Change in Sunflower Oil Quality and Safety Depending on Number of Deodorisation Cycles Used
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Their Refining and Deodorisation Conditions
2.2. Fatty Acids Composition and Content
2.3. Peroxide Value Measurement (PV)
2.4. Anisidine Value (AV)
2.5. Volatile Profile Analysis
2.5.1. Extraction of SFO’s Volatiles by HS-SPME
2.5.2. GC–FID and GC–MS Analysis
2.5.3. Identification of Volatiles
2.6. MCPD Esters and Glycidyl Esters Measurement
2.7. Statistics
3. Results
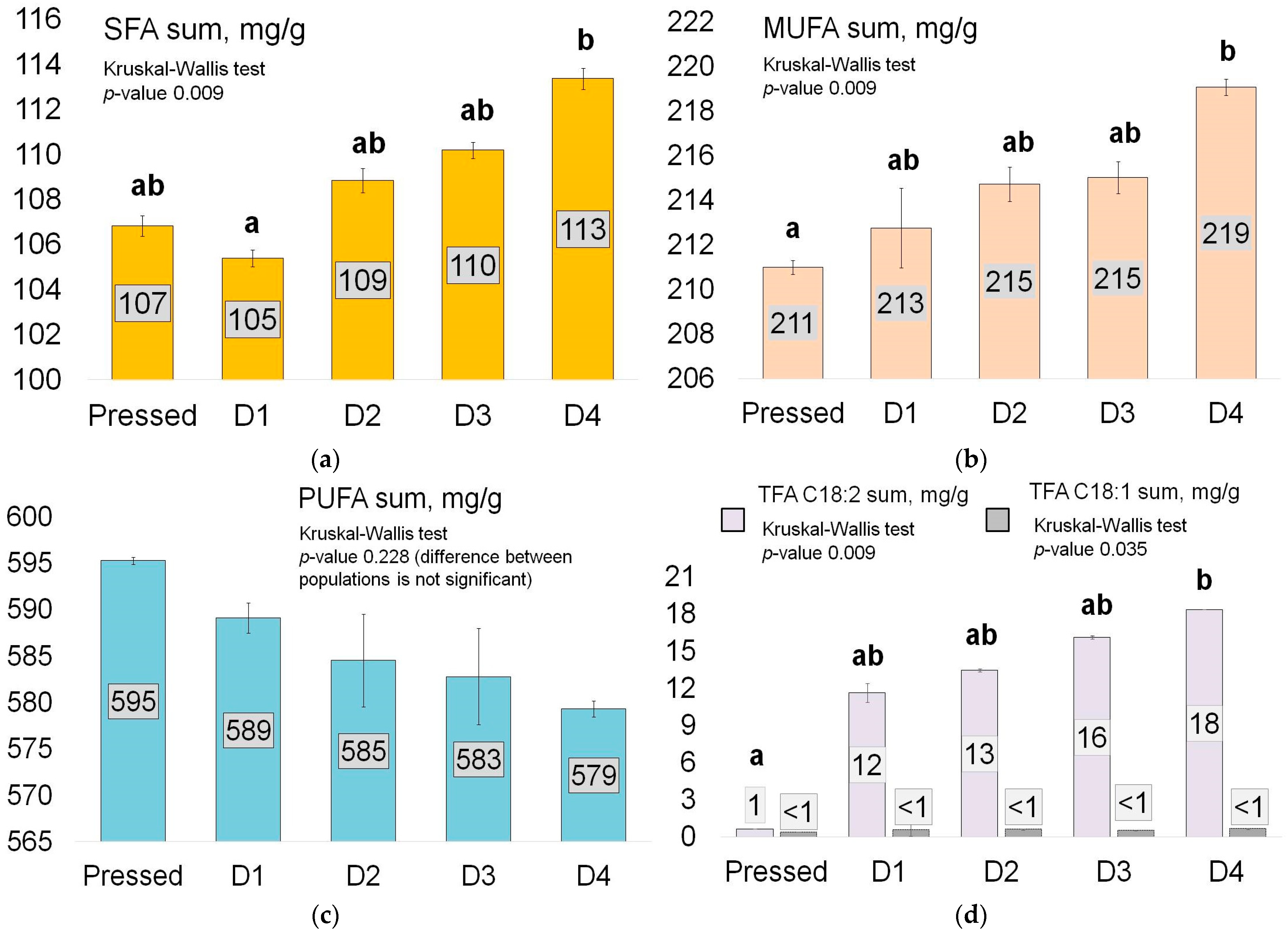
3.1. Change in Fatty Acids
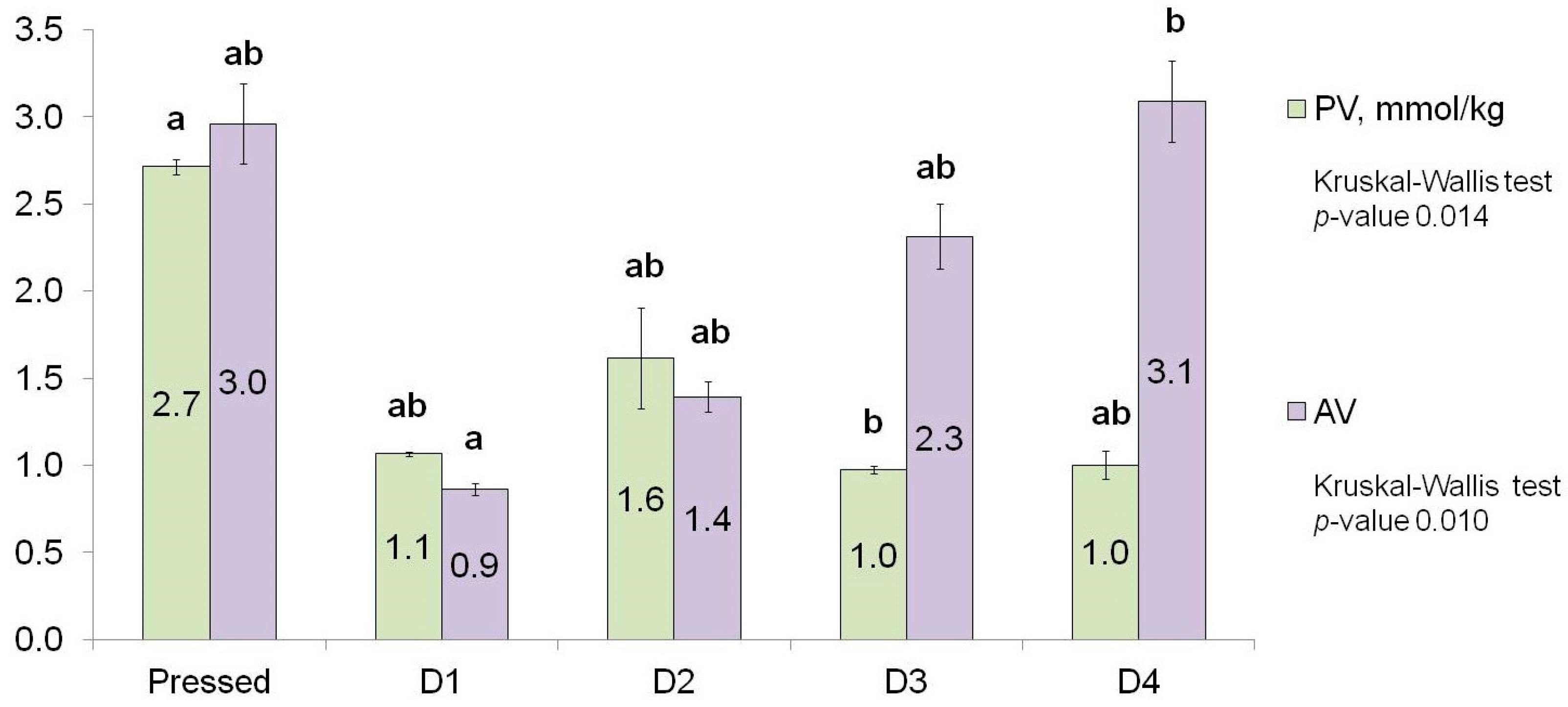
3.2. PV and AV Measurement
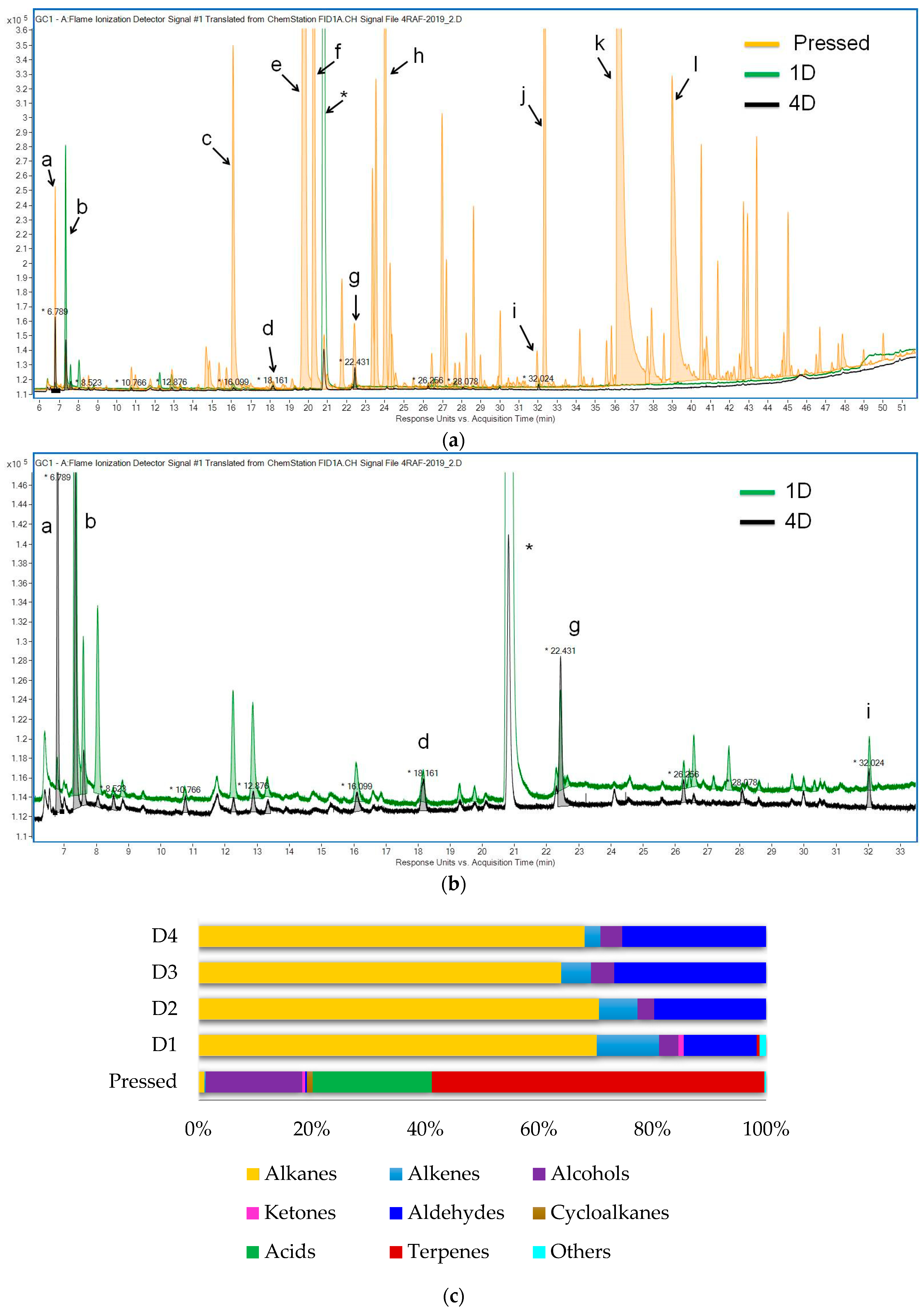
3.3. Change in Volatile Profile
3.4. MCPD Fatty Acid Esters and Glycidol Fatty Acid Esters Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vetter, W.; Schröder, M.; Lehnert, K. Differentiation of refined and virgin edible oils by means of the trans—And cis-phytol isomer distribution. J. Agric. Food Chem. 2012, 60, 6103–6107. [Google Scholar] [CrossRef]
- Durmaz, G.; Gökmen, V. Effect of refining on bioactive composition and oxidative stability of hazelnut oil. Food Res. Int. 2019, 116, 586–591. [Google Scholar] [CrossRef]
- FEDIOL Code of Practice for the Transport in Bulk of Oils into or within the European Union (Oils and Fats Which Are to Be (or likely to Be) Used for Human Consumption). FEDIOL The European Vegetable Oil and Proteinmeal Industry. 26 June 2014. Ref. 14COD152. Available online: https://www.fediol.eu/data/14COD152%20COP%20Transport%20in%20bulk%20of%20oils%20into%20or%20within%20the%20EU.pdf (accessed on 7 June 2024).
- Practical Guide to Previous Cargo(es) Restrictions for Means of Transport and Tank Lining as per FEDIOL Code of Practice for the Transport in Bulk into or within the European Union of Oils and Fats Which Are to be (or likely to Be) Used for Human Consumption. FEDIOL The European Vegetable Oil and Proteinmeal Industry. 26 June 2014. 14COD153. Available online: https://www.fediol.eu/data/314014COD153%20Practical%20guide%20to%20previous%20cargoes%20restrictions%20_1_.pdf (accessed on 7 June 2024).
- The Eurasian Economic Commission. The Decision “On Amendments to the Technical Regulations of the Customs Union “Technical Regulations for Fat and Oil Products” (TR CU 024/2011)” (in Russian). Available online: https://docs.eaeunion.org/pd/en-us/0104468/pd_18122019 (accessed on 15 August 2024).
- Oey, S.B.; van der Fels-Klerx, H.J.; Fogliano, V.; van Leeuwen, S.P.J. Effective physical refining for the mitigation of processing contaminants in palm oil at pilot scale. Food Res. Int. 2020, 138 Pt A, 109748. [Google Scholar] [CrossRef]
