K-Carrageenan/Locust Bean Gum Gels for Food Applications—A Critical Study on Potential Alternatives to Animal-Based Gelatin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rheology
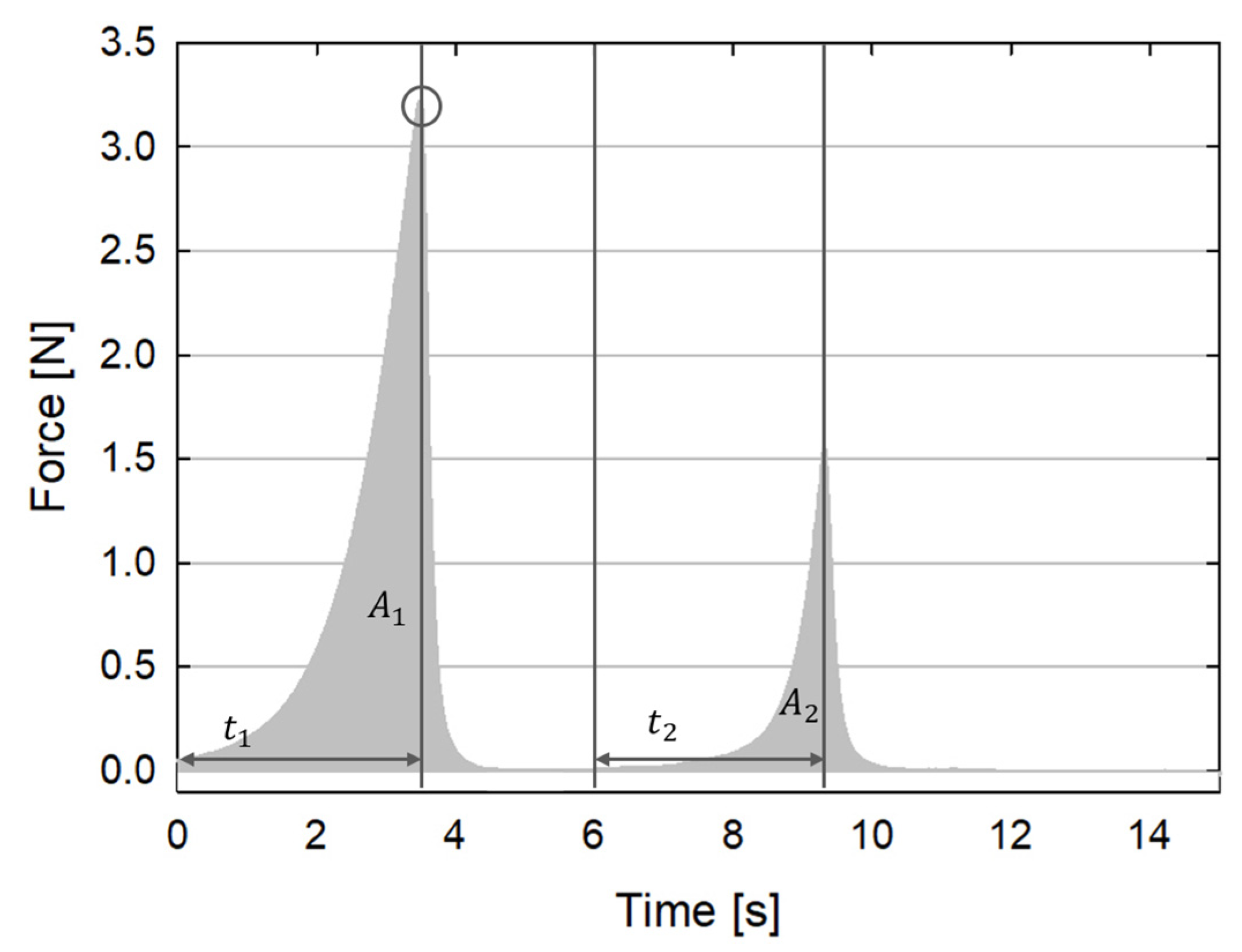
2.3. Texture Profile Analysis Procedure
- -
- Hardness, obtained by measuring the peak load reached during the first deformation cycle (the black circle in Figure 1). Hardness is related to the stiffness of the material.
- -
- Springiness, given by the ratio between the time interval needed to reach the peak load since the start of the second cycle and the same time interval for the first cycle (t2/t1 in Figure 1). Springiness is related to the recovery of the material and to its viscoelastic properties.
- -
- Cohesiveness, given by the ratio between the area under the time/force curve during the second cycle divided by the same area during the first cycle (A2/A1 in Figure 1). Cohesiveness is related to the consistency of the material. Its value varies from 0 to 1. The larger its value, the more the material keeps its texture unaltered.
