Enzymatic Deacidification and Aroma Characteristics Analysis of Rapeseed Oil Using Self-Made Immobilized Lipase CALB@MCM-41-C8
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Surface Functionalization of MCM-41 and Lipase Immobilization
2.3. Characterization of MCM-41, MCM-41-C8, and CALB@MCM-41-C8
2.4. Rapeseed Oil Deacidification Using CALB@MCM-41-C8
2.5. Oil Content and Composition Analysis by Gas Chromatograph
2.6. Oil Quality and Aroma Characteristics Analysis
3. Results
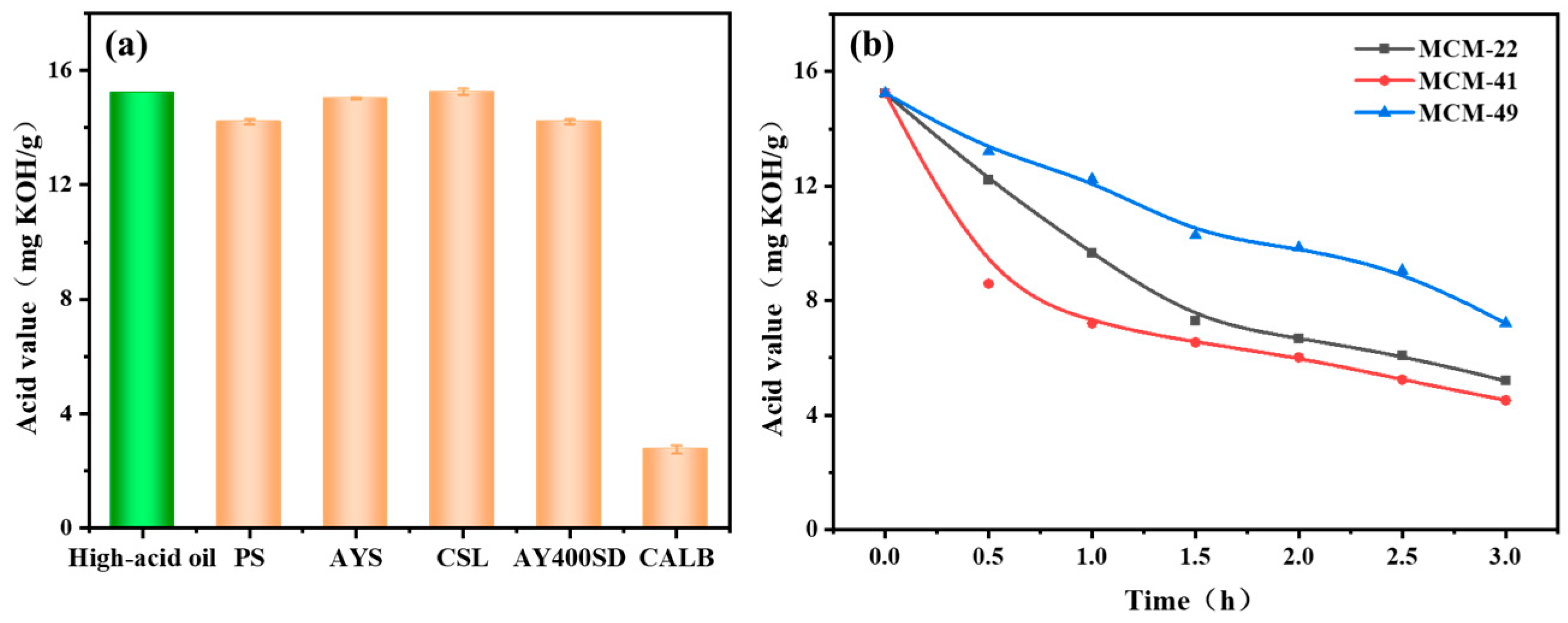
3.1. Screening of Lipase and Mesoporous Molecular Sieves
3.2. Characterization of CALB@MCM-41-C8
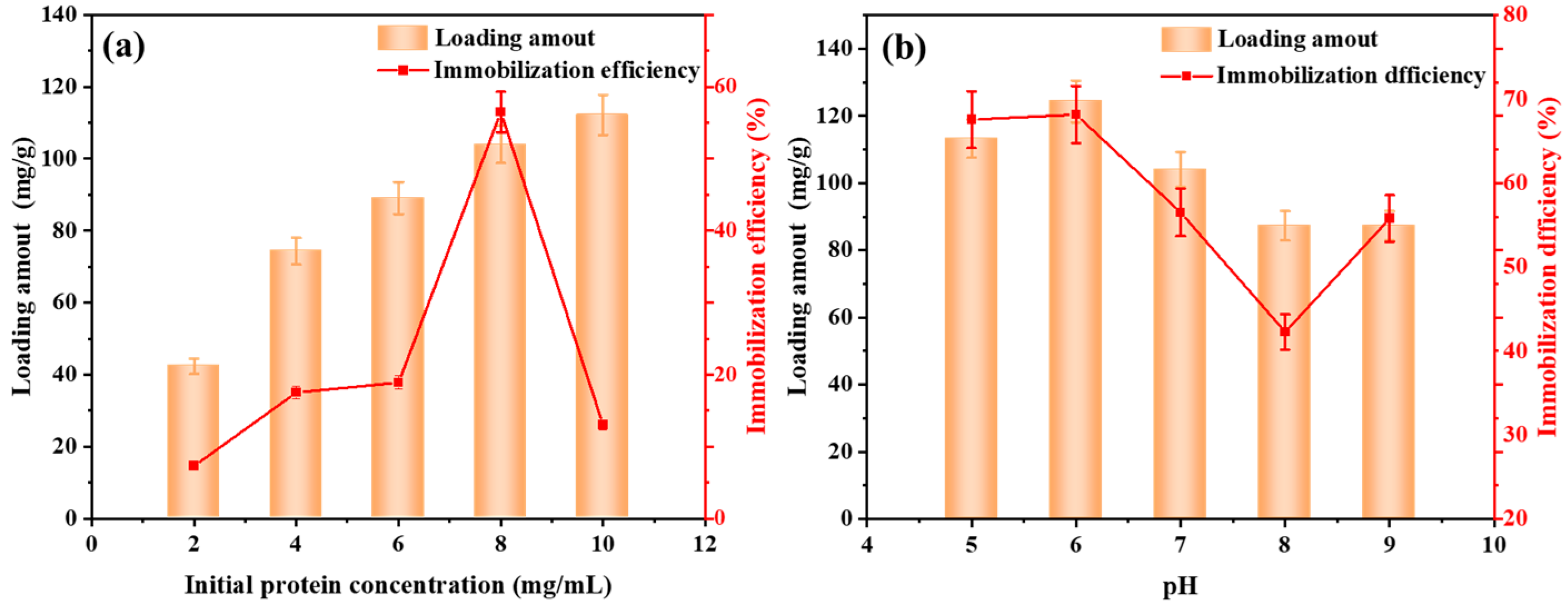
3.3. Optimization of Lipase Immobilization Conditions
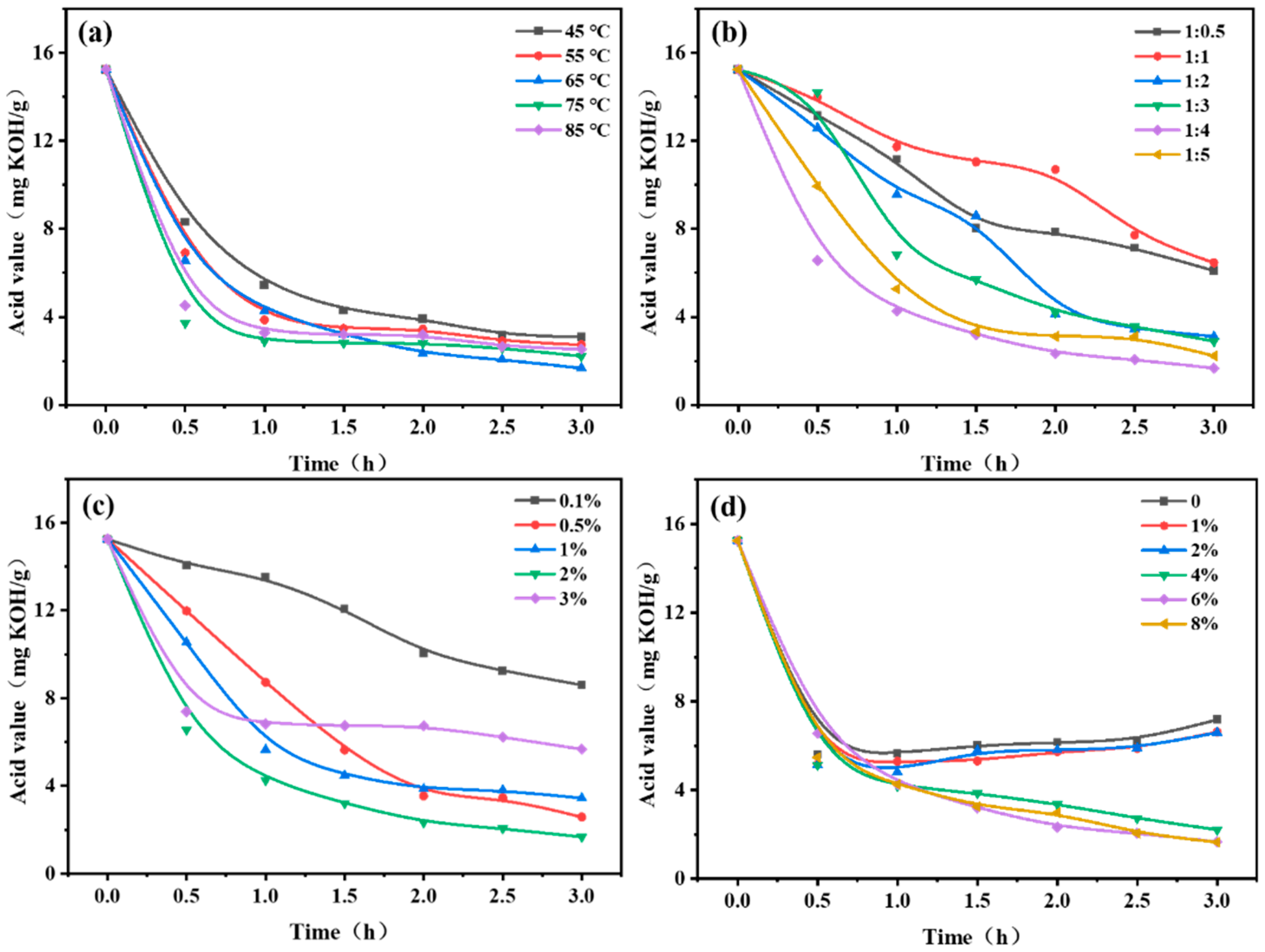
3.4. Optimization of Reaction Conditions
3.5. Oil Quality and Aroma Characteristics Evaluation
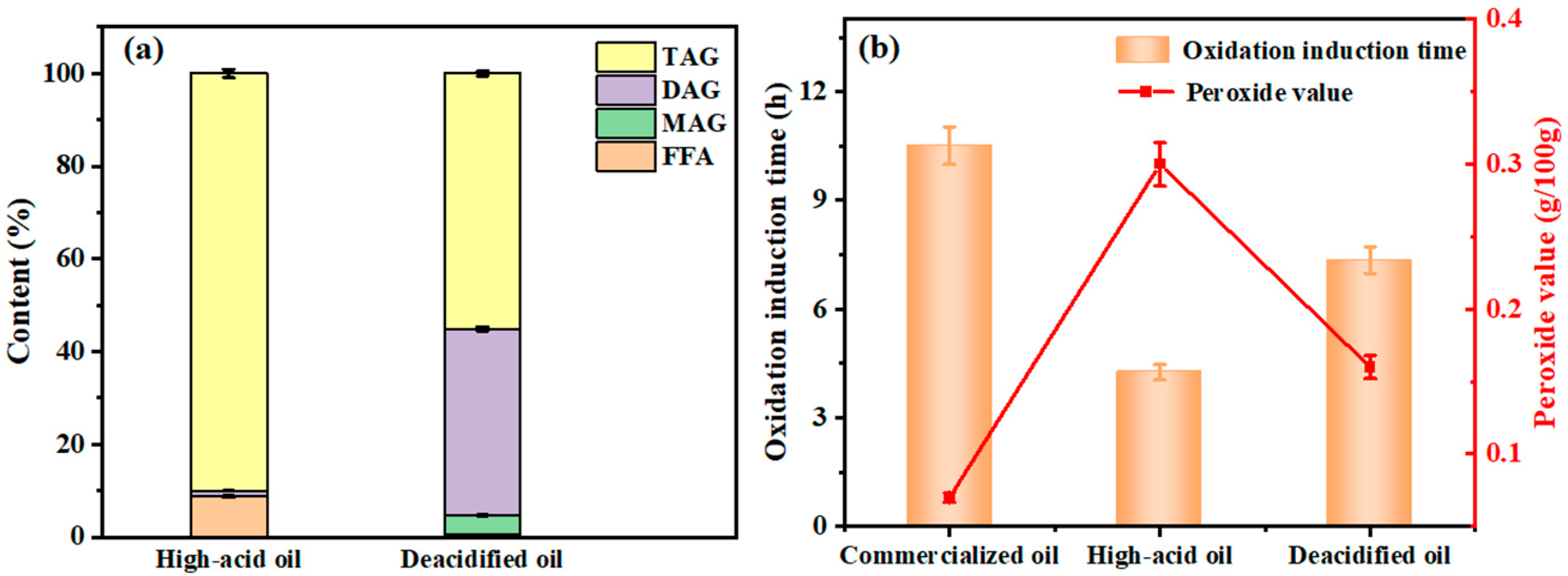
3.5.1. Lipid Composition in Rapeseed Oil
3.5.2. Analysis of Aroma Content in Rapeseed Oil
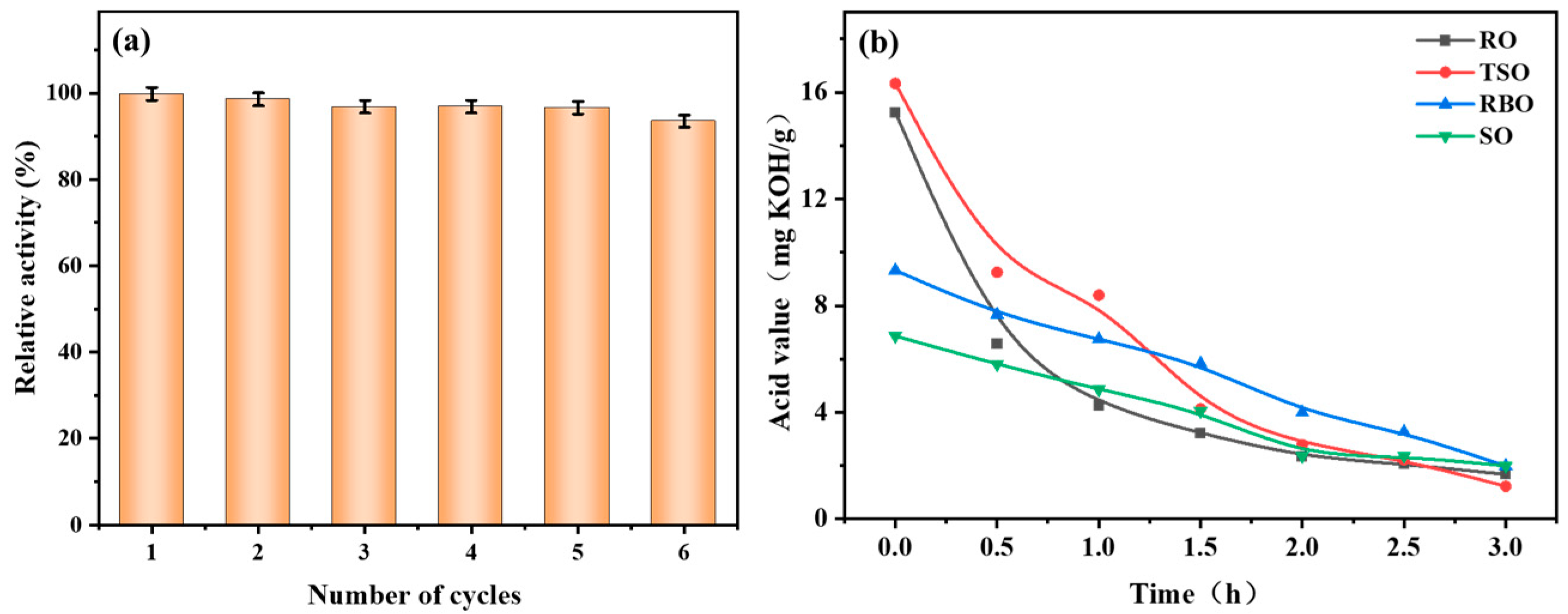
3.6. Reusability and Applicability of CALB@MCM-41-C8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Y.; Chen, S.; Yang, B.B.; Zhang, H.; Wang, X.G.; Granvogl, M.; Jin, Q.Z. Flavor of rapeseed oil: An overview of odorants, analytical techniques, and impact of treatment. Compr. Rev. Food Sci. Food Saf. 2021, 3983–4018. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Nian, B.; Shi, J.; Sun, X.; Du, R.; Tan, C.P.; Xu, Y.J.; Liu, Y. Influence of extraction technology on rapeseed oil functional quality: A study on rapeseed polyphenols. Food Funct. 2022, 13, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Siger, A.; Gawrysiak-Witulska, M.; Bartkowiak-Broda, I. Antioxidant (Tocopherol and canolol) content in rapeseed oil obtained from roasted yellow-seeded brassica napus. J. Am. Oil Chem. Soc. 2017, 94, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Koski, A.; Psomiadou, E.; Tsimidou, M.; Hopia, A.; Kefalas, P.; Heinonen, M. Oxidative stability and minor constituents of virgin olive oil and cold-pressed rapeseed oil. Eur. Food Res. Technol. 2002, 214, 294–298. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, C.; Zhou, Q.; Huang, F.H.; Liu, C.S.; Wang, H. Minor components and oxidative stability of cold-pressed oil from rapeseed cultivars in China. J. Food Compos. Anal. 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Rajendra, R.A.; Sudhakar, P.R.; Kumar, R.B.; Flyckt, K.S.; Shankhapal, A.R.; Oiha, R.; Everard, J.D.; Wanyne, L.L.; Ruddy, B.M.; Deonovic, B.; et al. Loss-of-function of triacylglycerol lipases are associated with low flour rancidity in pearl millet [Pennisetum glaucum (L.) R. Br.]. Front. Plant Sci. 2022, 13, 962667. [Google Scholar]
