Black Mulberries (Morus nigra L.) Modulate Oxidative Stress and Beta-Amyloid-Induced Toxicity, Becoming a Potential Neuroprotective Functional Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Black Mulberries and Extraction of Bioactive Compounds
2.3. Neuroprotection Models
2.3.1. Neuro-2a Cell Line
Cell Culture
N2a Treatments
Mitochondrial Activity by MTT Assay
Detection of Intracellular ROS
2.3.2. Caenorhabditis Elegans
Worm Strain and Maintenance
Amyloid-β Peptide Toxicity: Paralysis Assay
2.4. Artemia Salina Safety and Toxicity Assessment
2.5. FRAP Assay: Ferric Reducing/Antioxidant Power
2.6. Inhibition of MAO-A Bioassay
2.7. Statistical Analysis
3. Results
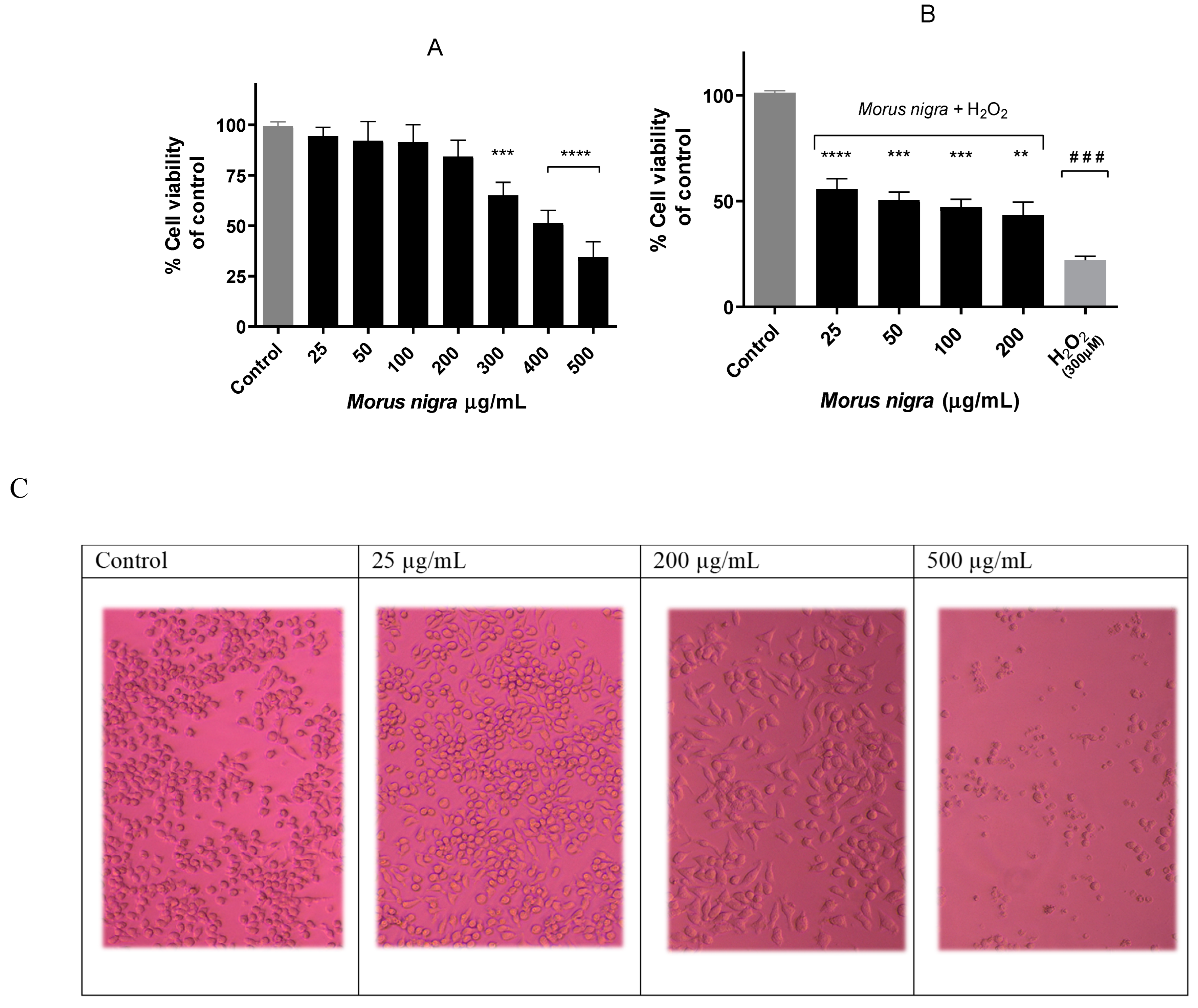
3.1. Effect of Black Mulberry Extract on Neuro-2a Cell Viability
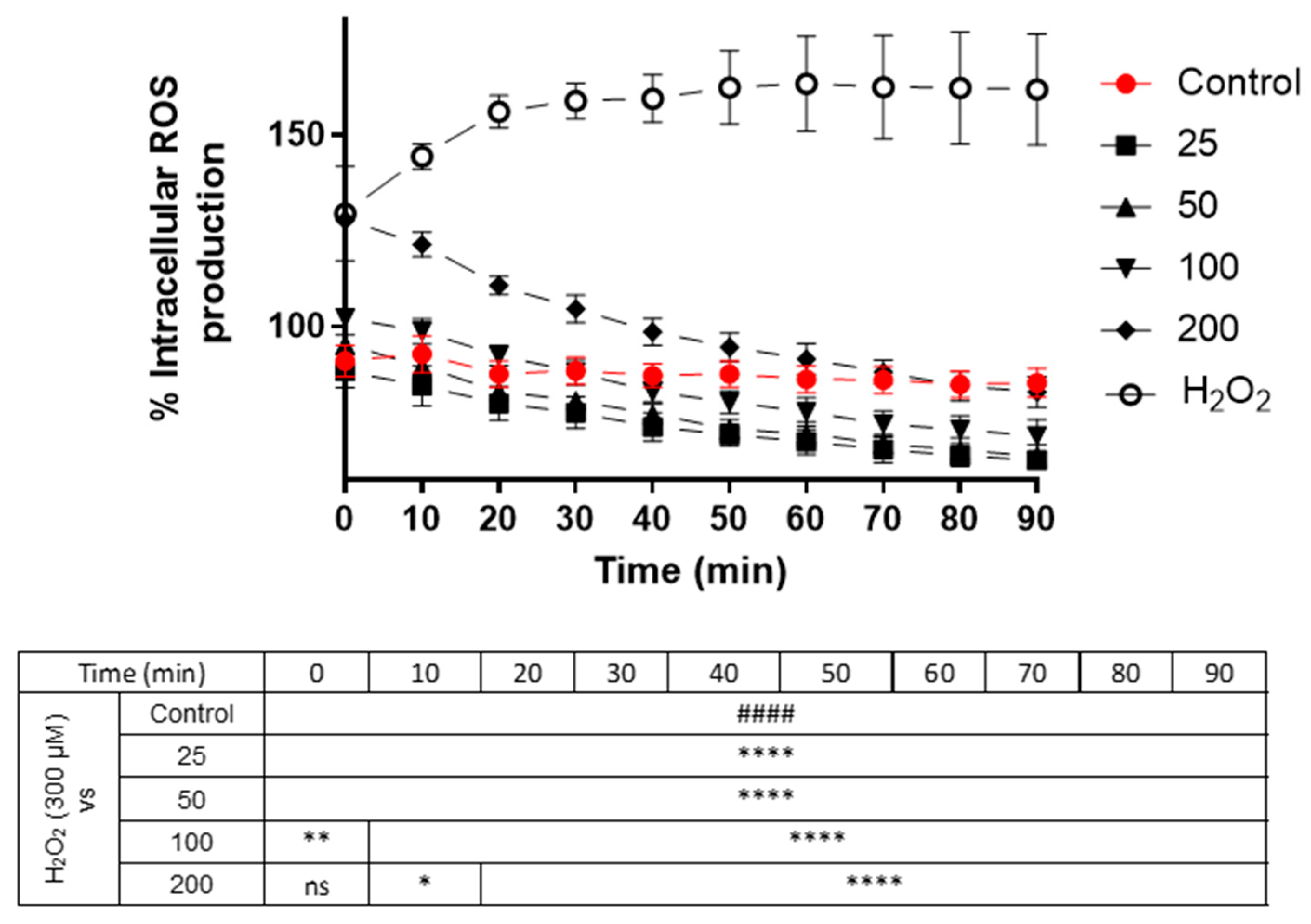
3.2. Effect of Black Mulberry Extract Concentrations on Intracellular ROS Production in Neuro-2a Cells
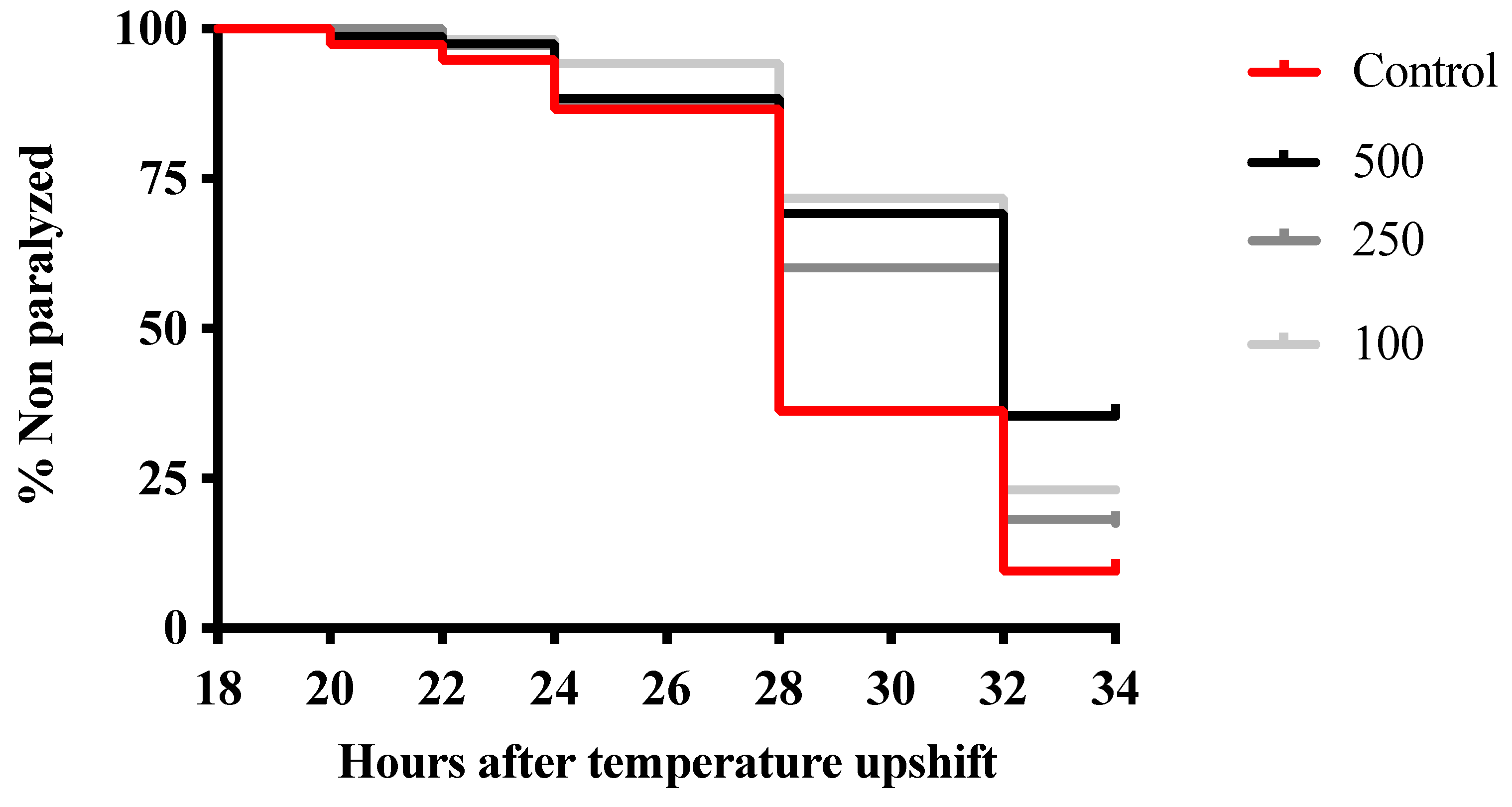
3.3. Morus Nigra Extract Prevents In Vivo Aβ Toxicity in C. elegans
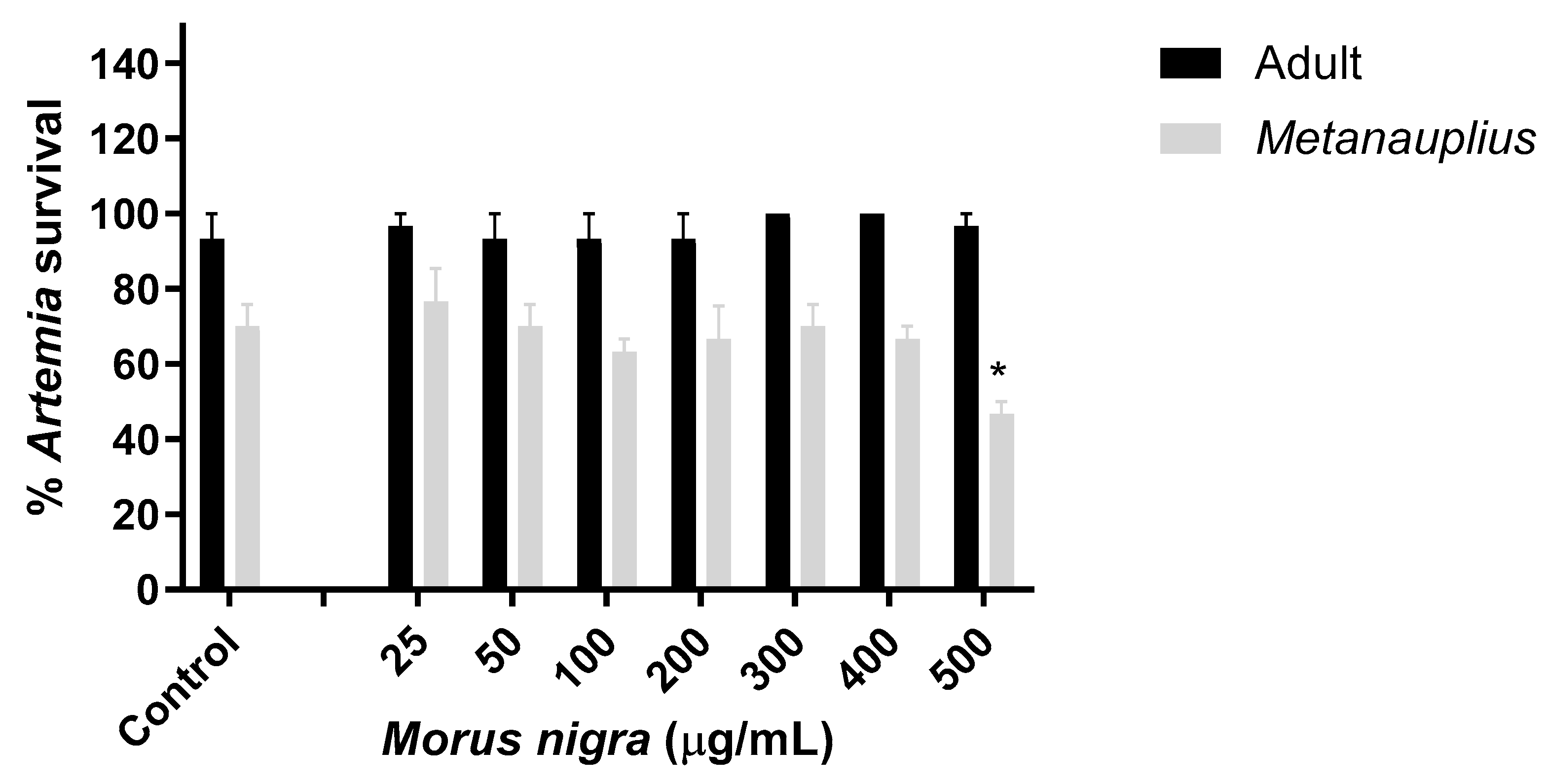
3.4. Effect of Morus Nigra Extract on Artemia Salina Viability
3.5. Evaluation of In Vitro Antioxidant Activity
