Anti-Inflammatory, Cytotoxic, and Genotoxic Effects of Soybean Oligopeptides Conjugated with Mannose
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation and Characterization of Soybean Oligopeptide (OT), Allulose-Conjugated OT (OT-AL), and D-Mannose-Conjugated OT (OT-Man)
2.3. Cell Culture
2.4. Cytotoxicity Assay
2.5. Hemolysis Assay
2.6. Measurement of Nitric Oxide (NO)/Nitrite
2.7. Pro-Inflammatory Cytokine Determination
2.8. Immunoblotting
2.9. Mutagenicity Testing of the Oligopeptide and Its Mannose-Conjugated Form Using the Salmonella Mutation Assay
2.10. In Vivo Genotoxicity Evaluation of the Oligopeptide and Its Mannose-Conjugated Form Using the Somatic Mutation and Recombination Test (SMART) in Drosophila melanogaster
2.11. Statistical Analysis
3. Results
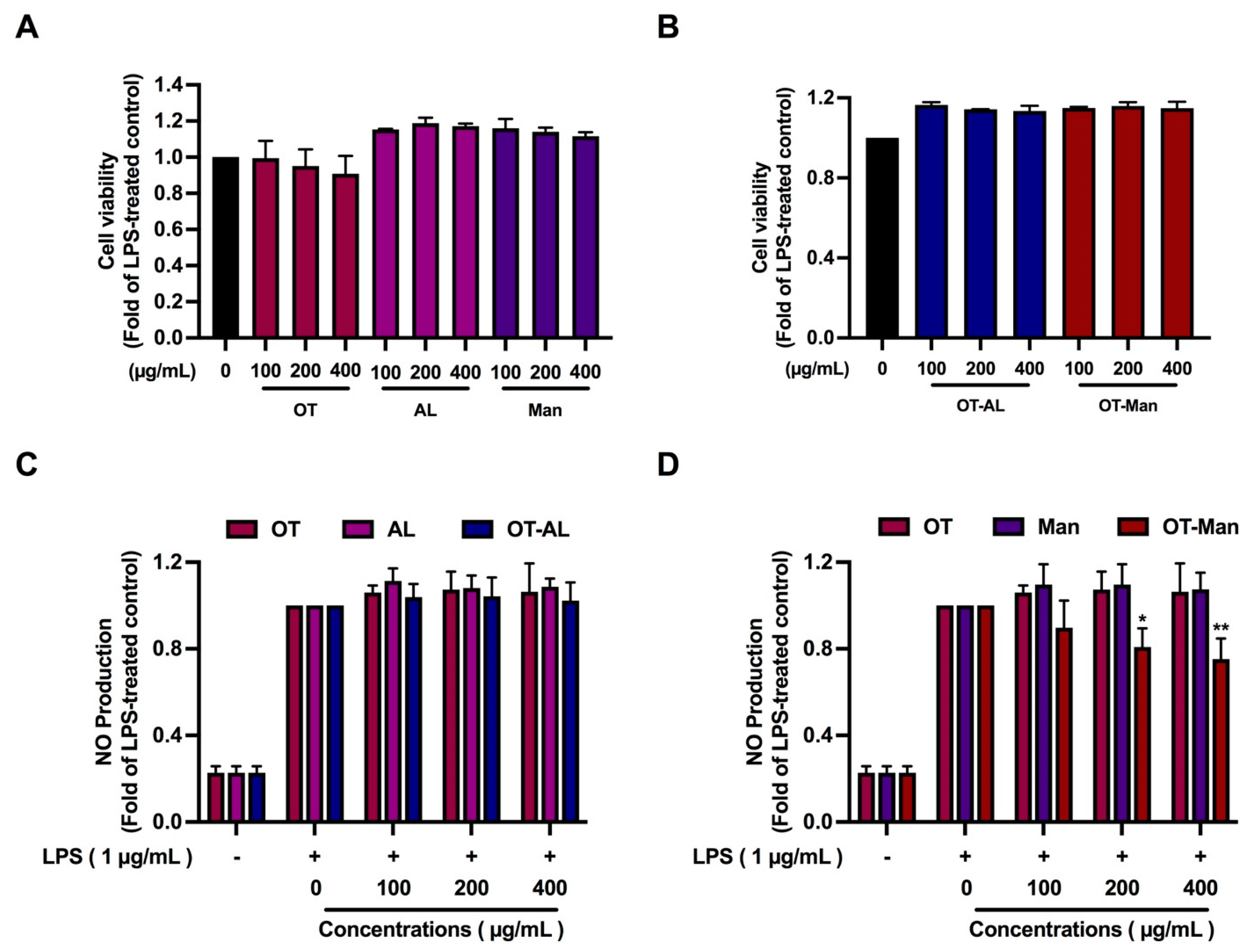
3.1. Cytotoxicity of the Oligopeptide and Its Conjugated Forms
3.2. The Effect of the Oligopeptide and Its Conjugated Forms on Nitric Oxide (NO) Production in LPS-Treated Macrophages
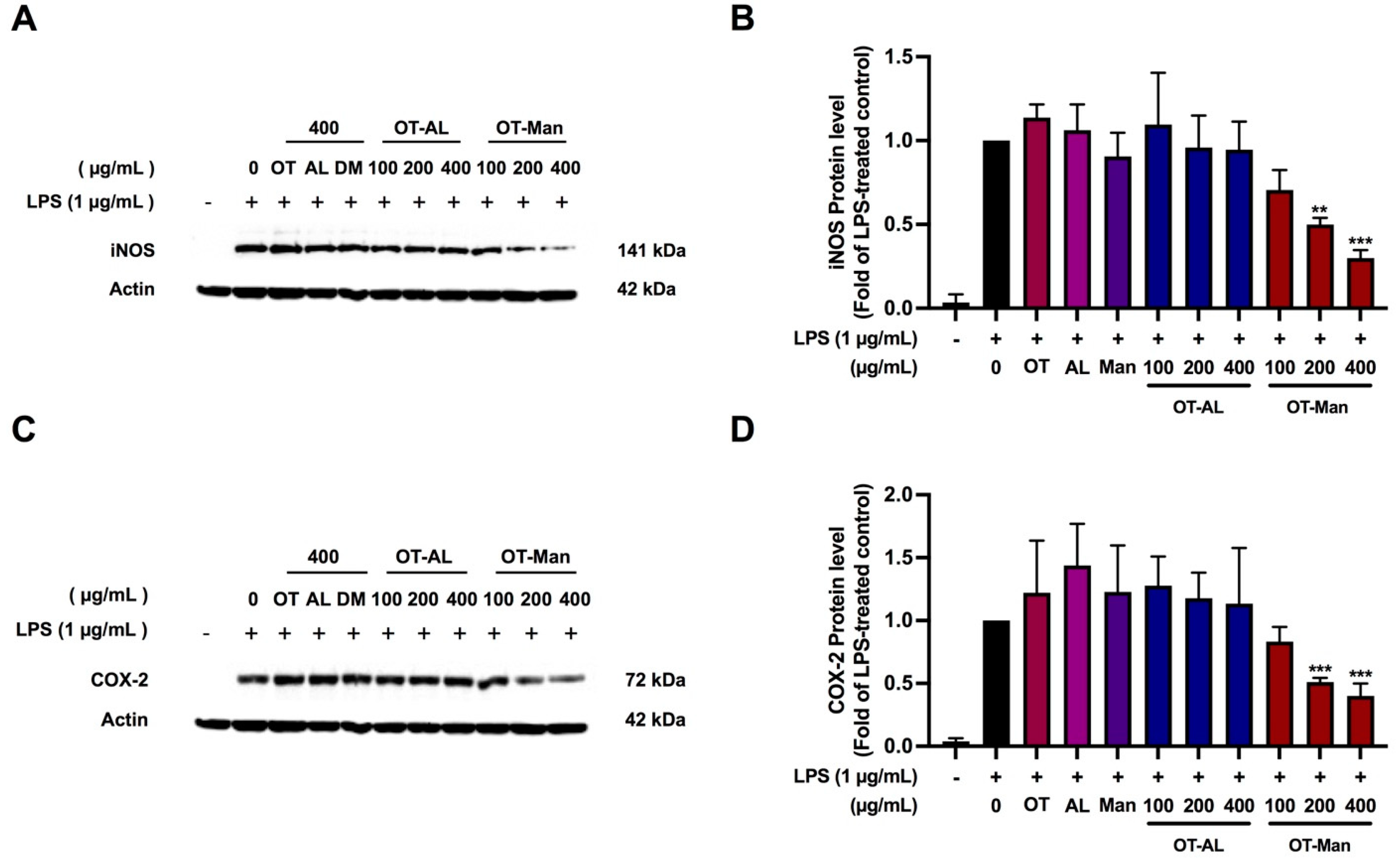
3.3. Effect of the Oligopeptide and Its Conjugated Forms on iNOS and COX-2 Levels in LPS-Treated RAW 264.7 Cells
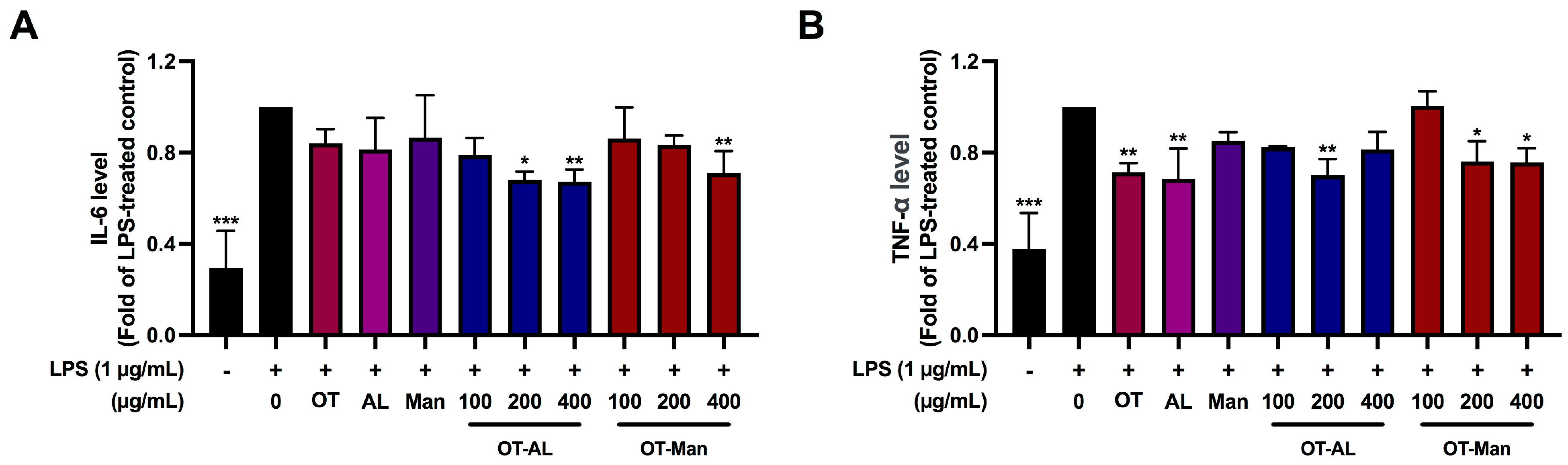
3.4. Effect of OT-AL and OT-Man on the Secretion of IL-6 and TNF-α in LPS-Treated RAW 264.7 Cells
3.5. Mutagenicity of Oligopeptide (OT), D-Mannose (Man), and Mannose Conjugated Oligopeptide (OT-Man)
3.6. In Vivo Genotoxicity Analysis in Drosophila Using SMART
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, D.; Gupta, R.; Joshi, I. Nutrient analysis of raw and processed soybean and development of value added soybean noodle. Inven. Rapid Life Style 2014, 1, 1–5. [Google Scholar]
- Subroto, E.; Qonit, M.A.H. Modification of soy protein for the production of bioactive peptides and their utilization. Int. J. Sci. Technol. Res. 2020, 9, 3121–3127. [Google Scholar]
- Kuiken, K.A.; Lyman, C.M. Essential Amino Acid Composition of Soy Bean Meals Prepared from Twenty Strains of Soy Beans. J. Biol. Chem. 1949, 177, 29–36. [Google Scholar] [CrossRef]
- Kudełka, W.; Kowalska, M.; Popis, M. Quality of Soybean Products in Terms of Essential Amino Acids Composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Bhathena, S.J. Role of dietary soy protein in obesity. Int. J. Med. Sci. 2007, 4, 72–82. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Lovati, M.R. Soy proteins and cardiovascular disease. Curr. Atheroscler. Rep. 2001, 3, 47–53. [Google Scholar] [CrossRef]
- Das, D.; Kabir, M.E.; Sarkar, S.; Wann, S.B.; Kalita, J.; Manna, P. Antidiabetic potential of soy protein/peptide: A therapeutic insight. Int. J. Biol. Macromol. 2022, 194, 276–288. [Google Scholar] [CrossRef]
