Olive Oil (Royal Cultivar) from Mill Obtained by Short Time Malaxation and Early Ripening Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Fruits and Olive Oil Mill Extraction
2.2. Olive Oil Quality Criteria and Photosynthetic Pigments
2.3. Fatty Acids
2.4. Phenolic Compounds
2.5. Volatile Compounds
2.6. Sensorial Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extraction Efficiency, Quality Criteria, and Pigment Composition
3.2. Fatty Acids
3.3. Phenolic Compounds
3.4. Volatile Compounds
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lama-Muñoz, A.; Contreras, M.d.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Content of phenolic compounds and mannitol in olive leaves extracts from six Spanish cultivars: Extraction with the Soxhlet method and pressurized liquids. Food Chem. 2020, 320, 126626. [Google Scholar] [CrossRef] [PubMed]
- Escuderos, M.E.; Sayago, A.; Morales, M.T.; Aparicio, R. Evaluation of α-tocopherol in virgin olive oil by a luminescent method. Grasas Aceites 2009, 60, 336–342. [Google Scholar] [CrossRef]
- Miho, H.; Moral, J.; Barranco, D.; Ledesma-Escobar, C.A.; Priego-Capote, F.; Díez, C.M. Influence of genetic and interannual factors on the phenolic profiles of virgin olive oils. Food Chem. 2021, 342, 128357. [Google Scholar] [CrossRef] [PubMed]
- EEC European Commission Regulation 432/2012, Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. 2012, L136, 1–40. Available online: http://data.europa.eu/eli/reg/2012/432/oj (accessed on 1 November 2023).
- Cevik, S.; Ozkan, G.; Kıralan, M. Optimization of malaxation process using major aroma compounds in virgin olive oil. Braz. Arc. Biol. Techn. 2016, 59, e16160356. [Google Scholar] [CrossRef]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Miho, H.; Muñoz, C.; Priego-Capote, F. Deciphering the influence of the cultivar on the phenolic content of virgin olive oil. J. Food Compos. Anal. 2024, 129, 106128. [Google Scholar] [CrossRef]
- Rivero-Pino, F. Oleocanthal—Characterization, production, safety, functionality and in vivo evidences. Food Chem. 2023, 425, 136504. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, C.; Domenici, V. Pigments in Extra-Virgin Olive Oils Produced in Tuscany (Italy) in Different Years. Foods 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Segura-Borrego, M.; Ríos-Reina, R.; Puentes-Campos, A.; Jiménez-Herrera, B.; Callejón, R.M. Influence of the Washing Process and the Time of Fruit Harvesting Throughout the Day on Quality and Chemosensory Profile of Organic Extra Virgin Olive Oils. Foods 2022, 11, 3004. [Google Scholar] [CrossRef] [PubMed]