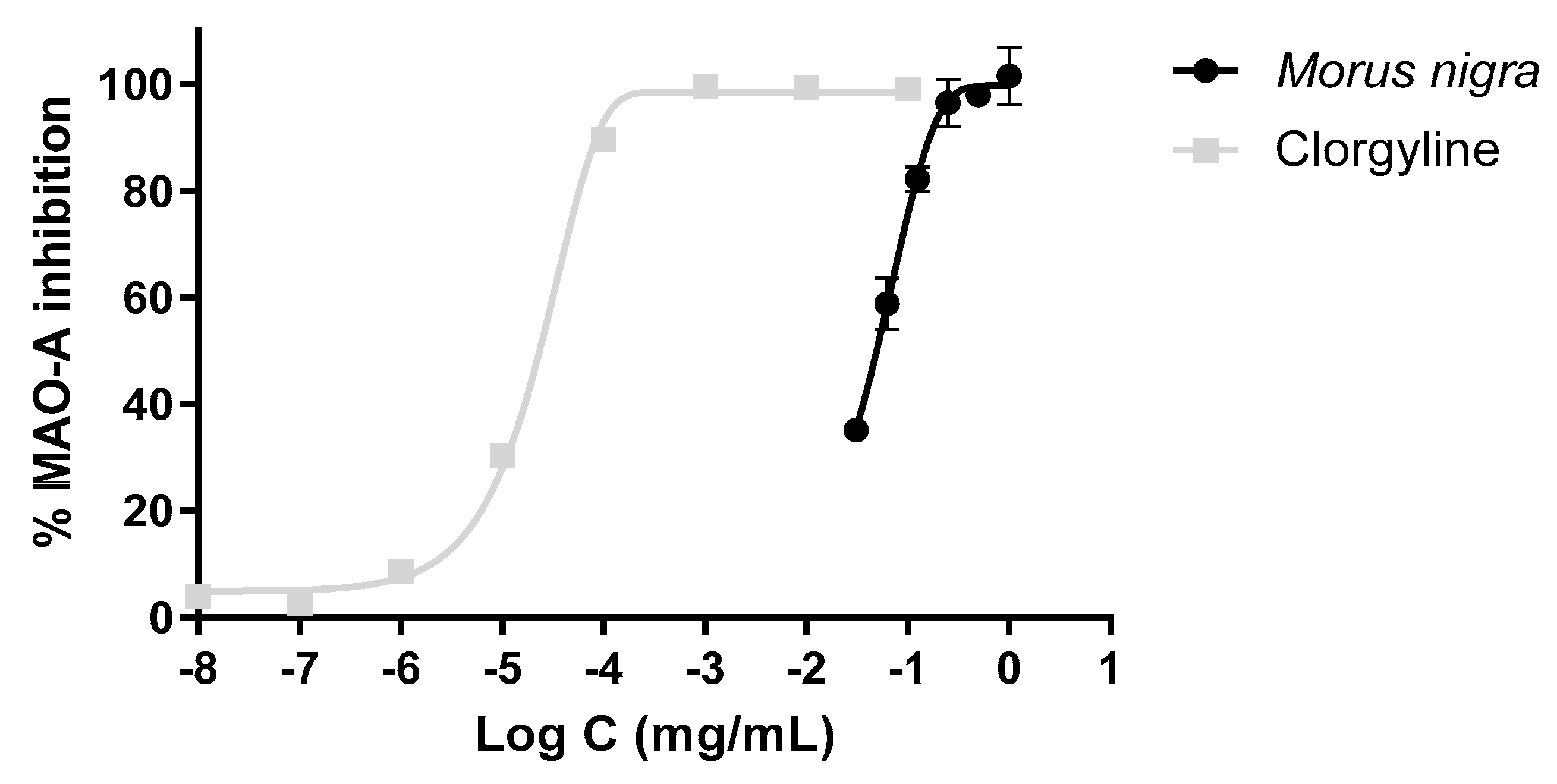
3.6. Inhibitory Effect of the Extract on the MAO-A Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, R.; Gangrade, T.; Punasiya, R.; Ghulaxe, C. Rubus fruticosus (blackberry) use as an herbal medicine. Pharmacogn. Rev. 2014, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Machii, H.; Koyama, A.; Yamanouchi, H. Mulberry Breeding, Cultivation and Utilization in Japan; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2000. [Google Scholar]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Rodrigues, E.L.; Marcelino, G.; Silva, G.T.; Figueiredo, P.S.; Garcez, W.S.; Corsino, J.; Guimarães, R.C.A.; Freitas, K.C. Nutraceutical and medicinal potential of the Morus species in metabolic dysfunctions. Int. J. Mol. Sci. 2019, 20, 301. [Google Scholar] [CrossRef] [PubMed]
- Özgen, M.; Serçe, S.; Kaya, C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci. Hortic. 2009, 119, 275–279. [Google Scholar] [CrossRef]
- Katayama, H.; Takano, R.; Sugimura, Y. Localization of mucilaginous polysaccharides in mulberry leaves. Protoplasma 2008, 233, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, M.; Muradoglu, F.; Sensoy RI, G.; Yilmaz, H. Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci. Hortic. 2011, 132, 37–41. [Google Scholar] [CrossRef]
