The Novel Concept of Synergically Combining: High Hydrostatic Pressure and Lytic Bacteriophages to Eliminate Vegetative and Spore-Forming Bacteria in Food Products
Abstract
:1. Introduction
2. High Hydrostatic Pressure—Innovative but Not New Technology
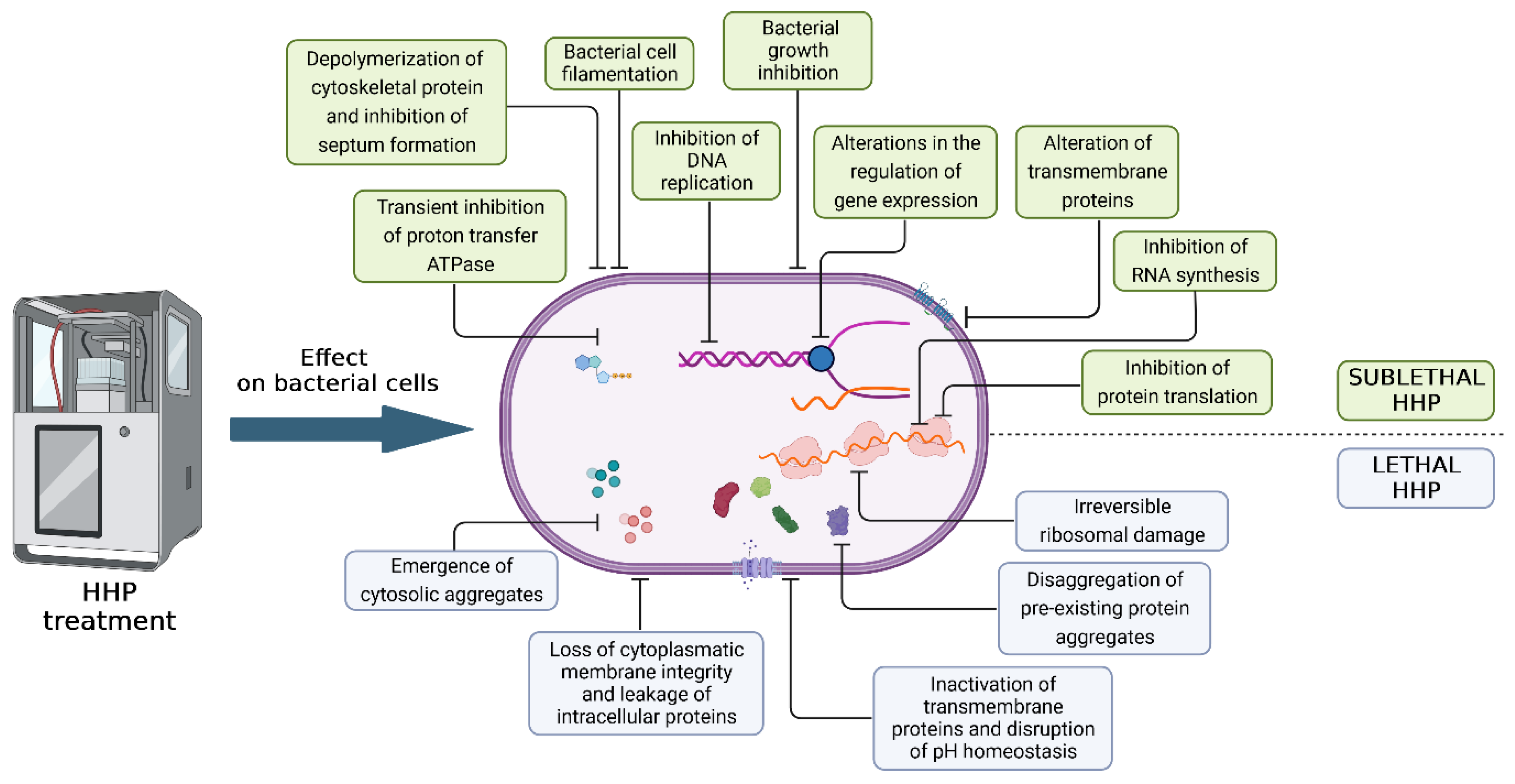
2.1. Mechanisms of Bacterial Cell Damage under the Influence of HHP
2.1.1. Injury to Vegetative Bacterial Cells
2.1.2. Bacterial Spore Damage
3. Bacteriophages—A Short History of Good Viruses
3.1. The Influence of HHP on the Phage Stability
3.2. Synergistic Effect of Phages and HHP in Bacterial Inactivation
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.W.; Hsu, C.P.; Wang, C.Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Muntean, M.-V.; Marian, O.; Barbieru, V.; Cătunescu, G.M.; Ranta, O.; Drocas, I.; Terhes, S. High Pressure Processing in Food Industry—Characteristics and Applications. Agric. Agric. Sci. Procedia 2016, 10, 377–383. [Google Scholar] [CrossRef]
- Lv, R.L.; Liu, D.H.; Zhou, J.W. Bacterial spore inactivation by non-thermal technologies: Resistance and inactivation mechanisms. Curr. Opin. Food Sci. 2021, 42, 31–36. [Google Scholar] [CrossRef]
- Reineke, K.; Mathys, A.; Heinz, V.; Knorr, D. Mechanisms of endospore inactivation under high pressure. Trends Microbiol. 2013, 21, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K. Food processing by high hydrostatic pressure. Biosci. Biotechnol. Biochem. 2017, 81, 672–679. [Google Scholar] [CrossRef] [PubMed]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Koutsoumanis, K.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. The efficacy and safety of high-pressure processing of food. EFSA J. 2022, 20, e07128. [Google Scholar]
- Tao, Y.; Sun, D.-W.; Hogan, E.; Kelly, A.L. High-Pressure Processing of Foods: An Overview. In Emerging Technologies for Food Processing, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 3–24. [Google Scholar]
- Balasubramaniam, V.M.; Martinez-Monteagudo, S.I.; Gupta, R. Principles and application of high pressure–based technologies in the food industry. Ann. Rev. Food Sci. Technol. 2015, 6, 435–462. [Google Scholar] [CrossRef]
- Aganovic, K.; Hertel, C.; Vogel, R.F.; Johne, R.; Schlüter, O.; Schwarzenbolz, U.; Jäger, H.; Holzhauser, T.; Bergmair, J.; Roth, A.; et al. Aspects of high hydrostatic pressure food processing: Perspectives on technology and food safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3225–3266. [Google Scholar] [CrossRef] [PubMed]
- Dehnad, D.; Emadzadeh, B.; Ghorani, B.; Rajabzadeh, G. High hydrostatic pressure (HHP) as a green technology opens up a new possibility for the fabrication of electrospun nanofibers: Part I-improvement of soy protein isolate properties by HHP. Food Hydrocoll. 2023, 140, 108659. [Google Scholar] [CrossRef]
- Carullo, D.; Barbosa-Cánovas, G.V.; Ferrari, G. Changes of Structural and Techno-Functional Properties of High Hydrostatic Pressure (HHP) Treated Whey Protein Isolate over Refrigerated Storage. LWT Food Sci. Technol. 2020, 137, 110436. [Google Scholar] [CrossRef]
- Sokołowska, B.; Skąpska, S.; Fonberg-Broczek, M.; Niezgoda, J.; Chotkiewicz, M.; Dekowska, A.; Rzoska, S. The Combined Effect of High Pressure and Nisin or Lysozyme on the Inactivation of Alicyclobacillus acidoterrestris Spores in Apple Juice. High Press. Res. 2012, 32, 119–127. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, H.W.; Hsu, C.P.; Yang, B.B. Recent advances in food processing using high hydrostatic pressure technology. Crit. Rev. Food Sci. Nutr. 2016, 56, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Podolak, R.; Whitman, D.; Black, D.G. Factors affecting microbial inactivation during high pressure processing in juices and beverages: A review. J. Food Protect. 2020, 83, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.J.; Lopes, R.P.; Delgadillo, I.; Saraiva, J.A. Microorganisms under high pressure-adaptation, growth and biotechnological potential. Biotechnol. Adv. 2013, 31, 1426–1434. [Google Scholar] [CrossRef]
- Pilavtepe-Çelik, M.; Yousef, A.; Alpas, H. Physiological changes of Escherichia coli O157:H7 and Staphylococcus aureus following exposure to high hydrostatic pressure. J. Verbrauch. Lebensm. 2013, 8, 175–183. [Google Scholar] [CrossRef]
- Inanoglu, S.; Barbosa-Canovas, G.V.; Sablani, S.S.; Zhu, M.J.; Keener, L.; Tang, J. High-pressure pasteurization of low-acid chilled ready-to-eat food. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4939–4970. [Google Scholar] [CrossRef]
