Influences of Depulping, Pod Storage and Fermentation Time on Fermentation Dynamics and Quality of Ghanaian Cocoa
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of Experiments
2.2. Pod Storage, Depulping, Fermentation, Drying and Sampling
2.3. Analyses during Fermentation and Drying, and of the Dried Cocoa Beans
2.3.1. Measurements of Pulp Content and pH of Cotyledon
2.3.2. Measurement of Temperatures in Fermentation Heaps
2.3.3. Sugar and Organic Acid Analysis by HPLC, during Fermentation and of the Dried Beans
2.3.4. Assessing the Viability of the Cocoa Embryo after Fermentation, and Cut-Tests of Dried Beans
2.3.5. Fermentation Index (FI) of the Dried Beans
2.4. Production and Analyses of Cocoa Liquor
2.4.1. Production of Cocoa Liquor
2.4.2. Sensory Analysis
2.4.3. Analysis of Selected Key-Aroma Compounds and Tastants in the Cocoa Liquor
Chemicals and Reagents
- Chemicals used for sample preparation, extraction and quantitation of selected polyphenols and alkaloids:
- Chemicals used for sample preparation, extraction and quantitation of selected aroma compounds:
Measurement of Selected Key Tastants
- Extraction for analysis of selected polyphenols and alkaloids
- Quantitation of selected polyphenols and alkaloids by HPLC-UV-MS
Measurement of Selected Key Aroma Compounds
- Sample Work-Up
- Quantitation of odorants by Gas Chromatography–Mass Spectrometry (GC-MS)
2.5. Statistics
3. Results
3.1. Post-Harvesting Process Monitoring and Quality of Dried Cocoa Beans
3.1.1. Pulp Content
3.1.2. Cotyledon pH during Fermentation and in Dried Beans
3.1.3. Temperature Development in Fermentation Heaps
3.1.4. Sugars and Organic Acids during Fermentation and in the Dried Beans
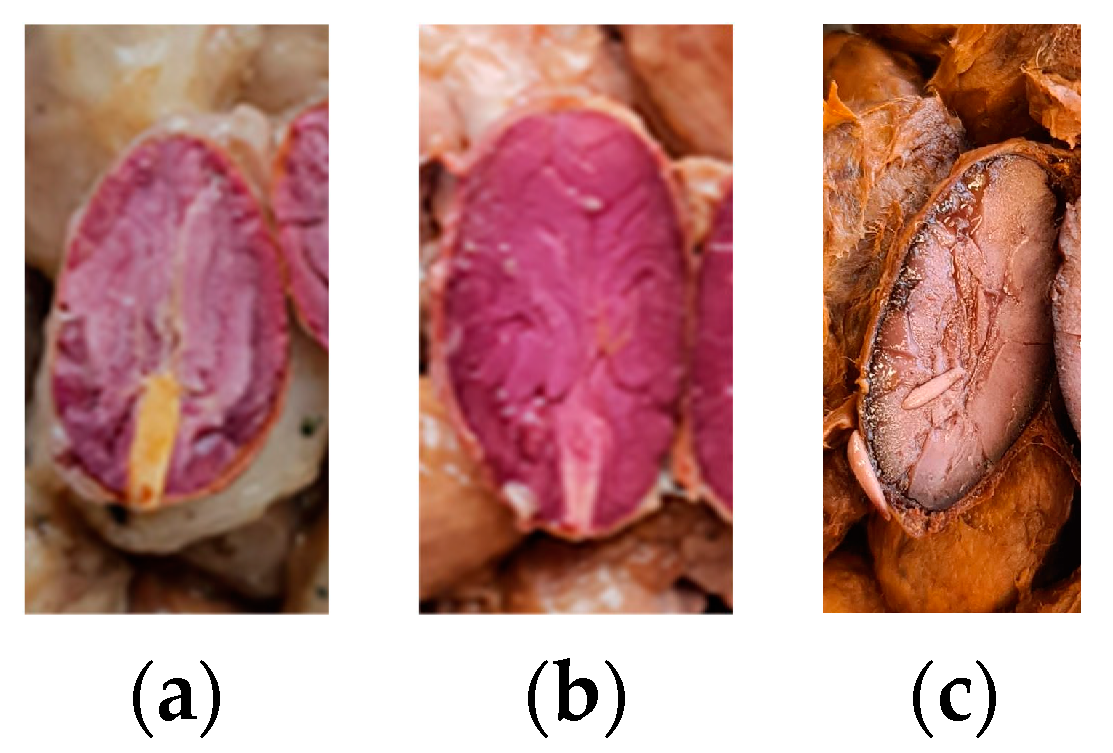
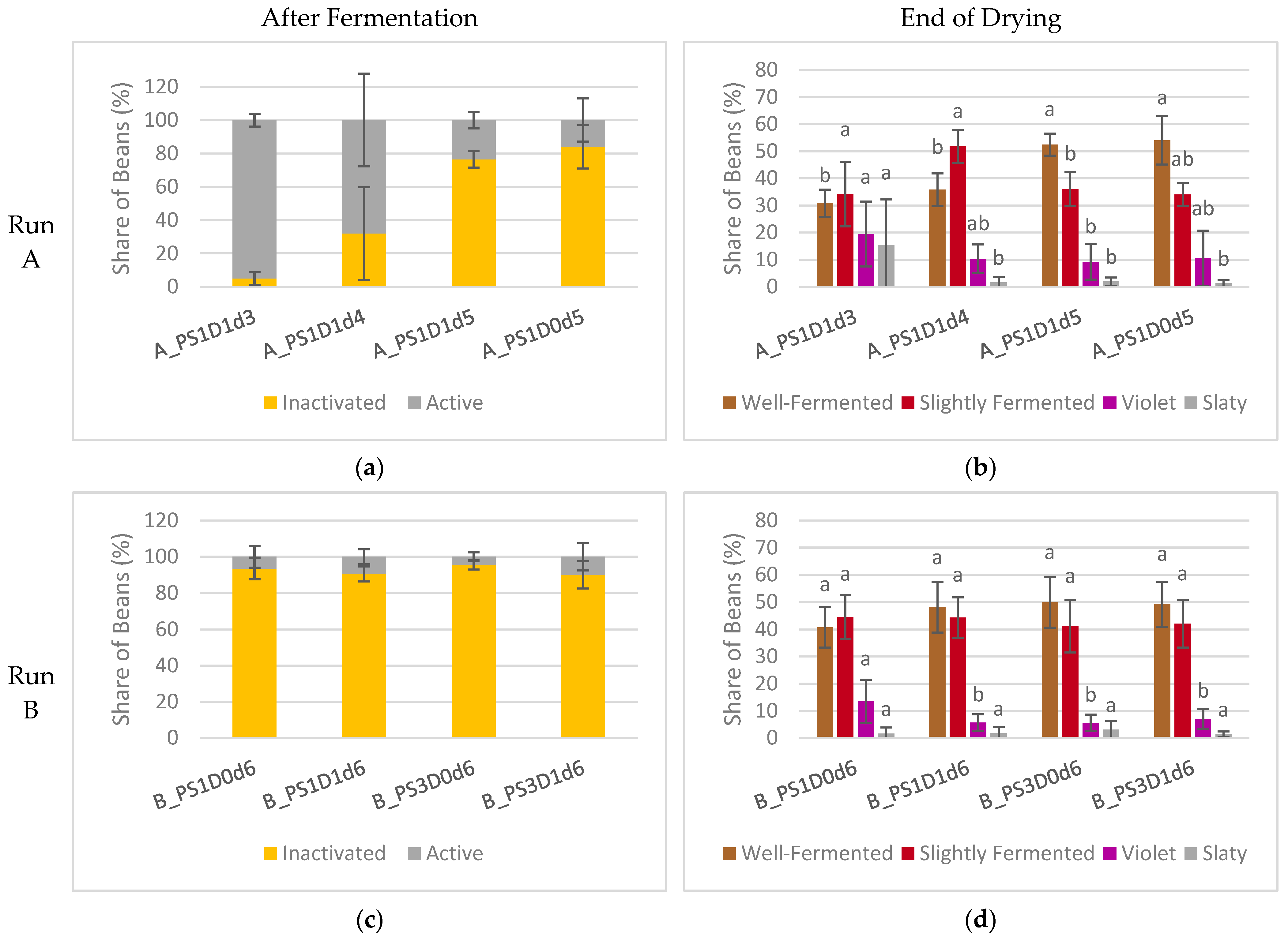
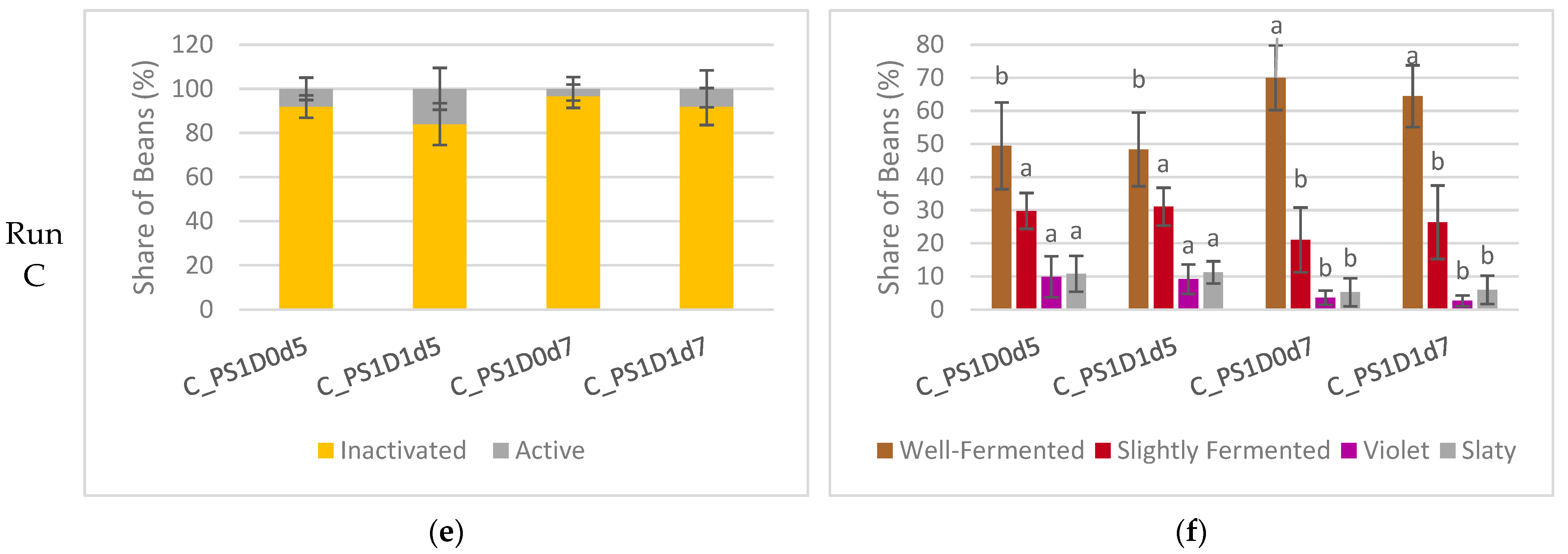
3.1.5. Viability of Cocoa Embryo after Fermentation and Cut-Test of Dried Beans
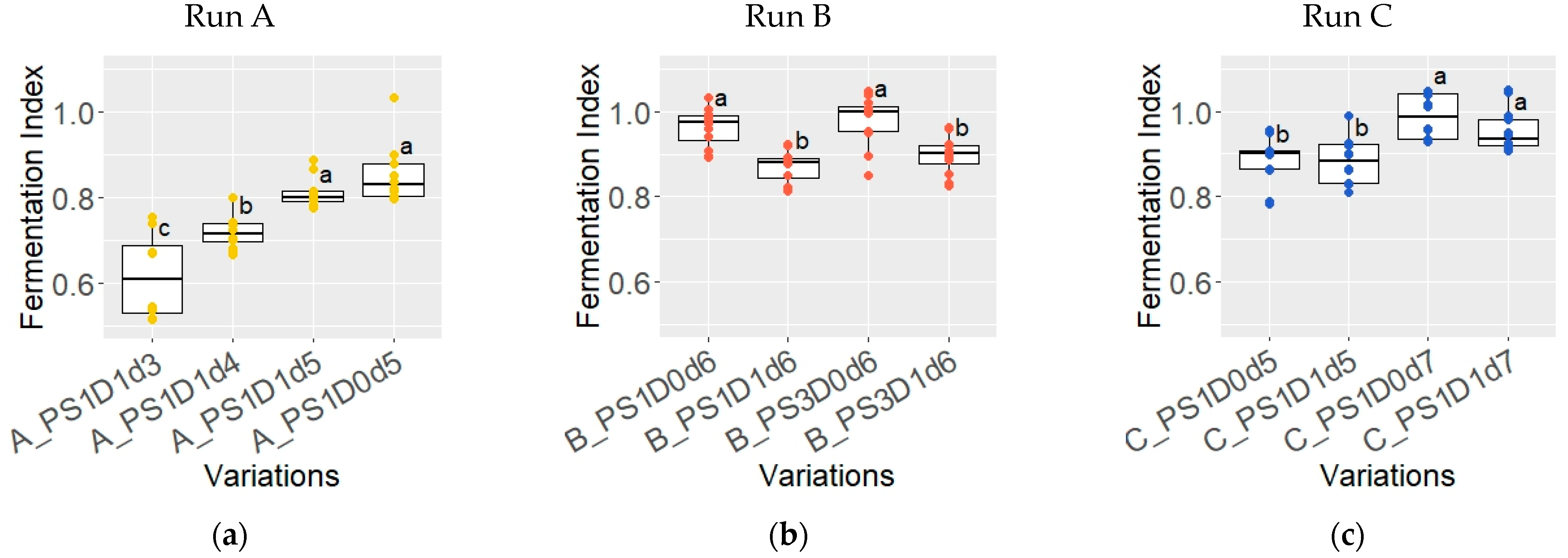
3.1.6. Fermentation Index of Dried Beans
3.2. Results of Cocoa Liquors Analyses
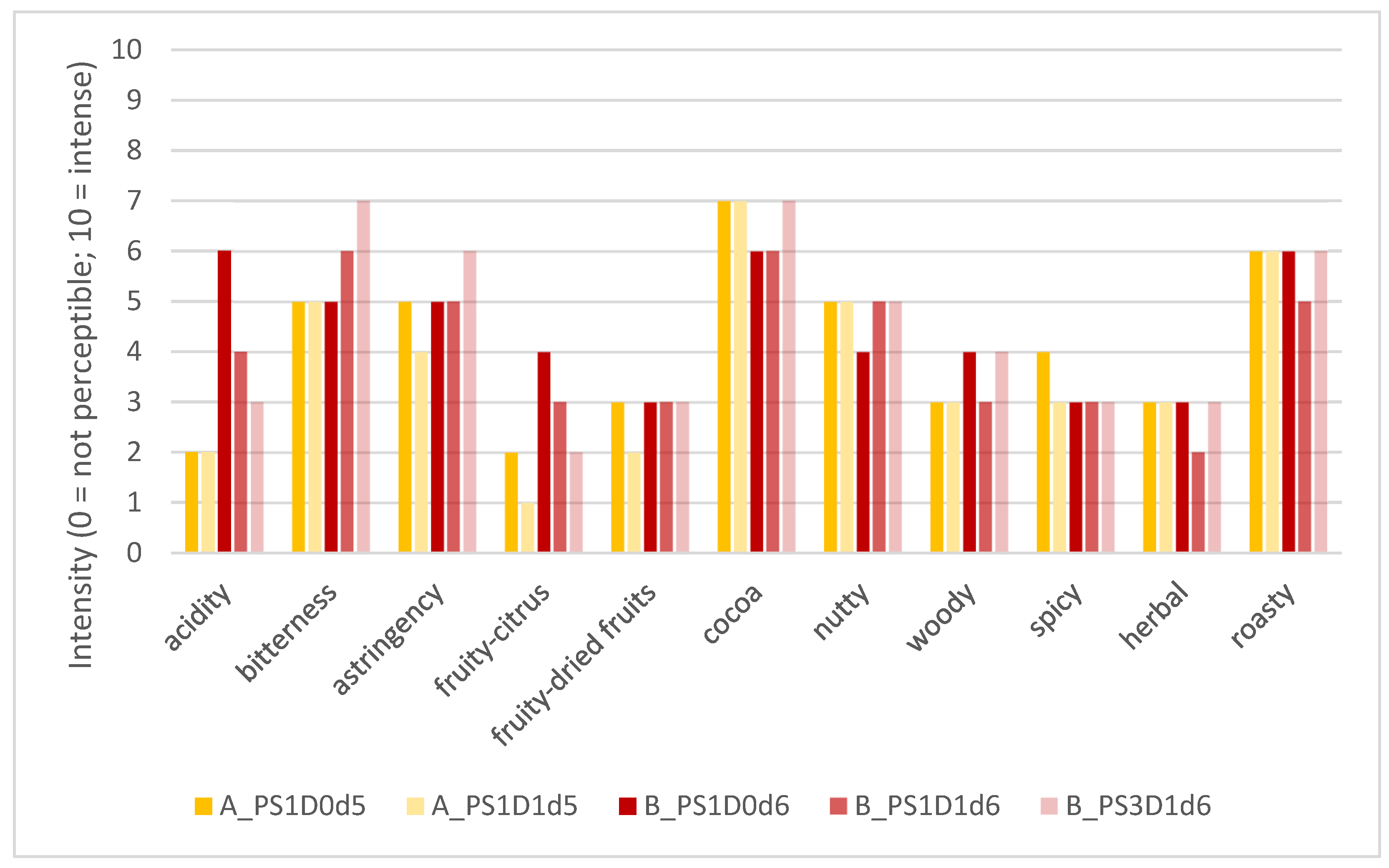
3.2.1. Sensory Evaluation
3.2.2. Chemical Analyses of Cocoa Liquor
Quantification of Selected Key Cocoa Tastants
Selected Key Aroma Compounds
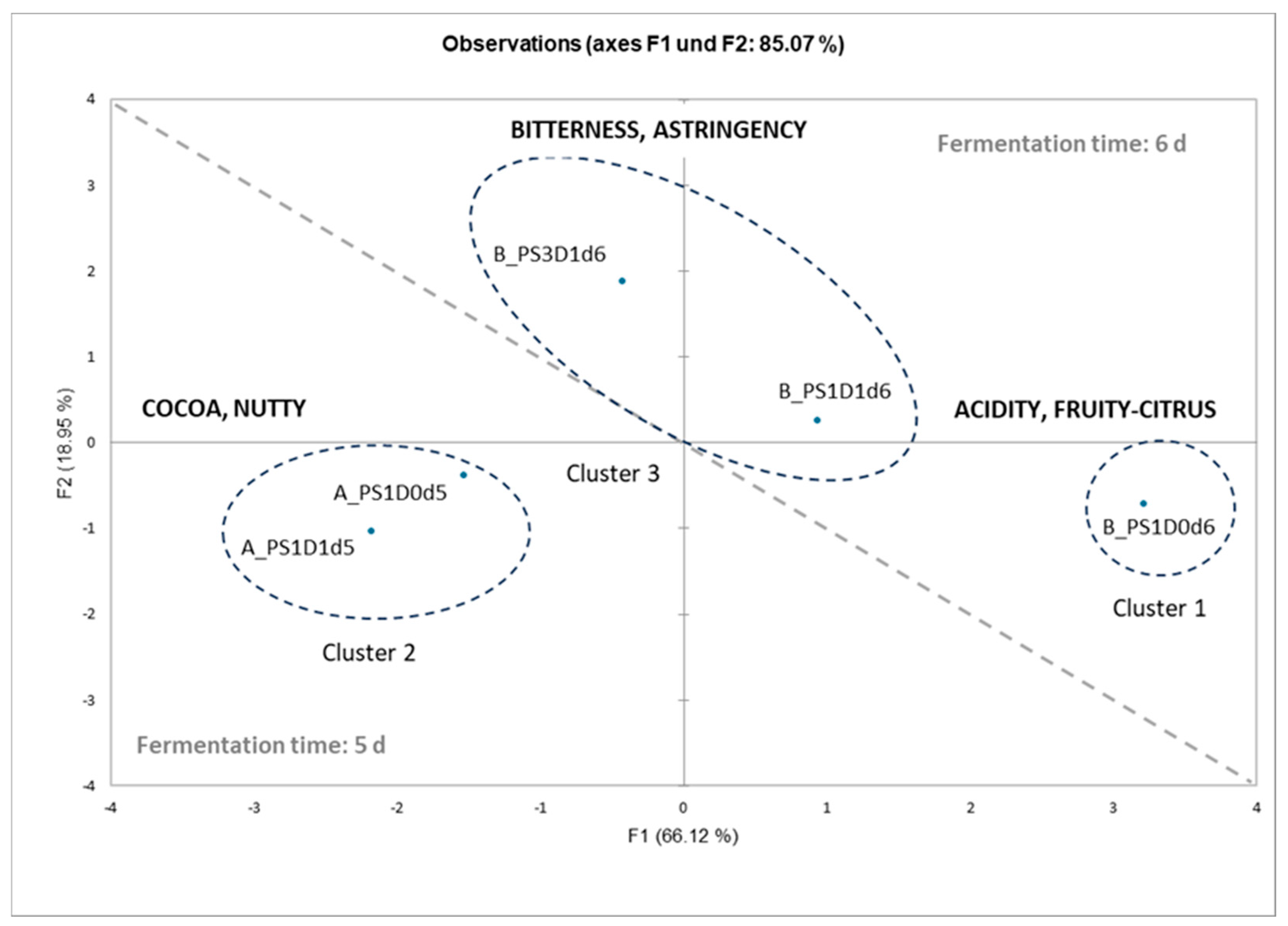
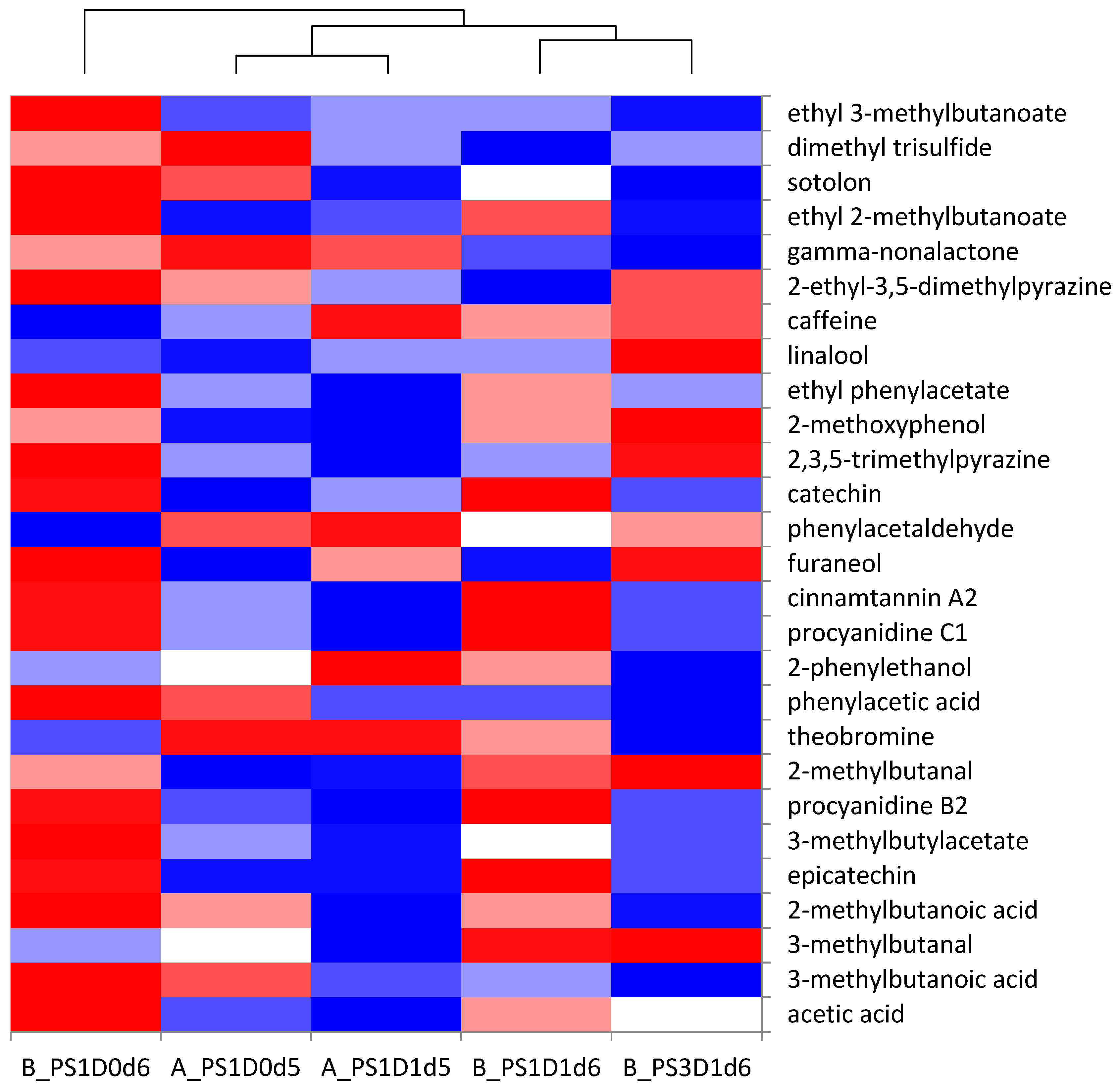
3.3. Multivariate Analyses