- Khalifa, I.; Zhu, W.; Li, K.-K.; Li, C.-M. Polyphenols of mulberry fruits as multifaceted compounds: Compositions, metabolism, health benefits, and stability—A structural review. J. Funct. Foods 2018, 40, 28–43. [Google Scholar] [CrossRef]
- Zeni AL, B.; Moreira, T.D.; Dalmagro, A.P.; Camargo, A.; Bini, L.A.; Simionatto, E.L.; Scharf, D.R. Evaluation of phenolic compounds and lipid-lowering effect of Morus nigra leaves extract. An. Da Acad. Bras. De Cienc. 2017, 89, 2805–2815. [Google Scholar] [CrossRef]
- Chen, H.; Pu, J.; Liu, D.; Yu, W.; Shao, Y.; Yang, G.; Xiang, Z.; He, N. Anti-inflammatory and antinociceptive properties of flavonoids from the fruits of black mulberry (Morus nigra L.). PLoS ONE 2016, 11, e0153080. [Google Scholar] [CrossRef]
- Abd El-Mawla AM, A.; Mohamed, K.M.; Mostafa, A.M. Induction of Biologically Active Flavonoids in Cell Cultures of Morus nigra and Testing their Hypoglycemic Efficacy. Sci. Pharm. 2011, 79, 951–961. [Google Scholar] [CrossRef]
