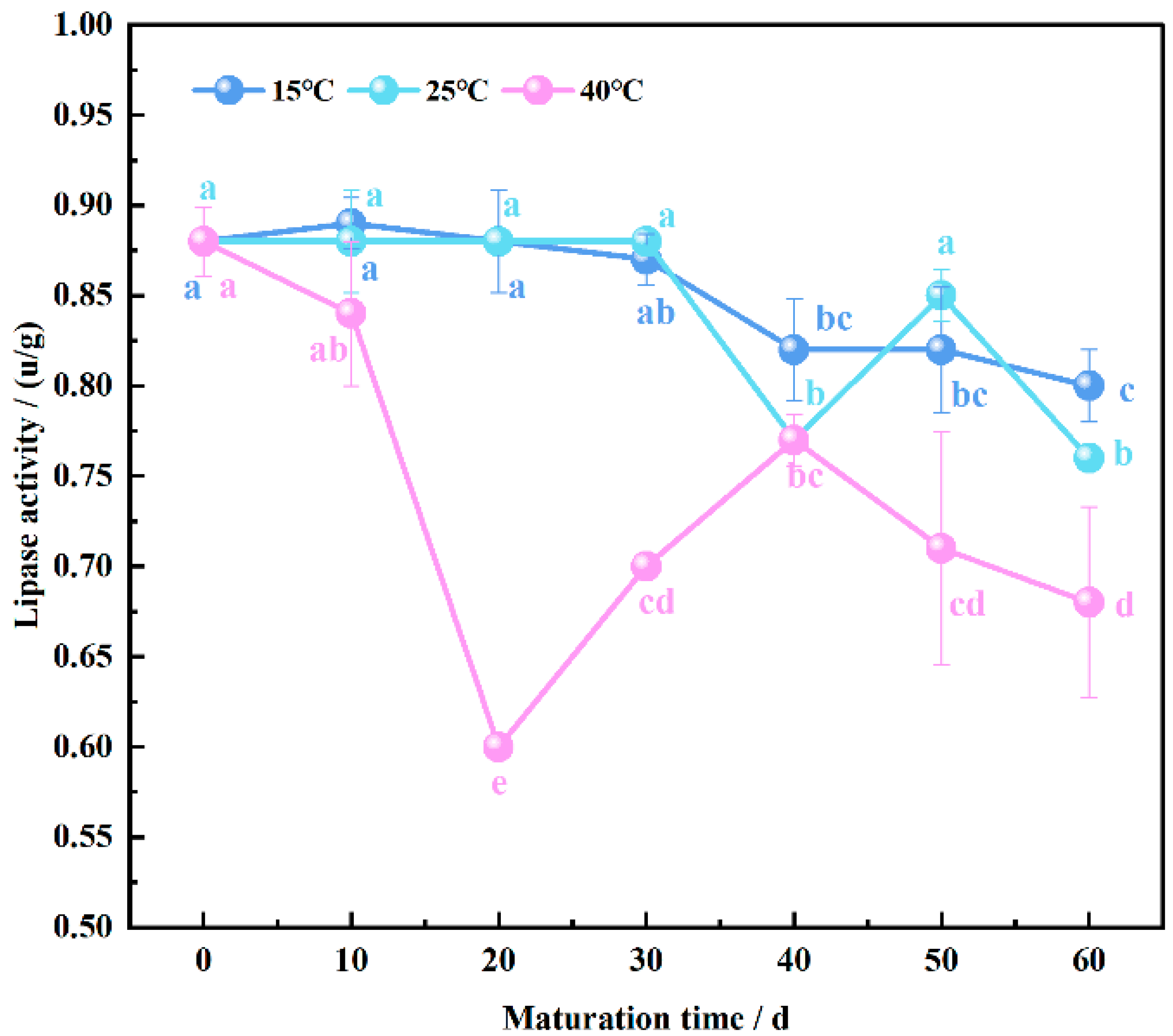
3.5. Endogenous Lipase Activity Patterns in Wheat Flour Maturation
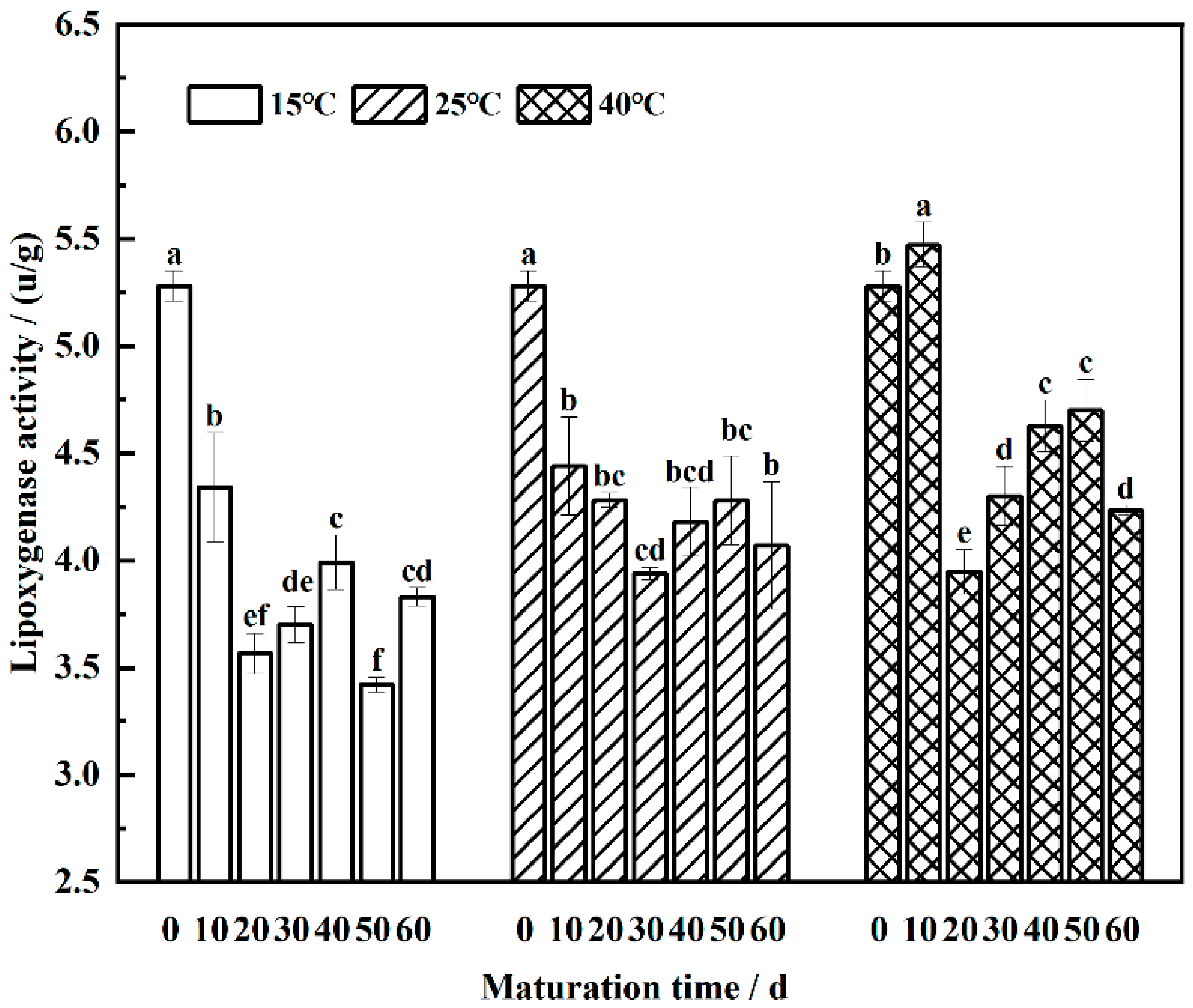
3.6. Lipoxygenase Activity Dynamics in the Maturation of Wheat Flour
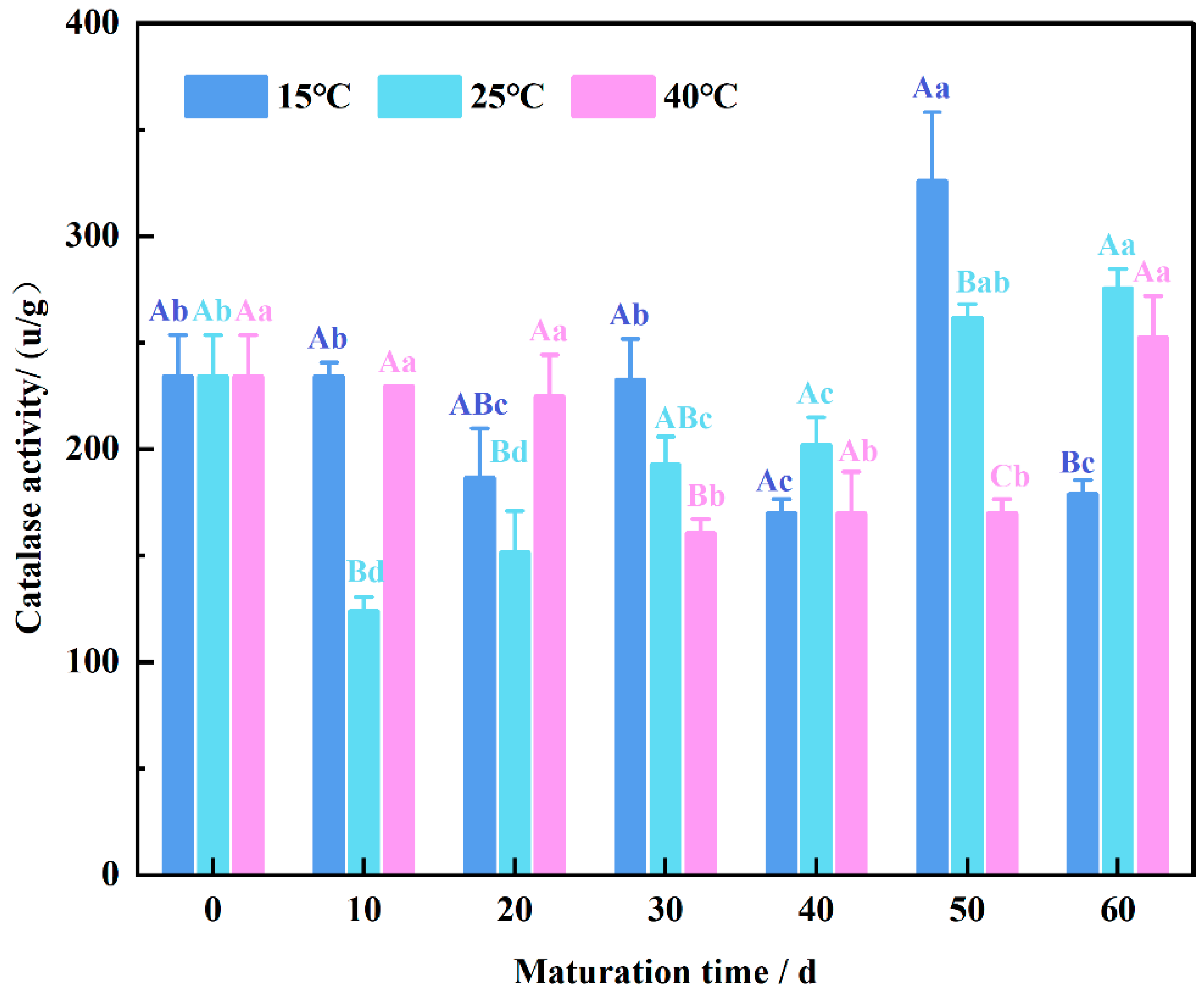
3.7. Changing Pattern of Endogenous Catalase Activity in Wheat Flour
3.8. Correlation Analysis of Quality Indicators
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.-J. Global trends in wheat production, consumption and trade. In Wheat Improvement: Food Security in a Changing Climate; Springer International Publishing: Cham, Switzerland, 2022; pp. 47–66. [Google Scholar]
- Lagrain, B.; Wilderjans, E.; Glorieux, C.; Delcour, J.A. Importance of Gluten and Starch for Structural and Textural Properties of Crumb from Fresh and Stored Bread. Food Biophys. 2012, 7, 173–181. [Google Scholar] [CrossRef]
- Wang, L.; Flores, R.A. The effects of storage on flour quality and baking performance. Food Rev. Int. 1999, 15, 215–234. [Google Scholar] [CrossRef]
- Mense, A.L.; Faubion, J.M. Effects of Aging New Crop Wheat and Flour on Breadmaking Quality and Lipid Composition. Cereal Foods World 2017, 62, 4–10. [Google Scholar] [CrossRef]
