Comprehensive Analysis of Allulose Production: A Review and Update
Abstract
1. Introduction
2. Recent Updates
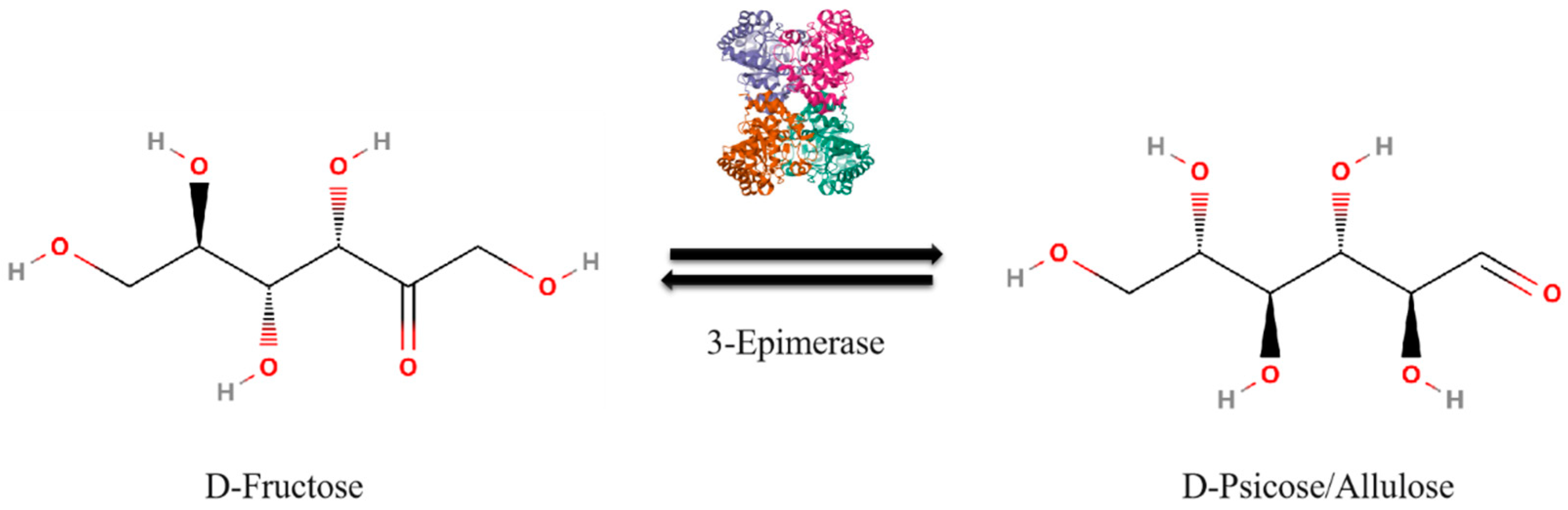
2.1. Enzymatic Conversion
2.1.1. Immobilization
2.1.2. Exploring Novel Industrial Enzymes
| No | Source | Temperature (°C) | pH | Metal Ions | Half-Life (min) | Equilibrium Ratio (D-Allulose to D-Fructose) | Specific Activity * (U/mg) | Kcat/Km (mM−1min−1) | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Agrobacterium sp. | 55–60 | 7.5–8.0 | Co2+ | 267(55 °C) | 30.0% | 253.0 | 19.5 | [23] |
| 2 | Bacillus sp. | 40 | 7.5 | Co2+ | 25 (50 °C) | 30.7% | 185.7 | - | [40] |
| 3 | Bacillus sp. KCTC 13219 | 55 | 8.0 | Mn2+ | 36,000 (50 °C), 1320 (55 °C) | 28.5% | 127.2 | - | [10] |
| 4 | Bacillus subtilis | 80 | 7.0 | Co2+ | 9900 (60 °C), 3240 (70 °C), 49 (80 °C) | 31.0% | 1.1 | - | [13] |
| 5 | Caballeronia fortuita | 65 | 7.5 | Co2+ | 63 (60 °C) | 29.5% (65 °C) | 270.0 | 432.6 | [41] |
| 6 | Christensenella minuta DSM 22607 | 50 | 6.0 | Ni2+ | 40 (50 °C) | 30% (50 °C) | 124.0 | [42] | |
| 7 | Clostridium cellulolyticum H10 | 70 | 8.0 | Co2+ | 537 (65 °C), 1573 (60 °C) A107P/D281G/C289R | 27.5% | 295.5 | - | [43] |
| 8 | Labedella endophytica | 80 | 6.0 | Ni2+ | 2262 (60 °C), 540 (65 °C), 276 (70 °C) | - | 110.7 | - | [21] |
| 9 | Novibacillus thermophilus | 70 | 7.0 | Co2+ | 846.3 (40 °C) 539.2 (50 °C), 47.8 (60 °C) | 28.3% | 146.0 | 1354.9 | [44] |
| 10 | Paenibacillus senegalensis | 55 | 8.0 | Mn2+ | 140 (60 °C) | 30% (55 °C) 30% (60 °C) | 25.2 | 39.0 | [36] |
| 11 | Pirellula sp. SH-Sr6A | 60 | 7.5 | Co2+ | 360 (60 °C) | 31.4% | - | - | [37] |
| 12 | Rhodopirellula baltica | 60 | 8.0 | Mn2+ | 52 (60 °C) | - | 37.6 | 43.3 | [46] |
| 13 | Sinorhizobium sp. | 50 | 8.0 | Mn2+ | - | 25.3% | - | 118.2 | [45] |
| 14 | Staphylococcus aureus | 70 | 8.0 | Mg2+ | 122.4 (70 °C) | 28.3% | 38.4 | - | [47] |
| 15 | Thermoclostridium caenicola | 65 | 7.5 | Co2+ | 120 (55 °C) | 28.0% | - | 4.9 | [22] |
2.1.3. Direct Evolution Study
2.1.4. Synthetic Biology Method
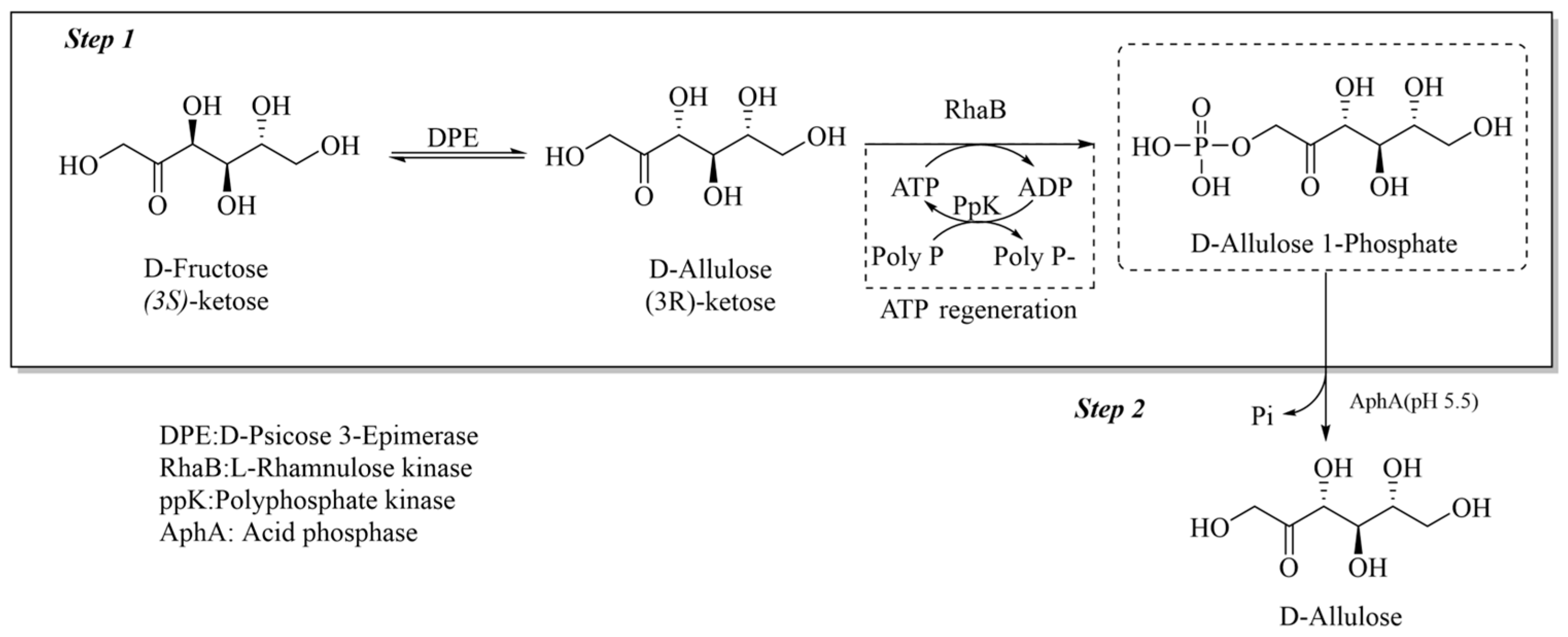
2.2. High-Throughput Screening Methods
3. Summary
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, C.; Chen, Z.; Gao, X.-D.; Li, Z. Recent Advances Regarding the Physiological Functions and Biosynthesis of D-Allulose. Front. Microbiol. 2022, 13, 881037. [Google Scholar] [CrossRef]