- Renne, M.F.; Ernst, R. Membrane Homeostasis beyond Fluidity: Control of Membrane Compressibility. Trends Biochem. Sci. 2023, 48, 963–977. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhu, J.; Liu, H.; Liu, T.; Yu, G.; Shao, M. Covalent Interaction between High hydrostatic pressure-pretreated rice bran protein hydrolysates and ferulic acid: Focus on antioxidant activities and emulsifying properties. J. Agric. Food Chem. 2021, 69, 7777–7785. [Google Scholar] [CrossRef]
- Inaoka, T. Characterization of high hydrostatic pressure-injured Bacillus subtilis cells. Biosci. Biotechnol. Biochem. 2017, 81, 1235–1240. [Google Scholar] [CrossRef]
- Yamamoto, K.; Zhang, X.; Inaoka, T.; Morimatsu, K.; Kimura, K.; Nakaura, Y. Bacterial injury induced by high hydrostatic pressure. Food Eng. Rev. 2021, 13, 442–453. [Google Scholar] [CrossRef]
- Schottroff, F.; Fröhling, A.; Zunabovic-Pichler, M.; Krottenthaler, A.; Schlüter, O.; Jäger, H. Sublethal injury and Viable but Non-culturable (VBNC) state in microorganisms during preservation of food and biological materials by non-thermal processes. Front. Microbiol. 2018, 9, 2773. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Niranjan, K. Opportunities and challenges in high pressure processing of foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef] [PubMed]
- Smelt, J.P.P. Recent Advances in the Microbiology of High Pressure Processing. Trends Food Sci. Technol. 1998, 9, 152–158. [Google Scholar] [CrossRef]
- Nasilowska, J.; Kocot, A.; Osuchowska, P.N.; Sokolowska, B. High-pressure-induced sublethal injuries of food pathogens-microscopic assessment. Foods 2021, 10, 2940. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Morimatsu, K.; Inaoka, T.; Yamamoto, K. Injury and recovery of Escherichia coli ATCC25922 cells treated by high hydrostatic pressure at 400–600 MPa. J. Biosci. Bioeng. 2017, 123, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kimura, K.; Inaoka, T.; Morimatsu, K.; Nakaura, Y. Injury and recovery in bacterial inactivation induced by high hydrostatic pressure. J. Jpn. Soc. Food Sci. 2018, 65, 154–162. [Google Scholar] [CrossRef]
- Durães-Carvalho, R.; Souza, A.R.; Martins, L.M.; Sprogis, A.C.; Bispo, J.A.; Bonafe, C.F.; Yano, T. Effect of high hydrostatic pressure on Aeromonas hydrophila AH 191 growth in milk. J. Food Sci. 2012, 77, M417–M424. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, B.; Skąpska, S.; Niezgoda, J.; Rutkowska, M.; Dekowska, A.; Rzoska, S.J. Inactivation and Sublethal Injury of Escherichia coli and Listeria innocua by High Hydrostatic Pressure in Model Suspensions and Beetroot Juice. High Press. Res. 2014, 34, 147–155. [Google Scholar] [CrossRef]
- Liu, H.B.; Li, P.; Sun, C.; Du, X.J.; Zhang, Y.; Wang, S. Inhibitor-assisted high-pressure inactivation of bacteria in skim milk. J. Food Sci. 2017, 82, 1672–1681. [Google Scholar] [CrossRef]
- Patterson, M.; Mackle, A.; Linton, M. Effect of high pressure, in combination with antilisterial agents, on the growth of Listeria monocytogenes during extended storage of cooked chicken. Food Microbiol. 2011, 28, 1505–1508. [Google Scholar] [CrossRef]
- Nemeth, C.; Dalmadi, I.; Friedrich, L.; Pásztor-Huszár, K.; Suhajda, Á.; Ivanics, J.; Balla, C. Pasteurization of liquid egg by high hydrostatic pressure (HHP) treatment. Afr. J. Microbiol. Res. 2016, 6, 660–664. [Google Scholar]
- Duru, I.C.; Andreevskaya, M.; Laine, P.; Rode, T.M.; Ylinen, A.; Løvdal, T.; Bar, N.; Crauwels, P.; Riedel, C.U.; Bucur, F.I.; et al. Genomic characterization of the most barotolerant Listeria monocytogenes RO15 strain compared to reference strains used to evaluate food high pressure processing. BMC Genom. 2020, 21, 455. [Google Scholar] [CrossRef] [PubMed]
- Koseki, S.; Yamamoto, K. Recovery of Escherichia coli ATCC 25922 in phosphate buffered saline after treatment with high hydrostatic pressure. Int. J. Food Microbiol. 2006, 110, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Nakaura, Y.; Morimatsu, K.; Inaoka, T.; Yamamoto, K. Listeria monocytogenes cells injured by high hydrostatic pressure and their recovery in nutrient-rich or -free medium during cold storage. High Press. Res. 2019, 39, 324–333. [Google Scholar] [CrossRef]
- Nasiłowska, J.; Sokołowska, B.; Fonberg-Broczek, M. Long-Term Storage of Vegetable Juices Treated by High Hydrostatic Pressure: Assurance of the Microbial Safety. Biomed. Res. Int. 2018, 2018, 7389381. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, H.; Yatuzuka, M.; Horie, N.; Furukawa, S.; Yamasaki, M. Synergistic effect of high hydrostatic pressure treatment and food additives on the inactivation of Salmonella enteritidis. Food Control 2009, 20, 963–966. [Google Scholar] [CrossRef]