- de Souza, M.M.; Bittar, M.; Cechinel-Filho, V.; Yunes, R.A.; Messana, I.; Monache, F.D.; Ferrari, F. Antinociceptive properties of morusin, a prenylflavonoid isolated from Morus nigra root bark. Z. Fur Naturforschung—Sect. C J. Biosci. 2000, 55, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.P.; Cheng, K.W.; Zhu, Q.; Wang, X.C.; Lin, Z.X.; Wang, M. Tyrosinase inhibitory constituents from the roots of Morus nigra: A structure-activity relationship study. J. Agric. Food Chem. 2010, 58, 5368–5373. [Google Scholar] [CrossRef]
- Tahir, L.; Aslam, A.; Ahmed, S. Antibacterial activities of Diospyros blancoi, Phoenix dactylifera and Morus nigra against dental caries causing pathogens: An in vitro study. Pak. J. Pharm. Sci. 2017, 30, 163–169. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28603127 (accessed on 1 July 2024).
- Cavalcante, A.; Lins, T.; Santos, J.; Barros, V.; Monte, A.; Barberino, R.S.; Almeida, J.; Matos, M. Supplemented Morus nigra extract-based medium associated with FSH enables the survival and growth of isolated ovine secondary ovarian follicles. Reprod. Domest. Anim. 2018, 53, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Dalmagro, A.P.; Camargo, A.; Zeni