- The Codex Alimentarius. Fats, Oils and Related Products, 2nd ed.; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2001; Available online: https://www.fao.org/3/y2774e/y2774e00.htm (accessed on 1 June 2024).
- Shahidi, F. (Ed.) Edible oil and fat products: Specialty oils and oil products. In Bailey’s Industrial Oil and Fat Products, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 3, p. 564. [Google Scholar]
- Özdikicierler, O.; Yemişçioğlu, F.; Başaran, N.; Önen, F. Multi-factor optimization of canola oil deodorisation parameters and evaluation of linolenic acid isomerization kinetics during pilot-scale deodorisation. J. Food Meas. Charact. 2021, 15, 604–619. [Google Scholar] [CrossRef]
- Yu, D.; Dong, T.; Zhang, L.; Zhou, X.; Wang, L.; Yang, F.; Liu, T. Effects of different deodorisation methods on the oxidation of sterol components in rice bran oil. Food Chem. 2023, 404, 134568. [Google Scholar] [CrossRef] [PubMed]
- List, G.R.; Evans, C.D.; Warner, K.; Beal, R.E.; Kwolek, W.F.; Black, L.T.; Moulton, K.J. Quality of oil from damaged soybeans. J. Am. Oil Chem. Soc. 1977, 54, 8–14. [Google Scholar] [CrossRef]
- ben Hammouda, I.; Freitas, F.; Ammar, S.; Da Silva, M.D.R.G.; Bouaziz, M. Comparison and characterization of volatile compounds as markers of oils stability during frying by HS–SPME-GC/MS and Chemometric analysis. J. Chromatogr. B 2017, 1068–1069, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.G.; Gah, C.; Steenbergen, H.; Rosing, E.; Spraul, M. A Fast GC-MS-Based Method for Efficacy Assessment of Natural Anti-Oxidants for Inhibiting Lipid Oxidation. J. AOAC Int. 2021, 104, 260–266. [Google Scholar] [CrossRef]
- Abraham, K.; Hielscher, J.; Kaufholz, T.; Mielke, H.; Lampen, A.; Monien, B. The hemoglobin adduct N-(2,3-dihydroxypropyl)-valine as biomarker of dietary exposure to glycidyl esters: A controlled exposure study in humans. Arch. Toxicol. 2019, 93, 331–340. [Google Scholar] [CrossRef]
- Huang, G.; Gao, B.; Xue, J.; Cheng, Z.; Sun, X.; Zhang, Y.; Yu, L. Toxicokinetics and metabolism of 3-monochloropropane 1,2-diol (3-MCPD) dipalmitate in Sprague Dawley rats. J. Agric. Food Chem. 2018, 66, 11672–11680. [Google Scholar] [CrossRef]
- Frenzel, F.; Oberemm, A.; Lampen, A.; Braeuning, A. Proteomic effects of repeated-dose oral exposure to 2-monochloropropanediol and its dipalmitate in rat testes. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 116 Pt B, 354–359. [Google Scholar] [CrossRef]