- Jahan, T. Comparative study of acid value and rancidity in commonly used oils and Fats. Int. J. Res. Appl. Sci. Eng. Technol. 2020, 8, 1429–1432. [Google Scholar] [CrossRef]
- Tuorila, H.; Cardello, A.V. Consumer response to an off flavor in juice in the presence of specific health claims. Food Qual. Prefer. 2002, 13, 561–569. [Google Scholar] [CrossRef]
- Haman, N.; Romano, A.; Asaduzzaman, M.; Ferrentino, G.; Biasioli, F.; Scampicchio, M. A Microcalorimetry study on the oxidation of linoleic acid and the control of rancidity. Talanta 2016, 164, 407–412. [Google Scholar] [CrossRef]
- Chiplunkar, P.P.; Pratap, A.P. Utilization of sunflower acid oil for synthesis of alkyd resin. Prog. Org. Coat. 2016, 93, 61–67. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, Y.W. Engine performance of high-acid oil-biodiesel through supercritical transesterification. ACS Omega 2024, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, S.; Yao, N.; Chu, C. A novel method for simultaneous degumming and deacidification of corn oil by miscella refining in one step. LWT-Food Sci. Technol. 2021, 137, 110480. [Google Scholar] [CrossRef]
- Pokorný, J. Book review: Bailey’s industrial oil & fat products. Edited by fereidoon shahidi. Eur. J. Lipid Sci. Technol. 2010, 108, 84–85. [Google Scholar]
- Xin, L.; Guo, L.; Edirs, S. An efficient deacidification process for safflower seed oil with high nutritional property through optimized ultrasonic-assisted technology. Molecules 2022, 27, 2305. [Google Scholar] [CrossRef] [PubMed]
- Tefan, N.G.; Iancu, P.; Pleu, V.; Călinescu, I.; Ignat, N.D. Highly efficient deacidification process for camelina sativa crude oil by molecular distillation. Sustainability 2021, 13, 2818. [Google Scholar] [CrossRef]
- Busto, M.; Carlos, R.V. Deacidification of vegetable oil by extraction with solvent recovery. Adsorption 2019, 25, 1397–1407. [Google Scholar] [CrossRef]
- Altuntas, A.H.; Ketenoglu, O.; Cetinbas, S.; Erdogdu, F.; Tekin, A. Deacidification of crude hazelnut oil using molecular distillation—Multiobjective optimization for free fatty acids and tocopherol. Eur. J. Lipid Sci. Technol. 2018, 1700369. [Google Scholar] [CrossRef]
- Rodrigues, C.E.; Gonçalves, C.B.; Marcon, E.C.; Batista, E.A.; Meirelles, A.J. Deacidification of rice bran oil by liquid–liquid extraction using a renewable solvent. Sep. Purif. Technol. 2014, 132, 84–92. [Google Scholar] [CrossRef]
- Wang, X.; Lu, J.; Liu, H.; Jin, Q.Z.; Wang, X.G. Improved deacidification of high acid rice bran oil by enzymatic esterification with phytosterol. Process Biochem. 2016, 1496–1502. [Google Scholar] [CrossRef]
