Metabolomic Approaches to Study the Potential Inhibitory Effects of Plantaricin Q7 against Listeria monocytogenes Biofilm
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Culture and Preparation of Plantaricin Q7
2.2. Determination of the Antimicrobial Activity of Plantaricin Q7
2.3. Metabolomic Sample Preparation
2.4. Metabolite Extraction
2.5. LC-MS/MS Analysis
2.6. Metabolite Identification
2.7. Statistical Analysis
3. Results and Discussion
3.1. Determination of Plantaricin Q7 Antimicrobial Activity
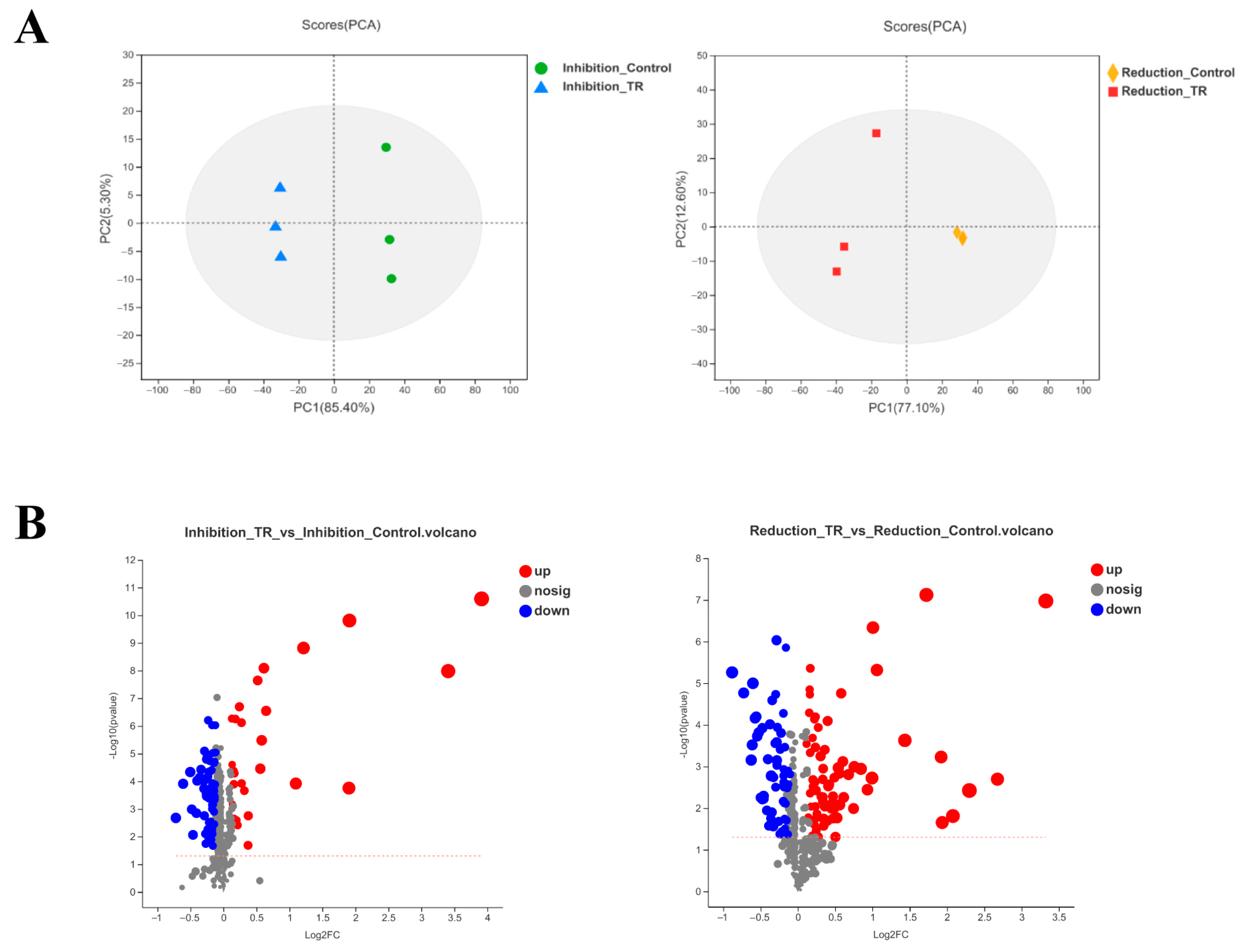
3.2. Metabolomic Samples’ Statistical Analysis
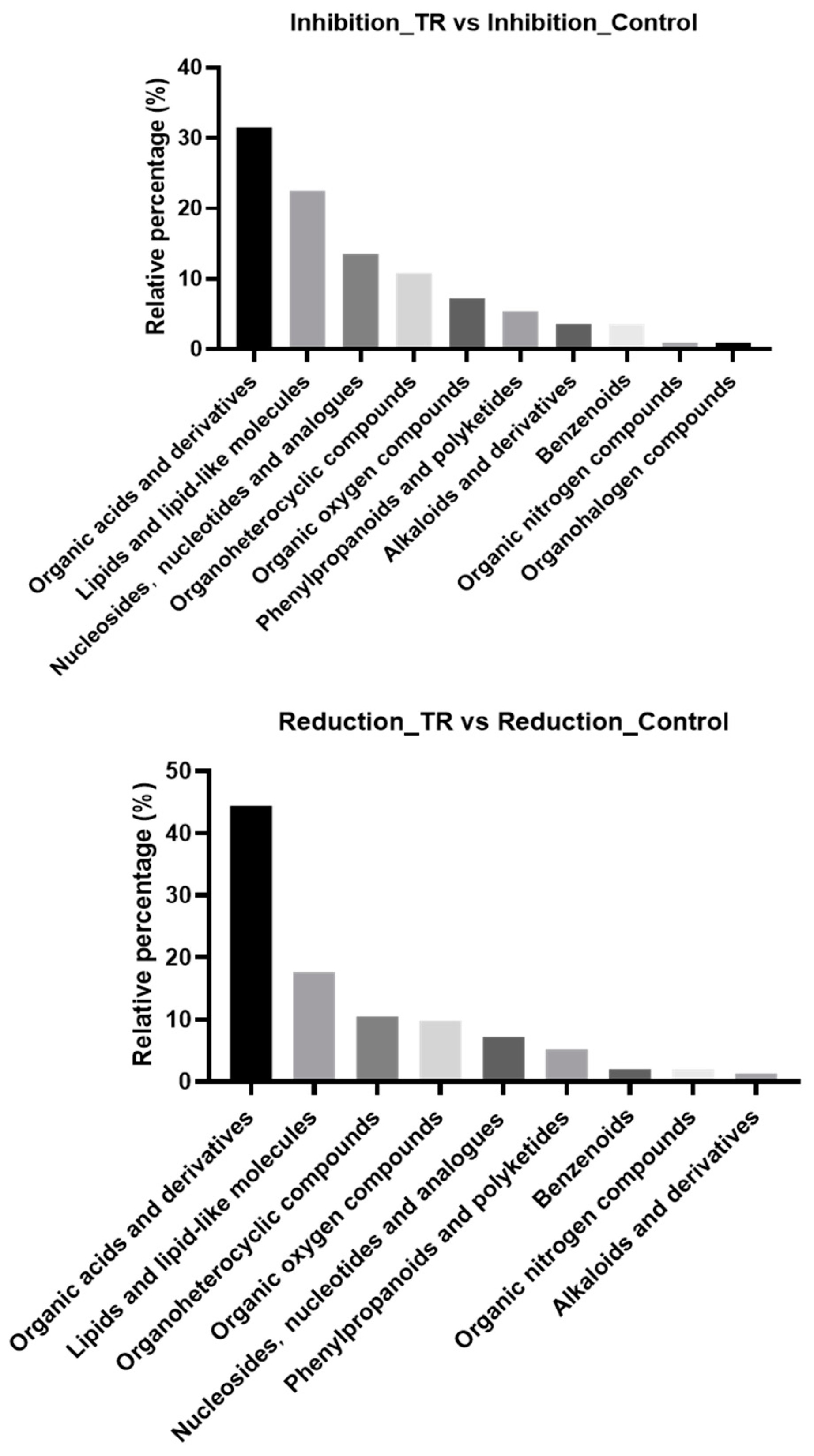
3.3. Differential Metabolite Classification
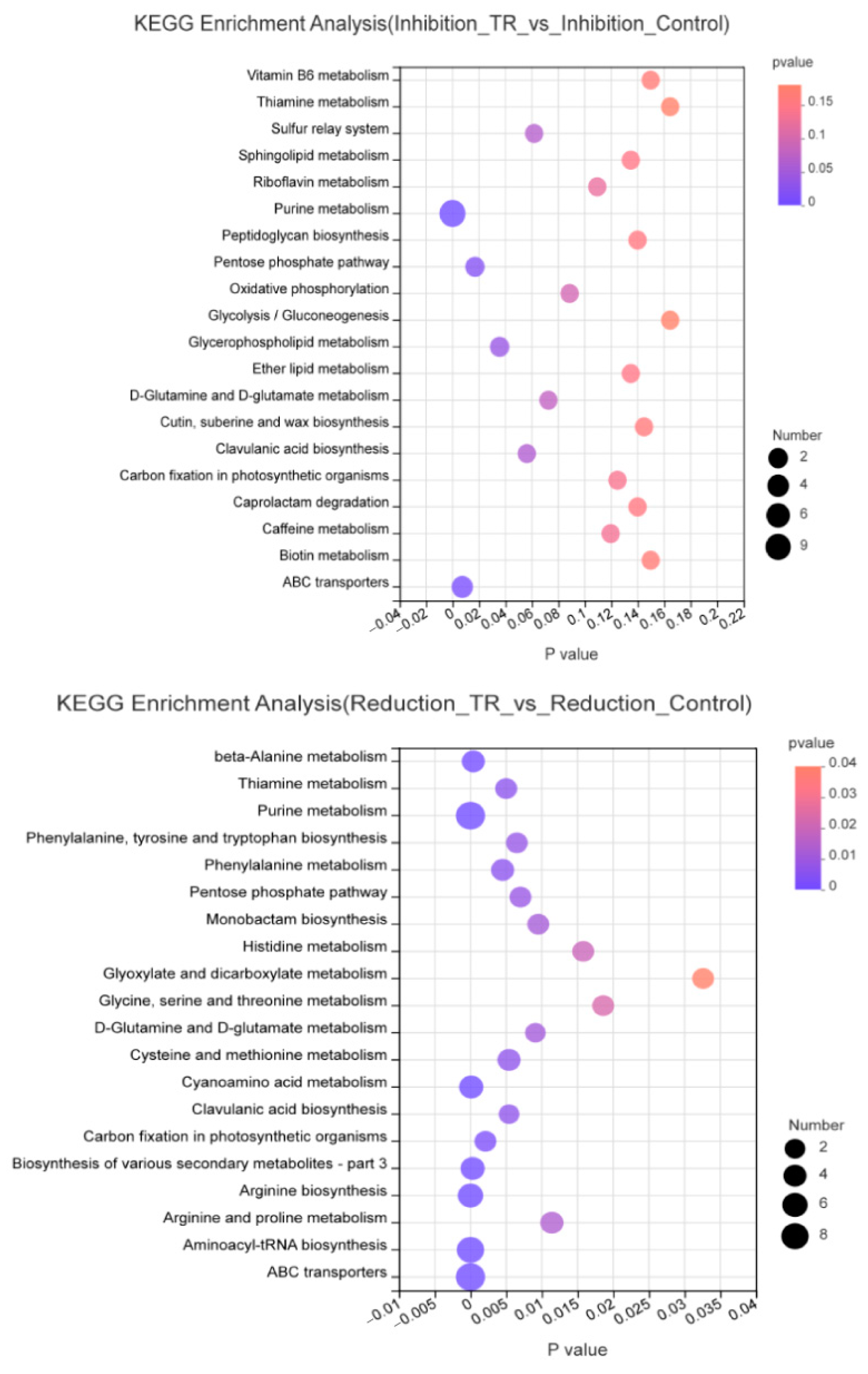
3.4. Metabolic Pathway Enrichment Analysis
3.5. Effect of Plantaricin Q7 on Purine Metabolism and Biofilm Formation of L. monocytogenes
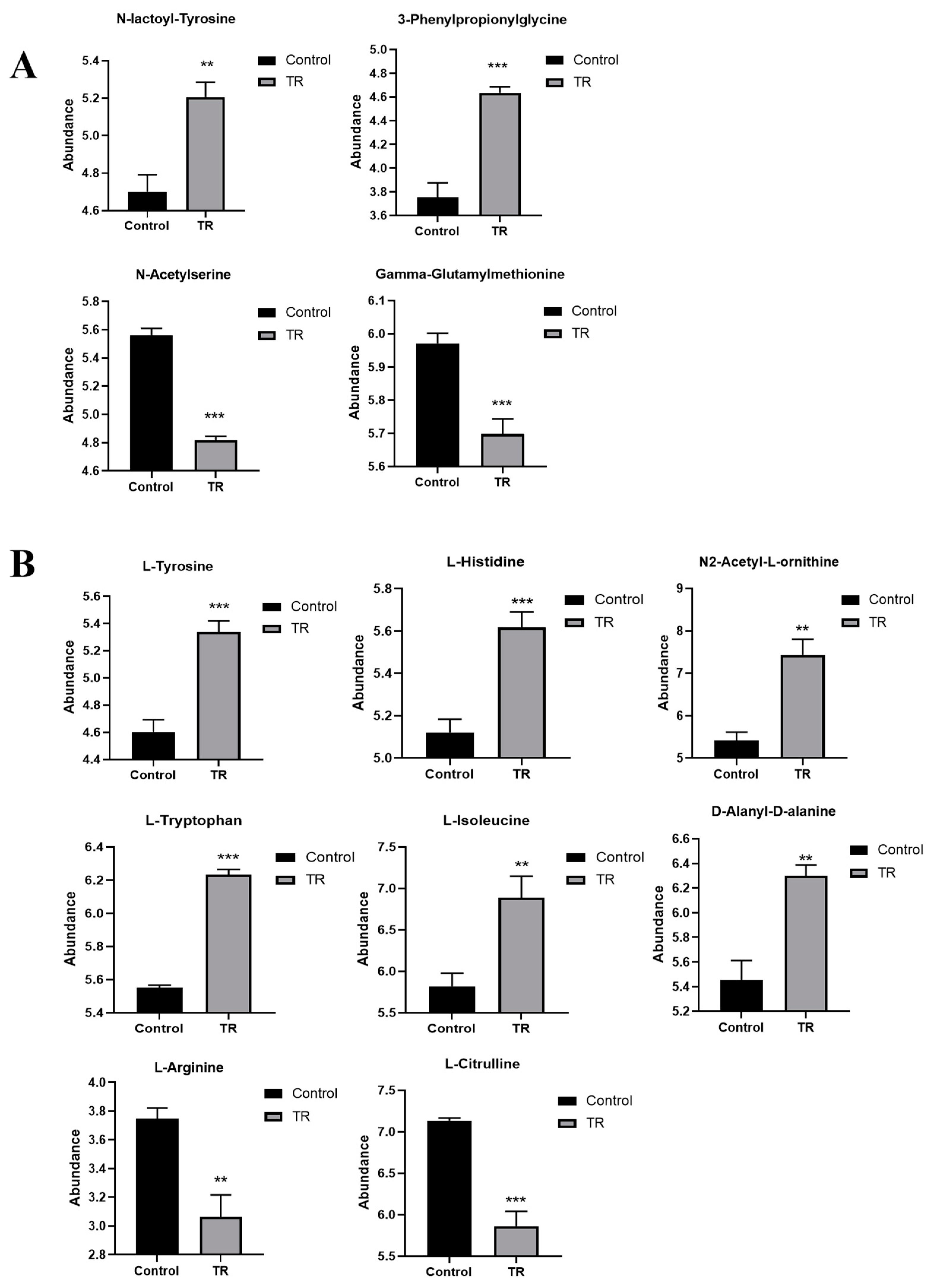
3.6. Effect of Plantaricin Q7 on Amino Acid Metabolism and Biofilm Formation of L. monocytogenes
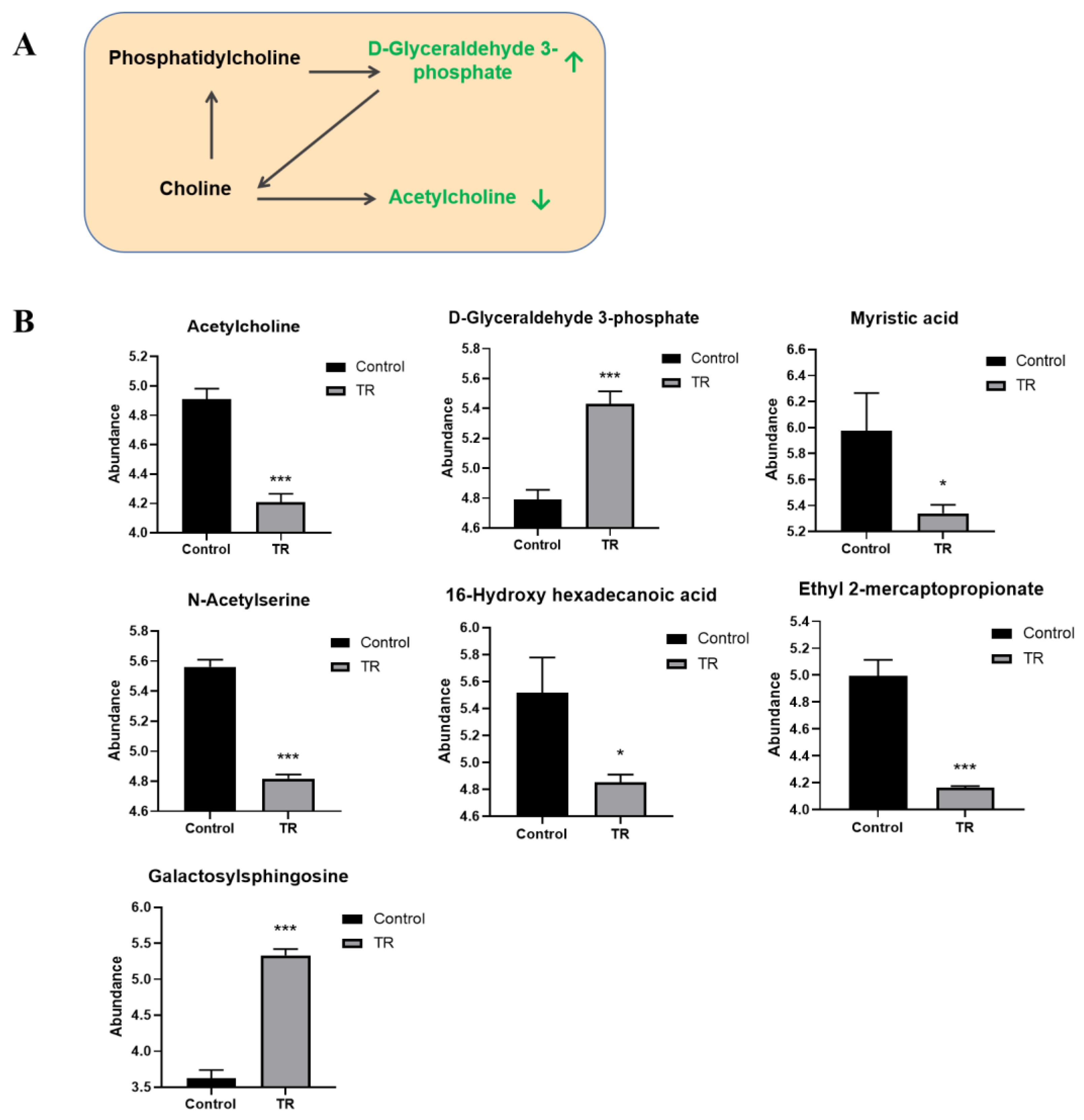
3.7. Effect of Plantaricin Q7 on Glycerol Phospholipid Metabolism and Biofilm Formation of L. monocytogenes
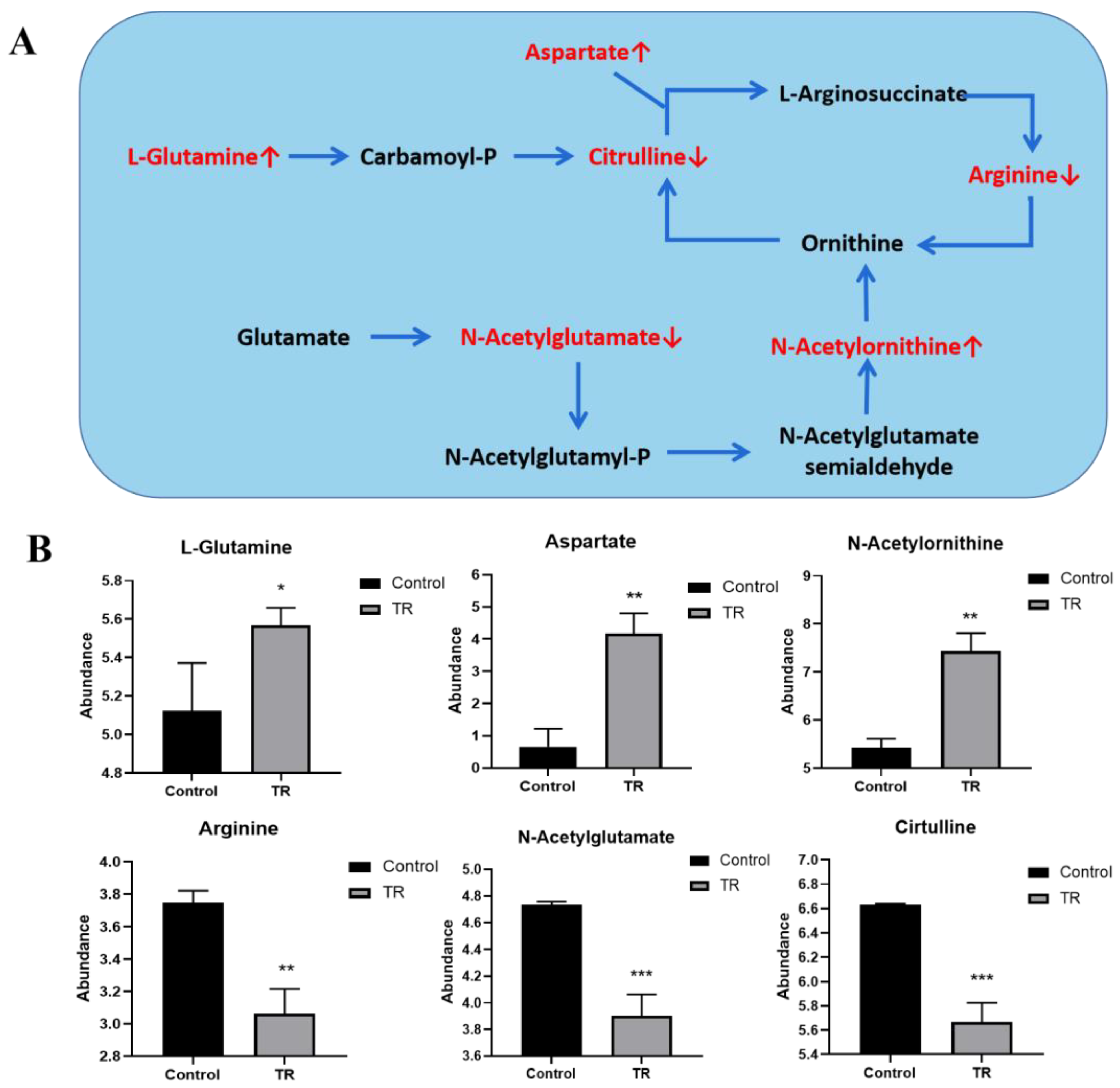
3.8. Effect of Plantaricin Q7 on Arginine Biosynthesis and Biofilm Formation of L. monocytogenes
3.9. Metabolic Pathways of L. monocytogenes Biofilm under Plantaricin Q7 Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.Y.; Lee, J.J.; Chien, C.C.; Tsai, W.C.; Lien, C.Y. High incidence of severe neurological manifestations and high mortality rate for adult Listeria monocytogenes meningitis in taiwan. J. Clin. Neurosci. 2019, 71, 177–185. [Google Scholar] [CrossRef]