AL, B. Morus nigra and its major phenolic, syringic acid, have antidepressant-like and neuroprotective effects in mice. Metab. Brain Dis. 2017, 32, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Hassanalilou, T.; Payahoo, L.; Shahabi, P.; Abbasi, M.M.; Jafar-Abadi, M.A.; Bishak, Y.K.; Khordadmehr, M.; Esnaashari, S.; Barzegar, A. The protective effects of Morus nigra L. leaves on the kidney function tests and histological structures in streptozotocin-induced diabetic rats. Biomed. Res. 2017, 28, 6113–6118. Available online: https://research.monash.edu/en/publications/the-protective-effects-of-morus-nigra-l-leaves-on-the-kidney-func (accessed on 1 July 2024).
- Tag, H.M. Hepatoprotective effect of mulberry (Morus nigra) leaves extract against methotrexate induced hepatotoxicity in male albino rat. BMC Complement. Altern. Med. 2015, 15, 252. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Choi, C.I. Pharmacological properties of Morus nigra L. (Black Mulberry) as a promising nutraceutical resource. Nutrients 2019, 11, 437. [Google Scholar] [CrossRef]
- Jiang, Y.; Nie, W.J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Thériault, M.; Caillet, S.; Kermasha, S.; Lacroix, M. Antioxidant, antiradical and antimutagenic activities of phenolic compounds present in maple products. Food Chem. 2006, 98, 490–501. [Google Scholar] [CrossRef]
- Haminiuk CW, I.; Maciel, G.M.; Plata-Oviedo MS, V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Mazzara, E.; Abouelenein, D.; Angeloni, S.; Nunez, S.; Sagratini, G.; López, V.; Cespi, M.; Vittori, S.; Caprioli, G.; et al. Optimization of Solvent-Free Microwave-Assisted Hydrodiffusion and Gravity Extraction of Morus nigra L. Fruits Maximizing Polyphenols, Sugar Content, and Biological Activities Using Central Composite Design. Pharmaceuticals 2022, 15, 99. [Google Scholar] [CrossRef]
- Liang, L.; Wu, X.; Zhu, M.; Zhao, W.; Li, F.; Zou, Y.; Yang, L. Chemical composition, nutritional value, and antioxidant activities of eight mulberry cultivars from China. Pharmacogn. Mag. 2012, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A. Anthocyanins as Natural Colorants and Bioactive Compounds. A Review. Acta Biol. Colomb. 2008, 13, 27–36. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20103054242 (accessed on 7 April 2022).