3. Results
3.1. Thermo-Reversibility: Melt-in-Mouth Requirements
3.2. Gel Elasticity: Shear Moduli at 20 °C
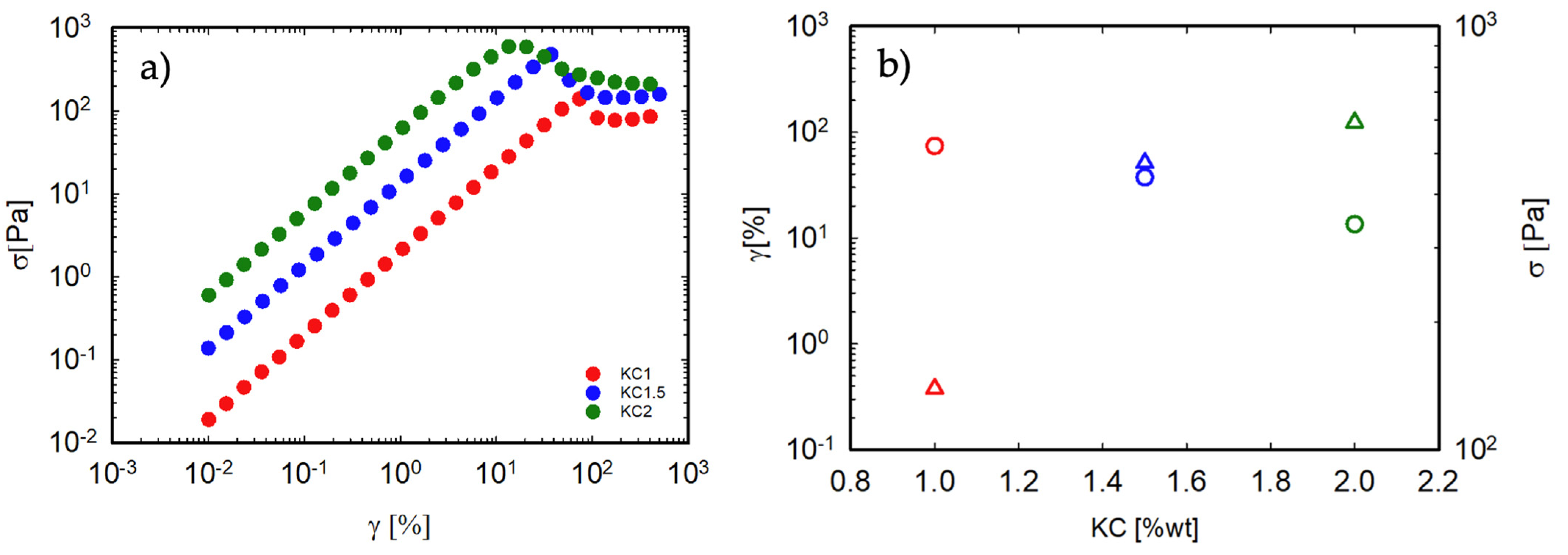
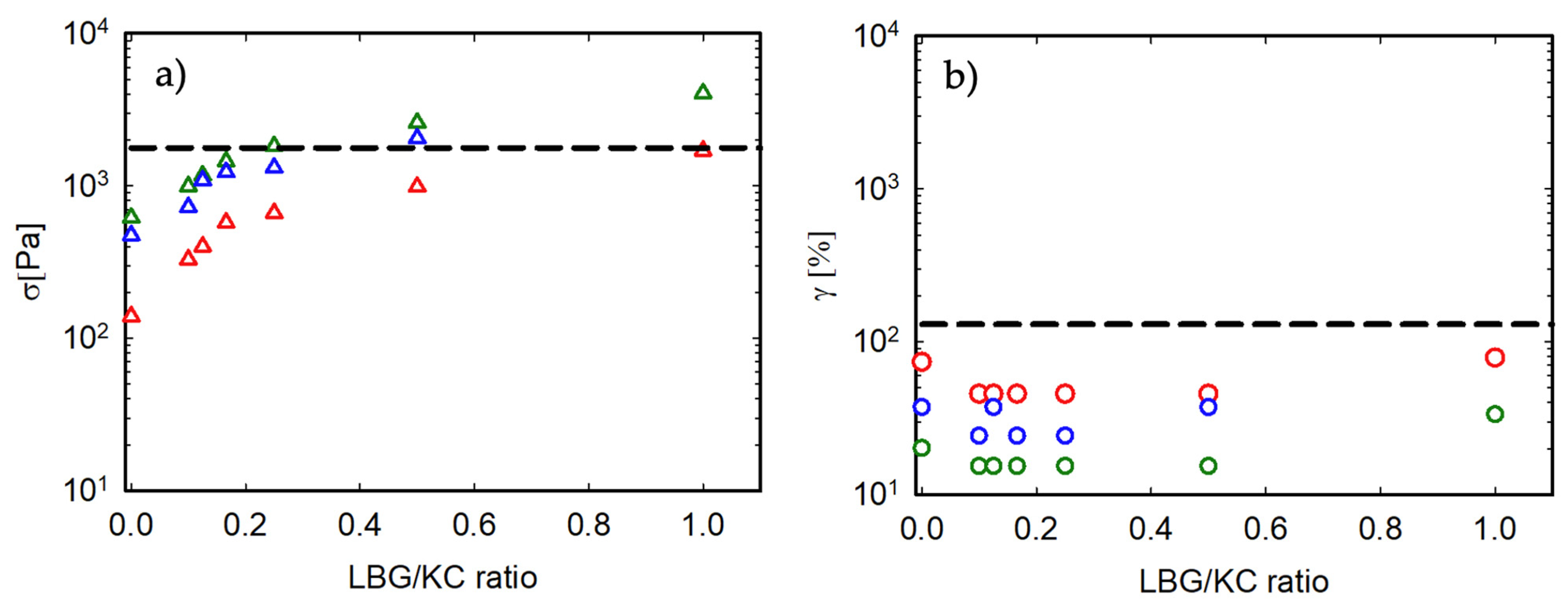
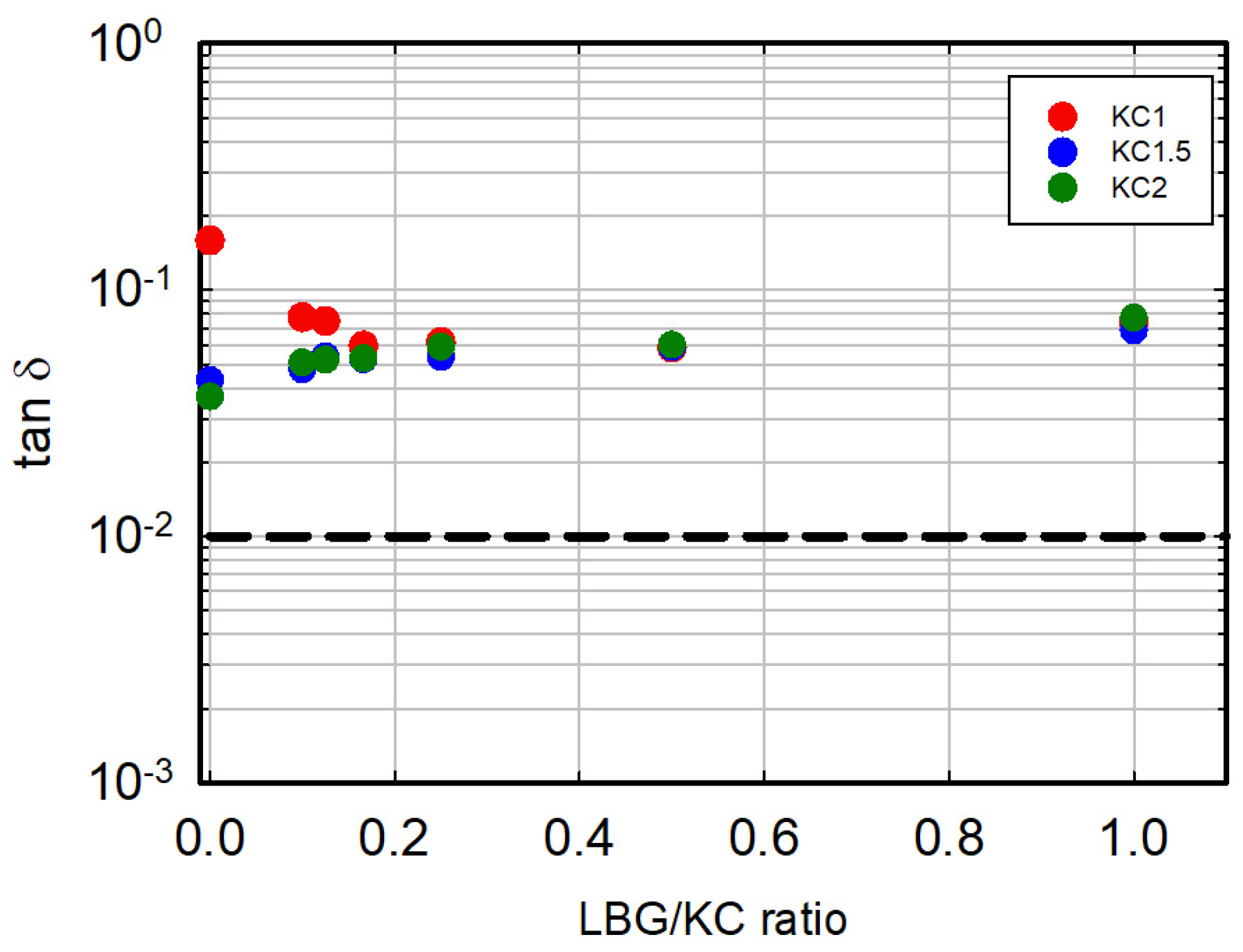
3.3. Non-Linear Response of the Gel
3.4. Comparison with Animal Gelatin
3.5. Texture Profile Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veis, A. The Physical Chemistry of Gelatin. In International Review of Connective Tissue Research; Hall, D.A., Ed.; Academic Press: New York, NY, USA, 1965; Volume 3, pp. 113–200. [Google Scholar]
- Boukid, F.; Lamri, F.; Nabi Dar, B.; Garron, M.; Castellari, M. Vegan Alternatives to Processed Cheese and Yogurt Launched in the European Market during 2020: A Nutritional Challenge? Foods 2021, 10, 2782. [Google Scholar] [CrossRef]
- Wang, T.; Masedunskas, A.; Willett, W.C.; Fontana, L. Vegetarian and vegan diets: Benefits and drawbacks. Eur. Heart J. 2023, 36, 3423–3439. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A.; Gazdecki, M.; Véha, M.; Szakály, M.; Szakály, Z. A Comprehensive Review of the Benefits and the Barriers to the Switch to a Plant-Based Diet. Sustainability 2020, 12, 4136. [Google Scholar] [CrossRef]
- Lu, W.; Li, X.; Fang, Y. Introduction to Food Hydrocolloids. In Food Hydrocolloids: Functionalities and Applications; Fang, Y., Zhang, H., Nishinari, K., Eds.; Springer: Singapore, 2021; pp. 1–28. [Google Scholar]
- Morrison, N.A.; Clark, R.C.; Chen, Y.L.; Talashek, T.; Sworn, G. Gelatin Alternatives for the Food Industry. In Progress in Colloid and Polymer Science; Nishinari, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 114, pp. 127–131. [Google Scholar]