- Abou, E.; Elsohaby, I.; Al-Mohammadi, A.; Seliem, M.; Tahoun, A.; Abousaty, A.; Algendy, R.M.; Mohamed, E.A.A.; El-Gazzar, N. Antibacterial and anti-biofilm activities of probiotic Lactobacillus plantarum against Listeria monocytogenes isolated from milk, chicken and pregnant women. Front. Microbiol. 2023, 14, 1201201. [Google Scholar] [CrossRef]
- Smith, M.K.; Draper, L.A.; Pieter-Jan, H.; Cotter, P.D.; Ross, R.P.; Colin, H. A bioengineered nisin derivative, m21a, in combination with food grade additives eradicates biofilms of Listeria monocytogenes. Front. Microbiol. 2016, 7, 1939. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Park, S. Whole-genome sequencing of Listeria monocytogenes isolated from the first listeriosis foodborne outbreak in South Korea. Front. Microbiol. 2023, 14, 1182090. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Cole, S.; Badel-Berchoux, S.; Guillier, L.; Felix, B.; Krezdorn, N.; Hébraud, M.; Bernardi, T.; Sultan, I.; Piveteau, P. Biofilm formation of Listeria monocytogenes strains under food processing environments and pan-genome-wide association study. Front. Microbiol. 2019, 10, 2698. [Google Scholar] [CrossRef] [PubMed]
- Maktabi, S.; Rashnavadi, R.; Tabandeh, M.R.; Sourestani, M.M. Effective Inhibition of Listeria monocytogenes Biofilm Formation by Satureja rechingeri Essential Oil: Mechanisms and Implications. Curr. Microbiol. 2024, 3, 81. [Google Scholar] [CrossRef]
- Laura, W.; Luyao, M.; Xiaonan, L. Impact of lactic acid bacteria on the control of Listeria monocytogenes in ready-to-eat foods. Food Qual. Saf. 2022, 6, fyac045. [Google Scholar] [CrossRef]