- Miyazawa, T.; Nakagawa, K.; Kudo, M.; Muraishi, K.; Someya, K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3- glucoside and cyanidin-3,5-diglucoside, into rats and humans. J. Agric. Food Chem. 1999, 47, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.N.; Chu, S.C.; Chiou, H.L.; Kuo, W.H.; Chiang, C.L.; Hsieh, Y.S. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006, 235, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.K.; Lee, H.J.; Shih, Y.W.; Chyau, C.C.; Wang, C.J. Mulberry anthocyanin extracts inhibit LDL oxidation and macrophage-derived foam cell formation induced by oxidative LDL. J. Food Sci. 2008, 73, H113-21. [Google Scholar] [CrossRef]
- Koyuncu, F. Organic acid composition of native black mulberry fruit. Chem. Nat. Compd. 2004, 40, 367–369. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC—Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.L.; Simon, J.E.; Lila, M.A.; Rochet, J.C. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson’s disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef]
- Cásedas, G.; González-Burgos, E.; Smith, C.; López, V.; Gómez-Serranillos, M.P. Regulation of redox status in neuronal SH-SY5Y cells by blueberry (Vaccinium myrtillus L.) juice, cranberry (Vaccinium macrocarpon A.) juice and cyanidin. Food Chem. Toxicol. 2018, 118, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Muriach, M.; Flores-Bellver, M.; Romero, F.J.; Barcia, J.M. Diabetes and the Brain: Oxidative Stress, Inflammation, and Autophagy. Oxidative Med. Cell. Longev. 2014, 2014, 102158. [Google Scholar] [CrossRef]
- Bickford, P.C.; Gould, T.; Briederick, L.; Chadman, K.; Pollock, A.; Young, D.; Shukitt-Hale, B.; Joseph, J. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000, 866, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.; Figueira, I.; MacEdo, D.; McDougall, G.J.; Leitão, M.C.; Vieira HL, A.; Stewart, D.; Alves, P.M.; Ferreira, R.B.; Santos, C.N. Neuroprotective effect of blackberry (Rubus sp.) polyphenols is potentiated after simulated gastrointestinal digestion. Food Chem. 2012, 131, 1443–1452. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; González-Burgos, E.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Evaluation of the Antioxidant Capacities and Cytotoxic Effects of Ten Parmeliaceae Lichen Species. Evid.-Based Complement. Altern. 2016, 201, 11. [Google Scholar] [CrossRef]
- Dostal, V.; Link, C.D. Assaying β-amyloid toxicity using a transgenic C. elegans model. J. Vis. Exp. JoVE 2010, 44, 2252. [Google Scholar] [CrossRef]
- Les, F.; Prieto, J.M.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Bioactive properties of commercialised pomegranate (Punica granatum) juice: Antioxidant, antiproliferative and enzyme inhibiting activities. Food Funct. 2015, 6, 2049–2057. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.T.; Stafford, G.I.; van Staden, J.; Christensen, S.B.; Jäger, A.K. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. J. Ethnopharmacol. 2008, 117, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Galli, R.L.; Shukitt-Hale, B.; Youdim, K.A.; Joseph, J.A. Fruit polyphenolics and brain aging: Nutritional interventions targeting age-related neuronal and behavioral deficits. Ann. N. Y. Acad. Sci. 2002, 959, 128–132. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.R.; Oliveira-Junior, R.G.; Diniz, T.C.; Branco, A.; Lima-Saraiva SR, G.; Guimarães, A.L.; Oliveira, A.P.; Pacheco AG, M.; Silva, M.G.; Moraes-Filho, M.O.; et al. Assessment of the antibacterial, cytotoxic and antioxidant activities of Morus nigra L. (Moraceae). Braz. J. Biol. 2018, 78, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lavens, P.; Sorgeloos, P. The history, present status and prospects of the availability of Artemia cysts for aquaculture. Aquaculture 2000, 181, 397–403. [Google Scholar] [CrossRef]
