The Application of Lophatherum Gracile Brongn Flavonoids in Wheat Flour Products: Effects on the Structural and Functional Characteristics of Wheat Dough
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
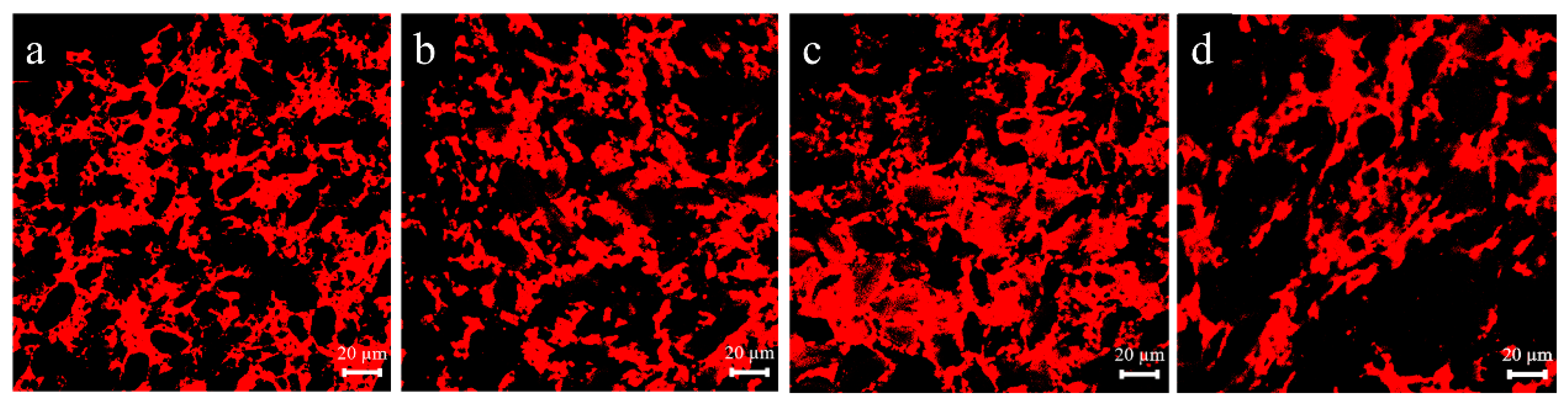
2.3. Determination of the Microscopic Morphology of the Dough Samples
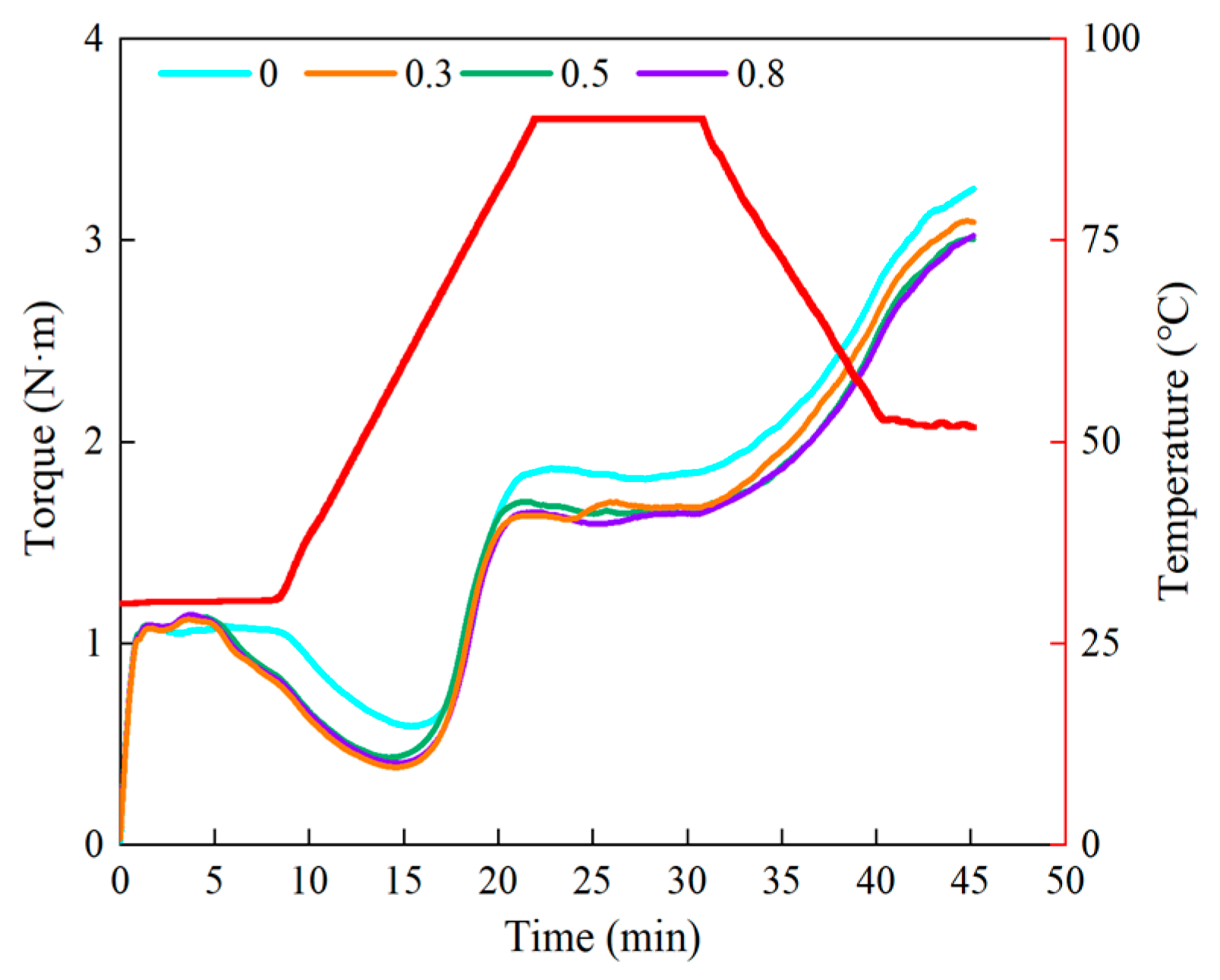
2.4. Determination of the Thermal-Mechanical Properties of the Dough Samples
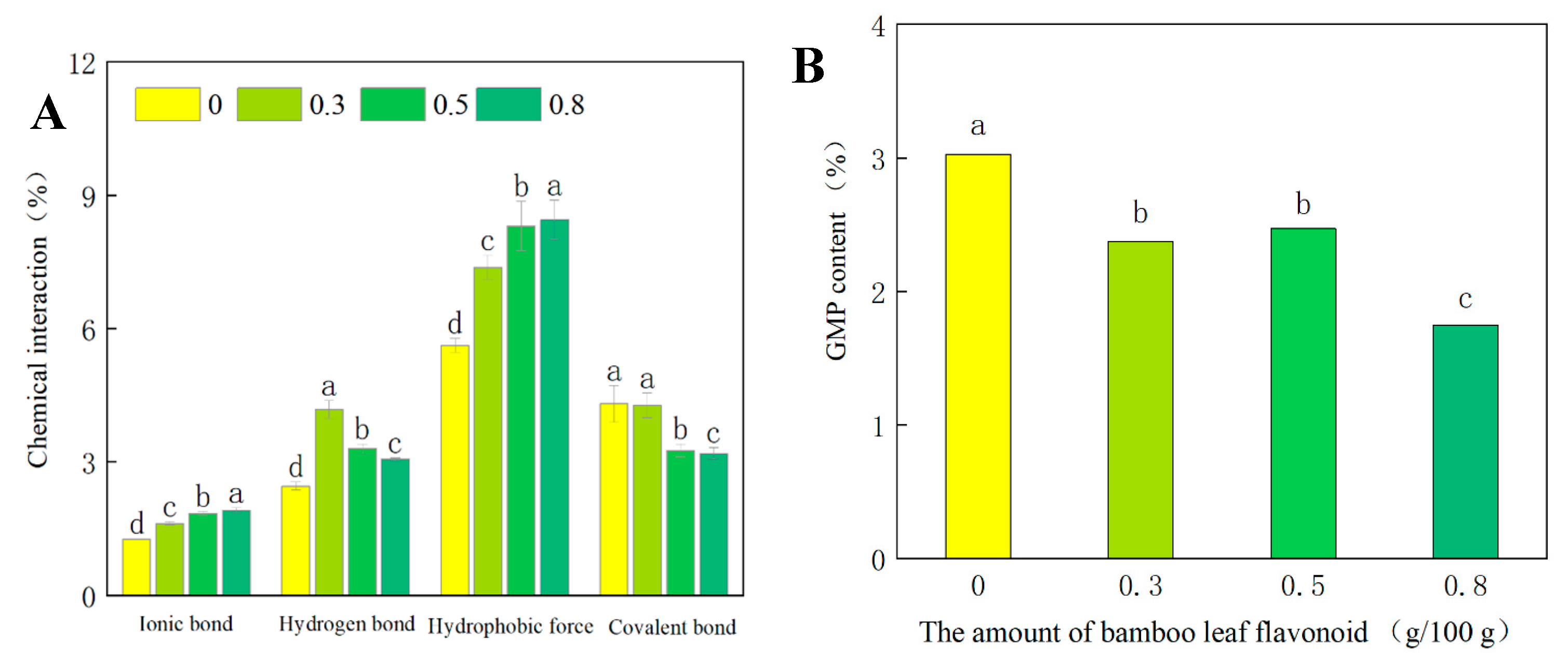
2.5. Determination of the Chemical Interactions in the Dough Samples
2.6. Determination of the Glutenin Macropolymer Content of the Dough Samples
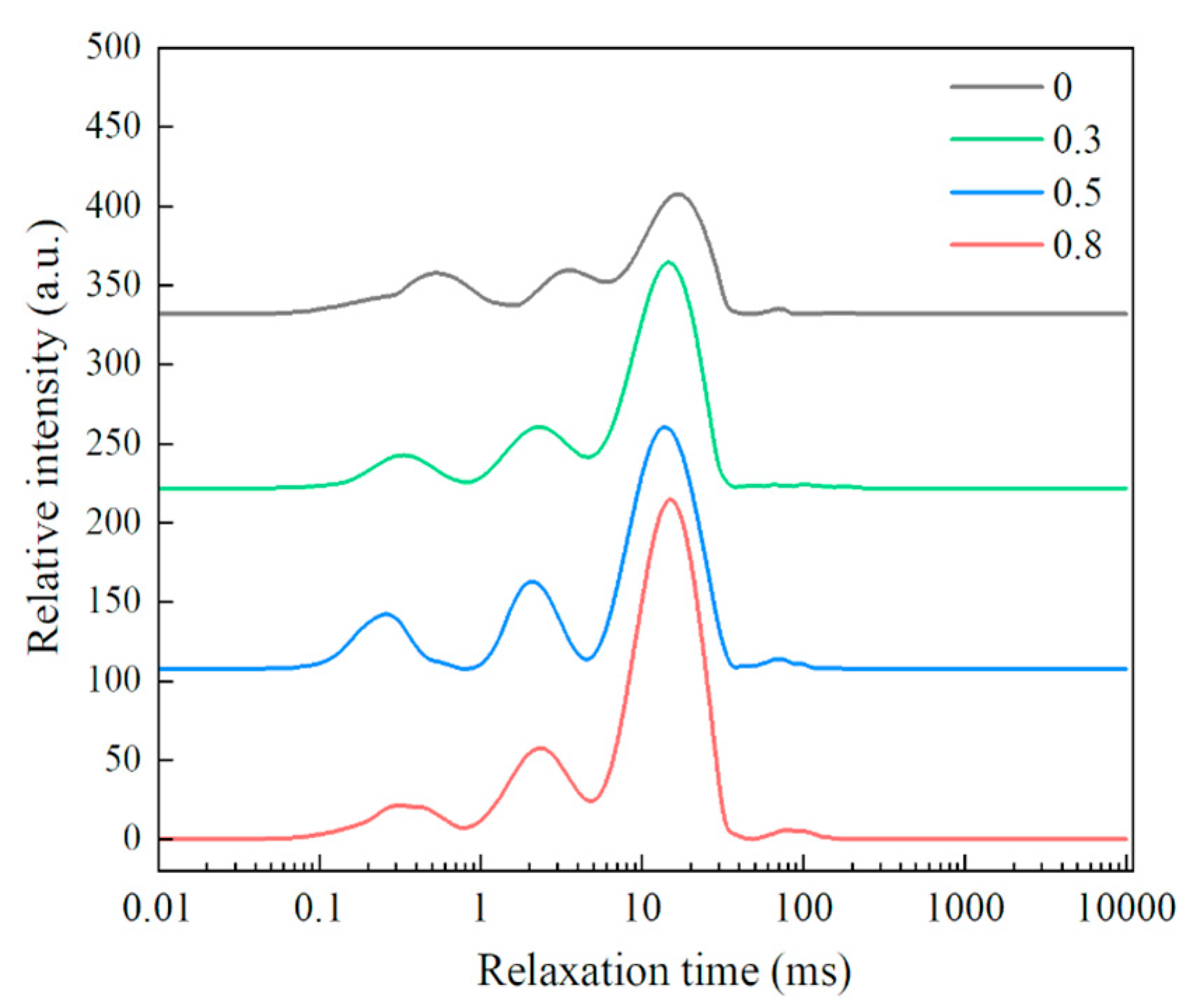
2.7. Determination of the Water Distribution in the Dough Samples
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Lophatherum Gracile Brongn Flavonoids on the Microstructure of the Dough
3.2. Effect of Lophatherum Gracile Brongn Flavonoids on the Thermal-Mechanical Properties of the Dough
3.3. Effect of Lophatherum Gracile Brongn Flavonoids on the Chemical Interaction Properties of the Dough
3.4. Effect of Lophatherum Gracile Brongn Flavonoids on the Glutenin Macropolymer Content of the Dough
3.5. Effect of Lophatherum Gracile Brongn Flavonoid on Water Distribution in the Dough
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, Z.; Chen, J.; Tufail, T.; Latif, A.; Arif, M.; Ullah, R.; Alqahtani, A.S.; Xu, B. Fundamental opportunities and challenges of nutraceutical noodles enriched with agri-food by-products. Trends Food Sci. Technol. 2023, 143, 104299. [Google Scholar] [CrossRef]
- Solanki, C.; Mridula, D.; Singh, R. Buckwheat processing and its utilization in value-added products: A comprehensive review. Pharma Innov. J. 2023, 12, 2746–2752. [Google Scholar]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Lai, K.H.; Chen, P.J.; Chen, C.C.; Yang, S.H.; El-Shazly, M.; Chang, Y.C.; Wu, Y.H.; Wu, Y.H.; Wang, Y.H.; Hsieh, H.L.; et al. Lophatherum gracile Brongn. attenuates neutrophilic inflammation through inhibition of JNK and calcium. J. Ethnopharmacol. 