- Higaki, S.; Inai, R.; Mochizuki, S.; Yoshihara, A.; Matsuo, T. Dietary dried sweetspire (Itea) powder reduces body fat accumulation in rats fed with a high-fat diet. J. Oleo Sci. 2022, 71, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Fernandes, P. Enzymes applied in sweeteners production. In Enzymatic Processes for Food Valorization; Elsevier: Amsterdam, The Netherlands, 2024; pp. 217–243. [Google Scholar]
- İlhan, E. Formulation and Characterization of Starch and Soy Protein Containing Low Calorie Soft Candy. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2019. [Google Scholar]
- Brewster, E. Solving for Sugar Reduction. Food Technol. 2021, 75, 39–47. [Google Scholar]
- Nachay, K.; Bartelme, M.Z. Ingredients for a Changing Consumer Landscape. IFT Food Technol. 2016, 6, 50–96. [Google Scholar]
- Jin, L.; Wan, Q.; Ouyang, S.; Zheng, L.; Cai, X.; Zhang, X.; Shen, J.; Jia, D.; Liu, Z.; Zheng, Y. Isomerase and epimerase: Overview and practical application in production of functional sugars. Critical Reviews in Food Science and Nutrition 2023, 1–16. [Google Scholar] [CrossRef]
- Jiang, S.; Xiao, W.; Zhu, X.; Yang, P.; Zheng, Z.; Lu, S.; Jiang, S.; Zhang, G.; Liu, J. Review on D-allulose: In vivo metabolism, catalytic mechanism, engineering strain construction, bio-production technology. Front. Bioeng. Biotechnol. 2020, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Lin, H.; Ning, Y.; Liu, G.; Ren, Y.; Li, C.; Zhang, C.; Lin, J.; Song, X.; Lin, J. D-Allulose (D-Psicose) Biotransformation From Allitol by a Newly Found NAD(P)-Dependent Alcohol Dehydrogenase from Gluconobacter frateurii NBRC 3264 and the Enzyme Characterization. Front. Microbiol. 2022, 13, 870168. [Google Scholar] [CrossRef] [PubMed]
- Narayan Patel, S.; Kaushal, G.; Singh, S.P. D-Allulose 3-epimerase of Bacillus sp. origin manifests profuse heat-stability and noteworthy potential of D-fructose epimerization. Microb. Cell Factories 2021, 20, 1–16. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, D.; Chen, J.; Xu, W.; Chen, Q.; Wu, H.; Guang, C.; Mu, W. D-allulose, a versatile rare sugar: Recent biotechnological advances and challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 5661–5679. [Google Scholar] [CrossRef]
- Wen, X.; Ning, Y.; Lin, H.; Ren, Y.; Li, C.; Liu, Y.; Zhang, C.; Lin, J.; Lin, J. D-Allulose (d-psicose) biotransformation from d-glucose, separation by simulated moving bed chromatography (SMBC) and purification by crystallization. Process Biochem. 2022, 119, 29–38. [Google Scholar] [CrossRef]
- Narayan Patel, S.; Kaushal, G.; Singh, S.P. A Novel d-Allulose 3-Epimerase Gene from the Metagenome of a Thermal Aquatic Habitat and d-Allulose Production by Bacillus subtilis Whole-Cell Catalysis. Appl. Environ. Microbiol. 2019, 86, e02605-19. [Google Scholar] [CrossRef]