- De Torres, A.; Espínola, F.; Moya, M.; Alcalá, S.; Vidal, A.M.; Castro, E. Assessment of phenolic compounds in virgin olive oil by response surface methodology with particular focus on flavonoids and lignans. LWT—Food Sci. Technol. 2018, 90, 22–30. [Google Scholar] [CrossRef]
- Romero, N.; Saavedra, J.; Tapia, F.; Sepúlveda, B.; Aparicio, R. Influence of agroclimatic parameters on phenolic and volatile compounds of Chilean virgin olive oils and characterization based on geographical origin, cultivar and ripening stage. J. Sci. Food Agric. 2015, 96, 583–592. [Google Scholar] [CrossRef]
- Clodoveo, M.L. Malaxation: Influence on virgin olive oil quality. Past, present and future—An overview. Trends Food Sci. Tech. 2012, 25, 13–23. [Google Scholar] [CrossRef]
- Veneziani, G.; Nucciarelli, D.; Taticchi, A.; Esposto, S.; Selvaggini, R.; Tomasone, R.; Pagano, M.; Servili, M. Application of Low Temperature during the Malaxation Phase of Virgin Olive Oil Mechanical Extraction Processes of Three Different Italian Cultivars. Foods 2021, 10, 1578. [Google Scholar] [CrossRef]
- Miho, H.; Moral, J.; López-González, M.A.; Díez, C.M.; Priego-Capote, F. The phenolic profile of virgin olive oil is influenced by malaxation conditions and determines the oxidative stability. Food Chem. 2020, 314, 126183. [Google Scholar] [CrossRef]
- Nardella, M.; Moscetti, R.; Bedini, G.; Bandiera, A.; Chakravartula, S.S.N.; Massantini, R. Impact of traditional and innovative malaxation techniques and technologies on nutritional and sensory quality of virgin olive oil—A review. Food Chem. Adv. 2023, 2, 100163. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Calcio Gaudino, E.; Binello, A.; Rego, D.; Pereira, M.; Martínez, M.; Cravotto, G. Combined Ultrasound and Pulsed Electric Fields in Continuous-flow Industrial Olive-Oil Production. Foods 2022, 11, 3419. [Google Scholar] [CrossRef]
- Espínola, F.; Moya, M.; Fernández, D.G.; Castro, E. Modelling of virgin olive oil extraction using response surface methodology. Int. J. Food Sci. Tech. 2011, 46, 2576–2583. [Google Scholar] [CrossRef]