- Tang, M.X.; Lei, Y.C.; Wang, Y.; Li, D.; Wang, L.J. Rheological and Structural Properties of Sodium Caseinate as Influenced by Locust Bean Gum and κ-Carrageenan. Food Hydrocoll. 2021, 112, 106251. [Google Scholar] [CrossRef]
- Ozmen, D.; Toker, O.S. Large-amplitude Oscillatory Shear Behavior of Xanthan Gum/Locust Bean Gum Mixture: Effect of Preparation Methods on Synergistic Interaction. J. Food Process Eng. 2022, 45, 14073. [Google Scholar] [CrossRef]
- Jo, W.; Bak, J.H.; Yoo, B. Rheological Characterizations of Concentrated Binary Gum Mixtures with Xanthan Gum and Galactomannans. Int. J. Biol. Macromol. 2018, 114, 263–269. [Google Scholar] [CrossRef]
- Lin, H.-T.V.; Tsai, J.-S.; Liao, H.-H.; Sung, W.-C. The Effect of Hydrocolloids on Penetration Tests and Syneresis of Binary Gum Gels and Modified Corn Starch-Gum Gels. Gels 2023, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Mezreli, G.; Kurt, A.; Akdeniz, E.; Ozmen, D.; Basyigit, B.; Toker, O.S. A New Synergistic Hydrocolloid with Superior Rheology: Locust Bean/Xanthan Gum Binary Solution Powdered by Different Drying Methods. Food Hydrocoll. 2024, 154, 110078. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, J.; Yin, J.; Nie, S. Structural Properties of Bletilla Striata Polysaccharide and the Synergistic Gelation of Polysaccharide and Xanthan Gum. Food Hydrocoll. 2023, 142, 108843. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, F.; Deng, Y.; Tang, X.; Li, P.; Zhao, Z.; Zhang, M.; Liu, G. Texture Characterization of 3D Printed Fibrous Whey Protein-starch Composite Emulsion Gels as Dysphagia Food: A Comparative Study on Starch Type. Food Chem. 2024, 458, 140302. [Google Scholar] [CrossRef]