- Zhong, N.; Kou, M.; Zhao, F.; Yang, F.H.; Lin, S.Y. Enzymatic Production of Diacylglycerols from High-Acid Soybean Oil. J. Am. Oil Chem. Soc. 2019, 967–974. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R. Immobilization of lipases—A review. Part I: Enzyme immobilization. ChemBioEng Rev. 2019, 6, 157–166. [Google Scholar] [CrossRef]
- Carballares, D.; Rocha-Martin, J.; Fernandez-Lafuente, R. Chemical amination of immobilized enzymes for enzyme coimmobilization: Reuse of the most stable immobilized and modified enzyme. Int. J. Biol. Macromol. 2022, 208, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, H.; Zhang, Z.; Yui, K.; Kobayashi, T.; Maeda, K. Transesterification of triolein and methanol with Novozym 435 using co-solvents. Fuel 2019, 263, 116600. [Google Scholar] [CrossRef]
- Hernández-Martín, E.; Otero, C. Different enzyme requirements for the synthesis of biodiesel: Novozym 435 and Lipozyme TL IM. Bioresour. Technol. 2008, 99, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Ungcharoenwiwat, P.; Canyuk, B.; H-Kittikun, A. Synthesis of jatropha oil based wax esters using an immobilized lipase from Burkholderia sp. EQ3 and Lipozyme RM IM. Process Biochem. 2016, 51, 392–398. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira Aría, L.; Barbosa, O.; Santos, J.; Rodrigues, R.C.; Berenguer-Murcia, A.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst. Catal. Sci. Technol. 2019, 2380–2420. [Google Scholar] [CrossRef]
- Reichardt, C.; Utgenannt, S.; Stahmann, K.P.; Klepel, O.; Barig, S. Highly stable adsorptive and covalent immobilization of thermomyces lanuginosus lipase on tailor-made porous carbon material. Biochem. Eng. J. 2018, 138, 63–73. [Google Scholar] [CrossRef]
- Matsuura, S.I.; Ikeda, T.; Chiba, M.; Yamamoto, K. Efficient production of γ-aminobutyric acid by glutamate decarboxylase immobilized on an amphiphilic organic-inorganic hybrid porous material. J. Biosci. Bioeng. 2021, 131, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. Struct. Funct. Interact. 2018, 120, 2530–2544. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Tang, J.J.; Wei, W.J.; Li, S.J. Recent progress in the syntheses and applications of multishelled hollow nanostructures. Mater. Chem. Front. 2020, 4, 1105–1149. [Google Scholar] [CrossRef]
- Atyaksheva, L.F.; Kasyanov, I.A.; Ivanova, I.I. Adsorptive immobilization of proteins on mesoporous molecular sieves and zeolites. Pet. Chem. 2019, 59, 327–337. [Google Scholar] [CrossRef]
- Lee, C.H.; Lin, T.S.; Mou, C.Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Bajda, T. A review on the application of zeolites and mesoporous silica materials in the removal of non-steroidal anti-inflammatory drugs and antibiotics from water. Materials 2021, 14, 4994. [Google Scholar] [CrossRef]
- Villa, C.C.; Galus, S.; Nowacka, M.; Magri, A.; Petriccione, M.; Gutiérrez, T.J. Molecular sieves for food applications: A review. Trends Food Sci. Technol. 2020, 102, 102–122. [Google Scholar] [CrossRef]