- EEC European Commission Regulation 2095/2016, Amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and olive-residue Oil and on the Relevant Methods of Analysis. 2016, L326, 1–6. Available online: http://data.europa.eu/eli/reg_del/2016/2095/oj (accessed on 1 November 2023).
- Polari, J.J.; Garcí-Aguirre, D.; Olmo-García, L.; Carrasco-Pancorbo, A.; Wang, S.C. Impact of industrial hammer mill rotor speed on extraction efficiency and quality of extra virgin olive oil. Food Chem. 2018, 242, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Minguez-Mosquera, I.M.; Rejano-Navarro, L.; Gandul-Rojas, B.; Sanchez-Gómez, A.H.; Garrido-Fernandez, J. Color-pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc. 1991, 68, 332–336. [Google Scholar] [CrossRef]
- Vidal, A.M.; Alcalá, S.; de Torres, A.; Moya, M.; Espínola, F. Characterization of Olive Oils from Superintensive Crops with Different Ripening Degree, Irrigation Management, and Cultivar: (Arbequina, Koroneiki, and Arbosana). Eur. J. Lipid Sci. Technol. 2019, 121, 1800360. [Google Scholar] [CrossRef]
- IOC Determination of Biophenols in Olive Oils by HPLC. COI/T.20/Doc No 29/Rev 2. 2022. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/06/Doc.-No-29-REV-2_ENK.pdf (accessed on 1 November 2023).
- Vidal, A.M.; Alcalá, S.; de Torres, A.; Moya, M.; Espínola, F. Use of talc in oil mills: Influence on the quality and content of minor compounds in olive oils. LWT—Food Sci. Technol. 2018, 98, 31–38. [Google Scholar] [CrossRef]
- EN ISO/IEC 17025:2017; Code for General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneve, Switzerland, 2017.
- Espínola, F.; Vidal, A.M.; Espínola, J.M.; Moya, M. Processing Effect and Characterization of Olive Oils from Spanish Wild Olive Trees (Olea europaea var. sylvestris). Molecules 2021, 26, 1304. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.M.; Alcalá, S.; de Torres, A.; Moya, M.; Espínola, F. Centrifugation, storage, and filtration of olive oil in an oil mill: Effect on the quality and content of minority compounds. J. Food Qual. 2019, 2019, 7381761. [Google Scholar] [CrossRef]
- Benito, M.; Abenoza, M.; Oria, R.; Sánchez-Gimeno, A.C. Physico-chemical, nutritional and sensory characterization of Verdeña, Verdilla and Royal varieties olive oil. Riv. Ital. Delle Sostanze Gr. 2012, 89, 40–46. [Google Scholar]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Valli, E.; Bendini, A.; Gallina Toschi, T.; Simal-Gandara, J. Characterization of virgin olive oils produced with autochthonous Galician varieties. Food Chem. 2016, 212, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Zribi, A.; Gargouri, B.; Jabeur, H.; Rebaï, A.; Abdelhedi, R.; Bouaziz, M. Enrichment of pan-frying refined oils with olive leaf phenolic-rich extract to extend the usage life. Eur. J. Lipid Sci. Technol. 