- European Food Safety Authority. Risks for human health related to the presence of 3- and 2-monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl fatty acid esters in food EFSA Panel on Contaminants in the Food Chain. EFSA J. 2016, 14, e04426. [Google Scholar]
- Omara, T.; Kigenyi, E.; Laker, F.; Adokorach, M.; Otim, G.; Kalukusu, R.; Musau, B.; Kagoya, S.; Victoria Nakabuye, B. Effects of Continuous Deep-fat Frying on the Physicochemical Properties of Assorted Brands of Edible Cooking Oils Sold in Greater Metropolitan Kampala. Asian J. Appl. Chem. Res. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Jurid, L.S.; Zubairi, S.I.; Kasim, Z.M.; Kadir, I.A.A. The effect of repetitive frying on physicochemical properties of refined, bleached and deodorised Malaysian tenera palm olein during deep-fat frying. Arab. J. Chem. 2020, 13, 6149–6160. [Google Scholar] [CrossRef]
- Golay, P.A.; Moulin, J.; Alewijn, M.; Braun, U.; Choo, L.F.; Cruijsen, H.; Delmonte, P.; Fontecha, J.; Holroyd, S.; Hostetler, G.; et al. Determination of labeled fatty acids content in milk products, infant formula, and adult/pediatric nutritional formula by capillary gas chromatography: Collaborative study, final action 2012.13. J. AOAC Int. 2016, 99, 210–222. [Google Scholar] [CrossRef] [PubMed]
- AOCS. Peroxide Value, Acetic Acid, Isooctane Method. AOCS Official Method Cd 8b-90 Reapproved 2017. 2009. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details/productId/111547 (accessed on 6 June 2024).
- IFRA Analytical Method. Determination of the Peroxide Value. 2019. Available online: https://ifrafragrance.org/docs/default-source/guidelines/20190910-revised-ifra-analytical-method-on-peroxide-value.pdf?sfvrsn=c4a931e2_0 (accessed on 30 May 2024).
- ISO 6885:2016; Animal and Vegetable Fats and Oils. Determination of Anisidine Value. International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/69593.html (accessed on 1 June 2024).
- National Library of Medicine. PubChem. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 1 June 2024).
- National Institute of Standards and Technology. NIST Chemistry WebBook, SRD 69. 2023. Available online: https://webbook.nist.gov/chemistry/name-ser/ (accessed on 1 June 2024).
- The Good Scents Company. (1980–2021). The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/index.html (accessed on 1 June 2024).
- Makarenko, M.A.; Malinkin, A.D.; Bessonov, V.V. Gas chromatography/tandem mass spectrometry with long-term alkaline transesterification for the determination of esters of monochloropropanediols and glycidol with fatty acids in edible vegetable oils and fats. Gig. Sanit. (Hyg. Sanit. Russ. J.) 2021, 100, 640–646. (In Russian) [Google Scholar] [CrossRef]
