Nutritional Quality Analysis and Classification Detection of Buckwheat in Different Harvest Periods
Abstract
:1. Introduction
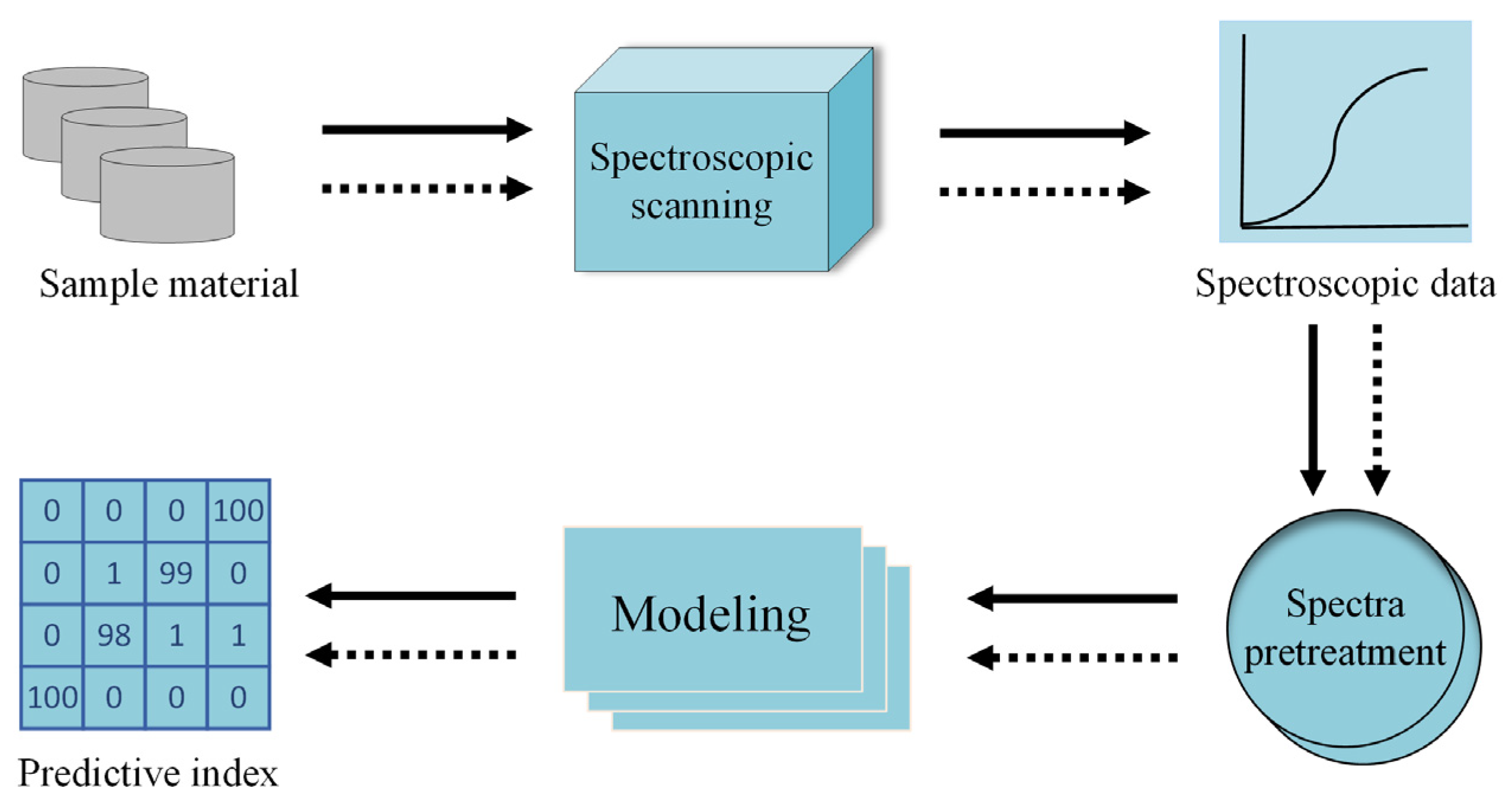
2. Materials and Methods
2.1. Test Materials
2.2. NIR Spectral Imaging Acquisition Equipment
2.3. Determination of Nutritional Quality of Buckwheat during Different Harvest Periods