3.3.1. Effects of Post-Harvesting Parameters on Selected Quality Parameters of Dried Beans
3.3.2. Principal Component Analysis for Sensory Analysis and Heat-Map Clustering for Chemical Analyses
4. Discussion
4.1. Influences of Depulping on Fermentation Dynamics and the Quality of the Dried Beans and the Cocoa Liquor
4.2. Influences of Pod Storage on Fermentation Dynamics and the Quality of Dried Beans and Cocoa Liquor
4.3. Influence of Fermentation Time on the Quality of Dried Beans and Cocoa Liquor
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICCO. Quarterly Bulletin of Cocoa Statistics 22–23 Cocoa Year; International Cocoa Organization: Abidjan, Côte d’Ivoire, 2024. [Google Scholar]
- Anim-Kwapong, G.J.; Frimpong, E.B. Vulnerability and Adaptation Assessment under the Netherlands Climate Change Studies Assistance Programme Phase 2 (NCCSAP2): Vulnerability of Agriculture to Climate Change-Impact of Climate Change on Cocoa Production; Cocoa Research Institute of Ghana: New Tafo-Akim, Ghana, 2005. [Google Scholar]
- COCOBOD Ghana Cocoa Board. Available online: https://cocobod.gh/cocoa-story (accessed on 24 April 2024).