2021, 264, 113224. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, C.Y.; Lai, K.H.; Chang, Y.C.; Hwang, T.L. Anti-inflammatory and antiviral activities of flavone C-glycosides of Lophatherum gracile for COVID-19. J. Funct. Foods 2023, 101, 105407. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Ge, W.; Cai, G.; Guo, Y.; Gong, J. Spectrum–effect relationship between ultra-high-performance liquid chromatography fingerprints and antioxidant activities of Lophatherum gracile Brongn. Food Sci. Nutr. 2022, 10, 1592–1601. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.F.; Wu, Q.L.; Guo, X.; Shang, Y.F.; Yang, S.H.; Niu, X.L.; Thakur, K.; Ma, Y.L.; Wei, Z.J. Lophatherum gracile Brongn.: A review on phytochemistry, bioactivity and food applications. Food Chem. Adv. 2024, 4, 100688. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, X.; Dai, T.; Wen, Z.; Guo, X.; Song, Y.; Long, L.; Li, Y.; Mei, X. Chloroplast genome structure and phylogenetic position of Lophatherum gracile. Mitochondrial DNA Part B 2021, 6, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.; Li, Y. Dough properties, bread quality, and associated interactions with added phenolic compounds: A review. J. Funct. Foods 2019, 52, 629–639. [Google Scholar] [CrossRef]
- Awika, J.M. Effect of bioactive components on dough rheology, baking and extrusion. In Fruit and Cereal Bioactives: Sources, Chemistry, and Application; Food Science & Technology; CRC Press: Boca Raton, FL, USA, 2011; pp. 337–344. [Google Scholar] [CrossRef]
- Czajkowska–González, Y.A.; Alvarez–Parrilla, E.; del Rocío Martínez–Ruiz, N.; Vázquez–Flores, A.A.; Gaytán–Martínez, M.; de la Rosa, L.A. Addition of phenolic compounds to bread: Antioxidant benefits and impact on food structure and sensory characteristics. Food Prod. Process. Nutr. 2021, 3, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Xu, F.; Han, R.; Quan, M.; Wang, L. Effect of Tremella fuciformis on dough structure and rheology, noodle flavor, and quality characteristics. Lwt 2022, 172, 114180. [Google Scholar] [CrossRef]