- Rayaprolu, S.J.; Hettiarachchy, N.S.; Horax, R.; Phillips, G.K.; Mahendran, M.; Chen, P. Soybean peptide fractions inhibit human blood, breast and prostate cancer cell proliferation. J. Food Sci. Technol. 2017, 54, 38–44. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Li, H.; Zhang, C.; Zhang, J.; Uddin, J.; Liu, X. Effect of soybean oligopeptide on the growth and metabolism of Lactobacillus acidophilus JCM 1132. RSC Adv. 2020, 10, 16737–16748. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liu, C.; Liu, X. The Beneficial Effects of Soybean Proteins and Peptides on Chronic Diseases. Nutrients 2023, 15, 1811. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, A.B.; Mellem, J.J.; Ijabadeniyi, O.A. Potential for enhanced soy storage protein breakdown and allergen reduction in soy-based foods produced with optimized sprouted soybeans. LWT 2018, 98, 540–545. [Google Scholar] [CrossRef]
- Kim, I.S.; Yang, W.S.; Kim, C.H. Beneficial Effects of Soybean-Derived Bioactive Peptides. Int. J. Mol. Sci. 2021, 22, 8570. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yan, L.; Yang, J.; He, Y.; Wu, L. Effect of Modification Strategies on the Biological Activity of Peptides/Proteins. ChemBioChem 2024, 25, e202300481. [Google Scholar] [CrossRef]
- Chu-Kung, A.F.; Bozzelli, K.N.; Lockwood, N.A.; Haseman, J.R.; Mayo, K.H.; Tirrell, M.V. Promotion of Peptide Antimicrobial Activity by Fatty Acid Conjugation. Bioconjug. Chem. 2004, 15, 530–535. [Google Scholar] [CrossRef]
- Sun, Y.; Hayakawa, S.; Ogawa, M.; Izumori, K. Evaluation of the Site Specific Protein Glycation and Antioxidant Capacity of Rare Sugar−Protein/Peptide Conjugates. J. Agric. Food Chem. 2005, 53, 10205–10212. [Google Scholar] [CrossRef]
- Liu, B.; Gou, Y.; Tsuzuki, T.; Yamada, T.; Iida, T.; Wang, S.; Banno, R.; Toyoda, Y.; Koike, T. d-Allulose Improves Endurance and Recovery from Exhaustion in Male C57BL/6J Mice. Nutrients 2022, 14, 404. [Google Scholar] [CrossRef] [PubMed]
- Cayabyab, K.B.; Shin, M.J.; Heimuli, M.S.; Kim, I.J.; D’Agostino, D.P.; Johnson, R.J.; Koutnik, A.P.; Bellissimo, N.; Diamond, D.M.; Norwitz, N.G.; et al. The Metabolic and Endocrine Effects of a 12-Week Allulose-Rich Diet. Nutrients 2024, 16, 1821. [Google Scholar] [CrossRef]
- Paurević, M.; Šrajer Gajdošik, M.; Ribić, R. Mannose Ligands for Mannose Receptor Targeting. Int. J. Mol. Sci. 2024, 25, 1370. [Google Scholar] [CrossRef]
- Junfeng, F.; Yanyan, Z.; Szesze, T.; Fengjuan, L.; Manyu, Z.; Saito, M.; Tatsumi, E.; Lite, L. Improving Functional Properties of Soy Protein Hydrolysate by Conjugation with Curdlan. J. Food Sci. 2006, 71, C285–C291. [Google Scholar] [CrossRef]
- Yu, M.; He, S.; Tang, M.; Zhang, Z.; Zhu, Y.; Sun, H. Antioxidant activity and sensory characteristics of Maillard reaction products derived from different peptide fractions of soybean meal hydrolysate. Food Chem. 2018, 243, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Kutzli, I.; Weiss, J.; Gibis, M. Glycation of plant proteins via maillard reaction: Reaction chemistry, technofunctional properties, and potential food application. Foods 2021, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Siddiquy, M.; JiaoJiao, Y.; Rahman, M.H.; Iqbal, M.W.; Al-Maqtari, Q.A.; Easdani, M.; Yiasmin, M.N.; Ashraf, W.; Hussain, A.; Zhang, L. Advances of protein functionalities through conjugation of protein and polysaccharide. Food Bioprocess. Technol. 2024, 17, 2077–2097. [Google Scholar] [CrossRef]