- Lancelot, E.; Fontaine, J.; Grua-Priol, J.; Le-Bail, A. Effect of long-term storage conditions on wheat flour and bread baking properties. Food Chem. 2021, 346, 128902. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.H. Effect of Storage Temperature and Periods on Some Characteristics of Wheat Flour Quality. J. Food Nutr. Sci. 2015, 6, 1148–1159. [Google Scholar] [CrossRef]
- Cenkowski, S.S.; Dexter, J.E.; Scanlon, M.G. Mechanical compaction of flour: The effect of storage temperature on dough rheological properties. J. Can. Agric. Eng. 2000, 42, 33–41. [Google Scholar]
- Nakagawa, M.; Tabara, A.; Ushijima, Y.; Matsunaga, K.; Seguchi, M. Hydrophobicity of stored (15, 35 °C), or dry-heated (120 °C) rice flour and deteriorated breadmaking properties baked with these treated rice flour/fresh gluten flour. Biosci. Biotechnol. Biochem. 2016, 80, 983–990. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mense, A.L. Effects of Aging New Crop Wheat and Whole Wheat Flour on Breadmaking Quality and Glycolipid Composition. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2012. [Google Scholar]
- Barden, L.; Decker, E.A. Lipid Oxidation in Low-moisture Food: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2467–2482. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Zhu, D.; Nystrom, L. Improving wholegrain product quality by selecting lipid-stable wheat varieties. Food Chem. 2021, 345, 128683. [Google Scholar] [CrossRef]
- Kanner, J. Dietary advanced lipid oxidation end products are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef]
- Melis, S.; Pauly, A.; Gerits, L.R.; Pareyt, B.; Delcour, J.A. Lipases as Processing Aids in the Separation of Wheat Flour into Gluten and Starch: Impact on the Lipid Population, Gluten Agglomeration, and Yield. J. Agric. Food Chem. 2017, 65, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, M.M.; Ipek, D.; Ghafoor, K.; Al Juhaimi, F.; Uslu, N.; Babiker, E.E.; Ahmed, I.A.M.; Alsawmahi, O.N. Physico-chemical and sensory properties of chips produced using different lupin (Lupinus albus L.) flour formulations and cooking methods. Int. J. Food Sci. Technol. 2021, 56, 2780–2788. [Google Scholar] [CrossRef]
- Cao, J.; Deng, L.; Zhu, X.M.; Fan, Y.; Hu, J.N.; Li, J.; Deng, Z.Y. Novel approach to evaluate the oxidation state of vegetable oils using characteristic oxidation indicators. J. Agric. Food Chem. 2014, 62, 12545–12552. [Google Scholar] [CrossRef]