2013, 115, 1443–1453. [Google Scholar] [CrossRef]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of olive oil antioxidants between oil and water phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Masella, P.; Parenti, A.; Spugnoli, P.; Calamai, L. Influence of vertical centrifugation on extra virgin olive oil quality. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 1137–1140. [Google Scholar] [CrossRef]
- Ranalli, A.; Pollastri, L.; Contento, S.; Iannucci, E.; Lucera, L. Effect of olive paste kneading process time on the overall quality of virgin olive oil. Eur. J. Lipid Sci. Technol. 2003, 105, 57–67. [Google Scholar] [CrossRef]
- Vekiari, S.A.; Koutsaftakis, A. The effect of different processing stages of olive fruit on the extracted olive oil polyphenol content. Grasas Aceites 2002, 53, 304–308. [Google Scholar] [CrossRef]
- Ben Brahim, S.; Marrakchi, F.; Gargouri, B.; Bouaziz, M. Optimization of malaxing conditions using CaCO3 as a coadjuvant: A method to increase yield and quality of extra virgin olive oil cv. Chemlali. LWT—Food Sci. Technol. 2015, 63, 243–252. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T. Characterization of olive ripeness by green aroma compounds of virgin olive oil. J. Agric. Food Chem. 1998, 46, 1116–1122. [Google Scholar] [CrossRef]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.; Bejaoui, M.A.; Quintero-Flores, A.; Jiménez, A.; Beltrán, G. Biosynthesis of volatile compounds by hydroperoxide lyase enzymatic activity during virgin olive oil extraction process. Food Res. Int. 2018, 111, 220–228. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Hbaieb, R.H.; Kotti, F.; Mugnozza, G.S.; Gargouri, M. Mechanical strategies to increase nutritional and sensory quality of virgin olive oil by modulating the endogenous enzyme activities. Compr. Rev. Food Sci. Food Saf. 2014, 13, 135–154. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood Jr, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Inarejos-García, A.M.; Salvador, M.D.; Fregapane, G. Effect of malaxation conditions on phenol and volatile profiles in olive paste and the corresponding virgin olive oils (Olea europaea L. Cv. cornicabra). J. Agric. Food Chem. 2009, 57, 3587–3595. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Gómez-Rico, A.; Salvador, M.D.; Fregapane, G. Influence of malaxation conditions on virgin olive oil yield, overall quality and composition. Eur. Food Res. Tech. 2009, 228, 671–677. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Influence of malaxation temperature and time on the quality of virgin olive oils. Food Chem. 2001, 72, 19–28. [Google Scholar] [CrossRef]
- Youssef, O.; Mokhtar, G.; Abdelly, C.; Mohamed, S.N.; Mokhtar, Z.; Guido, F. Changes in volatile compounds and oil quality with malaxation time of Tunisian cultivars of Olea europea. Int. J. Food Sci. Technol. 2013, 48, 74–81. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Improvements in the malaxation process to enhance the aroma quality of extra virgin olive oils. Food Chem. 2014, 158, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rico, A.; Fregapane, G.; Salvador, M.D. Effect of cultivar and ripening on minor components in Spanish olive fruits and their corresponding virgin olive oils. Food Res. Int. 2008, 41, 433–440. [Google Scholar] [CrossRef]
- Aparicio, R.; Luna, G. Characterisation of monovarietal virgin olive oils. Eur. J. Lipid Sci. Technol. 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Delahunty, C.M.; Eyres, G.; Dufour, J. Gas chromatography-olfactometry. J. Sep. Sci. 2006, 29, 2107–2125. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Fregapane, G.; Salvador, M.D.; Simal-Gándara, J. Characterisation of extra virgin olive oils from Galician autochthonous varieties and their co-crushings with Arbequina and Picual cv. Food Chem. 2015, 176, 493–503. [Google Scholar] [CrossRef]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterisation of 39 varietal virgin olive oils by their volatile compositions. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Virgin olive oil odour notes: Their relationships with volatile compounds from the lipoxygenase pathway and secoiridoid compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Reiners, J.; Grosch, W. Odorants of Virgin Olive Oils with Different Flavor Profiles. J. Agric. Food Chem. 1998, 46, 2754–2763. [Google Scholar] [CrossRef]
| Olive Characteristics * | ||||||
|---|---|---|---|---|---|---|
| Trial | Time, min | Temperature, °C | Maturity Index | Oil, % | Moisture, % | Solids, % |