- Asare, R.; Asare, R.A.; Asante, W.A.; Markussen, B.; Ræbild, A. INFLUENCES OF SHADING AND FERTILIZATION ON ON-FARM YIELDS OF COCOA IN GHANA. Exp. Agric. 2017, 53, 416–431. [Google Scholar] [CrossRef]
- Hinneh, M.; Semanhyia, E.; Van de Walle, D.; De Winne, A.; Tzompa-Sosa, D.A.; Scalone, G.L.L.; De Meulenaer, B.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; et al. Assessing the Influence of Pod Storage on Sugar and Free Amino Acid Profiles and the Implications on Some Maillard Reaction Related Flavor Volatiles in Forastero Cocoa Beans. Food Res. Int. 2018, 111, 607–620. [Google Scholar] [CrossRef]
- Opoku-Ameyaw, K.; Baah, F.; Gyedu-Akoto, E.; Anchirinah, V.; Dzahini-Obiatey, H.K.; Cudjoe, A.R.; Opoku, S. Cocoa Manual: A Source Book for Sustainable Cocoa Production; Cocoa Research Institute of Ghana: New Tafo-Akim, Ghana, 2010. [Google Scholar]
- Kongor, J.E.; Takrama, J.F.; Budu, A.S.; Mensah-Brown, H.; Afoakwa, O. Effects of Fermentation and Drying on the Fermentation Index and Cut Test of Pulp Pre-Conditioned Ghanaian Cocoa (Theobroma cacao) Beans. J. Food Sci. Eng. 2013, 3, 625–634. [Google Scholar]
- Afoakwa, E.O.; Jennifer, Q.; Agnes, S.B.; Jemmy, S.T.; Saalia, F. Influence of Pulp-Preconditioning and Fermentation on Fermentative Quality and Appearance of Ghanaian Cocoa (Theobroma cacao) Beans.Pdf. Int. Food Res. J. 2012, 19, 127–133. [Google Scholar]
- Streule, S.; Freimüller Leischtfeld, S.; Chatelain, K.; Miescher Schwenninger, S. Effect of Pod Storage and Drying Temperature on Fermentation Dynamics and Final Bean Quality of Cacao Nacional in Ecuador. Foods 2024, 13, 1536. [Google Scholar] [CrossRef] [PubMed]
- Bariah, S.K.; Tajul, A.Y. Effect of Cocoa Pods Storage on the Temperature and Physicochemical Changes during Shallow Box Fermentation. IJISET-Int. J. Innov. Sci. Eng. Technol. 2017, 4, 197–203. [Google Scholar]
- Meyer, B.; Biehl, B.; Said, M.B.; Samarakoddy, R.J. Post-harvest Pod Storage: A Method for Pulp Preconditioning to Impair Strong Nib Acidification during Cocoa Fermentation in Malaysia. J. Sci. Food Agric. 1989, 48, 285–304. [Google Scholar] [CrossRef]
- Biehl, B.; Meyer, B.; Crone, G.; Pollmann, L.; Said, M.B. Chemical and Physical Changes in the Pulp during Ripening and Post-harvest Storage of Cocoa Pods. J. Sci. Food Agric. 1989, 48, 189–208. [Google Scholar] [CrossRef]
- Afoakwa, E.O. Cocoa Production and Processing Technology, 1st ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2014. [Google Scholar]
- Ghana Cocoa Board. Manual for Cocoa Extension in Ghana. CCAFS manual. Ghana Cocoa Board (COCO-BOD). 2018. Available online: https://ccafs.cgiar.org/resources/publications/manual-cocoa-extension-ghana (accessed on 7 June 2024).
- Adams, F.; Mensah, J.O.; Etuah, S.; Appiah, G.B.; Mensah, A.; Aidoo, R.; Quaye, J.; Ofori, K. Compliance with Cocoa Quality Management Practices in Ghana; To What Extent Are Cocoa Farmers Complying? Heliyon 2024, 10, e23863. [Google Scholar] [CrossRef]
- Amoah, A.A.; Maalekuu, B.K.; Takrama, J.; Asamoah, M.; Appiah, F. Effect of Method and Duration of Fermentation on the Quality of Dried Cocoa Beans. Ghana J. Hortic. JHORT 2023, 16, 31–41. [Google Scholar]
- Streule, S.; Freimüller Leischtfeld, S.; Galler, M.; Motzer, D.; Poulose-Züst, M.; Miescher Schwenninger, S. Variations in Ecuadorian Cocoa Fermentation and Drying at Two Locations: Implications for Quality and Sensory. Foods 2023, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Hamdouche, Y.; Meile, J.C.; Lebrun, M.; Guehi, T.; Boulanger, R.; Teyssier, C.; Montet, D. Impact of Turning, Pod Storage and Fermentation Time on Microbial Ecology and Volatile Composition of Cocoa Beans. Food Res. Int. 2019, 119, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Camu, N.; González, Á.; De Winter, T.; Van Schoor, A.; De Bruyne, K.; Vandamme, P.; Takrama, J.S.; Addo, S.K.; De Vuyst, L. Influence of Turning and Environmental Contamination on the Dynamics of Populations of Lactic Acid and Acetic Acid Bacteria Involved in Spontaneous Cocoa Bean Heap Fermentation in Ghana. Appl. Environ. Microbiol. 2008, 74, 86–98. [Google Scholar] [CrossRef]
- Guehi, T.S.; Dabonne, S.; Ban-Koffi, L.; Kedjebo, D.K.; Zahouli, I.B. Effect of Turning Beans and Fermentation Method on the Acidity and Physical Quality of Raw Cocoa Beans. Adv. J. Food Sci. Technol. 2010, 2, 163–171. [Google Scholar]
- Figueroa, K.H.N.; García, N.V.M.; Vega, R.C. Cocoa By-products. In Food Wastes and By-products; Campos-Vega, R., Oomah, B.D., Vergara-Castañeda, H.A., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 373–411. ISBN 978-1-119-53410-5. [Google Scholar]
- Vásquez, Z.S.; De Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; De Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological Approaches for Cocoa Waste Management: A Review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef]
- Bickel Haase, T.; Naumann-Gola, S.; Ortner, E.; Zorn, H.; Schweiggert-Weisz, U. Thermal Stabilisation of Cocoa Fruit Pulp—Effects on Sensory Properties, Colour and Microbiological Stability. Curr. Res. Food Sci. 2023, 7, 100549. [Google Scholar] [CrossRef] [PubMed]
- Afedzi, A.E.K.; Obeng-Boateng, F.; Aduama-Larbi, M.S.; Zhou, X.; Xu, Y. Valorization of Ghanaian Cocoa Processing Residues as Extractives for Value-Added Functional Food and Animal Feed Additives—A Review. Biocatal. Agric. Biotechnol. 2023, 52, 102835. [Google Scholar] [CrossRef]
- Schwan, R.F.; Wheals, A.E. The Microbiology of Cocoa Fermentation and Its Role in Chocolate Quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef]
- Ziegleder, G.; Biehl, B. Analysis of Cocoa Flavour Components and Flavour Precursors. In Analysis of Nonalcoholic Beverages; Linskens, H.-F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 321–393. ISBN 978-3-642-83343-4. [Google Scholar]
- Kadow, D.; Niemenak, N.; Rohn, S.; Lieberei, R. Fermentation-like Incubation of Cocoa Seeds (Theobroma cacao L.)—Reconstruction and Guidance of the Fermentation Process. LWT Food Sci. Technol. 2015, 62, 357–361. [Google Scholar] [CrossRef]
- Chetschik, I.; Kneubühl, M.; Chatelain, K.; Schlüter, A.; Bernath, K.; Hühn, T. Investigations on the Aroma of Cocoa Pulp (Theobroma cacao L.) and Its Influence on the Odor of Fermented Cocoa Beans. J. Agric. Food Chem. 2018, 66, 2467–2472. [Google Scholar] [CrossRef]
- Eskes, B.; Ahnert, D.; Carrion, L.G.; Seguine, E.; Assemat, S.; Guarda, D. Evidence on the Effect of the Cocoa Pulp Flavour Environment during Fermentation on the Flavour Profile of Chocolates. Available online: https://agritrop.cirad.fr/568108/ (accessed on 7 June 2024).