- Yu, W.H.; Wu, X.W.; Cheng, B.H.; Tao, T.Y.; Min, X.; Mi, R.Y.; Huang, Z.H.; Fang, M.H.; Liu, Y.G. Synthesis and Applications of SAPO-34 Molecular Sieves. Chem. A Eur. J. 2022, 28, e202102787. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Travis, K.P.; Burton, N.A.; Harding, J.H. A new method for the generation of realistic atomistic models of siliceous MCM-41. Microporous Mesoporous Mater. 2016, 228, 215–223. [Google Scholar] [CrossRef]
- Rui, Y.; Wu, X.; Ma, B.; Xu, Y. Immobilization of acetylcholinesterase on functionalized SBA-15 mesoporous molecular sieve for detection of organophosphorus and carbamate pesticide. Chin. Chem. Lett. 2018, 29, 1387–1390. [Google Scholar] [CrossRef]
- Lei, S.J.; Xu, Y.X.; Fan, G.F.; Xiao, M.; Pan, S.Y. Immobilization of naringinase on mesoporous molecular sieve MCM-41 and its application to debittering of white grapefruit. Appl. Surf. Sci. 2011, 257, 4096–4099. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zhang, Y.; Dong, C.Y.; Zhong, H.Y.; Gu, T.T.; Goh, K.; Han, Z.M.; Zheng, M.M.; Zhou, Y.B. Green and efficient synthesis of highly liposoluble and antioxidant L-ascorbyl esters by immobilized lipases. J. Clean. Prod. 2022, 379, 134772. [Google Scholar] [CrossRef]
- Lai, Y.D.; Li, D.M.; Liu, T.L.; Wan, C.Y.; Zhang, Y.; Zhang, Y.F.; Zheng, M.M. Preparation of functional oils rich in diverse medium and long-chain triacylglycerols based on a broadly applicable solvent-free enzymatic strategy. Food Res. Int. 2023, 164, 112338. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Zhang, Y.; Zhen, M.M.; Zhong, H.Y.; Zhu, H.; Wang, H.X.; Goh, K.; Zhong, J.T. Efficient and sustainable preparation of cinnamic acid flavor esters by immobilized lipase microarray. LWT 2023, 173, 114322. [Google Scholar] [CrossRef]
- Xu, L.J.; Zhang, Y.; Zivkovic, V.; Zhen, M.M.; Zhang, Y.; Deng, Q.C.; Zhou, Q. Deacidification of high-acid rice bran oil by the tandem continuous-flow enzymatic reactors. Food Chem. 2022, 393, 133440. [Google Scholar] [CrossRef] [PubMed]
- AOCS Official. Methods and Recommended Practices of the American Oil Chemists’ Society; Association of Official Analytical Chemists: Arlington, VA, USA, 2009. [Google Scholar]
- Yang, Y.; Chao, W.; Chen, Y.S.; Zhen, M.M.; Zhang, Y.; Deng, Q.C.; Zhou, Q. Aroma characteristics of flaxseed milk via GC–MS-O and odor activity value calculation: Imparts and selection of different flaxseed varieties. Food Chem. 2024, 432, 137095. [Google Scholar]
- Xu, L.; Wang, J.; Huang, F.; Zhen, M.M. An efficient and robust continuous-flow bioreactor for the enzymatic preparation of phytosterol esters based on hollow lipase microarray. Food Chem. 2022, 372, 131256. [Google Scholar] [CrossRef]