| 1 | 15 | 32.6 ± 1.3 | 1.07 ± 0.01 | 14.33 ± 0.07 a | 60.06 ± 0.35 | 25.61 ± 0.42 |
| 2 | 30 | 31.6 ± 1.2 | 1.05 ± 0.02 | 13.52 ± 0.12 b | 60.52 ± 0.50 | 25.96 ± 0.40 |
| Trial 1 (15 min) | Trial 2 (30 min) | p-Value | Fisher’s LSD | |||
|---|---|---|---|---|---|---|
| Extraction efficiency (%) | 73.13 ± 0.28 a | 74.98 ± 0.31 b | 0.0016 | 0.67 | ||
| Oils | Decanter | Centrifuge | Decanter | Centrifuge | ||
| Acidity (%) | 0.31 ± 0.01 a | 0.30 ± 0.01 a | 0.28 ± 0.01 b | 0.24 ± 0.01 c | 0.0001 | 0.02 |
| Peroxides (mEq O2/kg) | 3.71 ± 0.20 a | 4.44 ± 0.24 b | 2.96 ± 0.12 c | 3.10 ± 0.14 c | 0.0000 | 0.35 |
| K232 | 1.70 ± 0.07 a | 1.57 ± 0.07 b | 1.73 ± 0.02 a | 1.58 ± 0.03 b | 0.0113 | 0.10 |
| K270 | 0.17 ± 0.02 a | 0.16 ± 0.03 a | 0.15 ± 0.02 a | 0.15 ± 0.02 a | 0.8711 | |
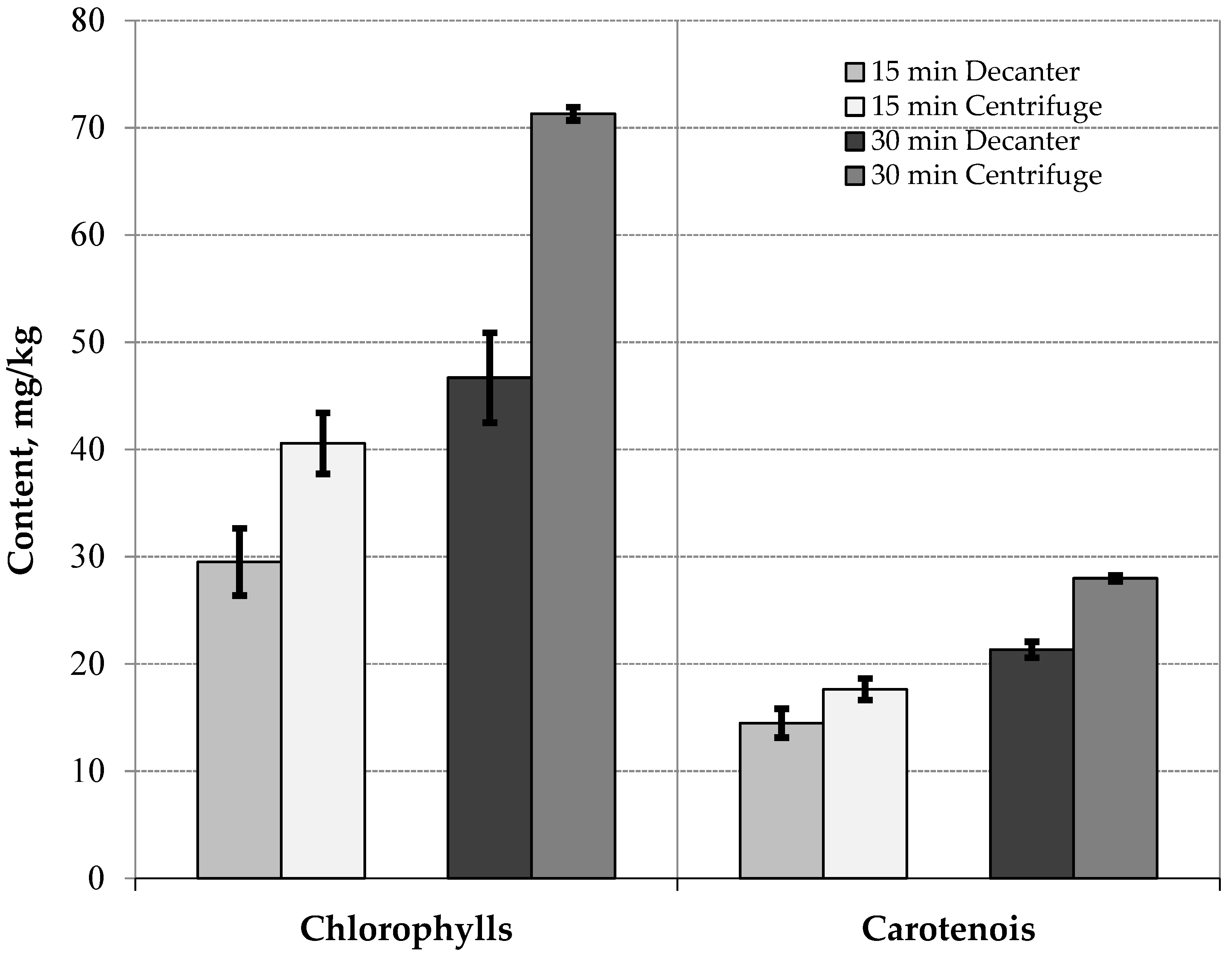
| Chlorophylls (mg/kg) | 29.51 ± 3.13 a | 40.57 ± 2.85 b | 46.70 ± 4.19 c | 71.31 ± 0.62 d | 0.0000 | 5.63 |
| Carotenoids (mg/kg) | 14.47 ± 1.35 a | 17.63 ± 1.01 b | 21.32 ± 0.74 c | 27.98 ± 0.27 d | 0.0000 | 1.76 |
| Trial 1 (15 min) | Trial 2 (30 min) | p-Value | Fisher’s LSD | |||
|---|---|---|---|---|---|---|
| Decanter | Centrifuge | Decanter | Centrifuge | |||
| Palmitic acid C16:0 | 14.40 ± 0.19 a | 14.47 ± 0.03 a | 14.16 ± 0.02 b | 14.28 ± 0.16 a,b | 0.0667 | 0.24 |
| Palmitoleic acid C16:1 | 1.27 ± 0.03 a,b | 1.29 ± 0.00 b | 1.23 ± 0.00 a | 1.25 ± 0.03 a,b | 0.0833 | 0.04 |
| Heptadecanoic acid C17:0 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | ||
| Heptadecenoic acid C17:1 | 0.09 ± 0.00 | 0.09 ± 0.00 | 0.09 ± 0.00 | 0.09 ± 0.00 | ||
| Stearic acid C18:0 | 1.41 ± 0.02 a,b | 1.43 ± 0.00 b | 1.39 ± 0.00 a | 1.40 ± 0.02 a,b | 0.0598 | 0.03 |
| Oleic acid C18:1 | 73.97 ± 1.68 a,b | 73.20 ± 0.21 a | 75.77 ± 0.06 b | 74.92 ± 1.18 a,b | 0.0690 | 1.95 |
| Linoleic acid C18:2 | 8.88 ± 0.06 a | 8.72 ± 0.19 a | 6.56 ± 0.05 b | 6.68 ± 0.03 b | 0.0000 | 0.26 |
| Linolenic acid C18:3 | 0.26 ± 0.00 | 0.26 ± 0.00 | 0.26 ± 0.00 | 0.26 ± 0.00 | ||
| Arachidic acid C20:0 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.05 ± 0.00 | 0.05 ± 0.01 | ||
| Eicosenoic acid C20:1 | 0.32 ± 0.00 | 0.32 ± 0.00 | 0.32 ± 0.00 | 0.32 ± 0.00 | ||
| Behenic acid C22:0 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.00 | ||
| Lignoceric acid C24:0 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | ||
| SFA | 16.03 ± 0.21 a | 16.12 ± 0.03 a | 15.77 ± 0.02 b | 15.91 ± 0.17 a,b | 0.0632 | 0.26 |