2.4. Extraction and Processing of Near-Infrared Spectral Data for Buckwheat in Different Harvest Periods
2.4.1. Data Extraction
2.4.2. Preprocessing of Near-Infrared Spectral Data for Buckwheat in Different Harvest Periods
2.4.3. Extraction of Characteristic Wavelengths from Near-Infrared Spectral Data of Buckwheat in Different Harvest Periods
2.4.4. Classification Models and Assessment Indicators
3. Results and Analysis
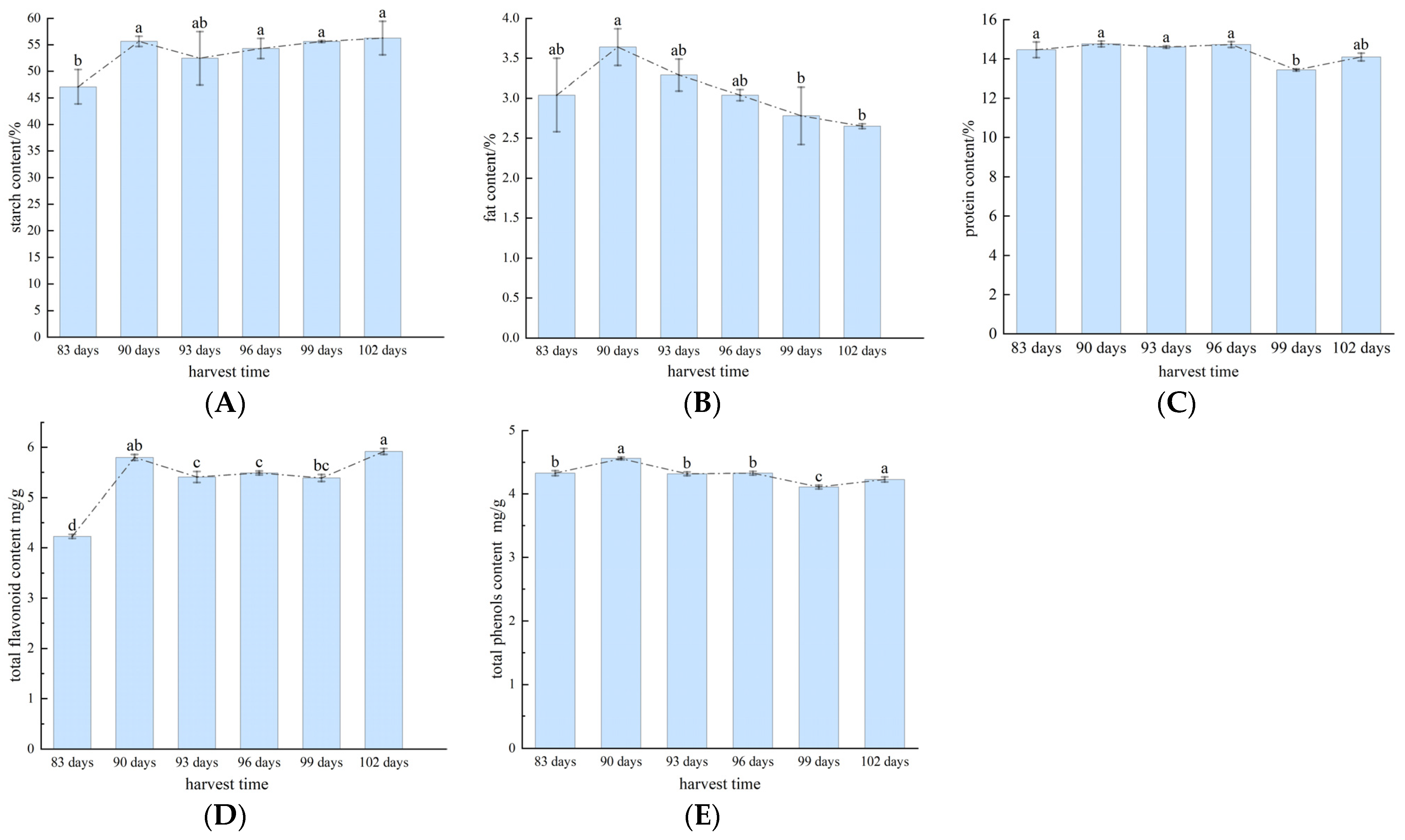
3.1. Analysis of Nutritional Quality Results for Buckwheat in Different Harvest Periods
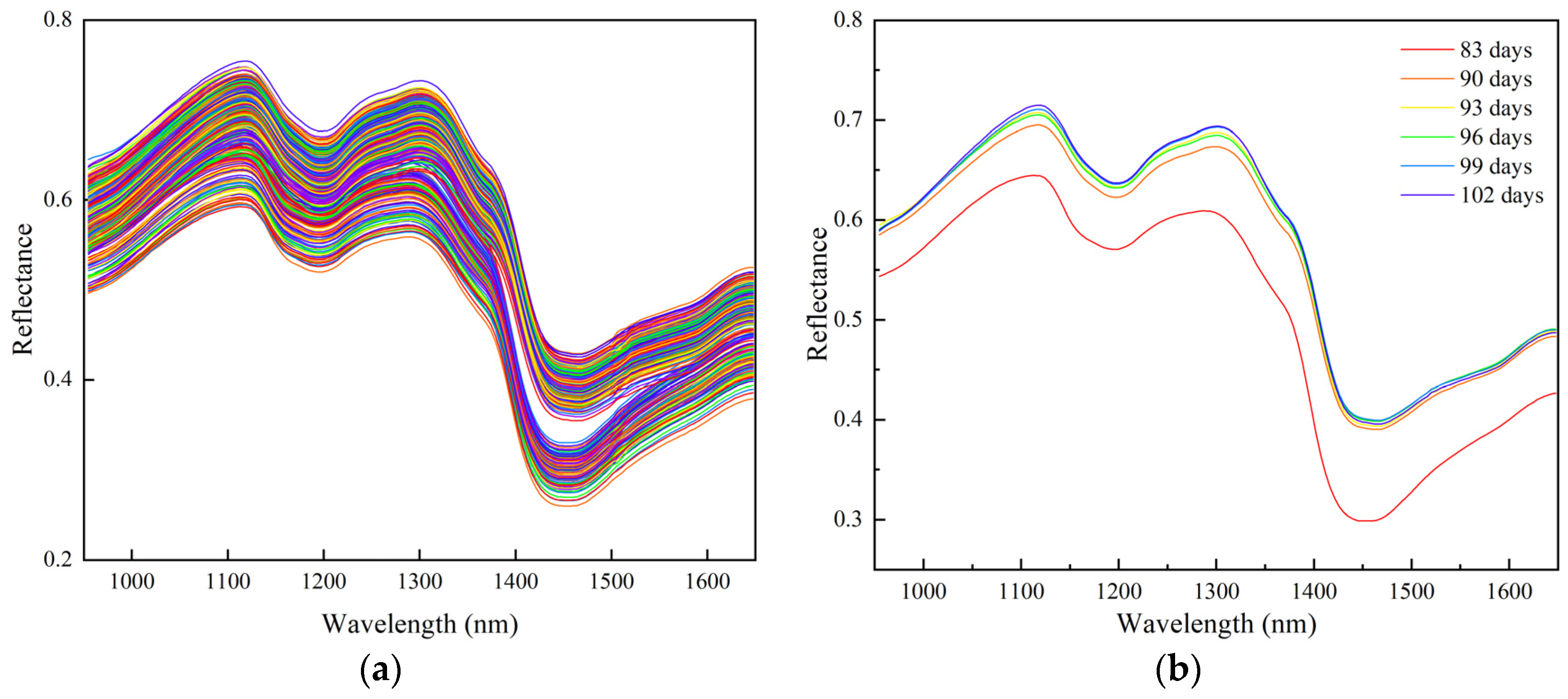
3.2. Analysis of Near-Infrared Spectral Results of Buckwheat in Different Harvest Periods
3.2.1. Spectral Characterization
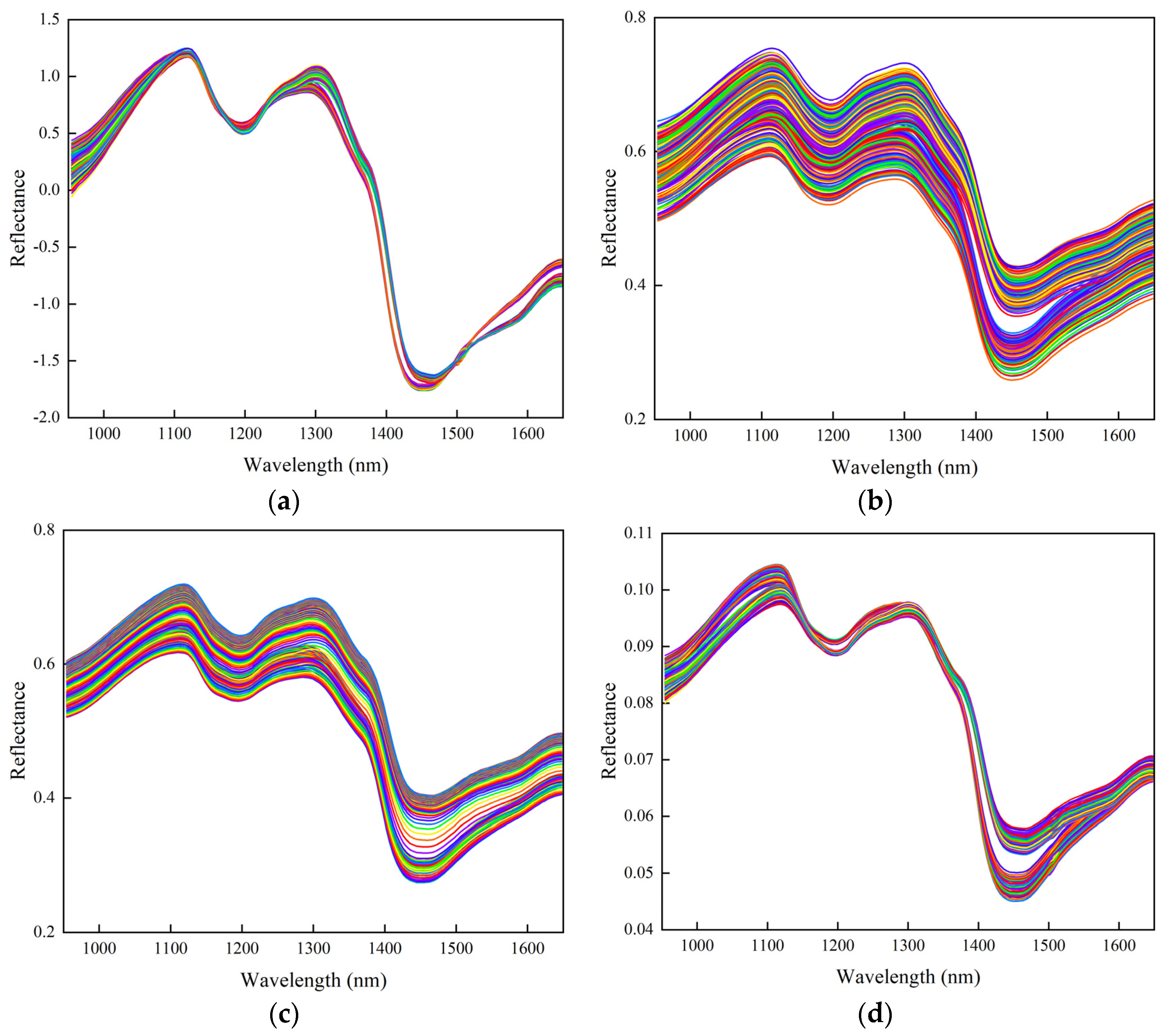
3.2.2. Analysis of Data Preprocessing Results