- Porębska, I.; Sokołowska, B. Analysis of the germination proteins in Alicyclobacillus acidoterrestris spores subjected to external factors. Acta Biochim. Pol. 2017, 64, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P.; Christie, G. What’s New and Notable in Bacterial Spore Killing! World J. Microbiol. Biotechnol. 2021, 37, 144. [Google Scholar] [CrossRef] [PubMed]
- Swarge, B.; Abhyankar, W.; Jonker, M.; Hoefsloot, H.; Kramer, G.; Setlow, P.; Brul, S.; de Koning, L.J. Integrative analysis of proteome and transcriptome dynamics during Bacillus subtilis spore revival. mSphere 2020, 5, e00463-20. [Google Scholar] [CrossRef]
- Mok, J.H.; Sun, Y.; Pyatkovskyy, T.; Hu, X.; Sastry, S.K. Mechanisms of Bacillus subtilis spore inactivation by single- and multi-pulse high hydrostatic pressure (MP-HHP). Innov. Food Sci. Emerg. Technol. 2022, 81, 103147. [Google Scholar] [CrossRef]
- Porębska, I.; Sokołowska, B.; Skąpska, S.; Rzoska, S.J. Treatment with high hydrostatic pressure and supercritical carbon dioxide to control Alicyclobacillus acidoterrestris spores in apple juice. Food Control. 2017, 73, 24–30. [Google Scholar] [CrossRef]
- Sokołowska, B.; Skapska, S.; Fonberg-Broczek, M.; Niezgoda, J.; Porebska, I.; Dekowska, A.; Rzoska, S.J. Germination and Inactivation of Alicyclobacillus acidoterrestris Spores Induced by Moderate Hydrostatic Pressure. Pol. J. Microbiol. 2015, 64, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Modugno, C.; Peltier, C.; Simonin, H.; Dujourdy, L.; Capitani, F.; Sandt, C.; Perrier-Cornet, J.M. Understanding the effects of high pressure on bacterial spores using synchrotron infrared spectroscopy. Front. Microbiol. 2020, 10, 3122. [Google Scholar] [CrossRef]
- Morimatsu, K.; Nakaura, Y.; Inaoka, T.; Kimura, K.; Yamamoto, K. Effects of solution pH and ions on suicidal germination of Bacillus subtilis spores induced by medium high temperature-medium high hydrostatic pressure treatment. Biocontrol Sci. 2019, 24, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Porębska, I.; Sokołowska, B.; Woźniak, Ł.; Skąpska, S.; Fonberg-Broczek, M.; Rzoska, S.J. DPA release and germination of Alicyclobacillus acidoterrestris spores under high hydrostatic pressure. J. Nutr. Food. Sci. 2015, 5, 1. [Google Scholar]
- Lyu, F.; Zhang, T.; Gui, M.; Wang, Y.; Zhao, L.; Wu, X.; Rao, L.; Liao, X. The Underlying Mechanism of Bacterial Spore Germination: An Update Review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2728–2746. [Google Scholar] [CrossRef]
- Paredes-Sabja, D.; Setlow, P.; Sarker, M.R. Germination of spores of Bacillales and Clostridiales species: Mechanisms and proteins involved. Trends Microbiol. 2011, 19, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sourri, P.; Argyri, A.A.; Nychas, G.J.E.; Tassou, C.C.; Panagou, E.Z. The Effect of Temperature-Assisted High Hydrostatic Pressure on the Survival of Alicyclobacillus acidoterrestris Inoculated in Orange Juice throughout Storage at Different Isothermal Conditions. Fermentation 2022, 8, 308. [Google Scholar] [CrossRef]
- Silva, F.V.; Tan, E.K.; Farid, M. Bacterial spore inactivation at 45–65 °C using high pressure processing: Study of Alicyclobacillus acidoterrestris in orange juice. Food Microbiol. 2012, 32, 206–211. [Google Scholar] [CrossRef]
- Vercammen, A.; Vivijs, B.; Lurquin, I.; Michiels, C.W. Germination and inactivation of Bacillus coagulans and Alicyclobacillus acidoterrestris spores by high hydrostatic pressure treatment in buffer and tomato sauce. Int. J. Food Microbiol. 2012, 152, 162–167. [Google Scholar] [CrossRef]
- Xu, J.; Janahar, J.J.; Park, H.W.; Balasubramaniam, V.M.; Yousef, A.E. Influence of water activity and acidity on Bacillus cereus spore inactivation during combined high pressure-thermal treatment. LWT-Food Sci. Technol. 2021, 146, 111465. [Google Scholar] [CrossRef]
- Sarker, M.R.; Akhtar, S.; Torres, J.A.; Paredes-Sabja, D. High Hydrostatic Pressure-Induced Inactivation of Bacterial Spores. Crit. Rev. Microbiol. 2015, 41, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Scepankova, H.; Pinto, C.A.; Estevinho, L.M.; Saraiva, J.A. High-Pressure-Based Strategies for the Inactivation of Bacillus subtilis Endospores in Honey. Molecules 2022, 27, 5918. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Thi Minh, H.; Dantigny, P.; Perrier-Cornet, J.M.; Gervais, P. Germination and inactivation of Bacillus subtilis spores induced by moderate hydrostatic pressure. Biotechnol. Bioeng. 2010, 107, 876–883. [Google Scholar] [CrossRef]