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in food industry equipment and premise. Int. J. Food Microbiol. 2011, 145, 5. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Circular and leaderless bacteriocins: Biosynthesis, mode of action, applications, and prospects. Front. Microbiol. 2018, 9, 2085–2102. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Yi, H.; Han, X.; Chi, C. Identification and characterization of plantaricin Q7, a novel plantaricin produced by Lactobacillus plantarum Q7. Lwt-Food Sci. Technol. 2016, 71, 386–390. [Google Scholar] [CrossRef]
- Bu, Y.; Liu, Y.; Liu, Y.; Wang, S.; Liu, Q.; Hao, H.; Yi, H. Screening and Probiotic Potential Evaluation of Bacteriocin-Producing Lactiplantibacillus plantarum In Vitro. Foods 2022, 11, 1575. [Google Scholar] [CrossRef] [PubMed]
- Liu, H. Screening of Antimicrobial Peptides from Lactic Acid Bacteria that Inhibit Cold-Resistant Bacteria and study of Their Structure and Antimicrobial Mechanism. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2016. [Google Scholar] [CrossRef]
- Liu, Y.; Bu, Y.; Li, J.; Liu, Y.; Liu, A.; Gong, P.; Liu, T.; Zhang, L.; Wang, S.; Yi, H. Inhibition activity of plantaricin Q7 produced by Lactobacillus plantarum Q7 against Listeria monocytogenes and its biofilm. Fermentation 2022, 8, 75. [Google Scholar] [CrossRef]
- Favre, L.; Ortalo-Magné, A.; Greff, S.; Pérez, T.; Thomas, O.P.; Martin, J.C.; Culioli, G. Discrimination of four marine biofilm-forming bacteria by LC-MS metabolomics and influence of culture parameters. J. Proteome Res. 2017, 16, 1962–1975. [Google Scholar] [CrossRef] [PubMed]