- Liu, X.; Zhou, S.; Jiang, Y.; Xu, X. Optimization of Deodorisation Design for Four Different Kinds of Vegetable Oil in Industrial Trial to Reduce Thermal Deterioration of Product. J. Am. Oil Chem. Soc. 2021, 98, 475–483. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Wen, S.; Sun, Y.; Chen, J.; Gao, Y.; Sagymbek, A.; Yu, X. Analytical methods for determining the peroxide value of edible oils: A mini-review. Food Chem. 2021, 358, 129834. [Google Scholar] [CrossRef]
- Shahidi, F. Bailey’s Industrial Oil and Fat Products, 6th ed.; Wiley: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring oxidative stability and changes in key volatile compounds in edible oils during ambient storage through HS-SPME/GC–MS. Int. J. Food Prop. 2018, 20 (Suppl. 3), S2926–S2938. [Google Scholar] [CrossRef]
- Ratcliffe, N.; Wieczorek, T.; Drabińska, N.; Gould, O.; Osborne, A.; De Lacy Costello, B. A mechanistic study and review of volatile products from peroxidation of unsaturated fatty acids: An aid to understanding the origins of volatile organic compounds from the human body. J. Breath Res. 2020, 14, 034001. [Google Scholar] [CrossRef]
- Warner, K.; Michael Eskin, N.A. Methods to Access Quality and Stability of Oils and Fat-Containing Foods, 1st ed.; AOCS Publishing: New York, NY, USA, 1995. [Google Scholar] [CrossRef]
- Oueslati, I.; Manaï, H.; Madrigal-Martínez, M.; Martínez-Cañas, M.A.; Sánchez-Casas, J.; Zarrouk, M.; Flamini, G. Volatile molecular markers of VOO Thermo-oxidation: Effect of heating processes, macronutrients composition, and olive ripeness on the new emitted aldehydic compounds. Food Res. Int. 2018, 106, 654–665. [Google Scholar] [CrossRef]
- Ivanova-Petropulos, V.; Mitrev, S.; Stafilov, T.; Markova, N.; Leitner, E.; Lankmayr, E.; Siegmund, B. Characterisation of traditional Macedonian edible oils by their fatty acid composition and their volatile compounds. Food Res. Int. 2015, 77, 506–514. [Google Scholar] [CrossRef]
- Santos, J.E.R.; Villarino, B.J.; Zosa, A.R.; Dayrit, F.M. Analysis of volatile organic compounds in virgin coconut oil and their sensory attibutes. Philipp. J. Sci. 2011, 140, 161–171. [Google Scholar]
- Kremer, J.I.; Pickard, S.; Stadlmair, L.F.; Glaß-Theis, A.; Buckel, L.; Bakuradze, T.; Eisenbrand, G.; Richling, E. Alkylpyrazines from Coffee are Extensively Metabolized to Pyrazine Carboxylic Acids in the Human Body. Mol. Nutr. Food Res. 2019, 63, 1801341. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Cabo, N.; Ibargoitia, M.L.; Ruiz, A. Study of both Sunflower Oil and Its Headspace throughout the Oxidation Process. Occur. Headspace Toxic Oxyg. Aldehydes. J. Agric. Food Chem. 2005, 53, 1093–1101. [Google Scholar] [CrossRef]