- Tian, C.; Yang, J.; Liu, C.; Chen, P.; Zhang, T.; Men, Y.; Ma, H.; Sun, Y.; Ma, Y. Engineering substrate specificity of HAD phosphatases and multienzyme systems development for the thermodynamic-driven manufacturing sugars. Nat. Commun. 2022, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Y.; Liu, Y.; Li, J.; Du, G.; Chen, J.; Lv, X.; Liu, L. Sustainable bioproduction of natural sugar substitutes: Strategies and challenges. Trends Food Sci. Technol. 2022, 129, 512–527. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Mu, W. Recent Advances in Ketose 3-Epimerase and Its Application for D-Allulose Production. Nov. Enzym. Funct. Carbohydr. Prod. 2021, 17–42. [Google Scholar] [CrossRef]
- Narayan Patel, S.; Sharma, S.; Singh, A.K.; Singh, S.P. Potential Microbial Bioresources for Functional Sugar Molecules. Microb. Bioreact. Ind. Mol. 2023, 211–236. [Google Scholar] [CrossRef]
- Taylor, J.A.; Palur, D.S.K.; Zhang, A.; Gonzales, J.N.; Arredondo, A.; Coulther, T.A.; Lechner, A.; Rodríguez, E.P.; Fiehn, O.; Didzbalis, J.; et al. Awakening the natural capability of psicose production in Escherichia coli. NPJ Sci. Food 2023, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; An, Y.; Iqbal, M.W.; Cong, H.; Zhang, G.; Zhang, Y.; Ravikumar, Y.; Zabed, H.M.; Zhao, M.; Zhou, H.; et al. The Characterization of a Novel D-allulose 3-Epimerase from Blautia produca and Its Application in D-allulose Production. Foods 2022, 11, 3225. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Shi, T.; Shi, T.; Han, P.; Han, P.; You, C. Chun You Thermodynamics-Driven Production of Value-Added d-Allulose from Inexpensive Starch by an In Vitro Enzymatic Synthetic Biosystem. ACS Catal. 2021, 11, 5088–5099. [Google Scholar] [CrossRef]
- Chen, D.; Chen, J.; Liu, X.; Guang, C.; Zhang, W.; Mu, W. Biochemical identification of a hyperthermostable l-ribulose 3-epimerase from Labedella endophytica and its application for d-allulose bioconversion. Int. J. Biol. Macromol. 2021, 189, 214–222. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Ke, M.; Ye, S.; Wang, X.; Zhang, W.; Mu, W. Characterization of a Recombinant d-Allulose 3-epimerase from Thermoclostridium caenicola with Potential Application in d-Allulose Production. Mol. Biotechnol. 2021, 63, 534–543. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, C.; Chen, X.; Ruan, X.; Zhang, Y.; Xu, H.; Guo, Y.; Xu, J.; Lv, P.; Wang, Z. Engineered Bacillus subtilis harbouring gene of d-tagatose 3-epimerase for the bioconversion of d-fructose into d-psicose through fermentation. Enzym. Microb. Technol. 2020, 136, 109531. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M. Safety evaluation of the food enzyme d-psicose 3-epimerase from the genetically modified Corynebacterium glutamicum strain FIS 002. EFSA J. 2021, 19, e06870. [Google Scholar] [PubMed]