- Perin, G.B.; Felisberti, M.I. Mechanism and kinetics of lipase-catalyzed polycondensation of glycerol and sebacic acid: Influence of solvent and temperature. Biomacromolecules 2022, 23, 2968–2975. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.J.; Park, J.B.; Park, S. Esterification of secondary alcohols and multi-hydroxyl compounds by candida antarctica lipase B and subtilisin. Biotechnol. Bioprocess Eng. 2019, 24, 41–47. [Google Scholar] [CrossRef]
- Liu, T.L.; Fang, J.; Wang, X.Q.; Fan, Y.C.; Yuan, M. Synthesis of titanium containing MCM-41 from industrial hexafluorosilicic acid as epoxidation catalyst. Catal. Today 2017, 297, S0920586117301347. [Google Scholar] [CrossRef]
- Borzouee, F.; Varshosaz, J.; Cohan, R.A.; Norouzian, D.; Pirposhteh, R.T. A comparative analysis of different enzyme immobilization nanomaterials: Progress, constraints and recent trends. Curr. Med. Chem. 2021, 28, 3980–4003. [Google Scholar] [CrossRef]
- Barbosa, T.S.B.; Barros, T.R.B.; Barbosa, T.L.A.; Rodrigues, M.G.F. Green synthesis for MCM-41 and SBA-15 Silica using the waste mother liquor. Silicon 2022, 14, 6233–6243. [Google Scholar] [CrossRef]
- Das, K.S.; Bhunia, K.M.; Sinha, K.A. Synthesis, characterization, and biofuel application of mesoporous zirconium oxophosphates. ACS Catal. 2011, 1, 493–501. [Google Scholar] [CrossRef]
- Arita, K.; Yarimizu, S.; Moriguchi, M.; Inoue, T.; Asahara, H. Synthesis of zwitterionic phospholipid-connected silane coupling agents and their hybridization with graphene oxide. J. Oleo Sci. 2024, 73, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, S.; Ha, C.S. In-situ addition of graphene oxide for improving the thermal stability of superhydrophobic hybrid materials. Polym. Int. J. Sci. Technol. Polym. 2017, 116, 412–422. [Google Scholar] [CrossRef]
- Liu, D.M.; Chen, J.; Shi, Y.P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends Anal. Chem. 2018, 102, S0165993618300360. [Google Scholar] [CrossRef]
- Coutino-Gonzalez, E.; Manriquez, J.; Robles, I.; Espejel-Ayala, F. Synthesis of MCM-41 material from acid mud generated in the aluminum extraction of kaolinite mineral. Environ. Prog. 2019, 38, 13069.1–13069.7. [Google Scholar] [CrossRef]
- Ajmal, M.; Fieg, G. Intensification of lipase-catalyzed esterification using ultrasound: Process engineering perspectives. Chemise Ing. Tech. 2017, 89, 1367–1373. [Google Scholar] [CrossRef]
- Sri Kaja, B.; Lumor, S.; Besong, S.; Taylor, B.; Ozbay, G. Investigating enzyme activity of immobilized candida rugosa lipase. J. Food Qual. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Zou, D.; Wang, T.; Liu, T.Y.; Wang, L.Q.; Elfalleh, W.; Jiang, L.Z. Immobilized CALB catalyzed transesterification of soybean oil and phytosterol. Food Biophys. 2018, 13, 208–215. [Google Scholar] [CrossRef]