| MUFA | 75.65 ± 1.65 a,b | 74.90 ± 0.21 a | 77.42 ± 0.06 b | 76.59 ± 1.15 a,b | 0.0688 | 1.90 |
| PUFA | 9.15 ± 0.04 a | 9.09 ± 0.19 a | 6.82 ± 0.05 b | 6.94 ± 0.02 b | 0.0000 | 0.33 |
| C18:1/C18:2 | 8.33 ± 0.19 a | 8.40 ± 0.21 a | 11.56 ± 0.10 b | 11.22 ± 0.18 c | 0.0000 | 0.33 |
| MUFA/PUFA | 8.27 ± 0.19 a | 8.34 ± 0.20 a | 11.36 ± 0.10 b | 11.03 ± 0.17 c | 0.0000 | 0.32 |
| Trial 1 (15 min) | Trial 2 (30 min) | p-Value | Fisher’s LSD | |||
|---|---|---|---|---|---|---|
| Decanter | Centrifuge | Decanter | Centrifuge | |||
| Phenolic alcohols | ||||||
| Hydroxytyrosol | 1.34 ± 0.57 a | 1.16 ± 0.18 a,b | 0.65 ± 0.14 a,b | 0.55 ± 0.17 b | 0.0963 | 0.75 |
| Tyrosol | 2.08 ± 0.17 a | 1.25 ± 0.18 b | 2.39 ± 0.15 c | 1.45 ± 0.06 b | 0.0002 | 0.34 |
| Phenolic acids | ||||||
| Vainillinc acid | 0.86 ± 0.09 a | 0.48 ± 0.09 b | 0.81 ± 0.06 a | 0.53 ± 0.09 b | 0.0019 | 0.15 |
| Vainillin | 1.80 ± 0.05 a | 1.31 ± 0.14 b | 2.07 ± 0.15 c | -- | 0.0000 | 0.24 |
| p-Coumaric acid | 1.51 ± 0.36 a | 2.44 ± 1.22 a,b | 6.94 ± 0.02 c | 4.20 ± 0.76 b | 0.0067 | 2.06 |
| Ferulic acid | 6.78 ± 0.11 a | 1.87 ± 0.14 b | 8.40 ± 0.37 c | 5.79 ± 0.46 d | 0.0000 | 0.83 |
| Secoiridoids | ||||||
| 3.4-DHPEA-EDA (oleacein) | 20.48 ± 2.88 a | 25.61 ± 1.05 a | 53.33 ± 1.33 b | 48.42 ± 7.01 b | 0.0004 | 8.22 |
| 3.4-DHPEA-EA | 17.80 ± 0.68 a | 16.37 ± 0.47 a | 21.38 ± 2.02 b | 21.06 ± 0.13 b | 0.0079 | 2.75 |
| p-HPEA-EDA (oleocanthal) | 18.45 ± 0.92 a | 17.08 ± 2.81 a | 28.71 ± 1.64 b | 24.48 ± 3.18 b | 0.0033 | 5.17 |
| p-HPEA-EA | 17.96 ± 2.25 a | 17.37 ± 2.40 a | 16.63 ± 0.59 a | 15.80 ± 2.51 a | 0.6787 | |
| Lignans | ||||||
| Pinoresinol + Acetoxypinoresinol | 8.85 ± 0.72 a,b | 6.93 ± 0.65 a | 7.83 ± 0.83 a,b | 9.08 ± 0.90 b | 0.0910 | 1.80 |
| Flavones | ||||||
| Luteolin | 1.13 ± 0.56 a | 0.56 ± 0.23 a | 1.22 ± 0.32 a | 1.77 ± 0.85 a | 0.1341 | |
| Apigenin | 8.01 ± 0.65 a | 4.40 ± 0.07 b | 5.45 ± 0.37 b | 7.46 ± 0.41 a | 0.0009 | 1.09 |
| Total HPLC Phenols | 105.54 ± 2.85 a | 96.99 ± 6.18 b | 156.38 ± 1.12 c | 138.86 ± 4.60 d | 0.0000 | 7.80 |
| Trial 1 (15 min) | Trial 2 (30 min) | p-Value | Fisher’s LSD | |||
|---|---|---|---|---|---|---|
| Decanter | Centrifuge | Decanter | Centrifuge | |||
| LOX pathway | ||||||
| Hexanal | 2.00 ± 0.18 a | 1.85 ± 0.04 a,b | 1.75 ± 0.09 b | 1.69 ± 0.08 b | 0.0397 | 0.21 |