- Wang, F.; Zhu, M.; Song, Z.; Li, C.; Wang, Y.; Zhu, Z.; Sun, D.; Lu, F.; Qin, H.-M. Reshaping the Binding Pocket of Lysine Hydroxylase for Enhanced Activity. ACS Catal. 2020, 10, 13946–13956. [Google Scholar] [CrossRef]
- Li, J.; Dai, Q.; Zhu, Y.; Xu, W.; Zhang, W.; Chen, Y.; Mu, W. Low-calorie bulk sweeteners: Recent advances in physical benefits, applications, and bioproduction. Crit. Rev. Food Sci. Nutr. 2023, 64, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Maeng, H.-J.; Yoon, J.-H.; Chun, K.-H.; Kim, S.T.; Jang, D.-J.; Park, J.-E.; Kim, Y.H.; Kim, S.-B.; Kim, Y.C. Metabolic stability of D-allulose in biorelevant media and hepatocytes: Comparison with fructose and erythritol. Foods 2019, 8, 448. [Google Scholar] [CrossRef] [PubMed]
- Demao, L.I.; Yan, Z.; Ju, Z.; Wanf, Q.; Sun, J.; Jiang, H.; Ma, Y. Regulation and guidance for marketing of food ingredients from biomanufacturing and policy suggestions for China. Bull. Chin. Acad. Sci. (Chin. Version) 2020, 35, 1041–1052. [Google Scholar]
- Cywińska-Antonik, M.; Chen, Z.; Groele, B.; Marszałek, K. Application of Emerging Techniques in Reduction of the Sugar Content of Fruit Juice: Current Challenges and Future Perspectives. Foods 2023, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-H.; Kwon, M.-C.; Kim, S.-W. Advanced Whole-cell Conversion for D-allulose Production Using an Engineered Corynebacterium glutamicum. Biotechnol. Bioprocess Eng. 2022, 27, 276–285. [Google Scholar] [CrossRef]
- Hu, M.; Li, M.; Jiang, B.; Tao, Z. Bioproduction of D-allulose: Properties, applications, purification, and future perspectives. Compr. Rev. Food Sci. Food Saf. 2021, 20, 6012–6026. [Google Scholar] [CrossRef]
- Nirwantono, R.; Trinugroho, J.P.; Sudigyo, D.; Ahmad Hidayat, A.; Mahesworo, B. Bens Pardamean Genome mining of potential enzyme from Chelatococcus composti for sustainable production of D-Allulose. IOP Conf. Ser. 2023, 1169, 012083. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Z.; Nakanishi, H.; Li, Z. Highly Efficient Synthesis of Rare Sugars from Glycerol in Endotoxin-Free ClearColi by Fermentation. Foods 2023, 12, 3078. [Google Scholar] [CrossRef]