- Lenz, C.A.; Reineke, K.; Knorr, D.; Vogel, R.F. High pressure thermal inactivation of Clostridium botulinum type E endospores—Kinetic modeling and mechanistic insights. Front Microbiol. 2015, 6, 652. [Google Scholar] [CrossRef] [PubMed]
- Evelyn; Silva, F.V.M. High pressure thermal processing for the inactivation of Clostridium perfringens spores in beef slurry. Innov. Food Sci. Emerg. Technol. 2016, 33, 26–31. [Google Scholar] [CrossRef]
- Kmiha, S.; Modugno, C.; Aouadhi, C.; Simonin, H.; Mejri, S.; Perrier-Cornet, J.; Maaroufi, A. Inhibitory effect of high hydrostatic pressure; nisin; and moderate heating on the inactivation of Paenibacillus sp. and Terribacillus aidingensis spores isolated from UHT milk. High Press. Res. 2021, 41, 328–340. [Google Scholar] [CrossRef]
- Georget, E.; Sevenich, R.; Reineke, K.; Mathys, A.; Heinz, V.; Callanan, M.; Rauh, C.; Knorr, D. Inactivation of Microorganisms by High Isostatic Pressure Processing in Complex Matrices: A Review. Innov. Food Sci. Emerg. Technol. 2015, 27, 1–14. [Google Scholar] [CrossRef]
- Wuytack, E.Y.; Boven, S.; Michiels, C.W. Comparative Study of Pressure-Induced Germination of Bacillus subtilis Spores at Low and High Pressures. Appl. Environ. Microbiol. 1998, 64, 3220–3224. [Google Scholar] [CrossRef]
- Zemmyo, D.; Yamamoto, M.; Miyata, S. Fundamental Study of Decellularization Method Using Cyclic Application of High Hydrostatic Pressure. Micromachines 2020, 11, 1008. [Google Scholar] [CrossRef]
- Sehrawat, R.; Kaur, B.P.; Nema, P.K.; Tewari, S.; Kumar, L. Microbial Inactivation by High Pressure Processing: Principle, Mechanism and Factors Responsible. Food Sci. Biotechnol. 2020, 30, 19–35. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Sonar, C.R.; Patel, J.; Albahr, Z.; Sablani, S.S. High pressure-assisted thermal sterilization of low-acid fruit and vegetable purees: Microbial safety, nutrient, quality, and packaging evaluation. Food Control 2020, 114, 107233. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Albero, B.; Tadeo, J.L.; Miguel, E.; Pérez, R.A. Rapid Determination of Antibiotic Residues in Cereals by Liquid Chromatography Triple Mass Spectrometry. Anal. Bioanal. Chem. 2019, 411, 6129–6139. [Google Scholar] [CrossRef]
- Treiber, F.M.; Beranek-Knauer, H. Antimicrobial Residues in Food from Animal Origin—A Review of the Literature Focusing on Products Collected in Stores and Markets Worldwide. Antibiotics 2021, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T. Bacteriophage Adsorption: Likelihood of Virion Encounter with Bacteria and Other Factors Affecting Rates. Antibiotics 2023, 12, 723. [Google Scholar] [CrossRef]
- Sanz-Gaitero, M.; Seoane-Blanco, M.; van Raaij, M.J. Structure and function of bacteriophages. In Bacteriophages; Harper, D.R., Abedon, S.T., Burrowes, B.H., McConville, M.L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 19–91. ISBN 978-3-319-41985-5. [Google Scholar]
- Dec, M.; Wernicki, A.; Urban-Chmiel, R. Efficacy of experimental phage therapies in livestock. Anim. Health Res. Rev. 2020, 21, 69–83. [Google Scholar] [CrossRef]
- Letarov, A.V. History of Early Bacteriophage Research and Emergence of Key Concepts in Virology. Biochemistry 2020, 85, 1093–1112. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, Y.J.; Lee, M.H.; Park, C.W.; Lee, S.M.; Soh, J.O.; Lee, J.H. M13 Bacteriophage-Based Bio-Nano Systems for Bioapplication. BioChip J. 2022, 16, 227–245. [Google Scholar] [CrossRef]
- Sun, L.; Young, L.N.; Zhang, X.; Boudko, S.P.; Fokine, A.; Zbornik, E.; Roznowski, A.P.; Molineux, I.J.; Rossmann, M.G.; Fane, B.A. Icosahedral bacteriophage ФX174 forms a tail for DNA transport during infection. Nature 2014, 505, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Aprea, G.; D’Angelo, A.R.; Prencipe, V.A.; Migliorati, G. Bacteriophage Morphological Characterization by Using Transmission Electron Microscopy. J. Life Sci. 2015, 9, 214–220. [Google Scholar]
- Nobrega, F.L.; Vlot, M.; de Jonge, P.A.; Dreesens, L.L.; Beaumont, H.J.E.; Lavigne, R.; Dutilh, B.E.; Brouns, S.J.J. Targeting mechanisms of tailed bacteriophages. Nat. Rev. Microbiol. 2018, 16, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Annapure, U.S. Bacteriophage biocontrol of foodborne pathogens. J. Food Sci. Technol. 2016, 53, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.L.; Klose, T.; Arisaka, F.; Speir, J.A.; Veesler, D.; Fokine, A.; Rossmann, M.G. Role of bacteriophage T4 baseplate in regulating assembly and infection. Proc. Natl. Acad. Sci. USA 2016, 113, 2654–2659. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, Z.; Abedon, S.T. Diversity of phage infection types and associated terminology: The problem with ‘Lytic or lysogenic’. Fems Microbiol. Lett. 2016, 363, fnw047. [Google Scholar] [CrossRef] [PubMed]