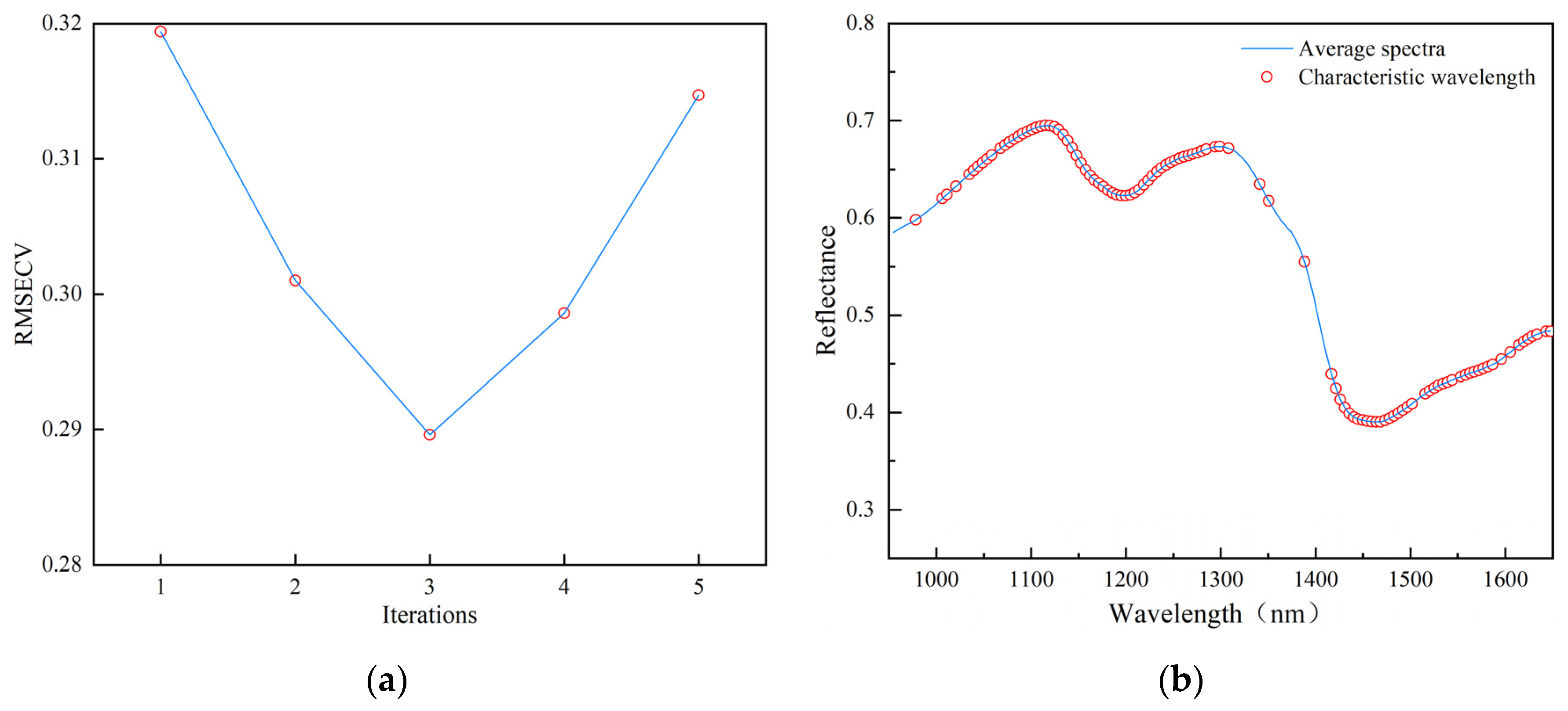
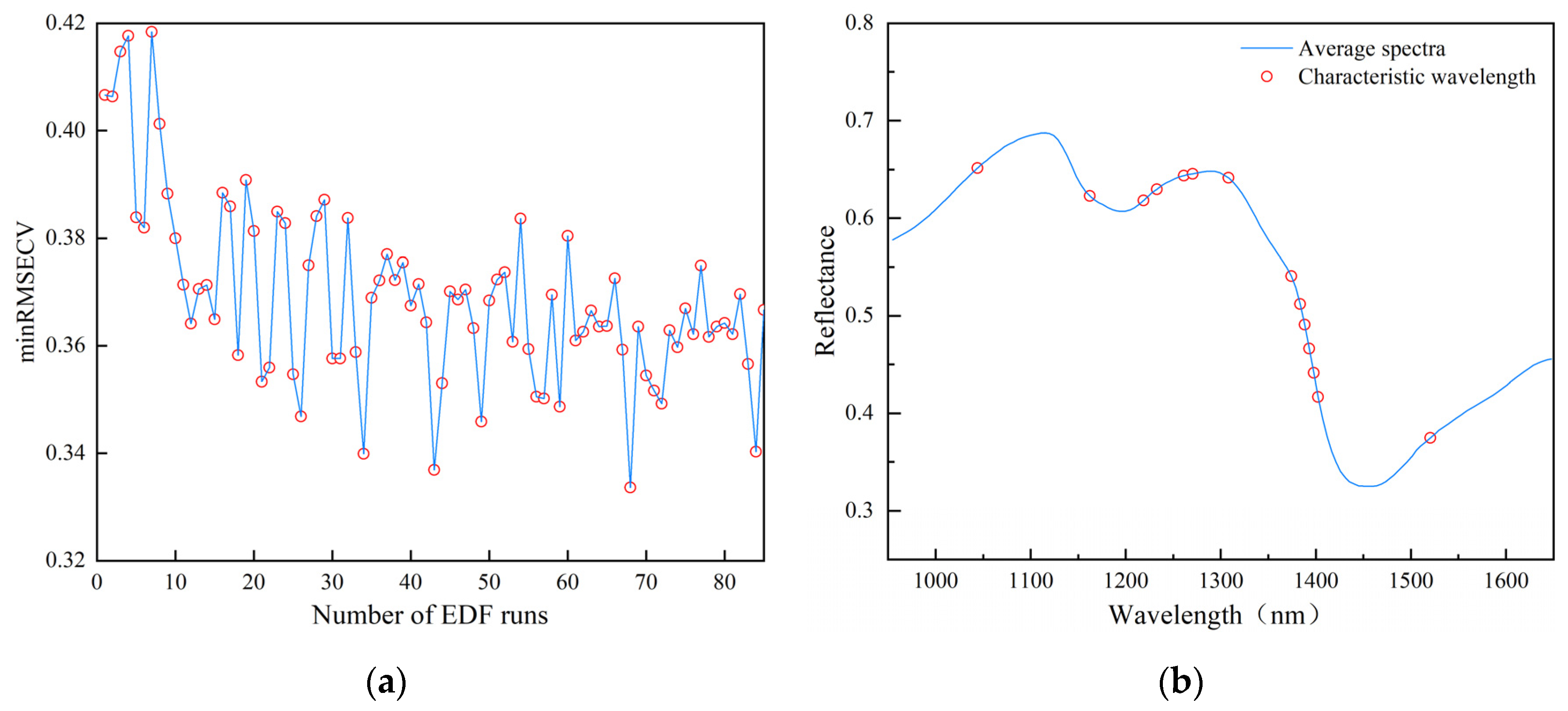
3.2.3. Analysis of Feature Wavelength Extraction Results
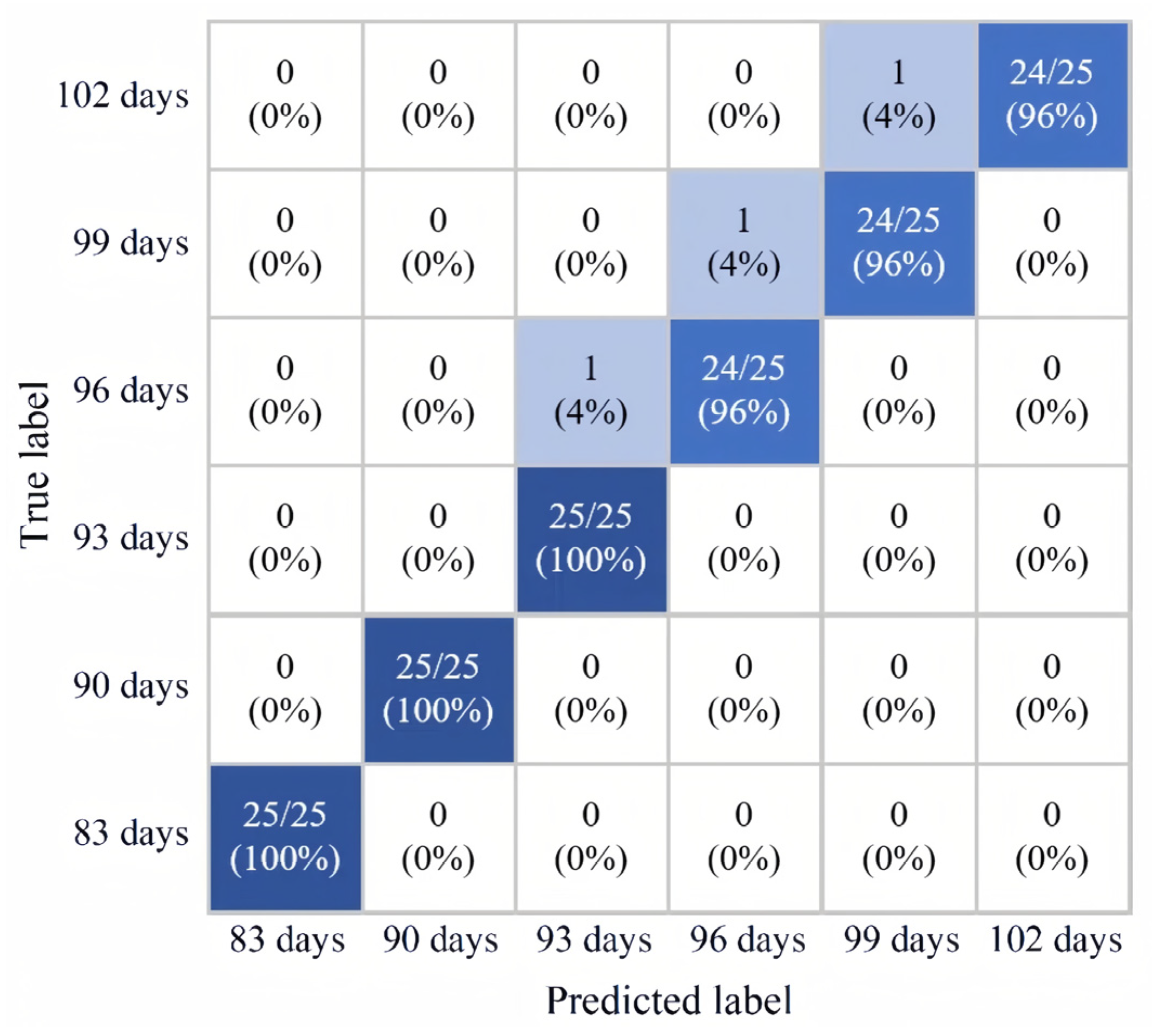
3.2.4. Analysis of Classification Results
4. Discussion
5. Conclusions
- (1)
- In this study, six buckwheat harvests (with 83-day, 90-day, 93-day, 96-day, 99-day and 102-day growth cycles) were investigated. The six different harvests of buckwheat kernels were analyzed using physicochemical tests to determine the buckwheat grain nutrient quality over the different harvest periods and the changes in buckwheat grain protein, fat, and starch contents. The total flavonoid and total phenol contents were higher in the 90-day cycle; thus, this was determined as the optimal buckwheat harvest period.