- Xia, Y.; Cheng, Q.; Mu, W.; Hu, X.; Sun, Z.; Qiu, Y.; Liu, X.; Wang, Z. Research advances of D-allulose: An overview of physiological functions, enzymatic biotransformation technologies, and production processes. Foods 2021, 10, 2186. [Google Scholar] [CrossRef] [PubMed]
- Ninchan, B.; Songbang, S.; Watthanasakphuban, N. Optimization and Comparative Methods for Efficient D-psicose Production Using Physicochemical and Enzymatic Processes. Sugar Tech 2024, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Tian, C.; Zhang, T.; Ren, C.; Zhu, Y.; Zeng, Y.; Men, Y.; Sun, Y.; Ma, Y. Development of food-grade expression system for d-allulose 3-epimerase preparation with tandem isoenzyme genes in Corynebacterium glutamicum and its application in conversion of cane molasses to D-allulose. Biotechnol. Bioeng. 2019, 116, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lei, L.; Li, L.; Feng, Z.; Guan, L.; Lu, F.; Qin, H.-M. Two-step biosynthesis of d-allulose via a multienzyme cascade for the bioconversion of fruit juices. Food Chem. 2021, 357, 129746. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Liu, C.-L.; Yang, Y.; Liu, X.; Zhan, J.; Bai, Z. Immobilization of d-allulose 3-epimerase into magnetic metal–organic framework nanoparticles for efficient biocatalysis. World J. Microbiol. Biotechnol. 2022, 38, 144. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.-C.; Chen, C.-N.; Hsu, C.-T.; Lee, H.-C.; Fang, H.-Y.; Wang, M.-J.; Wu, Y.-H.; Fang, T.-Y. Characterization of a recombinant D-allulose 3-epimerase from Agrobacterium sp. ATCC 31749 and identification of an important interfacial residue. Int. J. Biol. Macromol. 2018, 112, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-J.; Kwon, E.R.; Kim, S.J.; Choi, M.S.; Kim, Y.-S.; Park, C.-S. D-Allulose Production from d-fructose by Putative Dolichol Phosphate Mannose Synthase from Bacillus sp. with Potential d-allulose 3-epimrase Activity. Biotechnol. Bioprocess Eng. 2021, 26, 976–984. [Google Scholar] [CrossRef]
- Li, S.; Chen, Z.; Zhang, W.; Guang, C.; Mu, W. Characterization of a d-tagatose 3-epimerase from Caballeronia fortuita and its application in rare sugar production. Int. J. Biol. Macromol. 2019, 138, 536–545. [Google Scholar] [CrossRef]
- Wang, Y.; Ravikumar, Y.; Zhang, G.; Yun, J.; Zhang, Y.; Parvez, A.; Qi, X.; Sun, W. Biocatalytic Synthesis of D-Allulose Using Novel D-Tagatose 3-Epimerase From Christensenella minuta. Front. Chem. 2020, 8, 622325. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Kong, D.; Wu, J.; Liu, Z. Enhancing the thermostability of d-allulose 3-epimerase from Clostridium cellulolyticum H10 via directed evolution. Syst. Microbiol. Biomanuf. 2022, 2, 685–694. [Google Scholar] [CrossRef]