- Narisawa, T.; Sakai, K.; Nakajima, H.; Umino, M.; Yamashita, H.; Sugiyama, K.; Kiribuchi-Otobe, C.; Shiiba, K.; Yamada, M.; Asakura, T. Effects of fatty acid hydroperoxides produced by lipoxygenase in wheat cultivars during dough preparation on volatile compound formation. Food Chem. 2024, 443, 138566. [Google Scholar] [CrossRef]
- Karakoc, F.B.; Ertas, N.; Aslan, M. Storage stability, nutritional and qualitative attributes of biscuits enriched with terebinth, flaxseed and sesame seeds. Br. Food J. 2024, 126, 3263–3282. [Google Scholar] [CrossRef]
- Devi, A.; Sindhu, R.; Khatkar, B.S. Effect of fats and oils on pasting and textural properties of wheat flour. J. Food Sci. Technol. 2020, 57, 3836–3842. [Google Scholar] [CrossRef] [PubMed]
- Goffman, F.D.; Bergman, C. Hydrolytic Degradation of Triacylglycerols and Changes in Fatty Acid Composition in Rice Bran During Storage. Cereal Chem. 2003, 80, 459–461. [Google Scholar] [CrossRef]
- ISO 3656:2011; Animal and Vegetable Fats and Oils—Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. ISO: Geneva, Switzerland, 2011.
- ISO 6885:2016; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. ISO: Geneva, Switzerland, 2016.