- Ryu, J.; McClements, D.J. Control of Plant-Based Biopolymer Composite Gel Texture: Combining Potato Proteins with Different High Acyl-Low Acyl Gellan Gum Ratios. Food Hydrocoll. 2024, 149, 109636. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M. Instrumental Food Texture Evaluation in Relation to Human Perception. Food Hydrocoll. 2022, 124, 107253. [Google Scholar] [CrossRef]
- Pons, M.; Fiszmaw, S.M. Instrumental Texture Profile Analysis with Particular Reference to Gelled Systems. J. Texture Stud. 1996, 27, 597–624. [Google Scholar] [CrossRef]
- Breene, W.M. Application of Texture Profile Analysis to Instrumental Food Texture Evaluation. J. Texture Stud. 1975, 6, 53–82. [Google Scholar] [CrossRef]
- Friedman, H.H.; Whitney, J.E.; Szczesniak, A.S. The Texturometer-A New Instrument for Objective Texture Measurement. J. Food Sci. 1963, 28, 390–396. [Google Scholar] [CrossRef]
- Szczesniak, A.S.; Brandt, M.A.; Friedman, H.H. Development of Standard Rating Scales for Mechanical Parameters of Texture and Correlation between the Objective and the Sensory Methods of Texture Evaluation. J. Food Sci. 1963, 28, 397–403. [Google Scholar] [CrossRef]
- Civille, V.; Szczesniak, A. Guidelines to Training a Texture Profile Panel. J. Texture Stud. 1973, 4, 204–223. [Google Scholar] [CrossRef]
- Christensen’, O.; Trudsoe, J. Effect of Other Hydrocolloids on the Texture of Kappa Carrageenan Gels. J. Texture Stud. 1980, 11, 137–148. [Google Scholar] [CrossRef]
- Russo Spena, S.; Grizzuti, N.; Tammaro, D. Linking Processing Parameters and Rheology to Optimize Additive Manufacturing of K-Carrageenan Gel Systems. Gels 2022, 8, 493. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust Bean Gum: Processing, Properties and Food Applications-A Review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef]