- Izydorczyk, M.; Hussain, A.; MacGregor, A. Effect of barley and barley components on rheological properties of wheat dough. J. Cereal Sci. 2001, 34, 251–260. [Google Scholar] [CrossRef]
- Chlopicka, J.; Pasko, P.; Gorinstein, S.; Jedryas, A.; Zagrodzki, P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT-Food Sci. Technol. 2012, 46, 548–555. [Google Scholar] [CrossRef]
- Kalinina, I.; Fatkullin, R.; Naumenko, N.; Popova, N.; Stepanova, D. The Influence of flavonoid dihydroquercetin on the enzymatic processes of dough ripening and the antioxidant properties of bread. Fermentation 2023, 9, 263. [Google Scholar] [CrossRef]
- Wang, C.C.; Sheng, Z.; Zhang, Y.H.; Du, Q.Z.; Li, Q.; Jin, P.; Xie, D.C.; Min, W.H.; Zhang, H.H. Effects of epigallocatechin-3-gallate on the structural hierarchy of the gluten network in dough. Food Hydrocoll. 2023, 142, 108803. [Google Scholar] [CrossRef]
- Huang, W.; Li, L.; Wang, F.; Wan, J.; Tilley, M.; Ren, C.; Wu, S. Effects of transglutaminase on the rheological and Mixolab thermomechanical characteristics of oat dough. Food Chem. 2010, 121, 934–939. [Google Scholar] [CrossRef]
- Cao, F.H.; Li, X.J.; Luo, S.Z.; Mu, D.D.; Zhong, X.Y.; Jiang, S.T.; Zheng, Z.; Zhao, Y.Y. Effects of organic acid coagulants on the physical properties of and chemical interactions in tofu. LWT-Food Sci. Technol. 2017, 85, 58–65. [Google Scholar] [CrossRef]
- Han, X.; Zhang, M.; Zhang, R.; Huang, L.; Jia, X.; Huang, F.; Liu, L. Physicochemical interactions between rice starch and different polyphenols and structural characterization of their complexes. Lwt 2020, 125, 109227. [Google Scholar] [CrossRef]