- Lee, C.; Sandig, B.; Buchmeiser, M.R.; Haumann, M. Supported ionic liquid phase (SILP) facilitated gas-phase enzyme catalysis—CALB catalyzed transesterification of vinyl propionate. Catal. Sci. Technol. 2018, 8, 2460–2466. [Google Scholar] [CrossRef]
- Chen, W.Y.; Kou, M.M.; Lin, S.Y.; Zhong, N.J. Effects of solvents on the glycerolysis performance of the SBA-15 supported lipases. J. Oleo Sci. 2021, 70, 385–395. [Google Scholar] [CrossRef]
- Xing, Y.L.; Wang, J.X.; Chen, L.; Wang, A.Q.; Li, F.; Wang, C.; Zhong, E.Q. Synthesis and characterization of Cu–BiVO4/MCM-41 composite catalysts with enhanced visible light photocatalytic activities. J. Mater. Sci. Mater. Electron. 2016, 27, 8633–8640. [Google Scholar] [CrossRef]
- Elena, D.G.; Sonia, Á.; Susana, L.; Álvarez, J.R. Low-temperature hydrophilic pervaporation of lactic acid esterification reaction media. Membranes 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhou, Y.H.; Huang, C.Q.; Jian, L.Z.; Lin, Z.P.; Lin, L.; Li, C.Z.; Ye, Y. Insight into the influence of plant oils on the composition of diacylglycerol fabricated by glycerolysis and esterification. Ind. Crops Prod. 2023, 204, 117324. [Google Scholar]
- Menalla, E.; Serna, J.G.; Cantero, D.; Cocero, M.G. Hydrothermal hydrolysis of triglycerides: Tunable and intensified production of diglycerides, monoglycerides, and fatty acids. Chem. Eng. J. 2024, 493, 152391. [Google Scholar] [CrossRef]
- Lee, J.W.; Zhang, Z.; Lai, M.O.; Tan, C.P.; Wang, Y. Diacylglycerol in food industry: Synthesis methods, functionalities, health benefits, potential risks and drawbacks. Trends Food Sci. Amp; Technol. 2020, 97, 114–125. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Tang, T.K.; Phuah, E.T.; Tan, C.P.; Wang, Y.; Li, Y.; Cheong, L.Z.; Lai, O.M. Production, safety, health effects and applications of diacylglycerol functional oil in food systems: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1–17. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Cuomo, F.; Macciola, V.; Lopez, F. Influence of free fatty acid content on the oxidative stability of red palm oil. Rsc Adv. 2016, 6, 101098–101104. [Google Scholar] [CrossRef]
- Chen, S.S.; Luo, S.Z.; Zheng, Z.; Zhao, Y.Y.; Pang, M.; Jiang, S.T. Enzymatic lipophilization of epicatechin with free fatty acids and its effect on antioxidative capacity in crude camellia seed oil. J. Sci. Food Agric. 2017, 97, 686–874. [Google Scholar] [CrossRef]
- Gurbuz, O.; Odor, B.; Rouseff, R. Comparison of cold pressed and essence orange oil oxidative stability using TI GCO and GC-MS. Dev. Food Sci. 2006, 43, 297–300. [Google Scholar]