- Mohtar, F.; Perera, C.; Quek, S.-Y. Optimisation of Gelatine Extraction from Hoki (Macruronus Novaezelandiae) Skins and Measurement of Gel Strength and SDS-PAGE. Food Chem. 2010, 122, 307–313. [Google Scholar] [CrossRef]
- Russo Spena, S.; Visone, B.; Grizzuti, N. An Engineering Approach to the 3D Printing of K-Carrageenan/Konjac Glucomannan Hydrogels. Int. J. Food Sci. Technol. 2024, 59, 4122–4133. [Google Scholar] [CrossRef]
- Russo Spena, S.; Pasquino, R.; Sarrica, A.; Delmonte, M.; Yang, C.; Grizzuti, N. Kinetics of Acid Hydrolysis of K-Carrageenan by in Situ Rheological Follow-Up. Food Hydrocoll. 2023, 144, 108953. [Google Scholar] [CrossRef]
- Turquois, T.; Rochas, C.; Taravel, F.R. Rheological Studies of Synergistic Kappa Carrageenan-Carob Galactomannan. Carbohydr. Polym. 1992, 17, 263–268. [Google Scholar] [CrossRef]
- Stading, M.; Hermansson, A.M. Rheological Behaviour of Mixed Gels of K-Carrageenan-Locust Bean Gum. Carbohydr. Polym. 1993, 22, 49–56. [Google Scholar] [CrossRef]
- Tako, M.; Nakamura, S. Synergistic Interaction between Kappa-Carrageenan and Locust-Bean Gum in Aqueous Media. Agric. Biol. Chem. 1986, 50, 2817–2822. [Google Scholar]
- Fernandes, P.B.; Gonçalves, M.P.; Doublier, J.L. Influence of Locust Bean Gum on the Rheological Properties of Kappa-Carrageenan Systems in the Vicinity of the Gel Point. Carbohydr. Polym. 1993, 22, 99–106. [Google Scholar] [CrossRef]
- Fernandes, P.B.; Gonçalves, M.P.; Doublier, J.L. A Rheological Characterization of Kappa-Carrageenan/Galactomannan Mixed Gels: A Comparison of Locust Bean Gum Samples. Carbohydr. Polym. 1991, 16, 253–274. [Google Scholar] [CrossRef]