- Phongphisutthinant, R.; Wiriyacharee, P.; Boonyapranai, K.; Ounjaijean, S.; Taya, S.; Pitchakarn, P.; Pathomrungsiyounggul, P.; Utarat, P.; Wongwatcharayothin, W.; Somjai, C.; et al. Effect of Conventional Humid–Dry Heating through the Maillard Reaction on Chemical Changes and Enhancement of In Vitro Bioactivities from Soy Protein Isolate Hydrolysate–Yeast Cell Extract Conjugates. Foods 2024, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Facchin, B.M.; Dos Reis, G.O.; Vieira, G.N.; Mohr, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech. Dis. 2016, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Polaka, S.; Rajpoot, K.; Tekade, M.; Sharma, M.C.; Tekade, R.K. Chapter 6—Importance of toxicity testing in drug discovery and research. In Pharmacokinetics and Toxicokinetic Considerations; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 2, pp. 117–144. [Google Scholar]
- Buacheen, P.; Karinchai, J.; Inthachat, W.; Butkinaree, C.; Jankam, C.; Wongnoppavich, A.; Imsumran, A.; Chewonarin, T.; Pimpha, N.; Temviriyanukul, P.; et al. The Toxicological Assessment of Anoectochilus burmannicus Ethanolic-Extract-Synthesized Selenium Nanoparticles Using Cell Culture, Bacteria, and Drosophila melanogaster as Suitable Models. Nanomaterials 2023, 13, 2804. [Google Scholar] [CrossRef] [PubMed]
- Pitchakarn, P.; Ting, P.; Buacheen, P.; Karinchai, J.; Inthachat, W.; Chantong, B.; Suttisansanee, U.; Nuchuchua, O.; Temviriyanukul, P. Multi-Endpoint Toxicological Assessment of Chrysin Loaded Oil-in-Water Emulsion System in Different Biological Models. Nanomaterials 2024, 14, 1001. [Google Scholar] [CrossRef] [PubMed]
- Hagens, W.I.; Oomen, A.G.; de Jong, W.H.; Cassee, F.R.; Sips, A.J. What do we (need to) know about the kinetic properties of nanoparticles in the body? Regul. Toxicol. Pharmacol. 2007, 49, 217–229. [Google Scholar] [CrossRef]
- Thusyanthan, J.; Wickramaratne, N.S.; Senathilake, K.S.; Rajagopalan, U.; Tennekoon, K.H.; Thabrew, I.; Samarakoon, S.R. Cytotoxicity against Human Hepatocellular Carcinoma (HepG2) Cells and Anti-Oxidant Activity of Selected Endemic or Medicinal Plants in Sri Lanka. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 6407688. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guidance Document on Using Cytotoxicity Tests to Estimate Starting Doses for Acute Oral Systematic Toxicity Tests; OECD Environment Directorate: Paris, France, 2010. [Google Scholar]
- Phillips, D.H.; Arlt, V.M. Genotoxicity: Damage to DNA and its consequences. In Molecular, Clinical and Environmental Toxicology: Volume 1: Molecular Toxicology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 87–110. [Google Scholar]
- Ren, N.; Atyah, M.; Chen, W.-Y.; Zhou, C.-H. The various aspects of genetic and epigenetic toxicology: Testing methods and clinical applications. J. Transl. Med. 2017, 15, 110. [Google Scholar] [CrossRef]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- Inami, K.; Mochizuki, M. Chemical models for cytochrome P450 as a biomimetic metabolic activation system in mutation assays. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2002, 519, 133–140. [Google Scholar] [CrossRef]
- Baldrick, P. Genotoxicity test battery—An assessment of its utility in early drug development. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021, 868–869, 503388. [Google Scholar] [CrossRef]