- Leprince, A.; Mahillon, J. Phage Adsorption to Gram-Positive Bacteria. Viruses 2023, 15, 196. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, J.J.; Abedon, S.T. Adsorption: Phage acquisition of bacteria. In Bacteriophages: Biology, Technology, Therapy; Springer: Cham, Switzerland, 2021; pp. 93–117. [Google Scholar]
- Ballesté, E.; Blanch, A.R.; Muniesa, M.; García-Aljaro, C.; Rodríguez-Rubio, L.; Martín-Díaz, J.; Pascual-Benito, M.; Jofre, J. Bacteriophages in Sewage: Abundance, Roles, and Applications. FEMS Microbes 2022, 3, xtac009. [Google Scholar] [CrossRef] [PubMed]
- Sime-Ngando, T. Environmental bacteriophages: Viruses of microbes in aquatic ecosystems. Front. Microbiol. 2014, 5, 355. [Google Scholar] [CrossRef]
- Tzipilevich, E.; Habusha, M.; Ben-Yehuda, S. Acquisition of phage sensitivity by bacteria through exchange of phage receptors. Cell 2017, 168, 186–199. [Google Scholar] [CrossRef]
- Rakhuba, D.V.; Kolomiets, E.I.; Dey, E.S.; Novik, G.I. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Pol. J. Microbiol. 2010, 59, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Liu, J.; Hoover, T.R. Phylogenetic Distribution, Ultrastructure, and Function of Bacterial Flagellar Sheaths. Biomolecules 2020, 10, 363. [Google Scholar] [CrossRef]
- Li, J.; Tian, F.; Hu, Y.; Lin, W.; Liu, Y.; Zhao, F.; Ren, H.; Pan, Q.; Shi, T.; Tong, Y. Characterization and Genomic Analysis of BUCT549, a Novel Bacteriophage Infecting Vibrio alginolyticus With Flagella as Receptor. Front. Microbiol. 2021, 12, 668319. [Google Scholar] [CrossRef]
- Stone, E.; Campbell, K.; Grant, I.; McAuliffe, O. Understanding and Exploiting Phage-Host Interactions. Viruses 2019, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Federici, S.; Nobs, S.P.; Elinav, E. Phages and Their Potential to Modulate the Microbiome and Immunity. Cell. Mol. Immunol. 2021, 18, 889–904. [Google Scholar] [CrossRef]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Wei, F.; Yu, F.; Zhao, Z. Bactericidal activity of a holin-endolysin system derived from Vibrio alginolyticus phage HH109. Microb. Pathog. 2021, 159, 105135. [Google Scholar] [CrossRef]
- Cisek, A.; Dąbrowska, I.; Gregorczyk, K.; Wyżewski, Z. Phage therapy in bacterial infections treatment: One hundred years after the discovery of bacteriophages. Curr. Microbiol. 2016, 74, 277–283. [Google Scholar] [CrossRef]
- Ramisetty, B.C.M.; Sudhakari, P.A. Bacterial “grounded” prophages: Hotspots for genetic renovation and innovation. Front. Genet. 2019, 10, 12. [Google Scholar] [CrossRef]
- Tanouchi, Y.; Covert, M.W. Combining comprehensive analysis of off-site lambda phage integration with a CRISPR-based means of characterizing downstream physiology. mBio 2017, 8, e01038-17. [Google Scholar] [CrossRef]
- Venturini, C.; Zingali, T.; Wyrsch, E.R.; Bowring, B.; Iredell, J.; Partridge, S.R.; Djordjevic, S.P. Diversity of P1 phage-like elements in multidrug resistant Escherichia coli. Sci. Rep. 2019, 9, 18861. [Google Scholar] [CrossRef] [PubMed]
- Erez, Z.; Steinberger-Levy, I.; Shamir, M.; Doron, S.; Stokar-Avihail, A.; Peleg, Y.; Melamed, S.; Leavitt, A.; Savidor, A.; Albeck, S.; et al. Communication between viruses guides lysis–lysogeny decisions. Nature 2017, 541, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Hyman, P.J.C. Phage choice, isolation, and preparation for phage therapy. Curr. Pharm. Biotechnol. 2010, 11, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Hyman, P.; Abedon, S.T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 2010, 70, 217–248. [Google Scholar] [PubMed]
- Hamesch, K.; Borkham-Kamphorst, E.; Strnad, P.; Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab. Anim. 2015, 49 (Suppl. 1), 37–46. [Google Scholar] [CrossRef] [PubMed]
- Shymialevich, D.; Wójcicki, M.; Świder, O.; Średnicka, P.; Sokołowska, B. Characterization and Genome Study of a Newly Isolated Temperate Phage Belonging to a New Genus Targeting Alicyclobacillus acidoterrestris. Genes 2023, 14, 1303. [Google Scholar] [CrossRef] [PubMed]
- Wójcicki, M.; Świder, O.; Gientka, I.; Błażejak, S.; Średnicka, P.; Shymialevich, D.; Cieślak, H.; Wardaszka, A.; Emanowicz, P.; Sokołowska, B.; et al. Effectiveness of a Phage Cocktail as a Potential Biocontrol Agent against Saprophytic Bacteria in Ready-To-Eat Plant-Based Food. Viruses 2023, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.P.; Burgos, M.J.G.; Galvez, A.; López, R.L. Application of bacteriophages in post-harvest control of human pathogenic and food spoiling bacteria. Crit. Rev. Biotechnol. 2016, 36, 851–861. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Evaluation of the safety and efficacy of ListexTM P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA J. 2016, 14, e04565. [Google Scholar]