- Jia, D.-X.; Sun, C.-Y.; Jin, Y.-T.; Liu, Z.-Q.; Zheng, Y.-G.; Li, M.; Wang, H.-Y.; Chen, D.-S. Properties of d-allulose 3-epimerase mined from Novibacillus thermophilus and its application to synthesis of d-allulose. Enzym. Microb. Technol. 2021, 148, 109816. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, C.; Liu, X.; Gao, D.; Wang, X.; Tanokura, M.; Qin, H.-M.; Lu, F. Biochemical characterization and biocatalytic application of a novel D-tagatose 3-epimerase from Sinorhizobium sp. RSC Adv. 2019, 9, 2919–2927. [Google Scholar] [CrossRef]
- Mao, S.; Cheng, X.; Zhu, Z.; Chen, Y.; Li, C.; Zhu, M.; Liu, X.; Lu, F.; Qin, H.-M. Engineering a thermostable version of D-allulose 3-epimerase from Rhodopirellula baltica via site-directed mutagenesis based on B-factors analysis. Enzym. Microb. Technol. 2020, 132, 109441. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, D.; Li, C.; Chen, Y.; Zhu, M.; Liu, X.; Tanokura, M.; Qin, H.-M.; Lu, F. Redesign of a novel d -allulose 3-epimerase from Staphylococcus aureus for thermostability and efficient biocatalytic production of d -allulose. Microb. Cell Factories 2019, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Wang, T.; Li, H.; Li, C.; Guan, L.; Liu, W.; Wang, J.; Qin, H.-M.; Mao, S. Sequence- and Structure-Based Mining of Thermostable D-Allulose 3-Epimerase and Computer-Guided Protein Engineering To Improve Enzyme Activity. J. Agric. Food Chem. 2023, 71, 18431–18442. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Chen, Q.; Xu, W.; Zhang, W.; Mu, W. Computer-Aided Targeted Mutagenesis of Thermoclostridium caenicola d-Allulose 3-Epimerase for Improved Thermostability. J. Agric. Food Chem. 2022, 70, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; Zhao, J.; Li, H.; Wei, X.; Liu, J. Improved thermostability of D-allulose 3-epimerase from Clostridium bolteae ATCC BAA-613 by proline residue substitution. Protein Expr. Purif. 2022, 199, 106145. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Wang, T.; Qi, H.; Wei, M.; Lu, F.; Guan, L.; Mao, S.; Qin, H.-M. Engineering of Acid-Resistant d-Allulose 3-Epimerase for Functional Juice Production. J. Agric. Food Chem. 2022, 70, 16298–16306. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, L.; Zhang, W.; Li, C.; Mao, S.; Lu, F.; Qin, H.-M. Improving the enzyme property of D-allulose 3-epimerase from a thermophilic organism of Halanaerobium congolense through rational design. Enzym. Microb. Technol. 