- Pitchakarn, P.; Inthachat, W.; Karinchai, J.; Temviriyanukul, P. Human hazard assessment using Drosophila wing spot test as an alternative in vivo model for genotoxicity testing—A review. Int. J. Mol. Sci. 2021, 22, 9932. [Google Scholar] [CrossRef] [PubMed]
- Graf, U.; van Schaik, N. Improved high bioactivation cross for the wing somatic mutation and recombination test in Drosophila melanogaster. Mutat. Res. Environ. Mutagen. Relat. Subj. 1992, 271, 59–67. [Google Scholar] [CrossRef]
- Karinchai, J.; Budluang, P.; Temviriyanukul, P.; Ting, P.; Nuchuchua, O.; Wongnoppavich, A.; Imsumran, A.; Pitchakarn, P. Bioassay-guided study of the anti-inflammatory effect of Anoectochilus burmannicus ethanolic extract in RAW 264.7 cells. J. Ethnopharmacol. 2021, 280, 114452. [Google Scholar] [CrossRef]
- Bittersohl, H.; Steimer, W. Intracellular Concentrations of Immunosuppressants. In Personalized Immunosuppression in Transplantation; Elsevier: Amsterdam, The Netherlands, 2016; pp. 199–226. [Google Scholar]
- Park, C.-M.; Xian, M. Chapter Eight—Use of Phosphorodithioate-Based Compounds as Hydrogen Sulfide Donors. In Methods in Enzymology; Cadenas, E., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 554, pp. 127–142. [Google Scholar]
- Suganthy, N.; Muniasamy, S.; Archunan, G. Safety assessment of methanolic extract of Terminalia chebula fruit, Terminalia arjuna bark and its bioactive constituent 7-methyl gallic acid: In vitro and in vivo studies. Regul. Toxicol. Pharmacol. 2018, 92, 347–357. [Google Scholar] [CrossRef]
- Amin, K.; Dannenfelser, R.M. In vitro hemolysis: Guidance for the pharmaceutical scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Budluang, P.; Pitchakarn, P.; Ting, P.; Temviriyanukul, P.; Wongnoppawich, A.; Imsumran, A. Anti-inflammatory and anti-insulin resistance activities of aqueous extract from Anoectochilus burmannicus. Food Sci. Nutr. 2017, 5, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Inboot, W.; Taya, S.; Chailungka, A.; Meepowpan, P.; Wongpoomchai, R. Genotoxicity and antigenotoxicity of the methanol extract of Cleistocalyx nervosum var. paniala seed using a Salmonella mutation assay and rat liver micronucleus tests. Mol. Cell. Toxicol. 2012, 8, 19–24. [Google Scholar]
- Frei, H.; Würgler, F.E. Statistical methods to decide whether mutagenicity test data from Drosophila assays indicate a positive, negative, or inconclusive result. Mutat. Res. 1988, 203, 297–308. [Google Scholar] [CrossRef]
- Yi, G.; Li, H.; Liu, M.; Ying, Z.; Zhang, J.; Liu, X. Soybean protein-derived peptides inhibit inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4-mediated MAPK-JNK and NF-kappa B activation. J. Food Biochem. 2020, 44, e13289. [Google Scholar] [CrossRef]
- Han, Y.; Yoon, J.; Choi, M.-S. Tracing the Anti-Inflammatory Mechanism/Triggers of d-Allulose: A Profile Study of Microbiome Composition and mRNA Expression in Diet-Induced Obese Mice. Mol. Nutr. Food Res. 2020, 64, 1900982. [Google Scholar] [CrossRef]