- Munusamy, K.; Loke, M.F.; Vadivelu, J.; Tay, S.T. LC-MS analysis reveals biological and metabolic processes essential for Candida albicans biofilm growth. Microb. Pathog. 2020, 152, 104614. [Google Scholar] [CrossRef]
- Guo, R.; Lu, H. Targeted metabolomics revealed the regulatory role of manganese on small-molecule metabolism of biofilm formation in Escherichia coli. J. Anal. Test. 2020, 4, 226–237. [Google Scholar] [CrossRef]
- Dosler, S.; Karaaslan, E. Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides. Peptides 2014, 62, 32–37. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens wcs365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- Pisithkul, T.; Schroeder, J.W.; Trujillo, E.A.; Yeesin, P.; Stevenson, D.M.; Chaiamarit, T.; Coon, J.J.; Wang, J.D.; Amador-Noguez, D. Metabolic remodeling during biofilm development of Bacillus subtilis. mBio 2019, 10, e00623-e19. [Google Scholar] [CrossRef]
- Yan, H.; Wu, M.; Gao, B.; Bu, X.; Dong, Q.; Hirata, T.; Li, Z. Inhibition and eradication of Listeria monocytogenes biofilm using the combined treatment with nisin and sesamol. LWT 2024, 198, 116015. [Google Scholar] [CrossRef]
- Wang, N.; Gao, J.; Yuan, L.; Jin, Y.; He, G. Metabolomics profiling during biofilm development of Bacillus licheniformis isolated from milk powder. Int. J. Food Microbiol. 2021, 337, 108939. [Google Scholar] [CrossRef]
- Santi, L.; Beys-Da-Silva, W.O.; Berger, M.; Calzolari, D.; Guimaraes, J.A.; Moresco, J.J.; Yates, J.R. Proteomic profile of Cryptococcus neoformans biofilm reveals changes in metabolic processes. J. Proteome Res. 2014, 13, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Wang, Y.; Liu, Y.; Tan, F.; Wu, Y. Effects of exogenous glucose on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. MicrobiologyOpen 2019, 8, e933. [Google Scholar] [CrossRef] [PubMed]