- (2)
- In this study, near-infrared spectroscopy nondestructive testing technology was utilized to detect buckwheat content in six harvesting periods: 83 days, 90 days, 93 days, 96 days, 99 days, and 102 days. Through the comparison of six classification models, it was shown that the IVSO-RF model showed the best classification of different buckwheat harvests as the evaluation indexes were higher. When applying the IVSO-RF classification model to classify and validate a single buckwheat harvest period, the correctness rate of the training set for each harvest period reached 96%, and the correctness rate of the prediction set reached 100%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Wang, W.; Xing, X.; Sun, Y. Progress of Research on the Efficacy Components and Pharmacological Effects of Buckwheat. J. Shenyang Pharm. Univ. 2023, 1–12. [Google Scholar] [CrossRef]
- Luo, T.; Li, G.; You, J.; Tang, K.; Chen, Y. Nutritional Composition and Health Effects of Food and Medicine Crops Buckwheat. Spec. Econ. Anim. Plants 2024, 27, 178–181. [Google Scholar]
- Lu, Q.; Zheng, D.; Liu, Y.; Li, L.; Xu, B.; Ye, S. Problems and Thoughts on Mechanized Harvesting Technology of Buckwheat in China. J. Agric. Eng. 2020, 10, 6–9. [Google Scholar]
- Huang, X.; Zhang, W.; Dang, W.; Zhang, K.; Feng, S.; Liu, Z.; Yang, F. Buckwheat Harvesting Machinery Research Status and Development Trend. Farm Mach. 2018, 10, 84–90. [Google Scholar] [CrossRef]
- Wu, C.; Kong, L.; Wang, S.; Guo, Y. Near Infrared Spectroscopy (NIRS) Technology Applied in Millet Feature Extraction and Variety Identification. Afr. J. Agric. Res. 2017, 12, 2223–2231. [Google Scholar] [CrossRef]
- Wu, K.; Zhu, T.; Wang, Z.; Zhao, X.; Yuan, M.; Liang, D.; Li, Z. Identification of Varieties of Sorghum Based on a Competitive Adaptive Reweighted Sampling-Random Forest Process. Eur. Food Res. Technol. 2024, 250, 191–201. [Google Scholar] [CrossRef]
- Gong, K.; Li, Y.; Lu, C.; Zhou, L. Effects of Different Harvesting Times on the Nutritional and Flavour Quality and Starch R V A Characteristics of “Japonica 46”. Shanghai Agric. Sci. Technol. 2019, 25, 102–103. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Liu, J.; Wang, C.; Huang, J.; Yao, J.; Yan, S.; Wu, Z. Effect of Cultivars and Harvest Stages on the Maize Yield and Silage Quality. Acta Agrestia Sin. 2018, 26, 261–263. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, L.; Yao, S.; Zhao, Y. Integrated Evaluation on Yield and Nutritional Quality of Five Oat Varieties in Different Harvest Time. Shandong Agric. Sci. 2016, 48, 49–53. [Google Scholar]
- Liu, S.; Tan, X.; Liu, C.; Zhu, C.; Li, W.; Cui, S.; Du, Y.; Huang, D.; Xie, F. Recognition of Fusarium Head Blight Wheat Grain Based on Hyperspectral Data Processing Algorithm. Spectrosc. Spectr. Anal. 2019, 39, 3540–3546. [Google Scholar] [CrossRef]
- Larios, G.; Nicolodelli, G.; Ribeiro, M.; Canassa, T.; Reis, A.R.; Oliveira, S.L.; Alves, C.Z.; Marangoni, B.S.; Cena, C. Soybean Seed Vigor Discrimination by Using Infrared Spectroscopy and Machine Learning Algorithms. Anal. Methods 2020, 12, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; You, Z.; Cheng, F. Study on Identification of Immature Corn Seed Using Hyperspectral Imaging. Spectrosc. Spectr. Anal. 2016, 36, 4028–4033. [Google Scholar] [CrossRef]
- Singh, T.; Garg, N.M.; Iyengar, S.R.S. Nondestructive Identification of Barley Seeds Variety Using Near-Infrared Hyperspectral Imaging Coupled with Convolutional Neural Network. J. Food Process Eng. 2021, 44, e13821. [Google Scholar] [CrossRef]
- Determination of Starch in Foods. Available online: https://img59.chem17.com/1/20170726/636366583857616209893.pdf (accessed on 23 December 2016).
- Determination of Protein in Foods. Available online: https://www.antpedia.com/standard/pdf/1/1901/GB%205009.5-2016.pdf (accessed on 23 December 2016).
- Determination of Fat in Foods. Available online: https://www.anan.gov.cn/anan/cpbz/201801/ffddf722c4e44dcb9888d7e1b55c0146/files/163c21654559468aad3b88f429f07498.pdf (accessed on 23 December 2016).