- Suttisansanee, U.; Pitchakarn, P.; Ting, P.; Inthachat, W.; Thiyajai, P.; Rodthayoy, D.; Karinchai, J.; Chantarasuwan, B.; Nuchuchua, O.; Temviriyanukul, P. Health-promoting bioactivity and in vivo genotoxicity evaluation of a hemiepiphyte fig, Ficus dubia. Food Sci. Nutr. 2021, 9, 2269–2279. [Google Scholar] [CrossRef]
- Srichamnong, W.; Ting, P.; Pitchakarn, P.; Nuchuchua, O.; Temviriyanukul, P. Safety assessment of Plukenetia volubilis (Inca peanut) seeds, leaves, and their products. Food Sci. Nutr. 2018, 6, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, B.; Joe, G.-H.; Shimizu, Y.; Saeki, H. Glycation with uronic acid-type reducing sugar enhances the anti-inflammatory activity of fish myofibrillar protein via the Maillard reaction. Food Chem. 2023, 407, 135162. [Google Scholar] [CrossRef] [PubMed]
- Amigo, L.; Martínez-Maqueda, D.; Hernández-Ledesma, B. In Silico and In Vitro Analysis of Multifunctionality of Animal Food-Derived Peptides. Foods 2020, 9, 991. [Google Scholar] [CrossRef]
- Menaka, K.B.; Ramesh, A.; Thomas, B.; Kumari, N.S. Estimation of nitric oxide as an inflammatory marker in periodontitis. J. Indian. Soc. Periodontol. 2009, 13, 75–78. [Google Scholar] [CrossRef]
- Yousefpour, P.; Ni, K.; Irvine, D.J. Targeted modulation of immune cells and tissues using engineered biomaterials. Nat. Rev. Bioeng. 2023, 1, 107–124. [Google Scholar] [CrossRef]
- Dalle Vedove, E.; Costabile, G.; Merkel, O.M. Mannose and Mannose-6-Phosphate Receptor-Targeted Drug Delivery Systems and Their Application in Cancer Therapy. Adv. Health Mater. 2018, 7, e1701398. [Google Scholar] [CrossRef]
- Chu, S.; Tang, C.; Yin, C. Effects of mannose density on in vitro and in vivo cellular uptake and RNAi efficiency of polymeric nanoparticles. Biomaterials 2015, 52, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Hatami, E.; Mu, Y.; Shields, D.N.; Chauhan, S.C.; Kumar, S.; Cory, T.J.; Yallapu, M.M. Mannose-decorated hybrid nanoparticles for enhanced macrophage targeting. Biochem. Biophys. Rep. 2019, 17, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhu, C.; Yuan, W.; Wei, X.; Liu, C.; Huang, J.; Yuan, M.; Wu, Y.; Ling, Q.; Hoffmann, P.R.; et al. Mannose-rich Oligosaccharides-functionalized selenium nanoparticles mediates Macrophage reprogramming and inflammation resolution in ulcerative colitis. Chem. Eng. J. 2022, 435, 131715. [Google Scholar] [CrossRef]
- Cummings, R.D. The mannose receptor ligands and the macrophage glycome. Curr. Opin. Struct. Biol. 2022, 75, 102394. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesth. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, B.; Li, Y. Resolution of Cancer-Promoting Inflammation: A New Approach for Anticancer Therapy. Front. Immunol. 2017, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Bi, H.; Zhou, X.; Jiang, Y.; Zhu, H.; Fu, X.; Yang, B. Structure characterization of soybean peptides and their protective activity against intestinal inflammation. Food Chem. 2022, 387, 132868. [Google Scholar] [CrossRef]
- Torretta, S.; Scagliola, A.; Ricci, L.; Mainini, F.; Di Marco, S.; Cuccovillo, I.; Kajaste-Rudnitski, A.; Sumpton, D.; Ryan, K.M.; Cardaci, S. D-mannose suppresses macrophage IL-1β production. Nat. Commun. 2020, 11, 6343. [Google Scholar] [CrossRef]