- Harrington, N.E.; Sweeney, E.; Harrison, F. Building a better biofilm—Formation of in vivo-like biofilm structures by Pseudomonas aeruginosa in a porcine model of cystic fibrosis lung infection. Biofilm 2020, 2, 100024. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, S.; Dunn, M.R.; Chaput, J.C. An efficient and faithful in vitro replication system for threose nucleic acid. J. Am. Chem. Soc. 2013, 135, 3583–3591. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Han, J.; Bie, X.; Lu, Z.; Zhang, C.; Lv, F. Purification and characterization of plantaricin JLA-9: A novel bacteriocin against Bacillus spp. produced by Lactobacillus plantarum JLA-9 from suan-tsai, a traditional chinese fermented cabbage. J. Agric. Food Chem. 2016, 64, 2754–2764. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Lei, T.; Wang, Z.; He, M.; Zhang, J.; Wang, J.; Zeng, H.; Chen, M.; Xue, L.; Ye, Q.; et al. A novel gene vp0610 negatively regulates biofilm formation in Vibrio parahaemolyticus. Front. Microbiol. 2021, 12, 656380. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kong, F.; Liang, X.; Li, M.; Yang, N.; Cao, X.; Yang, M.; Tao, D.; Yue, X.; Zheng, Y. Label-free quantitative proteomics reveals the multitargeted antibacterial mechanisms of lactobionic acid against methicillin-resistant Staphylococcus aureus (MRSA) using SWATH-MS technology. J. Agric. Food Chem. 2019, 67, 12322–12332. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Li, J.; Liu, B.; Wang, Y. Advances in research on signal molecules regulating biofilms. World J. Microbiol. Biotechnol. 2019, 35, 130–138. [Google Scholar] [CrossRef]