- Zhou, Q.; Jia, X.; Yao, Y.Z.; Wang, B.; Wei, Q.C.; Zang, M.; Huang, F.H. Characterization of the aroma-active compounds in commercial fragrant rapeseed oils via monolithic material sorptive extraction. J. Agric. Food Chem. 2019, 67, 11454–11463. [Google Scholar] [CrossRef]
- Karangwa, E.; Zhang, X.; Murekatete, N.; Masamba, K.; Raymond, L.V.; Shabbar, A.; Zhang, Y.T.; Duhoranimana, E.; Muhoza, B.; Song, S.Q. Effect of substrate type on sensory characteristics and antioxidant capacity of sunflower Maillard reaction products. Eur. Food Res. Technol. 2015, 240, 939–960. [Google Scholar] [CrossRef]
- Matheis, K.; Granvogl, M. Characterisation of the key aroma compounds in commercial native cold-pressed rapeseed oil by means of the Sensomics approach. Eur. Food Res. Technol. Z. Fur Lebensm.-Unters. Und-Forschung. A 2016, 23, 1565–1575. [Google Scholar] [CrossRef]
- Zawistowski, J. Emulsions Comprising Comprising Non-Esterified Phytosterols in Tue Aqueous Puase: US11613818. U.S. Patent US20070141123A1, 29 June 2024. [Google Scholar]
- Zhang, L.Y.; Akhymetkan, S.; Chen, J.; Dong, Y.Y.; Gao, Y.; Yu, X.Z. Convenient method for the simultaneous production of high-quality fragrant rapeseed oil and recovery of phospholipids via electrolyte degumming. LWT 2022, 155, 112947. [Google Scholar] [CrossRef]
- Jiao, M.; Zhao, Y.Y.; Wu, R.L.; Hu, B.L.; Jia, C.H.; Rong, J.H.; Liu, R.; Zhao, S.M. Formation of AGEs in Penaeus vannamei fried with high oleic acid sunflower oil. Food Chem. X 2023, 19, 100869. [Google Scholar]
- Li, D.; Faiza, M.; Ali, S.; Wang, W.; Tan, C.P.; Yang, B.; Wang, Y. Highly efficient deacidification of high-acid rice bran oil using methanol as a novel acyl acceptor. Appl. Biochem. Biotechnol. 2018, 184, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
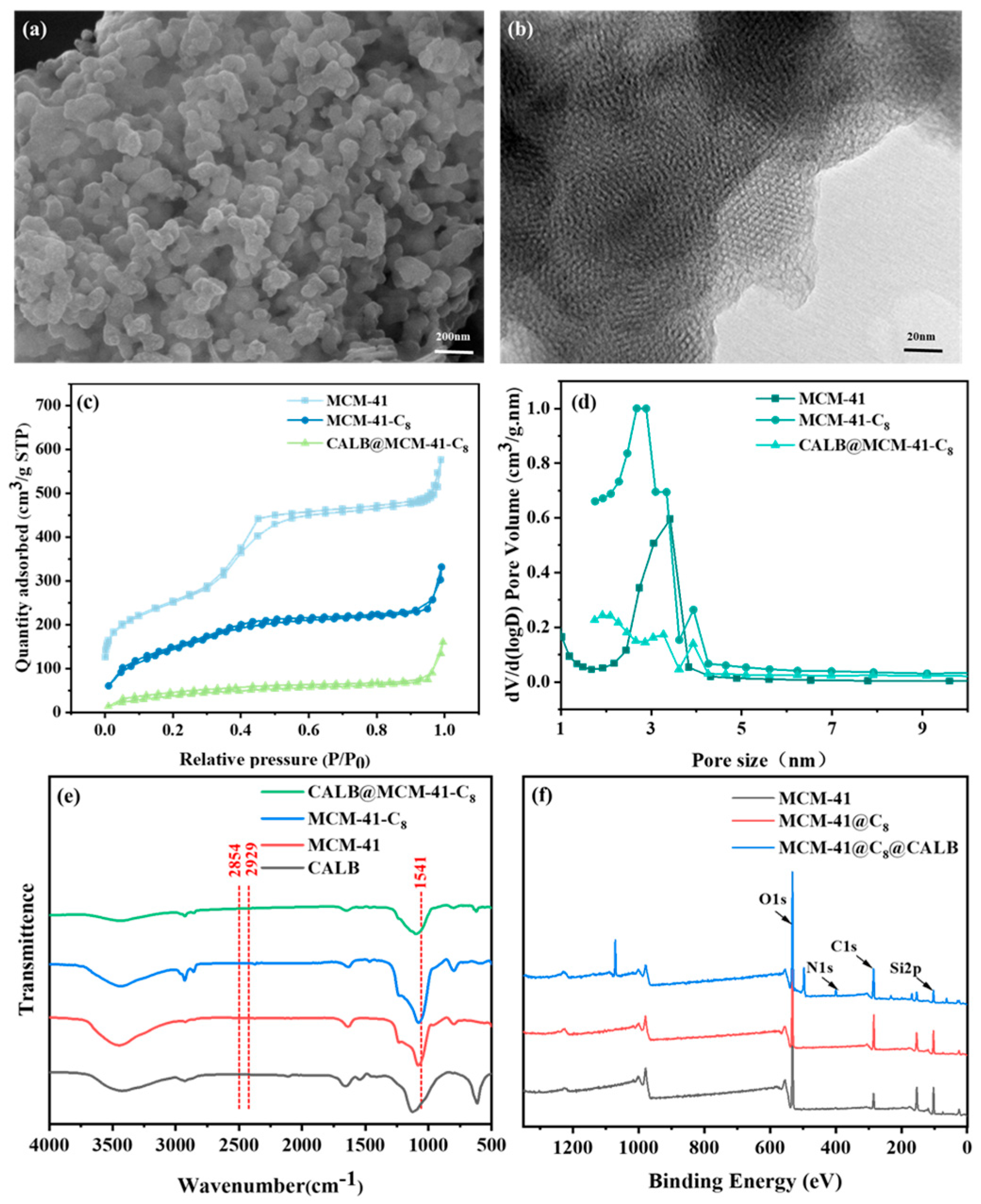
| Sample | Text Properties | Elemental Content (Atomic%) | |||||
|---|---|---|---|---|---|---|---|
| Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | C1s | N1s | O1s | Si2p | |
| MCM-41 | 1439.9 | 3.4 | 1.0 | 16.3 | 1.3 | 57.8 | 24.6 |
| MCM-41-C8 | 449.0 | 4.1 | 0.5 | 32.3 | 1.1 | 45.3 | 21.4 |
| CALB@MCM-41-C8 | 130.2 | 3.7 | 0.1 | 37.3 | 4.1 | 46.8 | 11.8 |
| Serial Number | Volatile Compounds | Odor Description | Aroma Content of Rapeseed Oil (μg/kg) | ||
|---|---|---|---|---|---|
| Commercialized Rapeseed Oil | High-Acid Rapeseed Oil | Deacidified Rapeseed Oil | |||
| Aldehydes | |||||
| 1 | Hexanal | Fatty, bitter almond aroma | |||
| 2 | Benzaldehyde | ||||
| 3 | Heptanal | n.d. | |||
| 4 | Furfural | ||||
| 5 | Benzeneacetaldehyde | ||||
| Subtotal | 5.00 | 5.74 | 37.63 | ||
| Sulfur-containing compounds | |||||
| 1 | Dimethyl sulfide | Smoky aroma | |||
| 2 | Disulfide, dimethyl | n.d. | |||
| 3 | Dimethyl sulfone | n.d. | |||
| Subtotal | 0.59 | 0.24 | 0.19 | ||