- Petersen, K.D.; Kleeberg, K.K.; Jahreis, G.; Fritsche, J. Assessment of the oxidative stability of conventional and high-oleic sunflower oil by means of solid-phase microextraction-gas chromatography. Int. J. Food Sci. Nutr. 2012, 63, 160–169. [Google Scholar] [CrossRef]
- Becalski, A.; Feng, S.; Lau, B.P.Y.; Zhao, T. A pilot survey of 2- and 3-monochloropropanediol and glycidol fatty acid esters in foods on the Canadian market 2011–2013. Journal of Food Composition and Analysis 2015, 37, 58–66. [Google Scholar] [CrossRef]
- German Federal Institute for Risk Assessment (BfR). Chemical Food Safety. A/2011. 2011. Available online: https://www.bfr.bund.de/en/presseinformation/2011/A/chemical_food_safety-59770.html (accessed on 30 May 2024).
- Guo, Q.; Ha, Y.; Li, Q.; Jin, J.; Deng, Z.; Li, Y.; Zhang, S. Impact of additives on thermally-induced trans isomers in 9c,12c linoleic acid triacylglycerol. Food Chem. 2015, 174, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, H.; Shoemaker, C.F.; Wang, S.C. The Effect of Different Cold Storage Conditions on the Compositions of Extra Virgin Olive Oil. J. Am. Oil Chem. Soc. 2014, 91, 1559–1570. [Google Scholar] [CrossRef]
- Goodrich, P.; O’Hara, E.; Atkins, M.P.; Hamer, C.K. Process for Removing Chloropropanols and/or Glycidol, or Their Fatty Acid Esters, from Glyceride Oil, and an Improved Glyceride Oil Refining Process Comprising the Same. U.S. Patent WO2016189328A1, 1 December 2016. [Google Scholar]
- Pudel, F.; Benecke, P.; Fehling, P.; Freudenstein, A.; Matthäus, B.; Schwaf, A. On the necessity of edible oil refining and possible sources of 3-MCPD and glycidyl esters. Eur. J. Lipid Sci. Technol. 2011, 113, 368–373. [Google Scholar] [CrossRef]
- Matthäus, B.; Pudel, F. Mitigation of MCPD and Glycidyl Esters in Edible Oils. In Processing Contaminants in Edible Oils: MCPD and Glycidyl Esters; AOCS Press: Champaign, IL, USA, 2014; pp. 23–55. [Google Scholar]
- Goodrich, P.; O’Hara, E.; Atkins, M.P.; Hamer, C.K. A Process for Refining Glyceride Oil Comprising a Basic Ionic Liquid Treatment. U.S. Patent WO2016189333A1, 1 December 2016. [Google Scholar]
- Official Journal of the European Union. Commission Regulation (EU) 2020/1322 of 23 September 2020 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of 3-Monochloropropanediol (3-MCPD), 3-MCPD Fatty Acid Esters and Glycidyl Fatty Acid Esters in Certain Foods. Available online: https://eur-lex.europa.eu/eli/reg/2020/1322/oj (accessed on 7 June 2024).