- Lee, D.; Han, Y.; Kwon, E.-Y.; Choi, M.-S. d-allulose Ameliorates Metabolic Dysfunction in C57BL/KsJ-db/db Mice. Molecules 2020, 25, 3656. [Google Scholar] [CrossRef] [PubMed]
- Guidance Document. The Declaration of Allulose and Calories from Allulose on Nutrition and Supplement Facts Labels: Guidance for Industry; Center for Food Safety and Applied Nutrition: College Park, MD, USA, 2019. [Google Scholar]
- Phé, V.; Pakzad, M.; Haslam, C.; Gonzales, G.; Curtis, C.; Porter, B.; Chataway, J.; Panicker, J.N. Open label feasibility study evaluating D-mannose combined with home-based monitoring of suspected urinary tract infections in patients with multiple sclerosis. Neurourol. Urodyn. 2017, 36, 1770–1775. [Google Scholar] [CrossRef]
- De Nunzio, C.; Bartoletti, R.; Tubaro, A.; Simonato, A.; Ficarra, V. Role of D-Mannose in the Prevention of Recurrent Uncomplicated Cystitis: State of the Art and Future Perspectives. Antibiotics 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Tan, S.S.; Tan, C.X. Soy protein, bioactive peptides, and isoflavones: A review of their safety and health benefits. PharmaNutrition 2023, 25, 100352. [Google Scholar] [CrossRef]
- OECD. Test No. 471: Bacterial Reverse Mutation Test; OECD Publishing: Paris, France, 2020. [Google Scholar]
- Chung, K.-T.; Kirkovsky, L.; Kirkovsky, A.; Purcell, W.P. Review of mutagenicity of monocyclic aromatic amines: Quantitative structure–activity relationships. Mutat. Res. Rev. Mutat. Res. 1997, 387, 1–16. [Google Scholar] [CrossRef]
- Jemnitz, K.; Veres, Z.; Torok, G.; Toth, E.; Vereczkey, L. Comparative study in the Ames test of benzo[a]pyrene and 2-aminoanthracene metabolic activation using rat hepatic S9 and hepatocytes following in vivo or in vitro induction. Mutagenesis 2004, 19, 245–250. [Google Scholar] [CrossRef]
- Kim, D.; Guengerich, F.P. Cytochrome P450 activation of arylamines and heterocyclic amines. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 27–49. [Google Scholar] [CrossRef]
- Wild, D. Mutagenicity of the food additive AF-2, a nitrofuran, in Escherichia coli and Chinese hamster cells in culture. Mutat. Res. Environ. Mutagen. Relat. Subj. 1975, 31, 197–199. [Google Scholar] [CrossRef]
- Marcos, R.; Sierra, L.M.; Gaivão, I. The SMART assays of Drosophila: Wings and eyes as target tissues. In Genotoxicity and DNA Repair: A Practical Approach; Springer: Berlin/Heidelberg, Germany, 2014; pp. 283–295. [Google Scholar]
- Lajovic, A.; Nagy, L.D.; Guengerich, F.P.; Bren, U. Carcinogenesis of Urethane: Simulation versus Experiment. Chem. Res. Toxicol. 2015, 28, 691–701. [Google Scholar] [CrossRef] [PubMed]
| Cell Lines | Oligopeptide (OT) (µg/mL) | Allulose (AL) (µg/mL) | D-mannose (Man) (µg/mL) | |||
|---|---|---|---|---|---|---|
| IC20 | IC50 | IC20 | IC50 | IC20 | IC50 | |
| HepG2 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| HEK293 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| LX-2 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| RAW 264.7 | 60 | 510 | >1000 | >1000 | >1000 | >1000 |