- Bangerter, U.; Beh, B.H.; Callis, A.B.; Pilkington, I.J. Treatment of Cocoa Beans for Improving Fermentation. U.S. Patent 5342632, 30 August 1994. [Google Scholar]
- Ho, V.T.T.; Zhao, J.; Fleet, G. Yeasts Are Essential for Cocoa Bean Fermentation. Int. J. Food Microbiol. 2014, 174, 72–87. [Google Scholar] [CrossRef]
- Wood, G.A.; Lass, R.A. Cocoa, 4th ed.; Blackwell: Oxford, UK, 2008; ISBN 978-0-632-06398-7. [Google Scholar]
- Haruna, L.; Abano, E.E.; Teye, E.; Tukwarlba, I.; Adu, S.; Agyei, K.J.; Kuma, E.; Yeboah, W.; Lukeman, M. Effect of Partial Pulp Removal and Fermentation Duration on Drying Behavior, Nib Acidification, Fermentation Quality, and Flavor Attributes of Ghanaian Cocoa Beans. J. Agric. Food Res. 2024, 17, 101211. [Google Scholar] [CrossRef]
- Pettipher, G.L. Analysis of Cocoa Pulp and the Formulation of a Standardised Artificial Cocoa Pulp Medium. J. Sci. Food Agric. 1986, 37, 297–309. [Google Scholar] [CrossRef]
- Mishra, K.; Green, A.; Burkard, J.; Gubler, I.; Borradori, R.; Kohler, L.; Meuli, J.; Krähenmann, U.; Bergfreund, J.; Siegrist, A.; et al. Valorization of Cocoa Pod Side Streams Improves Nutritional and Sustainability Aspects of Chocolate. Nat. Food 2024, 5, 423–432. [Google Scholar] [CrossRef]
- Ullrich, L.; Neiens, S.; Hühn, T.; Steinhaus, M.; Chetschik, I. Impact of Water on Odor-Active Compounds in Fermented and Dried Cocoa Beans and Chocolates Made Thereof. J. Agric. Food Chem. 2021, 69, 8504–8510. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, L.; Casty, B.; André, A.; Hühn, T.; Steinhaus, M.; Chetschik, I. Decoding the Fine Flavor Properties of Dark Chocolates. J. Agric. Food Chem. 2022, 70, 13730–13740. [Google Scholar] [CrossRef]
- Ullrich, L.; Casty, B.; André, A.; Hühn, T.; Chetschik, I.; Steinhaus, M. Influence of the Cocoa Bean Variety on the Flavor Compound Composition of Dark Chocolates. ACS Food Sci. Technol. 2023, 3, 470–477. [Google Scholar] [CrossRef]
- Romanens, E.; Näf, R.; Lobmaier, T.; Pedan, V.; Leischtfeld, S.F.; Meile, L.; Schwenninger, S.M. A Lab-Scale Model System for Cocoa Bean Fermentation. Appl. Microbiol. Biotechnol. 2018, 102, 3349–3362. [Google Scholar] [CrossRef]
- Romanens, E.; Pedan, V.; Meile, L.; Miescher Schwenninger, S. Influence of Two Anti-Fungal Lactobacillus Fermentum-Saccharomyces Cerevisiae Co-Cultures on Cocoa Bean Fermentation and Final Bean Quality. PLoS ONE 2020, 15, e0239365. [Google Scholar] [CrossRef] [PubMed]
- Streule, S.; Freimüller Leischtfeld, S.; Galler, M.; Miescher Schwenninger, S. Monitoring of Cocoa Post-Harvest Process Practices on a Small-Farm Level at Five Locations in Ecuador. Heliyon 2022, 8, e09628. [Google Scholar] [CrossRef]
- Gourieva, K.B.; Tserrevitinov, O.B. Method of Evaluating the Degree of Fermentation of Cocoa Beans 1979. U.S Patent 64654, 5 February 1979. [Google Scholar]
- DIN EN ISO 13299:2016; General Guidance for Establishing a Sensory Profile. International Organization for Standardization: Vernier, Switzerland, 2016.
- Schlüter, A.; André, A.; Hühn, T.; Rohn, S.; Chetschik, I. Influence of Aerobic and Anaerobic Moist Incubation on Selected Nonvolatile Constituents─Comparison to Traditionally Fermented Cocoa Beans. J. Agric. Food Chem. 2022, 70, 16335–16346. [Google Scholar] [CrossRef]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent Assisted Flavour Evaporation—A New and Versatile Technique for the Careful and Direct Isolation of Aroma Compounds from Complex Food Matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Chetschik, I.; Pedan, V.; Chatelain, K.; Kneubühl, M.; Hühn, T. Characterization of the Flavor Properties of Dark Chocolates Produced by a Novel Technological Approach and Comparison with Traditionally Produced Dark Chocolates. J. Agric. Food Chem. 2019, 67, 3991–4001. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor Formation and Character in Cocoa and Chocolate: A Critical Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef]
- Eskes, A.; Ahnert, D.; Assemat, S.; Seguine, E. Evidence for the Effect of the Cocoa Bean Flavour Environment during Fermentation on the Final Flavour Profile of Cocoa Liquor and Chocolate. ResearchGate 2009, 20, 1–6. Available online: https://www.researchgate.net/publication/228754936 (accessed on 7 June 2024).
- Rodriguez-Campos, J.; Escalona-Buendía, H.; Orozco, I.; Lugo, E.; Jaramillo-Flores, M. Dynamics of Volatile and Non-Volatile Compounds in Cocoa (Theobroma cacao L.) during Fermentation and Drying Processes Using Principal Components Analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Sarbu, I.; Csutak, O. 13—The Microbiology of Cocoa Fermentation. In Caffeinated and Cocoa Based Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 423–446. ISBN 978-0-12-815864-7. [Google Scholar]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products—An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Schlüter, A.; Hühn, T.; Kneubühl, M.; Chatelain, K.; Rohn, S.; Chetschik, I. Novel Time- and Location-Independent Postharvest Treatment of Cocoa Beans: Investigations on the Aroma Formation during “Moist Incubation” of Unfermented and Dried Cocoa Nibs and Comparison to Traditional Fermentation. J. Agric. Food Chem. 2020, 68, 10336–10344. [Google Scholar] [CrossRef]
- Schlüter, A.; Hühn, T.; Kneubühl, M.; Chatelain, K.; Rohn, S.; Chetschik, I. Comparison of the Aroma Composition and Sensory Properties of Dark Chocolates Made with Moist Incubated and Fermented Cocoa Beans. J. Agric. Food Chem. 2022, 70, 4057–4065. [Google Scholar] [CrossRef]
- Gutiérrez-Ríos, H.G.; Suárez-Quiroz, M.L.; Hernández-Estrada, Z.J.; Castellanos-Onorio, O.P.; Alonso-Villegas, R.; Rayas-Duarte, P.; Cano-Sarmiento, C.; Figueroa-Hernández, C.Y.; González-Rios, O. Yeasts as Producers of Flavor Precursors during Cocoa Bean Fermentation and Their Relevance as Starter Cultures: A Review. Fermentation 2022, 8, 331. [Google Scholar] [CrossRef]