- Oey, S.B.; van der Fels-Klerx, H.J.; Fogliano, V.; van Leeuwen, S.P.J. Mitigation Strategies for the Reduction of 2- and 3-MCPD Esters and Glycidyl Esters in the Vegetable Oil Processing Industry. Compr. Rev. Food Sci. Food Saf. 2019, 18, 349–361. [Google Scholar] [CrossRef] [PubMed]

| Pressed SFO | Refined and Deodorised SFO | ||||
|---|---|---|---|---|---|
| Sample code | Pressed | D1 | D2 | D3 | D4 |
| Number of refining cycles | 0 | 1 | 1 | 1 | 1 |
| Number of deodorisation cycles | 0 | 1 | 2 | 3 | 4 |
| PV, mmol/kg | 3.5 | 0.3 | 1.1 | 0.2 | 0.5 |
| Type of Oil | Pressed | D1 | D2 | D3 | D4 | Parent Fatty Acid | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound Name | CAS | IK | IKt | Aroma | T’R | ||||||
| Alkanes (sum) | 0.73 | 0.77 | 0.31 | 0.30 | 0.40 | ||||||
| Pentane | 109-66-0 | 499 | 500 | 0.43 | 0.41 | 0.02 | 0.14 | 0.18 | 0.16 | Ol/L | |
| Hexane | 110-54-3 | 601 | 600 | 0.99 | 0.22 | 0.74 | 0.16 | 0.11 | 0.23 | ||
| Heptane | 142-82-5 | 696 | 700 | sweet, ethereal | 2.17 | 0.02 | - | 0.00 1 | 0.00 1 | 0.00 1 | Ol |
| Octane | 111-65-9 | 800 | 800 | gasoline | 4.40 | 0.08 | 0.01 | 0.01 | 0.00 1 | 0.00 1 | Ol |
| Alkenes (sum) | 0.16 | 0.12 | 0.03 | 0.02 | 0.02 | ||||||
| 1-Heptene 2 | 592-76-7 | 749 | 731-748 | 0.66 | 0.01 | - | - | - | - | L | |
| 1-Octene | 111-66-0 | 851 | 822-892 | gasoline | 5.99 | - | 0.03 | 0.01 | 0.00 1 | 0.01 | |
| (Z)-2-Octene | 7642-04-8 | 868 | 862-882 | 6.51 | 0.12 | 0.07 | 0.02 | 0.01 | 0.01 | L | |
| (E)-2-Octene 2 | 13389-42-9 | 880 | 852-867 | 6.97 | 0.03 | 0.01 | 0.01 | 0.01 | 0.00 | ||
| Alcohols (sum) | 11.56 | 0.04 | 0.01 | 0.02 | 0.02 | ||||||
| Ethanol | 64-17-5 | 939 | 883-972 | 9.73 | 1.63 | 0.04 | 0.01 | 0.02 | 0.02 | ||
| 1-Propanol | 71-23-8 | 1064 | 988-1086 | alcoholic, fermented, fusel, mouldy | 13.92 | 3.75 | - | - | - | - | |
| 2-Methylpropanol | 78-83-1 | 1119 | 1043-1129 | ethereal, wine, bark | 16.05 | 0.35 | |||||
| 2-Propen-1-ol | 107-18-6 | 1141 | 1097-1167 | spicy, mustard | 16.99 | 0.39 | |||||
| 2-Methylbutanol | 137-32-6 | 1220 | 1158-1244 | fried, wine, onion, fruit, fusel, alcohol, whiskey | 20.62 | 1.09 | |||||
| 1-Pentanol | 71-41-0 | 1265 | 1200-1294 | fusel, oils, sweet | 22.26 | 0.62 | L | ||||
| 1-Hexanol 2 | 111-27-3 | 1368 | 1308-1349 | essential, fusel, oily, fruity, alcoholic, sweet, freshness | 25.98 | 3.16 | |||||
| 1-Heptanol | 111-70-6 | 1419 | 1405-1484 | musty, leafy, violet, herbal, green, sweet, woody, peony | 29.46 | 0.19 | |||||
| Ketones (sum) | 0.36 | 0.01 | |||||||||
| 2-Butanone | 78-93-3 | 915 | 866-950 | acetone, essential, fruity, camphor | 8.48 | 0.24 | |||||
| 2-Pentanone | 107-87-9 | 971 | 938-1015 | sweet, fruity, essential, wine, banana, woody | 11.68 | 0.07 | |||||
| 2-Heptanone | 110-43-0 | 1205 | 1145-1214 | fruity, spicy, sweet, herbal, coconut, woody | 20.08 | 0.01 | |||||
| 3-Octanone | 106-68-3 | 1280 | 1205-1300 | fresh, herbal, lavender, sweet, mushroom | 22.84 | 0.04 | |||||
| 2-Octanone | 111-13-7 | 1310 | 1262-1310 | earthy, natural, woody, herbal | 23.97 | 0.01 | 0.00 1 | ||||
| Aldehydes (sum) | 0.23 | 0.09 | 0.13 | 0.15 | |||||||
| incl. α,β-unsaturated | 0.03 | ||||||||||
| Acetaldehyde | 4124-63-4 | 714 | 689-744 | cabbage | 2.45 | 0.01 | 0.01 | 0.01 | 0.00 1 | L/Ln | |
| Propanal | 123-38-6 | 808 | 747-828 | earthy, alcoholic, wine, whiskey, cocoa, nutty | 4.62 | 0.04 | L/Ln | ||||
| 2-Methylbutanal | 96-17-3 | 925 | 880-963 | musty, cocoa, coffee, nutty | 8.99 | 0.13 | |||||
| Pentanal | 110-62-3 | 998 | 929-1013 | fermented, bakery, fruit, nutty, berry | 11.80 | 0.01 | 0.02 | 0.02 | 0.03 | L/Ln | |
| Hexanal | 66-25-1 | 1120 | 1034-1127 | herbal, oily | 16.07 | 0.06 | 0.04 | 0.08 | 0.09 | L | |