- Kahn, L.H.; Bergeron, G.; Bourassa, M.W.; Vegt, B.D.; Gill, J.; Gomes, F.; Malouin, F.; Opengart, K.; Ritter, G.D.; Singer, R.S.; et al. From farm management to bacteriophage therapy: Strategies to reduce antibiotic use in animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 31–39. [Google Scholar] [CrossRef]
- Wójcicki, M.; Świder, O.; Średnicka, P.; Shymialevich, D.; Ilczuk, T.; Koperski, Ł.; Cieślak, H.; Sokołowska, B.; Juszczuk-Kubiak, E. Newly Isolated Virulent Salmophages for Biocontrol of Multidrug-Resistant Salmonella in Ready-to-Eat Plant-Based Food. Int. J. Mol. Sci. 2023, 24, 10134. [Google Scholar] [CrossRef]
- Butala, M.; Dragoš, A. Unique Relationships between Phages and Endospore-Forming Hosts. Trends Microbiol. 2022, 31, 498–510. [Google Scholar] [CrossRef]
- Ahmadi, H.; Anany, H.; Walkling-Ribeiro, M.; Griffiths, M. Biocontrol of Shigella flexneri in Ground Beef and Vibrio cholerae in Seafood with Bacteriophage-Assisted High Hydrostatic Pressure (HHP) Treatment. Food Bioprocess Technol. 2015, 8, 1160–1167. [Google Scholar] [CrossRef]
- Kong, J.; Luoyizha, W.; Zhao, L.; Fan, C.; Li, H.; Li, H. Effects of high hydrostatic pressure treatment on bacterial composition in donkey milk studied by high throughput sequencing. Food Innov. Adv. 2023, 2, 85–94. [Google Scholar] [CrossRef]
- Pereira, C.; Marques, J.F.; Reis, S.; Costa, P.; Martins, A.P.; Pinto, C.A.; Saraiva, J.A.; Almeida, A. Combined Effect of Phage phT4A and Pressure-Based Strategies in the Inhibition of Escherichia coli. Antibiotics 2022, 11, 211. [Google Scholar] [CrossRef]
- Tabla, R.; Martinez, B.; Rebollo, J.E.; Gonzalez, J.; Ramirez, M.R.; Roa, I.; Rodriguez, A.; Garcia, P. Bacteriophage performance against Staphylococcus aureus in milk is improved by high hydrostatic pressure treatments. Int. J. Food Microbiol. 2012, 156, 209–213. [Google Scholar] [CrossRef]
- Maciel, C.; Campos, A.; Komora, N.; Pinto, C.A.; Fernandes, R.; Saraiva, J.A.; Teixeira, P. Impact of high hydrostatic pressure on the stability of lytic bacteriophages’ cocktail SalmonelexTM towards potential application on Salmonella inactivation. LWT-Food Sci. Technol. 2021, 151, 112108. [Google Scholar] [CrossRef]
- Komora, N.; Bruschi, C.; Ferreira, V.; Maciel, C.; Brandão, T.R.S.; Fernandes, R.; Saraiva, J.A.; Castro, S.M.; Teixeira, P. The protective effect of food matrices on Listeria lytic bacteriophage P100 application towards high pressure processing. Food Microbiol. 2018, 76, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Avsaroglu, M.D.; Buzrul, S.; Alpas, H.; Akcelik, M.; Bozoglu, F. Use of the Weibull model for lactococcal bacteriophage inactivation by high hydrostatic pressure. Int. J. Food Microbiol. 2006, 108, 78–83. [Google Scholar] [CrossRef]
- Guan, D.; Kniel, K.; Calci, K.R.; Hicks, D.T.; Pivarnik, L.F.; Hoover, D.G. Response of four types of coliphages to high hydrostatic pressure. Food Microbiol. 2006, 23, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qu, M.; Yao, J.; Wang, P.; Liao, X.; Hu, X.; Chen, F. Effect of high hydrostatic pressure on the viability of Streptococcus thermophilus bacteriophages isolated from cheese. Innov. Food Sci. Emerg. Technol. 2015, 29, 113–118. [Google Scholar] [CrossRef]
- Komora, N.; Maciel, C.; Amaral, R.A.; Fernandes, R.; Castro, S.M.; Saraiva, J.A.; Teixeira, P. Innovative hurdle system towards Listeria monocytogenes inactivation in a fermented meat sausage model—High pressure processing assisted by bacteriophage P100 and bacteriocinogenic Pediococcus acidilactici. Food Res. Int. 2021, 148, 110628. [Google Scholar] [CrossRef]

| Bacteria | Matrix | HHP Parameters | Inactivation (↓log CFU mL−1 or log CFU g−1) | Reference | ||
|---|---|---|---|---|---|---|
| Pressure (MPa) | Time (min) | Temperature (°C) | ||||
| Aeromonas hydrophila strain AH 191 | UHT whole milk | 250 | 10 | 25 | ~2.5 | [28] |
| 350 | 4 | ~7.0 | ||||
| Escherichia coli | beetroot juice (pH 4.18) | 400 | 10 | 20 | 6.2 | [29] |
| Escherichia coli | skim milk | 300 | 5 | nd | 2.1 | [30] |
| 400 | complete reduction | |||||
| Listeria innocua | beetroot juice (pH 4.18) | 400 | 10 | 20 | 7.0 after 1 min | [29] |
| Listeria monocytogenes | cooked chicken | 500 | 1 | 20 | 0.9 | [31] |
| 40 | 3.8 | |||||
| Listeria monocytogenes | skim milk | 300 | 5 | nd | 1.5 | [30] |
| 400 | 3.4 | |||||
| 500 | complete reduction | |||||
| Salmonella Enteritidis | liquid whole egg | 200 | 10 | nd | 4.89 | [32] |