- Ardhana, M.; Fleet, G.H. The Microbial Ecology of Cocoa Bean Fermentations in Indonesia. Int. J. Food Microbiol. 2003, 86, 87–99. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Ma, G.; Zhang, T.; Wang, L.; Pei, H.; Li, X.; Gao, L. Insights into Flavor and Key Influencing Factors of Maillard Reaction Products: A Recent Update. Front. Nutr. 2022, 9, 973677. [Google Scholar] [CrossRef]
- Wang, F.; Shen, H.; Liu, T.; Yang, X.; Yang, Y.; Guo, Y. Formation of Pyrazines in Maillard Model Systems: Effects of Structures of Lysine-Containing Dipeptides/Tripeptides. Foods 2021, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Andruszkiewicz, P.J.; D’Souza, R.N.; Altun, I.; Corno, M.; Kuhnert, N. Thermally-Induced Formation of Taste-Active 2,5-Diketopiperazines from Short-Chain Peptide Precursors in Cocoa. Food Res. Int. 2019, 121, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Kongor, J.; Takrama, J.; Budu, A.; Mensah-Brown, H. Effects of Pulp Preconditioning on Total Polyphenols, O-Diphenols and Anthocyanin Concentrations during Fermentation and Drying of Cocoa (Theobroma cacao) Beans. J. Food Sci. Eng. 2013, 3, 235–245. [Google Scholar]
- Wills, R.; McGlasson, B.; Graham, D.; Joyce, D. Postharvest: An Introduction to the Physiology & Handling of Fruit, Vegetables & Ornamentals; CAB INTERNATIONAL: Wallingford, CT, USA, 1998. [Google Scholar]
- Hinneh, M.; Abotsi, E.E.; Van de Walle, D.; Tzompa-Sosa, D.A.; De Winne, A.; Simonis, J.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; De Cooman, L.; et al. Pod Storage with Roasting: A Tool to Diversifying the Flavor Profiles of Dark Chocolates Produced from ‘Bulk’ Cocoa Beans? (Part I: Aroma Profiling of Chocolates). Food Res. Int. 2019, 119, 84–98. [Google Scholar] [CrossRef]
- De Vuyst, L.; Weckx, S. The Cocoa Bean Fermentation Process: From Ecosystem Analysis to Starter Culture Development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef]
- Calvo, A.M.; Botina, B.L.; García, M.C.; Cardona, W.A.; Montenegro, A.C.; Criollo, J. Dynamics of Cocoa Fermentation and Its Effect on Quality. Sci. Rep. 2021, 11, 16746. [Google Scholar] [CrossRef]
- Chávez, V.M.C.; Sopla, P.M.H.; Saldaña, V.V.; Guadalupe, G. Fermenter Type and Fermentation Time Effects in the Physicochemical and Organoleptic Characteristics of cocoa (Theobroma cacao L.). Soc. Sci. Learn. Educ. J. 2020, 5, 11. [Google Scholar]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Contreras-Ramos, S.M.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of Fermentation Time and Drying Temperature on Volatile Compounds in Cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef]
- Puerto, P.P.; Guerra, S.; Contreras, D. Changes in Physical and Chemical Characteristics of Fermented Cocoa (Theobroma cacao) Beans with Manual and Semi-Mechanized Transfer, between Fermentation Boxes. Sci. Agropecu. 2016, 07, 111–119. [Google Scholar] [CrossRef]
- Jinap, S.; Thien, J.; Yap, T.N. Effect of Drying on Acidity and Volatile Fatty Acids Content of Cocoa Beans. J. Sci. Food Agric. 1994, 65, 67–75. [Google Scholar] [CrossRef]
- Afoakwa, E.O. Chocolate Science and Technology; Wiley-Blackwell: Chichester, UK, 2010; ISBN 978-1-4051-9906-3. [Google Scholar]
- Schwan, R.F.; Pereira, G.V.d.M.; Fleet, G.H. Microbial Activities during Cocoa Fermentation. In Cocoa and Coffee Fermentations; Schwan, R.F., Fleet, G.H., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2014; pp. 129–192. [Google Scholar]
- Ackah, E.; Dompey, E. Effects of Fermentation and Drying Durations on the Quality of Cocoa (Theobroma cacao L.) Beans during the Rainy Season in the Juaboso District of the Western-North Region, Ghana. Bull. Natl. Res. Cent. 2021, 45, 175. [Google Scholar] [CrossRef]
- Gunam, I.B.W.; Suryanto, A.; Ganda Putra, G.P.; Kasa, I.W.; Permana, I.D.G.M.; Sarjana, P.; Putra, I.W.W.P.; Takama, T. The Effect of Mixed Inoculum Addition Concentration and Fermentation Time on the Characteristics of Dry Cocoa Beans (Theobroma cacao L.). Int. J. Adv. Sci. Eng. Inf. Technol. 2021, 11, 272–280. [Google Scholar] [CrossRef]
- Fowler, M.S. Cocoa Beans: From Tree to Factory. In Industrial Chocolate Manufacture and Use; Beckett, S.T., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 10–47. [Google Scholar]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]








| Experimental Run | Fermentation Variation | Total Repetitions 1 | Pod Storage (Days) | Depulping Process | Fermentation Time (Days) | Fermentation Time (h) |
|---|---|---|---|---|---|---|
| A | PS1D1d3 | n = 2 × 2 | 1 | with | 3 | 64 (run 1); 69 (run 2) |
| PS1D1d4 | n = 2 × 2 | 1 | with | 4 | 92 (run 1); 91 (run 2) | |
| PS1D1d5 | n = 2 × 2 | 1 | with | 5 | 116 (run 1); 115 (run 2) | |
| PS1D0d5 | n = 2 × 2 | 1 | without | 5 | ||
| B | PS1D0d6 | n = 2 × 2 | 1 | without | 6–6.5 | 156 (run 1); 147 (run 2) |
| PS1D1d6 | n = 2 × 2 | 1 | with | 6–6.5 | ||
| PS3D0d6 | n = 2 × 2 | 3 | without | 6–6.5 | ||
| PS3D1d6 | n = 2 × 2 | 3 | with | 6–6.5 | ||
| C | PS1D0d5 | n = 2 × 3 | 1 | without | 5 | 120 (run 1), 116 (run 2), 118 (run 3) |
| PS1D1d5 | n = 2 × 3 | 1 | with | 5 | ||
| PS1D0d7 | n = 2 × 3 | 1 | without | 7 | 169 (run 1), 169 (run 2), 165 (run 3) | |
| PS1D1d7 | n = 2 × 3 | 1 | with | 7 |
| Category | Attributes | Definition | Scale |
|---|---|---|---|
| Basic taste | Acidity | Basic taste, perception of the intensity of acidity like a solution of citric acid or acetic acid | Low-intense |
| Bitterness | Basic taste, perception of the intensity of bitterness like a caffeine solution | Low-intense | |
| Trigeminal sensation | Astringency | Dry and rough mouthfeel | Low-intense |
| Aroma (retronasal) | Fruity-citrus | Aroma perception, reminiscent of citrus fruits and bergamot | Low-intense |
| Fruity-dried fruits | Aroma perception, reminiscent of dried fruits, like raisins | Low-intense | |
| Cocoa | Aroma perception, reminiscent of cocoa powder | Low-intense | |
| Nutty | Aroma perception, reminiscent of ripe, unroasted nuts | Low-intense | |
| Woody | Aroma perception, reminiscent of dry wood | Low-intense | |