| 3T3-L1 Pre-adipocyte | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| 3T3-L1 Mature-adipocytes | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Cell Lines | Oligopeptide–Allulose (OT-AL) (µg/mL) | Oligopeptide–D-mannose (OT-Man) (µg/mL) | ||
|---|---|---|---|---|
| IC20 | IC50 | IC20 | IC50 | |
| HepG2 | >1000 | >1000 | >1000 | >1000 |
| HEK293 | >1000 | >1000 | >1000 | >1000 |
| LX-2 | >1000 | >1000 | >1000 | >1000 |
| RAW 264.7 | 270 | >1000 | 450 | >1000 |
| 3T3-L1 Pre-adipocyte | >1000 | >1000 | >1000 | >1000 |
| 3T3-L1 Mature-adipocytes | >1000 | >1000 | >1000 | >1000 |
| Extracts (µg/mL) | % Cell Viability (n = 5) |
|---|---|
| 0 | 100 |
| Oligopeptide (OT) | |
| 100 | 114 ± 20.76 |
| 200 | 110 ± 23.32 |
| 400 | 117 ± 20.97 |
| 800 | 120 ± 24.60 |
| Allulose | |
| 800 | 108 ± 17.96 |
| Oligopeptide–Allulose (OT-AL) | |
| 100 | 104 ± 19.21 |
| 200 | 110 ± 22.12 |
| 400 | 111 ± 25.05 |
| 800 | 113 ± 24.14 |
| D-mannose | |
| 800 | 106 ± 13.26 |
| Oligopeptide–D-mannose (OT-Man) | |
| 100 | 99 ± 12.60 |
| 200 | 111 ± 20.32 |
| 400 | 114 ± 17.88 |
| 800 | 120 ± 16.60 |
| Extracts (µg/mL) | % Hemolysis (n = 5) |
|---|---|
| 0.05% triton x-100 | 100 ± 0.00 |
| Oligopeptide (OT) | |
| 100 | 1.98 ± 0.11 |
| 200 | 2.01 ± 0.16 |
| 400 | 1.76 ± 0.13 |
| 800 | 1.65 ± 0.27 |
| Allulose | |
| 800 | 1.76 ± 0.26 |
| Oligopeptide–Allulose (OT-AL) | |
| 100 | 2.19 ± 0.23 |
| 200 | 2.26 ± 0.16 |
| 400 | 2.56 ± 0.26 |
| 800 | 2.75 ± 0.37 |
| D-mannose | |
| 800 | 1.84 ± 0.42 |
| Oligopeptide–D-mannose (OT-Man) | |
| 100 | 2.14 ± 0.09 |
| 200 | 2.38 ± 0.12 |
| 400 | 2.54 ± 0.23 |
| 800 | 2.98 ± 0.32 |
| Treatment | Dose (per Plate) | His+ Revertant Colonies per Plate | |||
|---|---|---|---|---|---|
| TA100 | TA98 | ||||
| +S9 | −S9 | +S9 | −S9 | ||
| DMSO | - | 131.3 ± 11.0 | 122.8 ± 6.2 | 38.5 ± 3.8 | 30.7 ± 5.0 |
| 2-AA | 0.5 µg | 599.7 ± 8.7 | - | 325.8 ± 16.2 | - |
| AF-2 | 0.01 µg | - | 527.3 ± 15.0 | - | - |
| AF-2 | 0.1 µg | - | - | - | 293.0 ± 10.3 |
| Oligopeptide (OT) | 0.04 mg | 133.3 ± 6.7 | 128.2 ± 4.5 | 34.8 ± 6.5 | 30.2 ± 1.8 |
| 0.2 mg | 144.0 ± 10.0 | 131.2 ± 3.8 | 38.2 ± 5.8 | 31.3 ± 7.0 | |
| 1 mg | 161.5 ± 6.5 | 147.5 ± 7.8 | 44.3 ± 6.3 | 34.5 ± 4.8 | |
| 5 mg | 228.2 ± 8.8 (k) | 191.8 ± 0.2 (k) | 62.8 ± 9.8 (k) | 43.5 ± 1.8 (k) | |
| D-Mannose (Man) | 0.04 mg | 127.5 ± 2.5 | 135.2 ± 5.2 | 37.7 ± 5.7 | 30.5 ± 2.5 |
| 0.2 mg | 140.3 ± 2.3 | 137.5 ± 4.5 | 37.8 ± 3.8 | 30.8 ± 4.8 | |
| 1 mg | 139.0 ± 0.7 | 135.2 ± 4.5 | 36.5 ± 7.5 | 27.8 ± 6.5 | |
| 5 mg | 138.5 ± 4.2 | 129.7 ± 4.7 | 38.5 ± 7.8 | 28.7 ± 6.0 | |
| Mannose-conjugated oligopeptide (OT-Man) | 0.04 mg | 139.5 ± 7.2 | 136.0 ± 5.7 | 37.5 ± 4.8 | 28.8 ± 6.2 |
| 0.2 mg | 137.3 ± 4.0 | 128.2 ± 5.2 | 39.3 ± 9.0 | 29.0 ± 4.7 | |
| 1 mg | 145.2 ± 4.8 | 127.2 ± 1.8 | 41.2 ± 8.2 | 32.5 ± 4.5 | |
| 5 mg | 146.3 ± 5.0 (k) | 140.8 ± 9.2 (k) | 45.0 ± 8.7 | 31.3 ± 4.7 | |
| Samples | Number of Wings | Frequency of Mutant Spots per Individual (Number of Spots) # | ||||
|---|---|---|---|---|---|---|
| Small Single (1–2 Cells) | Large Single (>2 Cells) | Twin | Total Spots | |||
| DI (negative control) | 40 | 0.63 (25) | 0.05 (2) | 0.00 (0) | 0.68 (27) | |