2021, 149, 109850. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, C.; Tang, M.; Xu, B.; Wang, L.; Qian, W.; He, J.; Zhao, Z.; Wu, Q.; Mu, Y. Improving the thermostability of Rhizopus chinensis lipase through site-directed mutagenesis based on B-factor analysis. Front. Microbiol. 2020, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, C.-Y.; Zheng, L.; Zheng, S.-H.; Zhang, Y.; Zhao, S.-Y.; Zheng, H.-D.; Fan, L.-H.; Lin, X.-C. Metabolically Engineered Escherichia coli for Conversion of D-Fructose to D-Allulose via Phosphorylation-Dephosphorylation. Front. Bioeng. Biotechnol. 2022, 10, 947469. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Feng, T.; Wang, Z.; Li, H.; Wei, X.; Chen, J.; Niu, D.; Liu, J. Phosphorylation-Driven Production of d-Allulose from d-Glucose by Coupling with an ATP Regeneration System. J. Agric. Food Chem. 2022, 70, 15539–15547. [Google Scholar] [CrossRef] [PubMed]
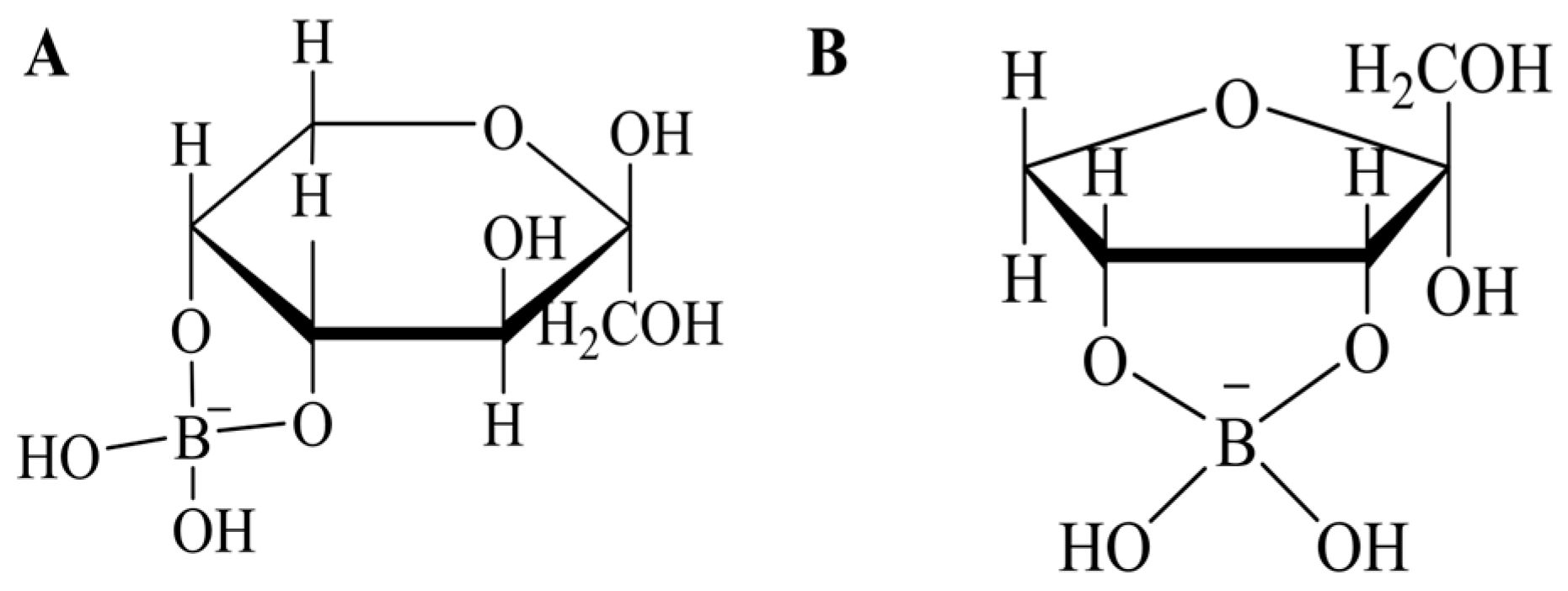
- Arimitsu, K.; Iwasaki, H.; Kimura, H.; Yasui, H. Strong binding affinity of D-allulose and allulosides to boronic acids and the structural characterization of their sugar-boronate complexes. Chem. Lett. 2021, 50, 1470–1474. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, Z.; Lv, J.; Li, Y.; Chen, J.; Cheng, X.; Li, N.; Liu, J. Irreversible biosynthesis of D-allulose from D-glucose in Escherichia coli through fine-tuning of carbon flux and cofactor regeneration engineering. J. Sci. Food Agric. 2023, 103, 5481–5489. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.; Wei, C.; Gao, X.; Mao, S.; Lu, F.; Qin, H.M. Continuous Spectrophotometric Assay for High-Throughput Screening of Predominant d-Allulose 3-Epimerases. J. Agric. Food Chem. 2021, 69, 11637–11645. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Cui, Y.; Lu, Y.; Zhao, Z. Comprehensive Analysis of Allulose Production: A Review and Update. Foods 2024, 13, 2572. https://doi.org/10.3390/foods13162572
Wang L, Cui Y, Lu Y, Zhao Z. Comprehensive Analysis of Allulose Production: A Review and Update. Foods. 2024; 13(16):2572. https://doi.org/10.3390/foods13162572
Chicago/Turabian StyleWang, Lei, Yun Cui, Yujie Lu, and Zongpei Zhao. 2024. "Comprehensive Analysis of Allulose Production: A Review and Update" Foods 13, no. 16: 2572. https://doi.org/10.3390/foods13162572
APA StyleWang, L., Cui, Y., Lu, Y., & Zhao, Z. (2024). Comprehensive Analysis of Allulose Production: A Review and Update. Foods, 13(16), 2572. https://doi.org/10.3390/foods13162572