- Pu, Y.; Pan, J.; Yao, Y.; Ngan, W.; Yang, Y.; Li, M.; Habimana, O. Ecotoxicological effects of erythromycin on a multispecies biofilm model, revealed by metagenomic and metabolomic approaches. Environ. Pollut. 2021, 276, 116737. [Google Scholar] [CrossRef]
- Shen, F.; Xing, M.; Liu, L.; Yuan, P.; Shi, Q.; Yu, L. Antimicrobial efficacy of the alkaloid harmaline alone and in combination with chlorhexidine digluconate against clinical isolates of Staphylococcus aureus grown in planktonic and biofilm cultures. Lett. Appl. Microbiol. 2012, 54, 475–482. [Google Scholar] [CrossRef]
- Miyamoto, T.; Katane, M.; Saitoh, Y.; Sekine, M.; Homma, H. Elucidation of the d-lysine biosynthetic pathway in the hyperthermophile Thermotoga maritima. Febs J. 2019, 286, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.T.K.; Frye, M.; Gagnon, P.; Vogel, J.P.; Chole, R. D-amino acids do not inhibit Pseudomonas aeruginosa biofilm formation. Laryngoscope Investig. Otolaryngol. 2016, 2, 4–9. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Kolodkin-Gal, I.; Foulston, L.; Kolter, R.; Aizenberg, J.; Losick, R. Inhibitory effects of D-amino acids on Staphylococcus aureus biofilm development. J. Bacteriol. 2011, 193, 5616–5622. [Google Scholar] [CrossRef]
- Leiman, S.A.; May, J.M.; Lebar, M.D.; Kahne, D.; Kolter, R.; Losick, R. D-amino acids indirectly inhibit biofilm formation in Bacillus subtilis by interfering with protein synthesis. J. Bacteriol. 2013, 195, 5391–5395. [Google Scholar] [CrossRef]
- Dat, N.M.; Phuong, T.M.; Thu, N.T.; Phong, T.K.; Uchino, T. Inhibition of bacterial adherence on stainless steel coupons by surface conditioning with selected polar lipids. J. Food Saf. 2022, 42, e12956. [Google Scholar] [CrossRef]