| Heptanal | 111-71-7 | 1209 | 1148-1219 | buttery, citrus, rancid | 20.22 | 0.03 | L/Ln | ||||
| (E)-2-Hexenal 2 | 6728-26-3 | 1251 | 1184-1236 | sweet, almond, fruity, fresh, leaves, apple, plum, vegetable | 21.73 | 0.01 | 0.01 | Ln | |||
| (E)-2-Heptenal | 18829-55-5 | 1359 | 1273-1366 | freshness, fatty | 25.67 | 0.02 | 0.01 | 0.02 | 0.02 | Ol/L | |
| (E,E)-2,4-Decadienal 2 | 2363-88-4 | 1859 | 1768-1858 | orange, sweet, fresh, citrus, greasy, freshness | 40.97 | 0.03 | Ol/L | ||||
| Cyclic (sum) | 0.49 | ||||||||||
| Methylcyclopentane 2 | 96-37-7 | 681 | 675 | 1.91 | 0.04 | ||||||
| Cyclohexane | 110-82-7 | 734 | 712-766 | 2.80 | 0.01 | ||||||
| 3-Methylfuran | 930-27-8 | 889 | 832-901 | 7.33 | 0.01 | ||||||
| 2-Pentylfuran | 3777-69-3 | 1255 | 1193-1265 | fruity, green, earthy, nutty, vegetable, metallic | 21.89 | 0.18 | |||||
| 2-Furanmethanol | 98-00-0 | 1687 | 1613-1698 | alcoholic, chemical, musty, sweet, caramel, bread, coffee | 36.15 | 0.09 | |||||
| Benzyl alcohol 2 | 100-51-6 | 1920 | 1821-1919 | floral, rose, phenolic, balsamic | 42.62 | 0.06 | |||||
| Benzene ethanol 2 | 60-12-8 | 1959 | 1856-1956 | floral, rose, dried rose, floral, rose water | 43.65 | 0.10 | |||||
| Acids (sum) | 14.60 | ||||||||||
| Acetic | 64-19-7 | 1481 | 1400-1498 | sharp, acrid, sour, acetic | 29.77 | 11.16 | |||||
| Propionic | 79-09-4 | 1571 | 1474-1486 | spicy, sour, cheesy, acetic | 32.63 | 3.27 | |||||
| Hexanoic | 142-62-1 | 1879 | 1797-1885 | sour, fatty, cheesy | 41.52 | 0.17 | |||||
| Terpene derivatives (sum) | 40.81 | ||||||||||
| Tricyclene | 508-32-7 | 1031 | 993-1047 | 12.80 | 0.04 | ||||||
| α-Pinene | 80-56-8 | 1050 | 989-1077 | fresh, camphor, sweet, pine, earthy, woody | 13.43 | 28.50 | |||||
| Terpene derivative 1 | 471-84-1 | 1095 | 15.04 | 0.04 | |||||||
| Camphene | 79-92-5 | 1104 | 1033-1115 | woody, herbal, fir, camphor, terpene | 15.24 | 0.44 | |||||
| β-Pinene | 127-91-3 | 1145 | 1065-1158 | dry, woody, fresh, pine, hay, freshness, resinous | 17.17 | 1.62 | |||||
| Sabinene | 3387-41-5 | 1155 | 1074-1156 | woody, terpene, citrus, pine, spices | 17.65 | 3.05 | |||||
| Terpene derivativee 2 | 36262-09-6 | 1160 | 17.91 | 0.49 | |||||||
| Verbenene | 4080-46-0 | 1162 | 18.00 | 0.19 | |||||||
| β-Myrcene | 123-35-3 | 1185 | 1113-1192 | pepper, terpene, pungent, balsamic, plastic | 19.12 | 0.02 | |||||
| Terpene derivative 3 | 1205 | 20.09 | 0.12 | ||||||||
| Limonene | 138-86-3 | 1226 | 1152-1245 | citrus, herbal, terpene, camphor | 20.84 | 0.46 | 0.01 | ||||
| Eucalyptol | 470-82-6 | 1239 | 1167-1253 | eucalyptus, herbal, camphor, medicinal | 21.30 | 0.11 | |||||
| γ-Terpinene | 99-85-4 | 1274 | 1200-1293 | oily, woody, terpene, lemon, lime, tropical herbs | 22.63 | 0.11 | |||||
| Kumene | 99-87-6 | 1300 | 1232-1322 | fresh, citrus, terpene, woody, special | 23.65 | 0.28 | |||||
| Terpene derivative 4 | 1313 | 24.09 | 0.05 | ||||||||
| α-Pinene epoxyde 2 | 1686-14-2 | 1421 | 1345-1384 | freshness | 27.81 | 0.21 | |||||
| Terpene derivative | 1464 | 29.21 | 0.17 | ||||||||
| Camfolenal | 4501-58-0 | 1537 | 1439-1793 | herbal, freshness, woody, amber, leafy | 31.56 | 0.43 | |||||
| Verbenol 1 | 1557 | 32.20 | 0.17 | ||||||||
| Verbenol 2 | 1620 | balsamic | 34.15 | 0.77 | |||||||
| Kalarene 2 | 17334-55-3 | 1649 | 1544 | 35.01 | 0.41 | ||||||
| Verbenol 3 | 1693 | fresh, pine, ozone | 36.36 | 0.70 | |||||||
| Pinocarveol 2 | 5947-36-4 | 1700 | 1632-1690 | camphor, woody-pine, balsamic | 36.56 | 0.67 | |||||
| Verbenol 4 | 1717 | balsamic | 37.04 | 0.75 | |||||||
| Terpene derivative 5 | 1768 | 38.46 | 0.15 | ||||||||