| Spicy | Aroma perception, reminiscent of vanilla or tobacco | Low-intense | |
| Herbal | Aroma perception, reminiscent of dried herbs, like vermouth | Low-intense | |
| Basic taste | Roasty | Aroma perception, reminiscent of roasted bread / toasted bread | Low-intense |
| Compound | Detection/Quantification | Linear Range (µg/mL) | Calibration Equation | R2 |
|---|---|---|---|---|
| Catechin | MS/EIC | 1–100 | y = 309,070x + 3 × 106 | 0.9923 |
| Epicatechin | MS/EIC | 1–100 | y = 293,036x + 3 × 106 | 0.9985 |
| Procyanidine B2 | MS/EIC | 1–25 | y = 247,405x + 212,827 | 0.9978 |
| Procyanidine C1 | MS/EIC | 1–25 | y = 93,519x + 16,054 | 0.9974 |
| Cinnamtannin A2 | MS/EIC | 1–100 | y = 21,900x + 15,453 | 0.9968 |
| Theobromine | UV/275 nm | 1–600 | y = 9.2645x + 56.775 | 0.9978 |
| Caffeine | UV/275 nm | 1–300 | y = 8.4225x − 0.5098 | 1.0000 |
| Variation | d0 | d1 | d2 | d3 | d4 | d5 | d6 | d7 1 | Dried | |
|---|---|---|---|---|---|---|---|---|---|---|
| Run A | PS1D1d3 | 6.57 ± 0.03 a | 6.44 ± 0.03 a | 6.33 ± 0.05 b | 6.10 ± 0.31 a | - | - | - | - | 5.87 ± 0.45 a |
| PS1D1d4 | 6.57 ± 0.03 a | 6.47 ± 0.02 a | 6.39 ± 0.03 b | 6.09 ± 0.22 a | 5.67 ± 0.55 a | - | - | - | 5.79 ± 0.16 a | |
| PS1D1d5 | 6.58 ± 0.02 a | 6.47 ± 0.05 a | 6.35 ± 0.04 b | 6.06 ± 0.20 a | 5.63 ± 0.50 a | 5.02 ± 0.32 a | - | - | 5.72 ± 0.32 a | |
| PS1D0d5 | 6.43 ± 0.19 a | 6.48 ± 0.03 a | 6.48 ± 0.04 a | 5.72 ± 0.47 a | 5.15 ± 0.29 a | 4.78 ± 0.26 a | - | - | 5.42 ± 0.24 a | |
| Run B | PS1D0d6 | 6.51 ± 0.02 b | 6.47 ± 0.02 a | 6.46 ± 0.03 a | 6.07 ± 0.21 a | 5.21 ± 0.24 a | 4.62 ± 0.02 a | 4.56 ± 0.04 a | 4.62 ± 0.00 * | 5.12 ± 0.08 b |
| PS1D1d6 | 6.53 ± 0.04 ab | 6.40 ± 0.03 ab | 6.21 ± 0.11 ab | 5.61 ± 0.71 a | 5.15 ± 0.62 a | 4.64 ± 0.13 a | 4.63 ± 0.11 a | 4.70 ± 0.15 * | 5.24 ± 0.10 ab | |
| PS3D0d6 | 6.54 ± 0.03 ab | 6.36 ± 0.07 ab | 6.23 ± 0.15 ab | 5.10 ± 0.29 a | 4.75 ± 0.22 a | 4.55 ± 0.07 a | 4.54 ± 0.05 a | 4.63 ± 0.22 * | 5.23 ± 0.06 ab | |
| PS3D1d6 | 6.59 ± 0.02 a | 6.33 ± 0.10 b | 5.75 ± 0.58 b | 5.16 ± 0.63 a | 4.87 ± 0.39 a | 4.68 ± 0.10 a | 4.73 ± 0.19 a | 4.69 ± 0.03 * | 5.45 ± 0.16 a | |
| Run C | PS1D0d5 | 6.44 ± 0.02 a | 6.36 ± 0.09 a | 6.08 ± 0.03 a | 4.84 ± 0.10 a | 4.75 ± 0.07 a | 4.83 ± 0.16 a | - | - | 5.23 ± 0.08 a |
| PS1D1d5 | 6.46 ± 0.06 a | 6.27 ± 0.07 a | 5.73 ± 0.31 a | 4.80 ± 0.13 a | 4.78 ± 0.13 a | 4.96 ± 0.16 a | - | - | 5.45 ± 0.15 a | |
| PS1D0d7 | 6.38 ± 0.03 a | 6.30 ± 0.08 a | 6.02 ± 0.09 a | 4.83 ± 0.07 a | 4.71 ± 0.20 a | 4.88 ± 0.30 a | 4.89 ± 0.16 a | 5.14 ± 0.49 a | 5.54 ± 0.23 a | |
| PS1D1d7 | 6.44 ± 0.07 a | 6.27 ± 0.06 a | 5.71 ± 0.36 a | 4.92 ± 0.19 a | 4.86 ± 0.04 a | 4.93 ± 0.22 a | 4.93 ± 0.07 a | 5.33 ± 0.23 a | 5.65 ± 0.16 a | |
| Variation | d0 | d1 | d2 | d3 | d4 | d5 | d6 | d7 1 | |
|---|---|---|---|---|---|---|---|---|---|
| Run A | PS1D1d3 | 29.2 ± 1.5 a | 33.2 ± 1.6 ab | 37.6 ± 4.1 a | 39.4 ± 5.4 b | - | - | - | - |
| PS1D1d4 | 30.1 ± 2.1 a | 34.3 ± 1.4 a | 39.6 ± 3.4 a | 44.4 ± 2.2 a | 44.0 ± 0.8 a | - | - | - | |
| PS1D1d5 | 29.4 ± 1.8 a | 33.3 ± 1.5 ab | 37.7 ± 3.5 a | 42.4 ± 3.0 ab | 43.7 ± 2.5 a | 43.4 ± 2.5 a | - | - | |
| PS1D0d5 | 28.1 ± 0.5 a | 32.3 ± 0.8 b | 36.5 ± 3.1 a | 43.4 ± 2.4 ab | 45.6 ± 1.3 a | 44.6 ± 1.5 a | - | - | |
| Ambient | 26.7 ± 2.8 (6 a.m.–6 p.m.) and 22.9 ± 0.7 (6 p.m.–6 a.m.) | ||||||||
| Run B | PS1D0d6 | 28.0 ± 2.7 a | 33.2 ± 1.5 b | 37.1 ± 1.7 b | 42.8 ± 2.3 a | 46.2 ± 1.8 ab | 47.2 ± 1.1 ab | 44.5 ± 0.6 a | 44.4 ± 0.3 a |
| PS1D1d6 | 29.3 ± 3.0 a | 35.0 ± 1.1 ab | 39.0 ± 2.7 ab | 43.9 ± 2.5 a | 45.0 ± 3.2 b | 46.3 ± 2.6 b | 43.9 ± 0.9 a | 43.0 ± 1.0 a | |
| PS3D0d6 | 30.3 ± 3.3 a | 35.7 ± 1.5 a | 40.7 ± 2.8 ab | 45.5 ± 1.6 a | 48.4 ± 1.0 a | 48.6 ± 0.9 a | 44.8 ± 0.7 a | 43.9 ± 1.8 a | |
| PS3D1d6 | 31.5 ± 1.3 a | 35.8 ± 1.7 a | 42.0 ± 3.1 a | 44.7 ± 3.0 a | 46.4 ± 1.4 ab | 47.0 ± 1.2 ab | 44.3 ± 1.3 a | 43.5 ± 2.5 a | |
| Ambient | 26.1 ± 4.1 (6 a.m.–6 p.m.) and 25.7 ± 4.1 (6 p.m.–6 a.m.) | ||||||||
| Run C | PS1D0d5 | 30.7 ± 1.9 a | 35.0 ± 2.5 c | 44.8 ± 2.8 a | 45.5 ± 1.6 a | 48.6 ± 1.3 a | 46.3 ± 2.0 a | - | - |
| PS1D1d5 | 32.2 ± 2.7 a | 38.0 ± 2.5 ab | 46.5 ± 2.2 a | 46.1 ± 1.5 a | 48.5 ± 1.2 a | 47.0 ± 1.6 a | - | - | |
| PS1D0d7 | 31.0 ± 1.3 a | 35.3 ± 1.9 bc | 44.9 ± 2.4 a | 45.3 ± 1.6 a | 48.7 ± 0.9 a | 46.9 ± 1.2 a | 47.8 ± 2.1 a | 46.2 ± 2.6 a | |
| PS1D1d7 | 32.5 ± 2.3 a | 38.3 ± 3.0 a | 46.3 ± 1.5 a | 45.7 ± 1.0 a | 48.0 ± 0.8 a | 47.2 ± 0.7 a | 46.9 ± 1.8 a | 46.0 ± 2.2 a | |
| Ambient | 29.6 ± 3.6 (6 a.m.–6 p.m.) and 24.4 ± 1.0 (6 p.m.–6 a.m.) | ||||||||
| Sample Variations/Key Tastants | A_PS1D0d5 | A_PS1D1d5 | B_PS1D0d6 | B_PS1D1d6 | B_PS3D1d6 | C_PS1D0d5 | C_PS1D1d5 | C_PS1D0d7 | C_PS1D1d7 | REML p-Values α = 5% | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Selected alkaloids—Average concentration ± standard deviation (g/kg ffdm) n = 3 | Depulping | Pod storage | |||||||||
| Theobromine | 29.93 ± 0.42 | 29.97 ± 0.12 | 29.02 ± 0.55 | 29.56 ± 0.44 | 28.49 ± 0.2 | 25.95 ± 0.19 | 27.56 ± 0.7 | 28.19 ± 1.24 | 27.49 ± 0.51 | 0.225 | 0.069 |
| Caffeine | 2.65 ± 0.05 | 2.71 ± 0.03 | 2.54 ± 0.03 | 2.68 ± 0.01 | 2.70 ± 0.01 | 3.21 ± 0.08 | 3.30 ± 0.13 | 3.11 ± 0.15 | 3.09 ± 0.08 | 0.051 | 0.318 |
| Selected polyphenols—Average concentration ± standard deviation (mg/kg ffdm) n = 3 | |||||||||||
| Catechin | 0.43 ± 0.02 | 0.60 ± 0.06 | 0.76 ± 0.02 | 0.83 ± 0.01 | 0.54 ± 0.05 | 1.04 ± 0.01 | 1.03 ± 0.1 | 0.66 ± 0.02 | 0.66 ± 0.03 | 0.024 | <0.0001 |
| Epicatechin | 2.06 ± 0.11 | 1.99 ± 0.2 | 3.14 ± 0.01 | 3.43 ± 0.13 | 2.18 ± 0.12 | 3.42 ± 0.05 | 3.05 ± 0.36 | 1.90 ± 0.03 | 1.80 ± 0.06 | 0.425 | <0.0001 |
| Procyanidine B2 | 1.65 ± 0.1 | 1.31 ± 0.15 | 2.67 ± 0.03 | 2.80 ± 0.08 | 1.71 ± 0.14 | 1.91 ± 0.04 | 1.89 ± 0.17 | 1.24 ± 0.02 | 1.17 ± 0.07 | 0.167 | <0.0001 |
| Procyanidine C1 | 1.50 ± 0.1 | 1.17 ± 0.15 | 2.09 ± 0.02 | 2.20 ± 0.09 | 1.38 ± 0.12 | 0.96 ± 0.03 | 1.04 ± 0.02 | 0.70 ± 0.03 | 0.74 ± 0.02 | 0.631 | <0.0001 |