- Dea, I.C.M.; Morrison, A. Chemistry and Interactions of Seed Galactomannas. Adv. Carbohydr. Chem. Biochem. 1975, 31, 241–312. [Google Scholar]
- Wu, D.; Yu, S.; Liang, H.; Eid, M.; Li, B.; Li, J.; Mao, J. An Innovative Konjac Glucomannan/κ-Carrageenan Mixed Tensile Gel. J. Sci. Food Agric. 2021, 101, 5067–5074. [Google Scholar] [CrossRef]
- Avallone, P.R.; Esposito, M.G.; Russo Spena, S.; Sarrica, A.; Delmonte, M.; Pasquino, R.; Grizzuti, N. Thermorheological Behavior of K-Carrageenan Hydrogels Modified with Xanthan Gum Thermorheological Behavior of k-Carrageenan Hydrogels Modified with Xanthan Gum. Fluids 2023, 8, 119. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Structure and Rheology of Gelatin Gels. Imaging Sci. J. 1997, 45, 205–209. [Google Scholar] [CrossRef]
- Song, X.; Chiou, B.S.; Xia, Y.; Chen, M.; Liu, F.; Zhong, F. The Improvement of Texture Properties and Storage Stability for Kappa Carrageenan in Developing Vegan Gummy Candies. J. Sci. Food Agric. 2022, 102, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Bertasa, M.; Dodero, A.; Alloisio, M.; Vicini, S.; Riedo, C.; Sansonetti, A.; Scalarone, D.; Castellano, M. Agar Gel Strength: A Correlation Study between Chemical Composition and Rheological Properties. Eur. Polym. J. 2019, 123, 109442. [Google Scholar] [CrossRef]
- Lai, M.-F.; Lii, C.-Y. Rheological and Thermal Characteristics of Gel Structures from Various Agar Fractions. Int. J. Biol. Macromol. 1997, 21, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Guan, X.; Qin, X.; Chen, Z.; Liu, X. Effects of Konjac Glucomannan with Different Degrees of Deacetylation on the Properties and Structure of Wheat Gluten Protein. Int. J. Biol. Macromol. 2024, in press. [CrossRef]