- Jensen, S.; Oestdal, H.; Clausen, M.R.; Andersen, M.L.; Skibsted, L.H. Oxidative stability of whole wheat bread during storage. LWT Food Sci. Technol. 2011, 44, 637–642. [Google Scholar] [CrossRef]
- Chew, S.-C.; Tan, C.-P.; Long, K.; Nyam, K.-L. Effect of chemical refining on the quality of kenaf (Hibiscus cannabinus) seed oil. Ind. Crops Prod. 2016, 89, 59–65. [Google Scholar] [CrossRef]
- Jia, W.T.; Yang, Z.; Guo, X.N.; Zhu, K.X. Effect of Superheated Steam Treatment on the Lipid Stability of Dried Whole Wheat Noodles during Storage. Foods 2021, 10, 1348. [Google Scholar] [CrossRef]
- Liu, X.; Chi, C.; Zhou, S.; Jiang, Y. Comparison of wheat germ and oil characteristics and stability by different stabilization techniques. LWT 2024, 191, 115664. [Google Scholar] [CrossRef]
- Tian, P.-P.; Lv, Y.-Y.; Yu, W.-J.; Zhang, S.-B.; Hu, Y.-S. Effect of artificial aging on wheat quality deterioration during storage. J. Stored Prod. Res. 2019, 80, 50–56. [Google Scholar] [CrossRef]
- Ali, S.; Khan, S.A.; Hamayun, M.; Lee, I.J. The Recent Advances in the Utility of Microbial Lipases: A Review. Microorganisms 2023, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhang, Z.; Emami, S.; Chen, J.; Leite Nobrega de Moura Bell, J.M.; Taha, A.Y. Triacylglycerols are preferentially oxidized over free fatty acids in heated soybean oil. npj Sci. Food 2021, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Koç, A.; Erbaş, M. Investigation of sorption isotherms of wheat germ for its effect on lipid oxidation. J. Food Sci. 2022, 87, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Topkafa, M.; Ayyildiz, H.F.; Kara, H. Impact of carotenoids on the oxidative stability and trans fatty acid content of sunflower oil by using central composite design. J. Iran. Chem. Soc. 2021, 18, 2261–2270. [Google Scholar] [CrossRef]
- Elisia, I.; Young, J.W.; Yuan, Y.V.; Kitts, D.D. Association between tocopherol isoform composition and lipid oxidation in selected multiple edible oils. Food Res. Int. 2013, 52, 508–514. [Google Scholar] [CrossRef]
- Nantanga, K.K.M.; Seetharaman, K.; de Kock, H.L.; Taylor, J.R.N. Thermal treatments to partially pre-cook and improve the shelf-life of whole pearl millet flour. J. Sci. Food Agric. 2008, 88, 1892–1899. [Google Scholar] [CrossRef]
- Cong, S.; Dong, W.; Zhao, J.; Hu, R.; Long, Y.; Chi, X. Characterization of the Lipid Oxidation Process of Robusta Green Coffee Beans and Shelf Life Prediction during Accelerated Storage. Molecules 2020, 25, 1157. [Google Scholar] [CrossRef]
- Bekdeşer, B.; Esin Çelik, S.; Bener, M.; Dondurmacıoğlu, F.; Yıldırım, E.; Nida Yavuz, E.; Apak, R. Determination of primary and secondary oxidation products in vegetable oils with gold nanoparticle based fluorometric turn-on nanosensor: A new total oxidation value. Food Chem. 2024, 434, 137426. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, H.; Lyu, X.; Chen, H.; Wei, F. Lipid oxidation in food science and nutritional health: A comprehensive review. Oil Crop Sci. 2023, 8, 35–44. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Lu, K.; Ma, X.; Tan, Z.; Long, B.; Chen, X.; Li, C.; Zhai, T.; Li, Y.; et al. Reactive aldehyde chemistry explains the missing source of hydroxyl radicals. Nat. Commun. 2024, 15, 1648. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, Y.; Tian, X.; Wang, X.; Liu, H.; Jia, F.; Zhang, X.; Wang, J. Effect of oil oxidation on aggregation of wheat gluten-peanut oil complexes during extrusion. Int. J. Food Sci. Technol. 2022, 57, 2467–2478. [Google Scholar] [CrossRef]