| Verbenon 2 | 1775 | 1676-1742 | camphor, menthol, celery | 38.68 | 0.56 | ||||||
| Terpene derivative 6 | 1828 | 40.15 | 0.06 | ||||||||
| Mirtenol 2 | 1835 | 1747-1831 | camphor, menthol, celery | 40.34 | 0.15 | ||||||
| Trans-carveol | 1197-07-5 | 1871 | 1801-1884 | mint, solvent, cumin | 41.31 | 0.08 | |||||
| Others (sum) | 0.20 | 0.01 | |||||||||
| Ethyl acetate | 103-45-7 | 902 | 856-917 | floral, pink, sweet, honey, fruity, tropical | 7.91 | 0.02 | 0.01 | ||||
| 2,5-Dimethylpyrazine | 123-32-0 | 1357 | 1274-1358 | cocoa, roasted nuts, roast beef, woody, herbs | 25.58 | 0.12 | |||||
| 2-Ethyl-5-methylpyrazine | 13360-64-0 | 1428 | 1341-1432 | coffee beans, nutty, herbal, roasted, earthy, powdery, cocoa, baked potatoes | 28.03 | 0.03 | |||||
| 2,3,5-Trimethylpyrazine 2 | 14667-55-1 | 1442 | 1341-1432 | nut peel, earthy, powdery, cocoa, baked potatoes, roasted peanuts, hazelnuts, musty | 28.48 | 0.03 | |||||
| Pressed | D1 | D2 | D3 | D4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quantiles | p-Value | Q1 | Me | Q3 | Q1 | Me | Q3 | Q1 | Me | Q3 | Q1 | Me | Q3 | Q1 | Me | Q3 |
| PV | 0.014 | 2.7 | 2.7 | 2.7 | 1.1 | 1.1 | 1.1 | 1.5 | 1.6 | 1.7 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| AV | 0.010 | 3.0 | 3.0 | 3.0 | 0.9 | 0.9 | 0.9 | 1.4 | 1.4 | 1.4 | 2.3 | 2.3 | 2.4 | 3.0 | 3.0 | 3.2 |
| 3-MCPD | 0.009 | 0.02 | 0.02 | 0.03 | 0.06 | 0.07 | 0.07 | 0.84 | 0.85 | 0.89 | 2.19 | 2.20 | 2.22 | 3.01 | 3.14 | 3.19 |
| 2-MCPD | 0.012 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.29 | 0.29 | 0.34 | 0.98 | 0.99 | 1.00 | 1.46 | 1.49 | 1.49 |
| Glycidol | 0.014 | 0.01 | 0.01 | 0.02 | 0.63 | 0.63 | 0.64 | 0.14 | 0.15 | 0.16 | 0.20 | 0.20 | 0.21 | 0.15 | 0.16 | 0.18 |
| TFA C18:2 | 0.009 | 1 | 1 | 1 | 11 | 12 | 12 | 13 | 14 | 14 | 16 | 16 | 16 | 18 | 18 | 18 |
| PUFA | 0.228 | 595 | 595 | 595 | 589 | 589 | 590 | 583 | 585 | 586 | 581 | 583 | 584 | 579 | 580 | 580 |
| MUFA | 0.009 | 211 | 211 | 211 | 212 | 213 | 213 | 214 | 215 | 215 | 215 | 216 | 216 | 219 | 219 | 219 |
| SFA | 0.009 | 107 | 107 | 107 | 105 | 106 | 106 | 108 | 109 | 109 | 110 | 110 | 110 | 113 | 114 | 114 |
| PV | AV | 3-MCPD | 2-MCPD | Glycidol | TFA C18:2 | MUFA | SFA | |
|---|---|---|---|---|---|---|---|---|
| Pr-D1 | 0.158 | 0.063 | 0.457 | 1.000 | 0.030 | 0.457 | 0.457 | 0.457 |
| Pr-D2 | 0.457 | 0.158 | 0.176 | 0.285 | 0.372 | 0.176 | 0.176 | 0.457 |
| Pr-D3 | 0.039 | 0.413 | 0.069 | 0.109 | 0.085 | 0.069 | 0.069 | 0.176 |
| Pr-D4 | 0.057 | 0.558 | 0.030 | 0.045 | 0.176 | 0.030 | 0.030 | 0.069 |
| D1-D2 | 0.413 | 0.457 | 0.457 | 0.285 | 0.085 | 0.457 | 0.457 | 0.176 |
| D1-D3 | 0.270 | 0.176 | 0.176 | 0.109 | 0.372 | 0.176 | 0.176 | 0.069 |
| D1-D4 | 0.413 | 0.033 | 0.069 | 0.045 | 0.176 | 0.069 | 0.069 | 0.030 |
| D2-D3 | 0.094 | 0.457 | 0.457 | 0.457 | 0.270 | 0.457 | 0.457 | 0.457 |
| D2-D4 | 0.142 | 0.077 | 0.176 | 0.176 | 0.558 | 0.176 | 0.176 | 0.176 |
| D3-D4 | 0.733 | 0.196 | 0.457 | 0.457 | 0.558 | 0.457 | 0.457 | 0.457 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarenko, M.A.; Malinkin, A.D.; Bessonov, V.V. Change in Sunflower Oil Quality and Safety Depending on Number of Deodorisation Cycles Used. Foods 2024, 13, 2555. https://doi.org/10.3390/foods13162555
Makarenko MA, Malinkin AD, Bessonov VV. Change in Sunflower Oil Quality and Safety Depending on Number of Deodorisation Cycles Used. Foods. 2024; 13(16):2555. https://doi.org/10.3390/foods13162555
Chicago/Turabian StyleMakarenko, Mariia Andreevna, Alexey Dmitrievich Malinkin, and Vladimir Vladimirovich Bessonov. 2024. "Change in Sunflower Oil Quality and Safety Depending on Number of Deodorisation Cycles Used" Foods 13, no. 16: 2555. https://doi.org/10.3390/foods13162555