- Du, X.; Li, J.; Chen, J.; Li, B. Effect of degree of deacetylation on physicochemical and gelation properties of konjac glucomannan. Food Res. Int. 2012, 46, 270–278. [Google Scholar] [CrossRef]
- Matsuo, M.; Takaya, T.; Miwa, S.; Moritaka, H.; Nishinari, K. The uniaxial compression test as a simulation of mastication for the texture evaluation of cooked rice. J. Jpn. Soc. Mastication Sci. Health Promot. 2002, 12, 11–25. [Google Scholar]
- Paredes, J.; Cortizo-Lacalle, D.; Imaz, A.M.; Aldazabal, J.; Vila, M. Application of Texture Analysis Methods for the Characterization of Cultured Meat. Nature 2022, 12, 3898. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, A.S. Texture Is a Sensory Property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef]
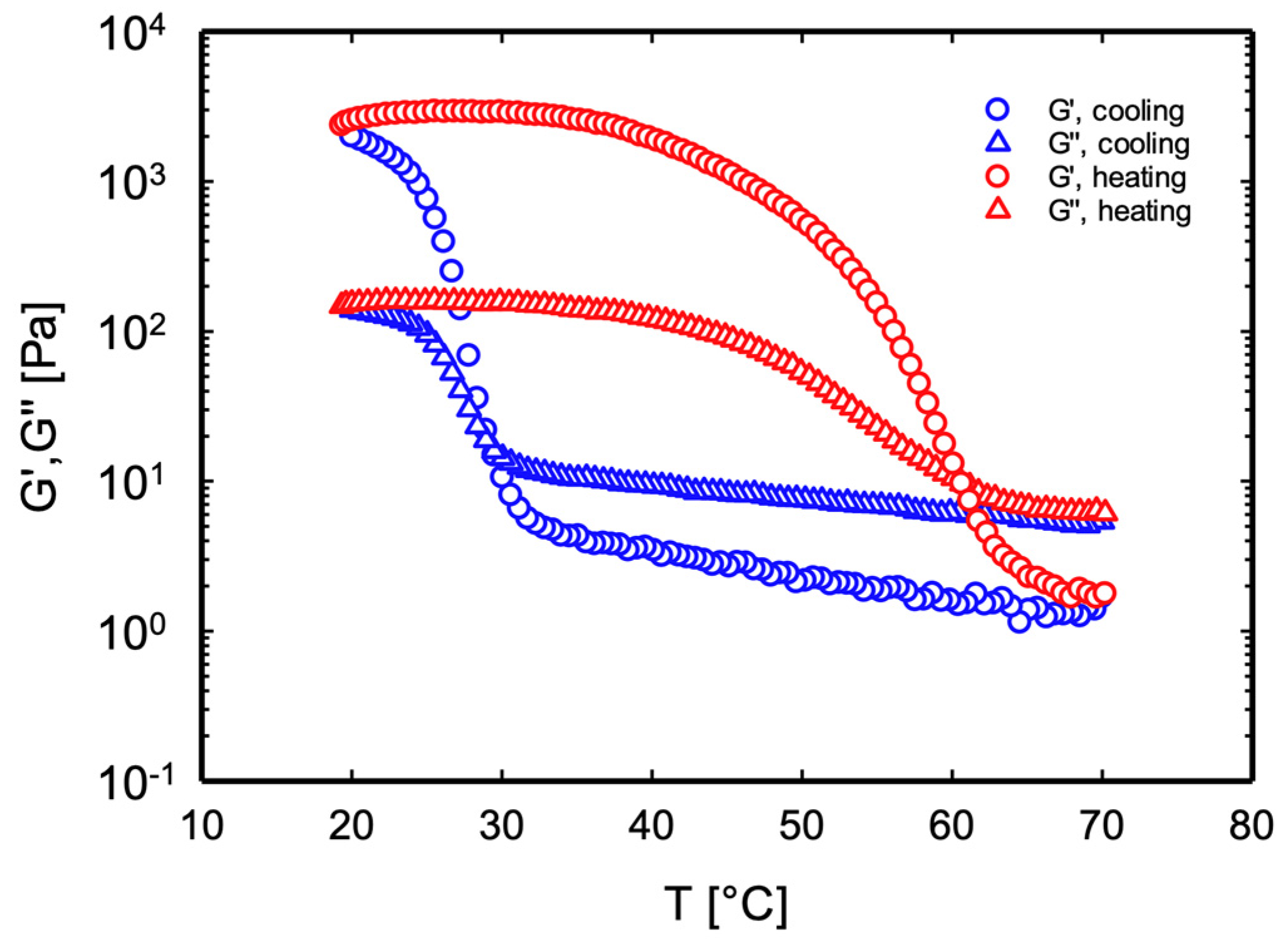
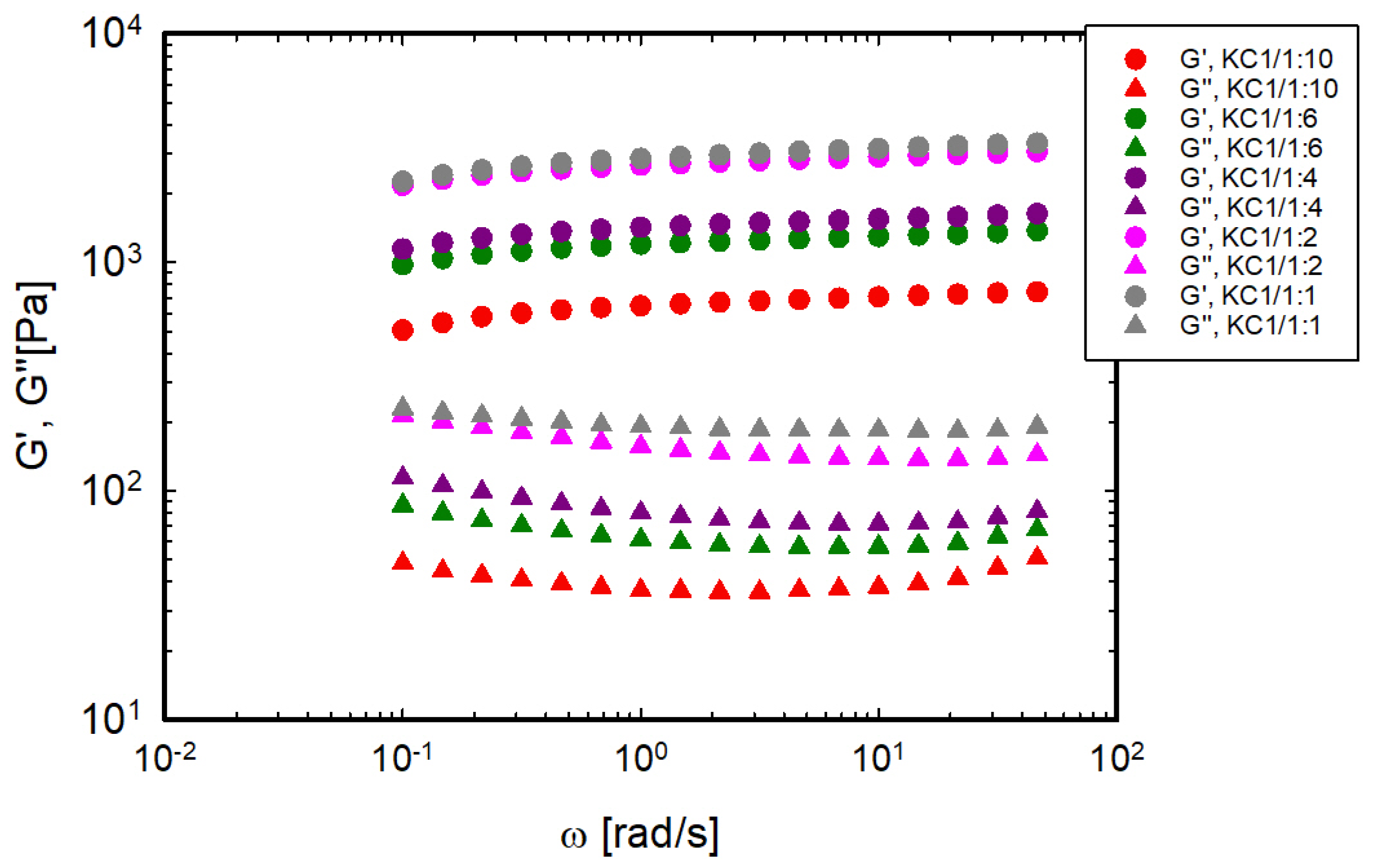
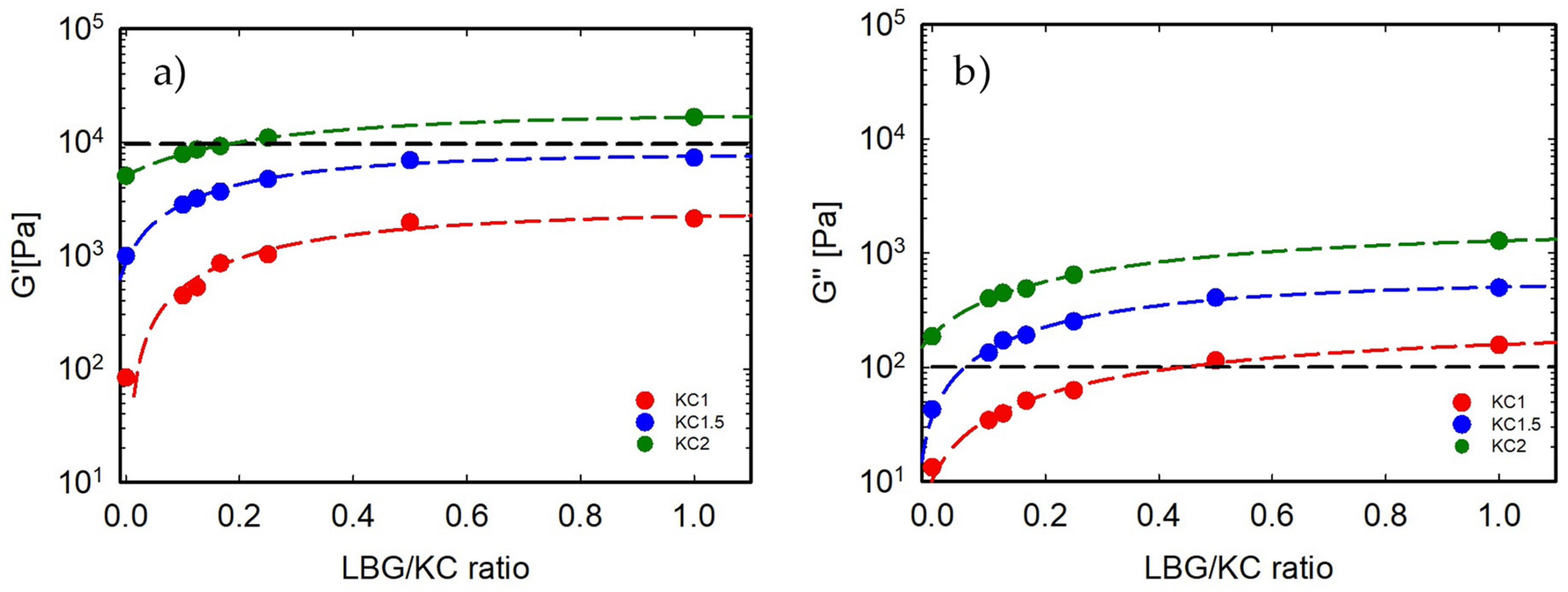
| Sample | TSOL-GEL [°C] | TGEL-SOL [°C] | Sample | TSOL-GEL [°C] | TGEL-SOL [°C] | Sample | TSOL-GEL [°C] | TGEL-SOL [°C] |
|---|---|---|---|---|---|---|---|---|
| κ-C 1 | 21.8 | 43.0 | κ-C 1.5 | 27.5 | 52.2 | κ-C 2 | 32.3 | 58.1 |
| κ-C 1/1:10 | 24.2 | 55.2 | κC1.5/1:10 | 28.4 | 60.6 | κ-C 2/1:10 | 34.0 | 64.8 |
| κ-C 1/1:6 | 24.4 | 56.2 | κ-C 1.5/1:6 | 29.2 | 60.6 | κ-C 2/1:6 | 34.2 | 65.9 |
| κ-C 1/1:4 | 25.6 | 56.4 | κ-C 1.5/1:4 | 30.3 | 61.7 | κ-C 2/1:4 | 35.3 | 66.5 |
| κ-C 1/1:2 | 26.1 | 56.7 | κ-C 1.5/1:2 | 30.2 | 61.5 | κ-C 2/1:2 | 35.8 | 66.7 |
| κ-C 1/1:1 AG | 26.9 16.3 | 56.7 29.5 | κ-C 1.5/1:1 | 35.6 | 65.4 | κ-C 2/1:1 | 38.4 | 69.2 |
| Sample | Hardness [N] | Springiness [%] | Cohesiveness [%] |