- Gerits, L.R.; Pareyt, B.; Delcour, J.A. A lipase based approach for studying the role of wheat lipids in bread making. Food Chem. 2014, 156, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Kublicki, M.; Koszelewski, D.; Brodzka, A.; Ostaszewski, R. Wheat germ lipase: Isolation, purification and applications. Crit. Rev. Biotechnol. 2022, 42, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Kapranchikov, V.S.; Zherebtsov, N.A.; Popova, T.N. Purification and Characterization of Lipase from Wheat (Triticum aestivum L.) Germ. Appl. Biochem. Microbiol. 2004, 40, 84–88. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Factories 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Singh, N.; Singh, S.; Vinutha, T.; Krishnan, V.; Goswami, S.; Kumar, B.; Jat, S.L.; Yogeesh, L.N.; Singh, S.P.; et al. Nutritional supremacy of pearl- and foxtail millets: Assessing the nutrient density, protein stability and shelf-life of flours in millets and cereals for developing nutri-stable foods. J. Plant Biochem. Biotechnol. 2022, 31, 837–852. [Google Scholar] [CrossRef]
- Ling, B.; Lyng, J.G.; Wang, S. Effects of hot air-assisted radio frequency heating on enzyme inactivation, lipid stability and product quality of rice bran. LWT 2018, 91, 453–459. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Miszczuk, E.; Bajguz, A.; Hayat, S. Specific Roles of Lipoxygenases in Development and Responses to Stress in Plants. Plants 2022, 11, 979. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Ahmad, P. Chapter 4—Catalase: A Versatile Antioxidant in Plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 131–148. [Google Scholar]
- Zhao, Y.; Han, G.; Li, Y.; Lv, H. Changes in quality characteristics and metabolites composition of wheat under different storage temperatures. J. Stored Prod. Res. 2024, 105, 102229. [Google Scholar] [CrossRef]
- Gebicka, L.; Krych-Madej, J. The role of catalases in the prevention/promotion of oxidative stress. J. Inorg. Biochem. 2019, 197, 110699. [Google Scholar] [CrossRef] [PubMed]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Zhou, H.H.; Mao, X.Y. Emerging Roles of 5-Lipoxygenase Phosphorylation in Inflammation and Cell Death. Oxid. Med. Cell. Longev. 2019, 2019, 2749173. [Google Scholar] [CrossRef]
| Variable | FFA | K268 | P-AV | TOTOX | Lipase | LOX | CAT |
|---|---|---|---|---|---|---|---|
| FFA | 1 | ||||||
| K268 | 0.053 | 1 | |||||
| P-AV | 0.312 | −0.266 | 1 | ||||
| TOTOX | 0.303 | 0.488 * | 0.530 * | 1 | |||
| Lipase | 0.028 | −0.819 ** | 0.199 | −0.548 * | 1 | ||
| LOX | −0.182 | 0.145 | −0.582 ** | −0.177 | 0.044 | 1 | |
| CAT | 0.005 | −0.037 | 0.111 | 0.171 | −0.066 | −0.176 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yu, Y.; An, X.; Zhang, H.; Gong, W.; Liang, Y.; Wang, J. Changes in Lipid Metabolites and Enzyme Activities of Wheat Flour during Maturation. Foods 2024, 13, 2537. https://doi.org/10.3390/foods13162537
Chen Y, Yu Y, An X, Zhang H, Gong W, Liang Y, Wang J. Changes in Lipid Metabolites and Enzyme Activities of Wheat Flour during Maturation. Foods. 2024; 13(16):2537. https://doi.org/10.3390/foods13162537
Chicago/Turabian StyleChen, Yanyan, Yingtao Yu, Xin An, Huihui Zhang, Wei Gong, Ying Liang, and Jinshui Wang. 2024. "Changes in Lipid Metabolites and Enzyme Activities of Wheat Flour during Maturation" Foods 13, no. 16: 2537. https://doi.org/10.3390/foods13162537
APA StyleChen, Y., Yu, Y., An, X., Zhang, H., Gong, W., Liang, Y., & Wang, J. (2024). Changes in Lipid Metabolites and Enzyme Activities of Wheat Flour during Maturation. Foods, 13(16), 2537. https://doi.org/10.3390/foods13162537