- Xue, H.; Deng, R.; Xu, Z.; Zhang, D. Sodium Nitrite-Aluminum Nitrate Colorimetric Assay for the Determination of Flavonoids in Buckwheat in Qianzhong. Guizhou J. Anim. Husb. Vet. Med. 2020, 44, 9–11. [Google Scholar]
- Cheng, Z.; Guo, H.; Hu, J.; He, Y.; Li, H.; Li, Y. Relationship Between Chlorophyll, Total Phenol, PPO and Browning of Dehulled Common Buckwheat Seeds. J. Chin. Cereals Oils Assoc. 2020, 35, 19–25. [Google Scholar]
- Song, X.; Du, G.; Li, Q.; Tang, G.; Huang, Y. Rapid Spectral Analysis of Agro-Products Using an Optimal Strategy: Dynamic Backward Interval PLS–Competitive Adaptive Reweighted Sampling. Anal. Bioanal. Chem. 2020, 412, 2795–2804. [Google Scholar] [CrossRef]
- Wrobel, T.P.; Liberda, D.; Koziol, P.; Paluszkiewicz, C.; Kwiatek, W.M. Comparison of the New Mie Extinction Extended Multiplicative Scattering Correction and Resonant Mie Extended Multiplicative Scattering Correction in Transmission Infrared Tissue Image Scattering Correction. Infrared Phys. Technol. 2020, 107, 103291. [Google Scholar] [CrossRef]
- Mishra, P.; Marini, F.; Biancolillo, A.; Roger, J.M. Improved Prediction of Fuel Properties with Near-Infrared Spectroscopy Using a Complementary Sequential Fusion of Scatter Correction Techniques. Talanta 2021, 223, 121693. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shen, W.; Yu, L.G.; Wang, Y.L.; Yang, J.Y.; Zhang, Z.W. Illumination Invariant Extraction for Face Recognition Using Neighboring Wavelet Coefficients. Pattern Recognit. 2012, 45, 1299–1305. [Google Scholar] [CrossRef]
- Chen, B.; Lu, B.; Lu, D. Parameter Optimization of Rapeseed Oil Content Model Using a Miniature Near-Infrared Spectrometer. Mod. Food Sci. Technol. 2015, 31, 286–292+267. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, R.; Chen, J.; Liu, L.; Matsushita, B. A Practical Approach to Reconstruct High-Quality Landsat NDVI Time-Series Data by Gap Filling and the Savitzky–Golay Filter. ISPRS J. Photogramm. Remote Sens. 2021, 180, 174–190. [Google Scholar] [CrossRef]
- Li, Y. Research on Nondestructive Detection of Soybean Seed Vigor and Viability Based on Hyperspectral Image Technology. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2021. [Google Scholar]
- Zhang, P.; Xu, Z.; Ma, H.; Cheng, W.; Li, X.; Tang, L.; Zhao, G.; Wu, Y.; Liu, Z.; Wang, Q. Novel Comprehensive Variable Selection Algorithm Based on Multi-Weight Vector Optimal Selection and Bootstrapping Soft Shrinkage. Infrared Phys. Technol. 2023, 133, 104800. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Zhang, Y.; Wang, K. Prediction of Compressive Strength Parallel to Grain of Birch Wood Using near Infrared Spectroscopy and SEPA-VISS-ARVM Model. J. For. Eng. 2022, 7, 52–58. [Google Scholar] [CrossRef]
- Georganos, S.; Grippa, T.; Gadiaga, A.N.; Linard, C.; Lennert, M.; Vanhuysse, S.; Mboga, N.; Wolff, E.; Kalogirou, S. Geographical Random Forests: A Spatial Extension of the Random Forest Algorithm to Address Spatial Heterogeneity in Remote Sensing and Population Modelling. Geocarto Int. 2021, 36, 121–136. [Google Scholar] [CrossRef]
- Dhanaraj, R.K.; Ramakrishnan, V.; Poongodi, M.; Krishnasamy, L.; Hamdi, M.; Kotecha, K.; Vijayakumar, V. Random Forest Bagging and X-Means Clustered Antipattern Detection from SQL Query Log for Accessing Secure Mobile Data. Wirel. Commun. Mob. Comput. 2021, 2021, 2730246. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, X.; Liao, S.; Zhan, B.; Luo, W.; Liu, S.; Liu, X.; Xie, C. Feasibility Study on Identification of Seeds of Hong Kong Seeds 49, October Red and September Fresh Cabbage Based on Visible/Shortwave Near-Infrared Spectroscopy of Partial Least Squares Discriminant (PLS-DA) and Least Squares Support Vector Machine (LS-SVM). Spectrosc. Spectr. Anal. 2024, 44, 1718–1723. [Google Scholar] [CrossRef]
- Yang, D.; Lu, A.; Ren, D.; Wang, J. Rapid Determination of Biogenic Amines in Cooked Beef Using Hyperspectral Imaging with Sparse Representation Algorithm. Infrared Phys. Technol. 2017, 86, 23–34. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Ferreira, M.M.C.; Salva, T.J.G. Chemometric Models for the Quantitative Descriptive Sensory Analysis of Arabica Coffee Beverages Using near Infrared Spectroscopy. Talanta 2011, 83, 1352–1358. [Google Scholar] [CrossRef]
- Lopes, L.C.; Brandão, I.V.; Sánchez, O.C.; Franceschi, E.; Borges, G.; Dariva, C.; Fricks, A.T. Horseradish Peroxidase Biocatalytic Reaction Monitoring Using Near-Infrared (NIR) Spectroscopy. Process Biochem. 2018, 71, 127–133. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, X.; Liu, X.; He, Y. Identification of Hybrid Okra Seeds Based on Near-Infrared Hyperspectral Imaging Technology. Appl. Sci. 2018, 8, 1793. [Google Scholar] [CrossRef]
- Shreeja, K.; Devi, T.S.; Gogoi, M.; Ahmed, P.; Sharma, M. Physico-Chemical and Nutritional Evaluation of Flour Made from an Under-Utilized Food Crop Buckwheat. J. Adv. Biol. Biotechnol. 2024, 27, 97–106. [Google Scholar] [CrossRef]
- Stacey, P.; O’Kiely, P.; Hackett, R.; Rice, B.; O’Mara, F.P. Changes in Yield and Composition of Barley, Wheat and Triticale Grains Harvested during Advancing Stages of Ripening. Ir. J. Agric. Food Res. 2006, 45, 197–209. Available online: https://www.jstor.org/stable/25562577 (accessed on 13 August 2024).