| 300 | ~5.20 | |||||
| 400 | 5.31 | |||||
| Salmonella Typhimurium | skim milk | 300 | 5 | nd | 2.8 | [30] |
| 400 | complete reduction | |||||
| Staphylococcus aureus | liquid whole egg | 200 | 10 | nd | 1.84 | [32] |
| 300 | ~2.00 | |||||
| 400 | 2.63 | |||||
| Staphylococcus aureus | skim milk | 300 | 5 | nd | 0.50 | [30] |
| 400 | 4.00 | |||||
| 500 | 5.85 | |||||
| Bacteria | Matrix | HHP Parameters | Inactivation (↓log CFU mL−1 or log CFU g−1) | Reference | ||
|---|---|---|---|---|---|---|
| Pressure (MPa) | Time (min) | Temperature (°C) | ||||
| Alicyclobacillus acidoterrestris | orange juice (pH 3.7, 11.45 °Brix) | 600 | 5 | 60 | 3.0 | [49] |
| 10 | 3.5 | |||||
| Alicyclobacillus acidoterrestris | orange juice (pH 3.8, 9.20 °Brix) | 600 | 10 | 45 | ~1.0 | [50] |
| Alicyclobacillus acidoterrestris | apple juice (pH 3.4, 11.20 °Brix) | 200 | 20 | 50 | 1.95 | [43] |
| 70 | 3.99 | |||||
| 500 | 70 | 6.13 | ||||
| Alicyclobacillus acidoterrestris | tomato pulp (pH 4.2) | 200 | 10 | 40 | 1.0–1.5 | [51] |
| 60 | ||||||
| 400 | 40 | |||||
| 60 | ||||||
| 600 | 40 | to 3.5 | ||||
| 60 | ||||||
| Bacillus cereus | CPB (aw = 0.92) | 600 | 5 | 70 | ~6.0 | [52] |
| CPB (aw = 0.85) | 3.0 | |||||
| CPB (aw = 0.80) | 1.5 | |||||
| Bacillus cereus | MES buffer | 600 | 100 | |||
| Bacillus coagulans | tomato pulp | 300 | 15 | 50 | 2.0 | [53] |
| 60 | 2.4 | |||||
| 600 | 50 | 3.1 | ||||
| 60 | 5.7 | |||||
| Bacillus coagulans | tomato sauce (pH 4.2) | 600 | 10 | 60 | 2.0 | [51] |
| Bacillus subtilis | honey (aw = 0.85) | 600 | 15 | 85 | 0 | [54] |
| Bacillus subtilis | distilled water | 350 | 10 | 40 | 1.0 | [55] |
| Clostridium botulinum | IPB (pH 7.0) | 450 | 10 | 45 | 1.0 | [56] |
| 600 | 75 | 2.1 | ||||
| 750 | 5.6 | |||||
| 900 | complete reduction | |||||
| 30 | 3.8 | |||||
| Clostridium perfringens | beef slurry (pH 6.5) | 600 | 20 | 75 | 2.2 | [57] |
| Paenibacillus sp. | UHT milk | 500 | 10 | 20 | 0.5 | [58] |
| 50 | 2.0 | |||||
| 600 | 20 | 1.1 | ||||
| 50 | 2.7 | |||||
| Terribacillus aidingensis | 500 | 10 | 20 | 2.1 | ||
| 50 | 2.2 | |||||
| 600 | 20 | 2.3 | ||||
| 50 | 2.2 | |||||
| Phage | Type of Genetic Material, Genome Size (bp) | Bacterial Host | Matrix | HHP Parameters | Phage Inactivation (↓log PFU mL−1 or log PFU g−1) | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Pressure (MPa) | Temperature (°C) | Time (min) | ||||||
| phiIPLA35 | dsDNA 45,344 | Staphylococcus aureus | pasteurized milk | 0–400 | 10 | 5 | no reduction | [109] |
| phiIPLA88 | dsDNA 42,526 | 500 | ~1.0 | |||||
| 600 | ~5.0 | |||||||
| 700 | complete reduction | |||||||
| phT4A | dsDNA 171,598 | Escherichia coli | TSB | 75 | ~21 | 5 | 0.29 | [108] |
| 20 | 0.46 | |||||||
| 30 | 0.61 | |||||||
| 200 | 5 | 2.44 | ||||||
| 20 | 2.56 | |||||||
| 30 | 2.71 | |||||||
| 300 | 5 | 3.13 | ||||||
| 20 | 3.45 | |||||||
| 30 | 4.26 | |||||||
| 400 | 5 | 4.94 | ||||||
| 20 | 6.26 | |||||||
| 30 | complete reduction | |||||||
| Bacteriophages cocktail SalmonellexTM | dsDNA | Salmonella Typhimurium strain DT104 | egg white | 200 | 10 | 5 | ~0.5 | [110] |
| 300 | <1.0 | |||||||
| 400 | 1.7 | |||||||
| 500 | 3.4 | |||||||
| 600 | 5.5 | |||||||
| Bacteriophages cocktail SalmonellexTM | dsDNA | Salmonella Typhimurium strain DT104 | egg yolk | 200 | 10 | 5 | ~0.5 | [110] |
| 300 | <1.0 | |||||||
| 400 | ~1.0 | |||||||
| 500 | 3.4 | |||||||
| 600 | 5.9 | |||||||
| liquid whole egg | 200 | ~0.5 | ||||||
| 300 | <1.0 | |||||||
| 400 | ~1.0 | |||||||
| 500 | 2.2 | |||||||
| 600 | 5.8 | |||||||
| PBS | 200 | <1.0 | ||||||
| 300 | 1.2 | |||||||
| 400 | 3.9 | |||||||
| 500 | 7.0 | |||||||
| 600 | 6.8 | |||||||
| phage stock solution | 200 | <1.0 | ||||||
| 300 | <1.0 | |||||||
| 400 | 1.0 | |||||||
| 500 | 1.5 | |||||||
| 600 | 9.8 | |||||||
| phage P100 | dsDNA 131,385 | Listeria monocytogenes | fermented sausage “Alheira” (pH 6.07) | 200 | 10 | 5 | <0.5 | [111] |
| 300 | 0.9 | |||||||
| 400 | complete reduction | |||||||
| cheese (pH 5.66) | 200 | <0.5 | ||||||
| 300 | 0.9 | |||||||
| 400 | complete reduction | |||||||
| PBS (pH 7.42) | 200 | <0.5 | ||||||
| 300 | 2.8 | |||||||
| 400 | complete reduction | |||||||
| UHT milk (pH 6.73) | 200 | <0.5 | ||||||
| 300 | 0.8 | |||||||
| 400 | complete reduction | |||||||
| apple juice (pH 3.41) | 200 | 3.0 | ||||||
| 300 | 7.0 | |||||||
| 400 | complete reduction | |||||||
| orange/carrot nectar (pH 3.54) | 200 | 3.0 | ||||||
| 300 | 7.0 | |||||||
| 400 | complete reduction | |||||||
| φLd66-36 | no data | Lactococcus lactis subsp. lactis | M17 broth | 300 | ~25 | 10 | ~2.5 | [112] |
| 20 | ~2.6 | |||||||