| Nitriles | |||||
| 1 | 2-Butenenitrile | Pickled vegetable aroma, Spicy and pungent | |||
| 2 | 3-Butenenitrile | n.d. | |||
| 3 | 4-Penenonitrile | n.d. | |||
| 4 | 5-Hexenonitrile | n.d. | |||
| 5 | 5-Methylthiopentonit-rile | n.d. | n.d. | ||
| 6 | Phenylpropionitrile | ||||
| 7 | n-heptonitrile | n.d. | n.d. | ||
| 8 | (E, E)-2,4-pentadienorile | ||||
| 9 | (E, Z)-2,4-pentadienorile | n.d. | n.d. | ||
| Subtotal | 19.36 | 16.37 | 2.59 | ||
| Heterocyclics | |||||
| 1 | 2,5-Dimethylpyrazine | Roasted aroma | |||
| 2 | 2-Amylfuran | n.d. | |||
| 3 | 2,6-Dimethylpyrazine | n.d. | |||
| 4 | 6-Methyl-2-ethylpyrazine | n.d. | |||
| 5 | 5-Methyl-2-ethylpyrazine | n.d. | |||
| 6 | 2,3,5-Trimethylpyrazine | ||||
| 7 | 2-Methyl-3,5-diethylpyrazine | ||||
| 8 | 2-Ethyl-3,5-dimethylpyrazine | ||||
| Subtotal | 10.61 | 5.29 | 1.52 | ||
| Acids | |||||
| 1 | Acetic acid | Acerbity | |||
| 2 | Propionic acid | n.d. | |||
| 3 | Hexanoic acid | n.d. | n.d. | ||
| 4 | Pelargonic acid | n.d. | n.d. | ||
| Subtotal | 7.24 | 8.07 | 0.32 | ||
| Ketones | |||||
| 1 | 5-Methylfuranone | Burnt, Meaty | n.d. | ||
| 2 | Furanone | ||||
| Subtotal | 0.6 | 0.28 | 0.09 | ||
| Alcohols | |||||
| 1 | Isopropanol | Mellow, malty | n.d. | n.d. | |
| 2 | Phenylethanol | n.d. | |||
| 3 | 5-Methyl-2-furanoformaldehyde | ||||
| 4 | Cyclobutanol | ||||
| Subtotal | 1.88 | 0.58 | 0.3 | ||
| Phenols | |||||
| 1 | Y-butyrolactone | Creamy, sweet | |||
| 2 | 4-Vinyl-2-methoxyphenol | n.d. | |||
| 3 | 4-Vinyl-2,6-dimethoxyphenol | n.d. | |||
| Subtotal | 1.84 | 3.07 | 1.75 | ||
| Total | 47.12 | 34.35 | 44.39 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Liu, T.; Liu, R.; Zhou, Q.; Zhou, Y.; Zhang, Y.; Zheng, M. Enzymatic Deacidification and Aroma Characteristics Analysis of Rapeseed Oil Using Self-Made Immobilized Lipase CALB@MCM-41-C8. Foods 2024, 13, 2539. https://doi.org/10.3390/foods13162539
Liu Z, Liu T, Liu R, Zhou Q, Zhou Y, Zhang Y, Zheng M. Enzymatic Deacidification and Aroma Characteristics Analysis of Rapeseed Oil Using Self-Made Immobilized Lipase CALB@MCM-41-C8. Foods. 2024; 13(16):2539. https://doi.org/10.3390/foods13162539
Chicago/Turabian StyleLiu, Zhonghui, Tieliang Liu, Run Liu, Qi Zhou, Yandaizi Zhou, Yi Zhang, and Mingming Zheng. 2024. "Enzymatic Deacidification and Aroma Characteristics Analysis of Rapeseed Oil Using Self-Made Immobilized Lipase CALB@MCM-41-C8" Foods 13, no. 16: 2539. https://doi.org/10.3390/foods13162539
APA StyleLiu, Z., Liu, T., Liu, R., Zhou, Q., Zhou, Y., Zhang, Y., & Zheng, M. (2024). Enzymatic Deacidification and Aroma Characteristics Analysis of Rapeseed Oil Using Self-Made Immobilized Lipase CALB@MCM-41-C8. Foods, 13(16), 2539. https://doi.org/10.3390/foods13162539