- Assifaoui, A.; Champion, D.; Chiotelli, E.; Verel, A. Characterization of water mobility in biscuit dough using a low-field 1H NMR technique. Carbohydr. Polym. 2006, 64, 197–204. [Google Scholar] [CrossRef]
- Li, L.; Zhou, W.; Wu, A.; Qian, X.; Xie, L.; Zhou, X.; Zhang, L. Effect of ginkgo biloba powder on the physicochemical properties and quality characteristics of wheat dough and fresh wet noodles. Foods 2022, 11, 698. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, H.M.; Zhu, K.X.; Guo, X.N.; Peng, W. Water cooking stability of dried noodles enriched with different particle size and concentration green tea powders. Foods 2020, 9, 298. [Google Scholar] [CrossRef]
- Rosell, C.M.; Collar, C.; Haros, M. Assessment of hydrocolloid effects on the thermo-mechanical properties of wheat using the Mixolab. Food Hydrocoll. 2007, 21, 452–462. [Google Scholar] [CrossRef]
- Pasqualone, A.; Costantini, M.; Labarbuta, R.; Summo, C. Production of extruded-cooked lentil flours at industrial level: Effect of processing conditions on starch gelatinization, dough rheological properties and techno-functional parameters. Lwt 2021, 147, 111580. [Google Scholar] [CrossRef]
- Švec, I.; Hrušková, M. The Mixolab parameters of composite wheat/hemp flour and their relation to quality features. LWT-Food Sci. Technol. 2015, 60, 623–629. [Google Scholar] [CrossRef]
- KolesÃ, A.; BojňanskÃ, T.; KopÄ ekovÃ, J.; KolesÃ, A. The influence of non-traditional fruits and elder flowers on rheological properties of the dough. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e4671. [Google Scholar]
- Hadnađev, T.D.; Torbica, A.; Hadnađev, M. Rheological properties of wheat flour substitutes/alternative crops assessed by Mixolab. Procedia Food Sci. 2011, 1, 328–334. [Google Scholar] [CrossRef]
- Yuan, T.; Zhao, S.; Yang, J.; Niu, M.; Xu, Y. Structural characteristics of β-glucans from various sources and their influences on the short-and long-term starch retrogradation in wheat flour. Int. J. Biol. Macromol. 2024, 264, 130561. [Google Scholar] [CrossRef]