- Migliore, L.; Coppedè, F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat. Res. 2009, 674, 73–84. [Google Scholar] [CrossRef]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Chung, J.I.; Kim, M.O. Anthocyanins Improve Hippocampus-Dependent Memory Function and Prevent Neurodegeneration via JNK/Akt/GSK3β Signaling in LPS-Treated Adult Mice. Mol Neurobiol 2019, 56, 671–687. [Google Scholar] [CrossRef]
- Joseph, J.A.; Shukitt-Hale, B.; Brewer, G.J.; Weikel, K.A.; Kalt, W.; Fisher, D.R. Differential protection among fractionated blueberry polyphenolic families against DA-, Aβ42- and LPS-Induced decrements in Ca2+ buffering in primary hippocampal cells. J. Agric. Food Chem. 2010, 58, 8196–8204. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.; Link, C.D.; Butterfield, D.A. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid β-peptide (1-42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging 2003, 24, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wang, N.; Zou, Y.; Fahim, M.; Zhou, Y.; Yang, H.; Liu, Y.; Li, H. Black mulberry (Morus nigra) fruit extract alleviated AD-Like symptoms induced by toxic Aβ protein in transgenic Caenorhabditis elegans via insulin DAF-16 signaling pathway. Food Res. Int. 2022, 160, 111696. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.G.; Dragicevic, N.B.; Deng, J.H.; Bai, Y.; Dimayuga, E.; Ding, Q.; Chen, Q.; Bruce-Keller, A.J.; Keller, J.N. Proteasome inhibition alters neural mitochondrial homeostasis and mitochondria turnover. J. Biol. Chem. 2004, 279, 20699–20707. [Google Scholar] [CrossRef] [PubMed]
- Dreiseitel, A.; Korte, G.; Schreier, P.; Oehme, A.; Locher, S.; Domani, M.; Hajak, G.; Sand, P.G. Berry anthocyanins and their aglycons inhibit monoamine oxidases A and B. Pharmacol. Res. 2009, 59, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Augusto, T.R.; Scheuermann Salinas, E.S.; Alencar, S.M.; D’Arce MA, B.R.; de Camargo, A.C.; de Souza Vieira, T.M.F. Phenolic compounds and antioxidant activity of hydroalcoholic extractsof wild and cultivated murtilla (Ugni molinae turcz.). Food Sci. Technol. 2015, 34, 667–673. [Google Scholar] [CrossRef]
- Isabelle, M.; Bee, L.L.; Choon, N.O.; Liu, X.; Huang, D. Peroxyl radical scavenging capacity, polyphenolics, and lipophilic antioxidant profiles of mulberry fruits cultivated in southern China. J. Agric. Food Chem. 2008, 56, 9410–9416. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Anwar, F.; Abbas, M.; Saari, N. Effect of maturity on phenolics (Phenolic acids and flavonoids) profile of strawberry cultivars and mulberry species from Pakistan. Int. J. Mol. Sci. 2012, 13, 4591–4607. [Google Scholar] [CrossRef]
- Suriyaprom, S.; Kaewkod, T.; Promputtha, I.; Desvaux, M.; Tragoolpua, Y. Evaluation of Antioxidant and Antibacterial Activities of White Mulberry (Morus alba L.) Fruit Extracts. Plants 2021, 10, 2736. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cásedas, G.; Moliner, C.; Abad-Longas, A.; Núñez, S.; Gómez-Rincón, C.; Maggi, F.; López, V. Black Mulberries (Morus nigra L.) Modulate Oxidative Stress and Beta-Amyloid-Induced Toxicity, Becoming a Potential Neuroprotective Functional Food. Foods 2024, 13, 2577. https://doi.org/10.3390/foods13162577
Cásedas G, Moliner C, Abad-Longas A, Núñez S, Gómez-Rincón C, Maggi F, López V. Black Mulberries (Morus nigra L.) Modulate Oxidative Stress and Beta-Amyloid-Induced Toxicity, Becoming a Potential Neuroprotective Functional Food. Foods. 2024; 13(16):2577. https://doi.org/10.3390/foods13162577
Chicago/Turabian StyleCásedas, Guillermo, Cristina Moliner, Alba Abad-Longas, Sonia Núñez, Carlota Gómez-Rincón, Filippo Maggi, and Víctor López. 2024. "Black Mulberries (Morus nigra L.) Modulate Oxidative Stress and Beta-Amyloid-Induced Toxicity, Becoming a Potential Neuroprotective Functional Food" Foods 13, no. 16: 2577. https://doi.org/10.3390/foods13162577