| Cinnamtannin A2 | 1.50 ± 0.13 | 1.07 ± 0.19 | 2.08 ± 0.02 | 2.25 ± 0.09 | 1.38 ± 0.16 | 0.62 ± 0.05 | 0.76 ± 0.01 | 0.53 ± 0.04 | 0.60 ± 0.02 | 0.862 | <0.0001 |
| Total flavanols DP 1–4 | 7.14 ± 0.46 | 6.14 ± 0.75 | 10.75 ± 0.01 | 11.51 ± 0.41 | 7.20 ± 0.58 | 7.96 ± 0.09 | 7.77 ± 0.65 | 5.03 ± 0.04 | 4.98 ± 0.15 | - | - |
| Sample | A_PS1D0d5 | A_PS1D1d5 | B_PS1D0d6 | B_PS1D1d6 | B_PS3D1d6 | C_PS1D0d5 | C_PS1D1d5 | C_PS1D0d7 | C_PS1D1d7 | REML p-Values α = 5% | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acids | Depulping | Pod storage | |||||||||
| Acetic acid a | 1556.71 ± 69.30 | 1170.80 ± 92.85 | 2967.25 ± 98.17 | 2105.89 ± 117.88 | 1953.89 ± 130.89 | 3688.26 ± 55.23 | 3013.61 ± 103.78 | 2360.99 ± 44.18 | 2151.32 ± 38.19 | <0.0001 | 0.0077 |
| 2-Methylbutanoic acid a | 12.70 ± 0.56 | 9.90 ± 0.29 | 14.39 ± 0.98 | 12.52 ± 0.16 | 10.11 ± 1.03 | 20.86 ± 0.23 | 18.82 ± 0.46 | 15.82 ± 0.35 | 14.30 ± 0.27 | <0.0001 | <0.0001 |
| 3-Methylbutanoic acid a | 35.05 ± 1.42 | 27.56 ± 0.76 | 39.28 ± 1.00 | 30.36 ± 0.79 | 25.11 ± 0.76 | 37.25 ± 0.22 | 33.97 ± 1.10 | 30.40 ± 0.46 | 29.69 ± 0.31 | <0.0001 | <0.0001 |
| Phenylacetic acid a | 7.93 ± 0.57 | 7.18 ± 0.23 | 8.66 ± 0.17 | 7.17 ± 0.33 | 6.74 ± 0.26 | 79.79 ± 4.32 | 65.32 ± 3.87 | 52.44 ± 1.98 | 56.09 ± 3.20 | 0.0685 | 0.8835 |
| Strecker—Aldehydes | |||||||||||
| 3-Methylbutanal a | 40.62 ± 4.26 | 37.44 ± 1.35 | 39.60 ± 1.88 | 42.20 ± 2.07 | 42.66 ± 4.07 | 50.77 ± 0.80 | 59.47 ± 5.77 | 79.49 ± 3.06 | 66.78 ± 6.04 | 0.7258 | 0.6935 |
| 2-Methylbutanal a | 3.85 ± 0.24 | 3.99 ± 0.18 | 4.65 ± 0.18 | 4.98 ± 0.21 | 5.30 ± 0.02 | 10.15 ± 0.05 | 11.69 ± 1.41 | 14.05 ± 0.70 | 14.04 ± 0.89 | 0.0661 | 0.6225 |
| Phenylacetaldehyde a | 7.66 ± 0.72 | 7.89 ± 0.59 | 6.01 ± 0.35 | 7.16 ± 0.42 | 7.50 ± 0.47 | 4.91 ± 0.07 | 5.03 ± 0.19 | 5.06 ± 0.04 | 5.01 ± 0.10 | 0.0458 | 0.0243 |
| Esters | |||||||||||
| 3-Methylbutyl acetate a | 2.33 ± 0.10 | 1.29 ± 0.07 | 5.88 ± 0.13 | 2.81 ± 0.07 | 1.73 ± 0.10 | 1.08 ± 0.05 | 0.79 ± 0.03 | 0.94 ± 0.04 | 0.70 ± 0.03 | <0.0001 | 0.0001 |
| Ethyl 2-methylbutanoate b | 32.19 ± 4.60 | 35.03 ± 3.33 | 55.63 ± 8.10 | 45.28 ± 2.30 | 32.20 ± 3.69 | 16.02 ± 0.42 | 20.57 ± 0.63 | 15.71 ± 0.27 | 16.00 ± 0.35 | 0.6999 | <0.0001 |
| Ethyl 3-methylbutanoate b | 46.64 ± 6.84 | 51.19 ± 6.17 | 77.97 ± 10.85 | 50.52 ± 3.70 | 43.82 ± 5.93 | 19.34 ± 1.00 | 20.04 ± 1.27 | 18.36 ± 1.15 | 16.10 ± 1.28 | 0.0670 | 0.0052 |
| Ethyl phenylacetate b | 415.17 ± 25.66 | 291.50 ± 24.44 | 669.26 ± 35.12 | 498.49 ± 4.22 | 426.43 ± 25.86 | 342.32 ± 11.10 | 263.71 ± 5.27 | 242.64 ± 4.78 | 232.59 ± 2.62 | <0.0001 | 0.0003 |
| Pyrazines | |||||||||||
| 2,3,5-Trimethylpyrazine b | 424.39 ± 16.99 | 299.14 ± 12.32 | 574.89 ± 15.58 | 415.75 ± 19.09 | 561.17 ± 3.90 | 1376.35 ± 17.19 | 1141.55 ± 21.91 | 1156.36 ± 18.49 | 1108.41 ± 37.96 | <0.0001 | 0.0002 |
| 2-Ethyl-3,5-dimethylpyrazine b | 353.73 ± 25.71 | 331.71 ± 22.22 | 387.70 ± 9.53 | 281.12 ± 15.72 | 373.53 ± 29.53 | 315.37 ± 12.85 | 270.22 ± 2.42 | 293.26 ± 11.03 | 282.43 ± 8.79 | <0.0001 | 0.0018 |
| Furans, furanones | |||||||||||
| Furaneol a | 7.93 ± 0.28 | 8.45 ± 0.26 | 8.76 ± 0.25 | 8.03 ± 0.22 | 8.69 ± 0.35 | 55.18 ± 4.43 | 54.62 ± 3.41 | 55.52 ± 2.18 | 51.37 ± 0.61 | 0.8636 | 0.9439 |
| Sotolone b | 27.27 ± 2.47 | 19.93 ± 1.08 | 29.97 ± 4.78 | 24.00 ± 1.92 | 19.11 ± 0.87 | 41.55 ± 2.89 | 36.97 ± 2.35 | 34.75 ± 1.10 | 33.10 ± 2.82 | <0.0001 | 0.0057 |
| 2-Methyl-3-(methyldithio)furan c | 725.98 ± 63.19 | 553.00 ± 55.39 | 747.25 ± 42.97 | 501.72 ± 57.76 | 493.64 ± 66.29 | 2098.98 ± 52.84 | 2419.00 ± 186.34 | 2874.61 ± 272.22 | 2823.19 ± 333.31 | 0.6185 | 0.4108 |
| Others | |||||||||||
| 2-Phenylethanol a | 14.12 ± 1.12 | 16.57 ± 1.30 | 13.55 ± 1.30 | 14.26 ± 0.52 | 10.90 ± 0.50 | 7.78 ± 0.31 | 8.03 ± 0.19 | 7.51 ± 0.42 | 7.99 ± 0.35 | 0.0072 | <0.0001 |
| 2-Methoxyphenol b | 38.90 ± 0.42 | 16.35 ± 1.30 | 138.67 ± 2.27 | 140.27 ± 7.14 | 214.46 ± 16.59 | 209.59 ± 3.04 | 257.06 ± 6.33 | 390.27 ± 13.29 | 301.06 ± 1.53 | 0.1470 | 0.0002 |
| Dimethyl trisulfide b | 48.76 ± 4.74 | 36.27 ± 6.49 | 41.49 ± 6.37 | 30.29 ± 3.20 | 37.24 ± 1.06 | 87.53 ± 1.18 | 102.51 ± 0.52 | 128.91 ± 3.92 | 142.72 ± 0.66 | 0.8264 | 0.8564 |
| Linalool b | 873.51 ± 15.27 | 1017.69 ± 62.98 | 966.13 ± 68.22 | 1042.53 ± 17.71 | 1642.57 ± 117.73 | 1773.14 ± 43.08 | 1630.96 ± 16.15 | 1374.28 ± 28.87 | 1762.93 ± 28.85 | 0.0124 | <0.0001 |
| Gamma-nonalactone b | 118.81 ± 5.78 | 113.41 ± 16.81 | 106.41 ± 8.15 | 84.62 ± 11.81 | 67.55 ± 4.37 | 45.71 ± 5.03 | 47.35 ± 0.71 | 53.09 ± 4.43 | 56.77 ± 6.38 | 0.0211 | 0.0005 |
| 4-Methylphenol c | 409.42 ± 38.06 | 583.31 ± 22.38 | 332.60 ± 11.46 | 448.90 ± 30.35 | 403.12 ± 3.03 | 16.88 ± 0.64 | 16.03 ± 0.42 | 17.49 ± 0.23 | 17.09 ± 0.33 | 0.0002 | 0.4392 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streule, S.; André, A.; Freimüller Leischtfeld, S.; Chatelain, K.; Gillich, E.; Chetschik, I.; Miescher Schwenninger, S. Influences of Depulping, Pod Storage and Fermentation Time on Fermentation Dynamics and Quality of Ghanaian Cocoa. Foods 2024, 13, 2590. https://doi.org/10.3390/foods13162590
Streule S, André A, Freimüller Leischtfeld S, Chatelain K, Gillich E, Chetschik I, Miescher Schwenninger S. Influences of Depulping, Pod Storage and Fermentation Time on Fermentation Dynamics and Quality of Ghanaian Cocoa. Foods. 2024; 13(16):2590. https://doi.org/10.3390/foods13162590
Chicago/Turabian StyleStreule, Stefanie, Amandine André, Susette Freimüller Leischtfeld, Karin Chatelain, Elodie Gillich, Irene Chetschik, and Susanne Miescher Schwenninger. 2024. "Influences of Depulping, Pod Storage and Fermentation Time on Fermentation Dynamics and Quality of Ghanaian Cocoa" Foods 13, no. 16: 2590. https://doi.org/10.3390/foods13162590
APA StyleStreule, S., André, A., Freimüller Leischtfeld, S., Chatelain, K., Gillich, E., Chetschik, I., & Miescher Schwenninger, S. (2024). Influences of Depulping, Pod Storage and Fermentation Time on Fermentation Dynamics and Quality of Ghanaian Cocoa. Foods, 13(16), 2590. https://doi.org/10.3390/foods13162590