- Gujral, H.S.; Sharma, B.; Khatri, M. Influence of replacing wheat bran with barley bran on dough rheology, digestibility and retrogradation behavior of chapatti. Food Chem. 2018, 240, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of bread dough with added fiber polysaccharides and phenolic antioxidants: A review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of wheat gluten proteins: Qualitative composition. Cereal Chem. 2023, 100, 23–35. [Google Scholar] [CrossRef]
- Lindsay, M.P.; Skerritt, J.H. Examination of the structure of the glutenin macropolymer in wheat flour and doughs by stepwise reduction. J. Agric. Food Chem. 1998, 46, 3447–3457. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, L.; Jiang, L.; Wang, Y.; Yang, G.; He, G. Effects of tannic acid on gluten protein structure, dough properties and bread quality of Chinese wheat. J. Sci. Food Agric. 2010, 90, 2462–2468. [Google Scholar] [CrossRef] [PubMed]
- Dufour, M.; Chaunier, L.; Lourdin, D.; Réguerre, A.L.; Hugon, F.; Dugué, A.; Kansou, K.; Saulnier, L.; Della Valle, G. Unravelling the relationships between wheat dough extensional properties, gluten network and water distribution. Food Hydrocoll. 2024, 146, 109214. [Google Scholar] [CrossRef]
- Kuo, M.I.; Gunasekaran, S.; Johnson, M.; Chen, C. Nuclear magnetic resonance study of water mobility in pasta filata and non-pasta filata Mozzarella. J. Dairy Sci. 2001, 84, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Liu, S.; Kong, S.; Wang, Z.; Shi, Z.; Cai, J. Effect of Sarcoplasmic Protein Solutions Dried at Different Times and Rates on Water Migration in Lamb Myofibril In Vitro. Foods 2024, 13, 930. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, C.; Tan, Y.; McClements, D.J.; Wang, L. Insight of rheology, water distribution and in vitro digestive behavior of starch based-emulsion gel: Impact of potato starch concentration. Food Hydrocoll. 2022, 132, 107859. [Google Scholar] [CrossRef]