- Cukkemane, N.; Bikker, F.J.; Nazmi, K.; Brand, H.S.; Sotres, J.; Lindh, L.; Arnebrant, T.; Veerman, E.C.I. Anti-adherence and bactericidal activity of sphingolipids against Streptococcus mutans. Eur. J. Oral. Sci. 2015, 123, 221–227. [Google Scholar] [CrossRef]
- Lattif, A.A.; Mukherjee, P.K.; Chandra, J.; Roth, M.R.; Welti, R.; Rouabhia, M.; Ghannoum, M.A. Lipidomics of Candida albicans biofilms reveals phase-dependent production of phospholipid molecular classes and role for lipid rafts in biofilm formation. Microbiology 2011, 157, 3232–3242. [Google Scholar] [CrossRef]
- Nwaiwu, O.; Wong, H.T.L.; Lad, M.; Macnaughtan, B.; Rees, C. Properties of the extracellular polymeric substance layer from minimally grown planktonic cells of Listeria monocytogenes. Biomolecules 2021, 11, 331. [Google Scholar] [CrossRef]
- Wang, N.; Sadiq, F.A.; Li, S.; He, G.; Yuan, L. Tandem mass tag-based quantitative proteomics reveals the regulators in biofilm formation and biofilm control of Bacillus licheniformis. Food Control 2020, 110, 107029. [Google Scholar] [CrossRef]
- Burrell, M.; Hanfrey, C.C.; Murray, E.J.; Stanley-Wall, N.R.; Michael, A.J. Evolution and multiplicity of arginine decarboxylases in polyamine biosynthesis and essential role in Bacillus subtilis biofilm formation. J. Biol. Chem. 2010, 285, 39224–39238. [Google Scholar] [CrossRef]