| Hexan-1-ol | 1.28 ± 0.05 a | 1.31 ± 0.06 a | 1.03 ± 0.05 b | 1.08 ± 0.07 b | 0.0008 | 0.11 |
| (E)-2-Hexenal | 5.03 ± 0.24 | 5.12 ± 0.40 | 4.63 ± 0.29 | 4.94 ± 0.09 | 0.3357 | |
| (E)-2-Hexen-1-ol | 1.36 ± 0.11 | 1.32 ± 0.12 | 1.40 ± 0.03 | 1.37 ± 0.08 | 0.7338 | |
| (Z)-3-Hexen-1-ol | 2.85 ± 0.09 a | 2.97 ± 0.16 a | 2.50 ± 0.24 b | 2.94 ± 0.12 a | 0.0285 | 0.31 |
| (Z)-3-Hexenyl acetate | 1.69 ± 0.07 a | 1.90 ± 0.16 b | 1.48 ± 0.08 c | 1.44 ± 0.04 c | 0.0044 | 0.22 |
| 1-Penten-3-ol | 1.16 ± 0.20 | 1.08 ± 0.18 | 1.09 ± 0.13 | 1.11 ± 0.05 | 0.9190 | |
| 1-Penten-3-one | 2.27 ± 0.09 a,b | 2.28 ± 0.11 a,b | 2.03 ± 0.24 a | 2.38 ± 0.01 b | 0.0793 | 0.27 |
| (Z)-2-Penten-1-ol | 1.08 ± 0.07 a,b | 1.15 ± 0.05 b | 0.97 ± 0.12 a | 1.22 ± 0.03 b | 0.0234 | 0.15 |
| Total LOX, mg/kg | 18.72 ± 0.64 a | 18.98 ± 0.58 a | 16.90 ± 0.88 b | 18.16 ± 0.47 a | 0.0197 | 1.24 |
| Other compounds | ||||||
| Acetic acid | 0.65 ± 0.07 a | 1.03 ± 0.14 a,b | 1.00 ± 0.19 a,b | 0.96 ± 0.03 b | 0.1379 | 0.32 |
| (E)-2-Pentenal | 0.78 ± 0.05 a,b | 0.80 ± 0.04 a,b | 0.73 ± 0.09 a | 0.85 ± 0.04 b | 0.1919 | 0.11 |
| Pentan-3-one | 0.47 ± 0.04 | 0.47 ± 0.04 | 0.46 ± 0.06 | 0.53 ± 0.02 | 0.3032 | |
| Octanal | 0.75 ± 0.08 | 0.75 ± 0.07 | 0.73 ± 0.07 | 0.78 ± 0.07 | 0.9009 | |
| Trial 1 (15 min) | Trial 2 (30 min) | p-Value | Fisher’s LSD | |||
|---|---|---|---|---|---|---|
| Decanter | Centrifuge | Decanter | Centrifuge | |||
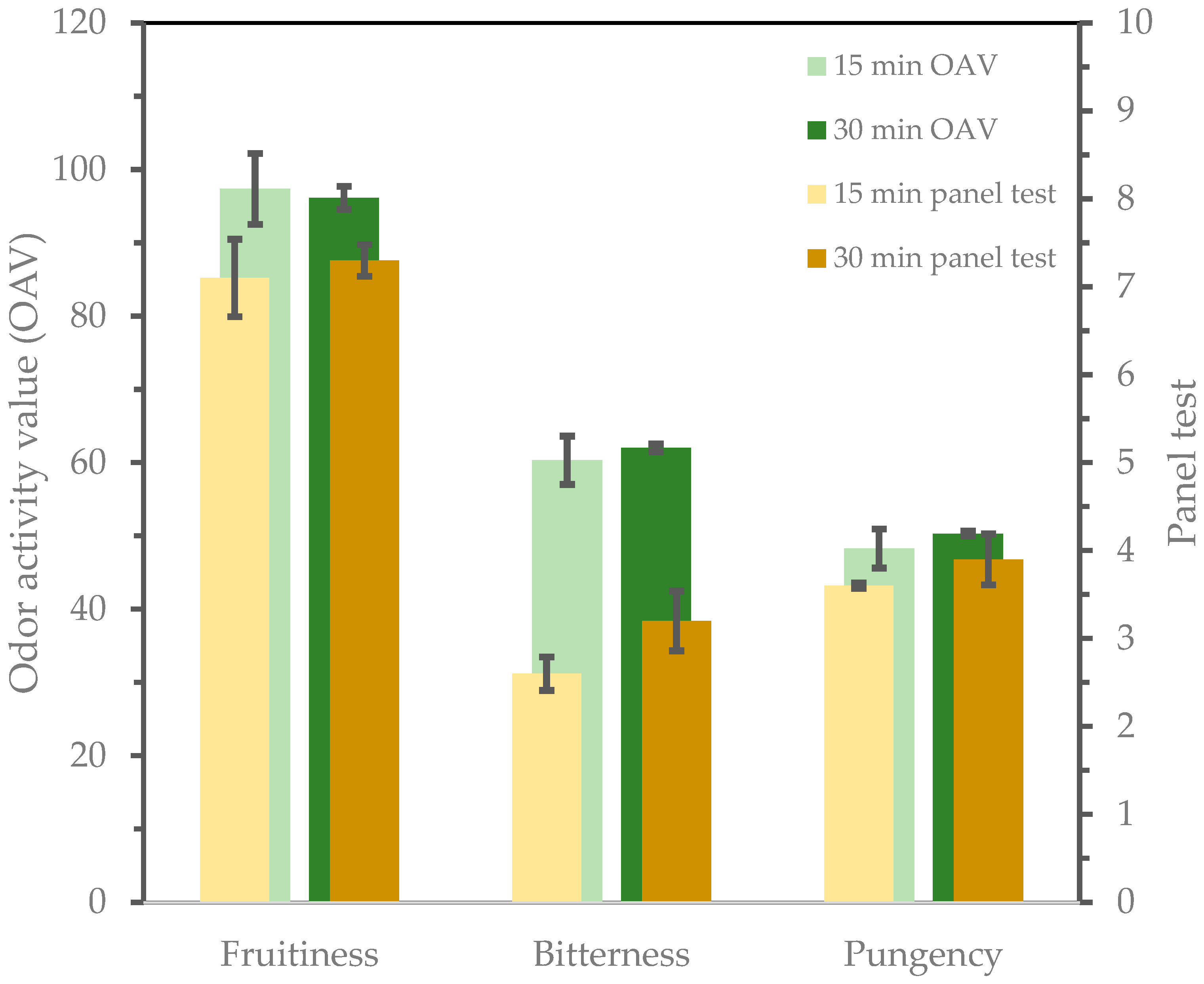
| Fruitiness | 6.0 ± 0.43 a | 7.1 ± 0.44 b | 6.5 ± 0.72 a,b | 7.3 ± 0.18 b | 0.0393 | 0.91 |
| Bitterness | 2.7 ± 0.27 a,b | 2.6 ± 0.19 a | 3.4 ± 0.32 c | 3.2 ± 0.34 b,c | 0.0243 | 0.54 |
| Pungency | 3.5 ± 0.20 a | 3.6 ± 0.03 a | 3.9 ± 0.24 a | 3.9 ± 0.29 a | 0.1091 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peralta, R.; Espínola, F.; Vidal, A.M.; Moya, M. Olive Oil (Royal Cultivar) from Mill Obtained by Short Time Malaxation and Early Ripening Stage. Foods 2024, 13, 2588. https://doi.org/10.3390/foods13162588
Peralta R, Espínola F, Vidal AM, Moya M. Olive Oil (Royal Cultivar) from Mill Obtained by Short Time Malaxation and Early Ripening Stage. Foods. 2024; 13(16):2588. https://doi.org/10.3390/foods13162588
Chicago/Turabian StylePeralta, Raúl, Francisco Espínola, Alfonso M. Vidal, and Manuel Moya. 2024. "Olive Oil (Royal Cultivar) from Mill Obtained by Short Time Malaxation and Early Ripening Stage" Foods 13, no. 16: 2588. https://doi.org/10.3390/foods13162588