- Fu, H.; Cao, D.; Hu, W.; Guan, Y.; Fu, Y.; Fang, Y.; Hu, J. Studies on Optimum Harvest Time for Hybrid Rice Seed. J. Sci. Food Agric. 2017, 97, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Molska, M.; Reguła, J.; Zielińska-Dawidziak, M.; Tomczak, A.; Świeca, M. Starch and Protein Analysis in Buckwheat (Fagopyrum Esculentum Moench) Sprouts Enriched with Probiotic Yeast. Learn. Technol. 2022, 168, 113903. [Google Scholar] [CrossRef]
- Fan, J. Influence of Harvesting Time on the Quality of Rice “Japonica 9108”. Agric. Eng. Technol. 2022, 42, 16–17. [Google Scholar] [CrossRef]
- Chai, Y.; Yu, Y.; Zhu, H.; Li, Z.; Dong, H.; Yang, H. Identification of Common Buckwheat (Fagopyrum Esculentum Moench) Adulterated in Tartary Buckwheat (Fagopyrum Tataricum (L.) Gaertn) Flour Based on near-Infrared Spectroscopy and Chemometrics. Curr. Res. Food Sci. 2023, 7, 100573. [Google Scholar] [CrossRef] [PubMed]
- Platov, Y.T.; Metlenkin, D.A.; Platova, R.A.; Rassulov, V.A.; Vereshchagin, A.I.; Marin, V.A. Correction to: Buckwheat Identification by Combined UV-VIS-NIR Spectroscopy and Multivariate Analysis. J. Appl. Spectrosc. 2022, 88, 1331. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.; Zhu, H. Research on Determination Method of Starch, Protein and Total Flavonoids Content in Buckwheat by Near-Infrared Spectroscopy. Sens. Lett. 2014, 12, 888–891. [Google Scholar] [CrossRef]
- Zhu, L.; Damaris, R.N.; Lv, Y.; Du, Q.; Shi, T.; Deng, J.; Chen, Q. Construction and Application of Detection Model for Leucine and Tyrosine Content in Golden Tartary Buckwheat Based on Near Infrared Spectroscopy. Appl. Sci. 2022, 12, 11051. [Google Scholar] [CrossRef]

| Harvest Period | Starch/% | Fat/% | Protein/% | Total Flavonoids (mg/g) | Total Phenols (mg/g) |
|---|---|---|---|---|---|
| 83 days | 47.12 ± 3.22 b | 3.04 ± 0.46 ab | 14.46 ± 0.46 a | 4.23 ± 0.04 d | 4.33 ± 0.04 b |
| 90 days | 55.66 ± 0.97 a | 3.64 ± 0.23 a | 14.76 ± 0.15 a | 5.80 ± 0.06 ab | 4.56 ± 0.02 a |
| 93 days | 52.49 ± 5.01 ab | 3.29 ± 0.20 ab | 14.60 ± 0.06 a | 5.41 ± 0.11 c | 4.32 ± 0.03 b |
| 96 days | 54.32 ± 1.90 a | 3.04 ± 0.07 ab | 14.73 ± 0.15 a | 5.49 ± 0.04 c | 4.33 ± 0.03 b |
| 99 days | 55.63 ± 0.17 a | 2.78 ± 0.36 b | 13.43 ± 0.06 b | 5.39 ± 0.07 bc | 4.11 ± 0.03 c |
| 102 days | 56.28 ± 3.17 a | 2.65 ± 0.03 b | 14.09 ± 0.20 ab | 5.92 ± 0.06 a | 4.23 ± 0.04 a |
| SD | 3.44 | 0.36 | 0.51 | 0.60 | 0.15 |
| Mean | 53.58 | 3.07 | 14.35 | 5.37 | 4.31 |
| CV | 6.43% | 11.60% | 3.56% | 11.18% | 3.43% |
| Model | Preprocessing Method | Training Set Correctness (%) | Prediction Set Correctness (%) |
|---|---|---|---|
| RF | S-G | 85.11 | 69.33 |
| SNV | 94 | 92 | |
| DWT | 92.67 | 89 | |
| normaliz | 90.44 | 87.33 | |
| LS-SVM | S-G | 40.67 | 36 |
| SNV | 55.78 | 64.67 | |
| DWT | 42.89 | 44 | |
| normaliz | 44.22 | 42.67 |
| Model | Number | Total | NFP | Accuracy/% | Error Rate/% | Precision/% | Recall/% | F1 Score/% |
|---|---|---|---|---|---|---|---|---|
| IVSO-RF | 106 | 150 | 5 | 96.67 | 3.33 | 96.62 | 89.16 | 92.58 |
| VCPA-RF | 14 | 150 | 25 | 83.33 | 16.67 | 83.94 | 81.23 | 82.99 |
| VISSA-RF | 77 | 150 | 7 | 95.33 | 4.67 | 95.54 | 89.03 | 92.43 |
| IVSO-LS-SVM | 106 | 150 | 79 | 47.33 | 52.67 | 54.94 | 66.95 | 44.31 |
| VCPA-LS-SVM | 14 | 150 | 103 | 31.33 | 68.67 | 37.58 | 29.18 | 27.60 |
| VISSA-LS-SVM | 77 | 150 | 81 | 46 | 54 | 53.99 | 50.25 | 41.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, P.; Liu, Y.; Yang, L.; Yan, H.; Feng, S.; Zheng, D. Nutritional Quality Analysis and Classification Detection of Buckwheat in Different Harvest Periods. Foods 2024, 13, 2576. https://doi.org/10.3390/foods13162576
Xin P, Liu Y, Yang L, Yan H, Feng S, Zheng D. Nutritional Quality Analysis and Classification Detection of Buckwheat in Different Harvest Periods. Foods. 2024; 13(16):2576. https://doi.org/10.3390/foods13162576
Chicago/Turabian StyleXin, Peichen, Yun Liu, Lufei Yang, Haoran Yan, Shuai Feng, and Decong Zheng. 2024. "Nutritional Quality Analysis and Classification Detection of Buckwheat in Different Harvest Periods" Foods 13, no. 16: 2576. https://doi.org/10.3390/foods13162576