| 30 | ~3.9 | |||||||
| 40 | ~3.8 | |||||||
| 50 | ~4.7 | |||||||
| 60 | ~6.8 | |||||||
| φLd66-36 | no data | Lactococcus lactis subsp. lactis | M17 broth | 350 | ~25 | 5 | ~2.2 | [112] |
| 10 | ~4.3 | |||||||
| 15 | ~5.0 | |||||||
| 20 | ~6.3 | |||||||
| φX174 | ssDNA 5386 | Escherichia coli | PBS | 450 | 21 | 5 | ~0.7 | [113] |
| 10 | ~0.5 | |||||||
| 15 | ~0.6 | |||||||
| 20 | ~0.6 | |||||||
| 60 | 0.8 | |||||||
| 600 | 5 | ~0.6 | ||||||
| 10 | ~0.5 | |||||||
| 20 | ~0.6 | |||||||
| 60 | ~0.7 | |||||||
| 350 | 21 | 5 | ~0.8 | |||||
| 400 | ~0.7 | |||||||
| 450 | ~0.7 | |||||||
| 500 | ~0.4 | |||||||
| 550 | ~0.4 | |||||||
| 600 | ~0.6 | |||||||
| 600 | 4 | 5 | ~0.6 | |||||
| 10 | ~0.6 | |||||||
| 21 | ~0.6 | |||||||
| 30 | ~0.5 | |||||||
| 40 | ~0.6 | |||||||
| MS2 | ssRNA 3569 | Escherichia coli | PBS | 450 | 21 | 5 | 0.2 | [113] |
| 10 | ~0.5 | |||||||
| 15 | 0.8 | |||||||
| 20 | ~1.1 | |||||||
| 60 | ~1.1 | |||||||
| 600 | 5 | ~3.4 | ||||||
| 10 | ~3.5 | |||||||
| 20 | ~3.7 | |||||||
| 60 | 4.0 | |||||||
| 350 | 21 | 5 | 0.5 | |||||
| 400 | ~0.3 | |||||||
| 450 | ~0.2 | |||||||
| 500 | ~0.7 | |||||||
| 550 | 1.5 | |||||||
| 600 | ~3.4 | |||||||
| 600 | 4 | 5 | 4.0 | |||||
| 10 | 4.4 | |||||||
| 21 | ~3.4 | |||||||
| 30 | ~3.4 | |||||||
| 40 | ~3.4 | |||||||
| ϕAbc2 | dsDNA 34,882 | Streptococcus thermophilus | M17 broth | 400 | ~33 | 5 | ~0.6 | [114] |
| 10 | ~1.4 | |||||||
| 20 | ~2.4 | |||||||
| 30 | ~3.0 | |||||||
| 500 | ~35 | 5 | ~5.1 | |||||
| 10 | ~5.4 | |||||||
| 20 | ~7.3 | |||||||
| 600 | ~38 | 5 | complete reduction | |||||
| ALQ13.2 | dsDNA 35,525 | 400 | ~33 | 5 | ~0.8 | |||
| 10 | ~1.2 | |||||||
| 20 | ~1.7 | |||||||
| 30 | ~2.0 | |||||||
| 500 | ~35 | 5 | ~1.2 | |||||
| 10 | ~2.1 | |||||||
| 20 | ~2.0 | |||||||
| 30 | ~2.8 | |||||||
| 600 | ~38 | 5 | 3.7 | |||||
| 10 | ~6.0 | |||||||
| 20 | ~7.8 | |||||||
| DT1 | dsDNA 34,820 | Streptococcus thermophilus | M17 broth | 400 | ~33 | 5 | ~0.6 | [114] |
| 10 | ~1.4 | |||||||
| 20 | ~2.6 | |||||||
| 30 | ~3.6 | |||||||
| 500 | ~35 | 5 | ~4.9 | |||||
| 10 | ~6.4 | |||||||
| 20 | ~8.3 | |||||||
| 600 | ~38 | 5 | complete reduction | |||||
| Bacterial Host (Contamination Level) | Matrix | Phage | HHP Parameters | Storage | Inactivation (↓log CFU mL−1 or log CFU g−1) | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Pressure (MPa) | Temperature (°C) | Time (min) | Temperature (°C) | Time (Day or Hours) | |||||
| Listeria monocytogenes strain ScottA (5.0 log CFU g−1) | fermented meat sausage “Alheira” (pH 6.07) | Listex™ P100 | 300 | 10 | 5 | 4 | 0 h | 3.2 | [111] |
| 14 d | 3.2 | ||||||||
| 60 d | 3.2 | ||||||||
| – | 7 d | 0.3 | |||||||
| 14 d | 0.3 | ||||||||
| 60 d | 0.3 | ||||||||
| Listeria monocytogenes strain 1942 (5.0 log CFU g−1) | Listex™ P100 | 0 h | 3.2 | ||||||
| 14 d | 3.2 | ||||||||
| 60 d | 3.2 | ||||||||
| – | 7 d | 0.3 | |||||||
| 14 d | 0.3 | ||||||||
| 60 d | 0.3 | ||||||||
| Shigella flexneri (3.9 log CFU g−1) | ground beef | AG10 | 250 | ~25 | 5 | ~21 | 0 h | complete reduction | [106] |
| – | 2.5 | ||||||||
| Staphylococcus aureus (4.0 log CFU mL−1) | pasteurized whole milk | phiIPLA35 phiIPLA88 | 400 | 10 | 5 | 25 | 4 h | ~2.0 | [109] |
| 1 d | complete reduction | ||||||||
| 2 d | complete reduction | ||||||||
| – | 2 h | ~2.0 | |||||||
| 1 d | ~1.5 | ||||||||
| 2 d | ~1.5 | ||||||||
| Vibrio cholerae (3.8 log CFU g−1) | mussels | JA-1 | 250 | ~25 | 5 | ~21 | 0 h | 2.6 | [106] |
| 300 | 13 | complete reduction | |||||||
| – | 250 | 5 | 2.2 | ||||||
| 300 | 13 | complete reduction | |||||||
| salmon fillet | JA-1 | 250 | 5 | 2.3 | |||||
| 300 | 13 | complete reduction | |||||||
| – | 250 | 5 | 2.1 | ||||||
| 300 | 13 | complete reduction | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shymialevich, D.; Wójcicki, M.; Sokołowska, B. The Novel Concept of Synergically Combining: High Hydrostatic Pressure and Lytic Bacteriophages to Eliminate Vegetative and Spore-Forming Bacteria in Food Products. Foods 2024, 13, 2519. https://doi.org/10.3390/foods13162519
Shymialevich D, Wójcicki M, Sokołowska B. The Novel Concept of Synergically Combining: High Hydrostatic Pressure and Lytic Bacteriophages to Eliminate Vegetative and Spore-Forming Bacteria in Food Products. Foods. 2024; 13(16):2519. https://doi.org/10.3390/foods13162519
Chicago/Turabian StyleShymialevich, Dziyana, Michał Wójcicki, and Barbara Sokołowska. 2024. "The Novel Concept of Synergically Combining: High Hydrostatic Pressure and Lytic Bacteriophages to Eliminate Vegetative and Spore-Forming Bacteria in Food Products" Foods 13, no. 16: 2519. https://doi.org/10.3390/foods13162519