| BLF Amount (g/100 g Wheat Flour) | Water Absorption (%) | Formation Time (min) | Stable Time (min) | C3–C2 (Nm) | C3–C4 (Nm) | C5–C4 (Nm) |
|---|---|---|---|---|---|---|
| 0 | 60.70 ± 0.00 a | 5.70 ± 0.03 a | 9.12 ± 0.14 a | 1.29 ± 0.01 a | 0.07 ± 0.01 a | 1.50 ± 0.01 a |
| 0.3 | 60.50 ± 0.00 b | 4.40 ± 0.05 b | 5.45 ± 0.05 b | 1.27 ± 0.01 a | 0.08 ± 0.00 a | 1.46 ± 0.02 b |
| 0.5 | 60.40 ± 0.00 b | 3.64 ± 0.10 c | 4.95 ± 0.04 c | 1.25 ± 0.01 b | 0.09 ± 0.00 a | 1.43 ± 0.01 b |
| 0.8 | 60.10 ± 0.00 c | 3.57 ± 0.08 c | 4.87 ± 0.05 d | 1.24 ± 0.01 b | 0.14 ± 0.01 b | 1.40 ± 0.01 c |
| BLF Amount (g/100 g Wheat Flour) | Vicinal Water | Multilayer Water | Entrapped Water | Free Water |
|---|---|---|---|---|
| 0 | 0.2133 ± 0.0528 a | 0.1797 ± 0.0615 a | 0.5703 ± 0.0250 b | 0.0125 ± 0.0050 a |
| 0.3 | 0.1332 ± 0.0194 b | 0.1827 ± 0.0137 a | 0.6687 ± 0.0119 a | 0.0127 ± 0.0054 a |
| 0.5 | 0.1256 ± 0.0487 b | 0.1843 ± 0.0235 a | 0.6886 ± 0.0480 a | 0.0146 ± 0.0040 a |
| 0.8 | 0.0867 ± 0.0287 b | 0.1847 ± 0.0134 a | 0.7173 ± 0.0434 a | 0.0155 ± 0.0039 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, Y.; Bao, H.; Zhang, H. The Application of Lophatherum Gracile Brongn Flavonoids in Wheat Flour Products: Effects on the Structural and Functional Characteristics of Wheat Dough. Foods 2024, 13, 2556. https://doi.org/10.3390/foods13162556
Li Q, Liu Y, Bao H, Zhang H. The Application of Lophatherum Gracile Brongn Flavonoids in Wheat Flour Products: Effects on the Structural and Functional Characteristics of Wheat Dough. Foods. 2024; 13(16):2556. https://doi.org/10.3390/foods13162556
Chicago/Turabian StyleLi, Qin, Yi Liu, Huimei Bao, and Haihua Zhang. 2024. "The Application of Lophatherum Gracile Brongn Flavonoids in Wheat Flour Products: Effects on the Structural and Functional Characteristics of Wheat Dough" Foods 13, no. 16: 2556. https://doi.org/10.3390/foods13162556