| 20 mM urethane | 40 | 6.10 (244) + | 3.40 (136) + | 0.68 (27) + | 10.18 (407) + | |
| OT | 62.5 µg/mL | 40 | 0.50 (20) − | 0.00 (0) − | 0.00 (0) − | 0.50 (20)− |
| 125 µg/mL | 40 | 0.50 (20) − | 0.00 (0) − | 0.00 (0) − | 0.50 (20) − | |
| 250 µg/mL | 40 | 0.28 (11) − | 0.00 (0) − | 0.00 (0) − | 0.28 (11) − | |
| 500 µg/mL | 40 | 0.28 (11) − | 0.03 (1) i | 0.00 (0) − | 0.30 (12) − | |
| 1000 µg/mL | 40 | 0.43 (17) − | 0.00 (0) − | 0.00 (0) − | 0.43 (17) − | |
| 2000 µg/mL | 40 | 0.45 (18) − | 0.03 (1) i | 0.00 (0) − | 0.48 (19) − | |
| 5000 µg/mL | 40 | 0.35 (14) − | 0.03 (1) i | 0.00 (0) − | 0.38 (15) − | |
| Man | 62.5 µg/mL | 40 | 0.40 (16) − | 0.03 (1) i | 0.00 (0) − | 0.43 (17)- |
| 125 µg/mL | 40 | 0.50 (20) − | 0.03 (1) i | 0.00 (0) − | 0.53 (21) − | |
| 250 µg/mL | 40 | 0.43 (17) − | 0.03 (1) i | 0.00 (0) − | 0.45 (18) − | |
| 500 µg/mL | 40 | 0.25 (10) − | 0.03 (1) i | 0.00 (0) − | 0.28 (11) − | |
| 1000 µg/mL | 40 | 0.25 (10) − | 0.03 (1) i | 0.00 (0) − | 0.28 (11) − | |
| 2000 µg/mL | 40 | 0.23 (9) − | 0.00 (0) − | 0.00 (0) − | 0.23 (9) − | |
| 5000 µg/mL | 40 | 0.18 (7) − | 0.00 (0) − | 0.00 (0) − | 0.18 (7) − | |
| OT-Man | 62.5 µg/mL | 40 | 0.25 (10) − | 0.00 (0) − | 0.00 (0) − | 0.25 (10) − |
| 125 µg/mL | 40 | 0.25 (10) − | 0.00 (0) − | 0.00 (0) − | 0.25 (10) − | |
| 250 µg/mL | 40 | 0.30 (12) − | 0.00 (0) − | 0.00 (0) − | 0.30 (12) − | |
| 500 µg/mL | 40 | 0.25 (10) − | 0.00 (0) − | 0.00 (0) − | 0.25 (10) − | |
| 1000 µg/mL | 40 | 0.43 (17) − | 0.00 (0) − | 0.00 (0) − | 0.43 (17) − | |
| 2000 µg/mL | 40 | 0.13 (5) − | 0.00 (0) − | 0.00 (0) − | 0.13 (5) − | |
| 5000 µg/mL | 40 | 0.18 (7)− | 0.00 (0)− | 0.00 (0)− | 0.18 (7)− | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitchakarn, P.; Buacheen, P.; Taya, S.; Karinchai, J.; Temviriyanukul, P.; Inthachat, W.; Chaipoot, S.; Wiriyacharee, P.; Phongphisutthinant, R.; Ounjaijean, S.; et al. Anti-Inflammatory, Cytotoxic, and Genotoxic Effects of Soybean Oligopeptides Conjugated with Mannose. Foods 2024, 13, 2558. https://doi.org/10.3390/foods13162558
Pitchakarn P, Buacheen P, Taya S, Karinchai J, Temviriyanukul P, Inthachat W, Chaipoot S, Wiriyacharee P, Phongphisutthinant R, Ounjaijean S, et al. Anti-Inflammatory, Cytotoxic, and Genotoxic Effects of Soybean Oligopeptides Conjugated with Mannose. Foods. 2024; 13(16):2558. https://doi.org/10.3390/foods13162558
Chicago/Turabian StylePitchakarn, Pornsiri, Pensiri Buacheen, Sirinya Taya, Jirarat Karinchai, Piya Temviriyanukul, Woorawee Inthachat, Supakit Chaipoot, Pairote Wiriyacharee, Rewat Phongphisutthinant, Sakaewan Ounjaijean, and et al. 2024. "Anti-Inflammatory, Cytotoxic, and Genotoxic Effects of Soybean Oligopeptides Conjugated with Mannose" Foods 13, no. 16: 2558. https://doi.org/10.3390/foods13162558
APA StylePitchakarn, P., Buacheen, P., Taya, S., Karinchai, J., Temviriyanukul, P., Inthachat, W., Chaipoot, S., Wiriyacharee, P., Phongphisutthinant, R., Ounjaijean, S., & Boonyapranai, K. (2024). Anti-Inflammatory, Cytotoxic, and Genotoxic Effects of Soybean Oligopeptides Conjugated with Mannose. Foods, 13(16), 2558. https://doi.org/10.3390/foods13162558