|---|---|---|---|
| κ-C 1 | Too weak gel | Too weak gel | Too weak gel |
| κ-C 1/1:10 | 1.1 | 33% | 15% |
| κ-C 1/1:6 | 2.9 | 48% | 26% |
| κ-C 1/1:4 | 2.7 | 48% | 32% |
| κ-C 1/1:2 | 3 | 53% | 43% |
| κ-C 1/1:1 | 4.8 | 75% | 48% |
| κ-C 1.5 | 3 | 50% | 30% |
| κ-C 1.5/1:10 | 4.8 | 63% | 33% |
| κ-C 1.5/1:6 | 5.23 | 68% | 39% |
| κ-C 1.5/1:4 | 6.3 | 71% | 40% |
| κ-C 1.5/1:2 | 6.5 | 80% | 46% |
| κ-C 1.5/1:1 | 7 | 84% | 58% |
| κ-C 2 | 7.5 | 75% | 39% |
| κ-C 2/1:10 | 8.2 | 78% | 39.56% |
| κ-C 2/1:6 | 9 | 78% | 41.8% |
| κ-C 2/1:4 | 9.3 | 83% | 46% |
| κ-C 2/1:2 | 10.2 | 85% | 48.36% |
| κ-C 2/1:1 | 11 | 87% | 54.7% |
| AG | 3 | 94% | 88% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo Spena, S.; Pasquino, R.; Grizzuti, N. K-Carrageenan/Locust Bean Gum Gels for Food Applications—A Critical Study on Potential Alternatives to Animal-Based Gelatin. Foods 2024, 13, 2575. https://doi.org/10.3390/foods13162575
Russo Spena S, Pasquino R, Grizzuti N. K-Carrageenan/Locust Bean Gum Gels for Food Applications—A Critical Study on Potential Alternatives to Animal-Based Gelatin. Foods. 2024; 13(16):2575. https://doi.org/10.3390/foods13162575
Chicago/Turabian StyleRusso Spena, Simona, Rossana Pasquino, and Nino Grizzuti. 2024. "K-Carrageenan/Locust Bean Gum Gels for Food Applications—A Critical Study on Potential Alternatives to Animal-Based Gelatin" Foods 13, no. 16: 2575. https://doi.org/10.3390/foods13162575
APA StyleRusso Spena, S., Pasquino, R., & Grizzuti, N. (2024). K-Carrageenan/Locust Bean Gum Gels for Food Applications—A Critical Study on Potential Alternatives to Animal-Based Gelatin. Foods, 13(16), 2575. https://doi.org/10.3390/foods13162575