- García-Almendárez, B.E.; Cann, I.K.O.; Martin, S.E.; Guerrero-Legarreta, I.; Regalado, C. Effect of lactococcus lactis uq2 and its bacteriocin on Listeria monocytogenes biofilms. Food Control 2008, 19, 670–680. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Tian, S.; Han, B. Understanding the interactions between Staphylococcus aureus and the raw-meat-processing environment isolate Klebsiella oxytoca in dual-species biofilms via discovering an altered metabolic profile. Microorganisms 2021, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Wahwah, N.; Dhar, D.; Chen, H.; Zhuang, S.; Chan, A.; Casteel, D.E.; Kalyanaraman, H.; Pilz, R.B.; Boss, G.R. Metabolic interaction between amino acid deprivation and cisplatin synergistically reduces phosphoribosyl-pyrophosphate and augments cisplatin cytotoxicity. Sci. Rep. 2020, 10, 19907. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, Y.; Hao, L.; Cao, J.; Jiang, L.; Yi, H. Metabolomic Approaches to Study the Potential Inhibitory Effects of Plantaricin Q7 against Listeria monocytogenes Biofilm. Foods 2024, 13, 2573. https://doi.org/10.3390/foods13162573
Liu Y, Liu Y, Hao L, Cao J, Jiang L, Yi H. Metabolomic Approaches to Study the Potential Inhibitory Effects of Plantaricin Q7 against Listeria monocytogenes Biofilm. Foods. 2024; 13(16):2573. https://doi.org/10.3390/foods13162573
Chicago/Turabian StyleLiu, Yinxue, Yisuo Liu, Linlin Hao, Jiayuan Cao, Lu Jiang, and Huaxi Yi. 2024. "Metabolomic Approaches to Study the Potential Inhibitory Effects of Plantaricin Q7 against Listeria monocytogenes Biofilm" Foods 13, no. 16: 2573. https://doi.org/10.3390/foods13162573
APA StyleLiu, Y., Liu, Y., Hao, L., Cao, J., Jiang, L., & Yi, H. (2024). Metabolomic Approaches to Study the Potential Inhibitory Effects of Plantaricin Q7 against Listeria monocytogenes Biofilm. Foods, 13(16), 2573. https://doi.org/10.3390/foods13162573





