Effect of Corynebacterium glutamicum Fermentation on the Volatile Flavors of the Enzymatic Hydrolysate of Soybean Protein Isolate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Three Seasoning Samples
2.3. Sensory Evaluation
2.4. E-Nose Measurement
2.5. GC–MS Analysis
2.6. ROAV
2.7. Statistical Analysis
3. Results and Discussion
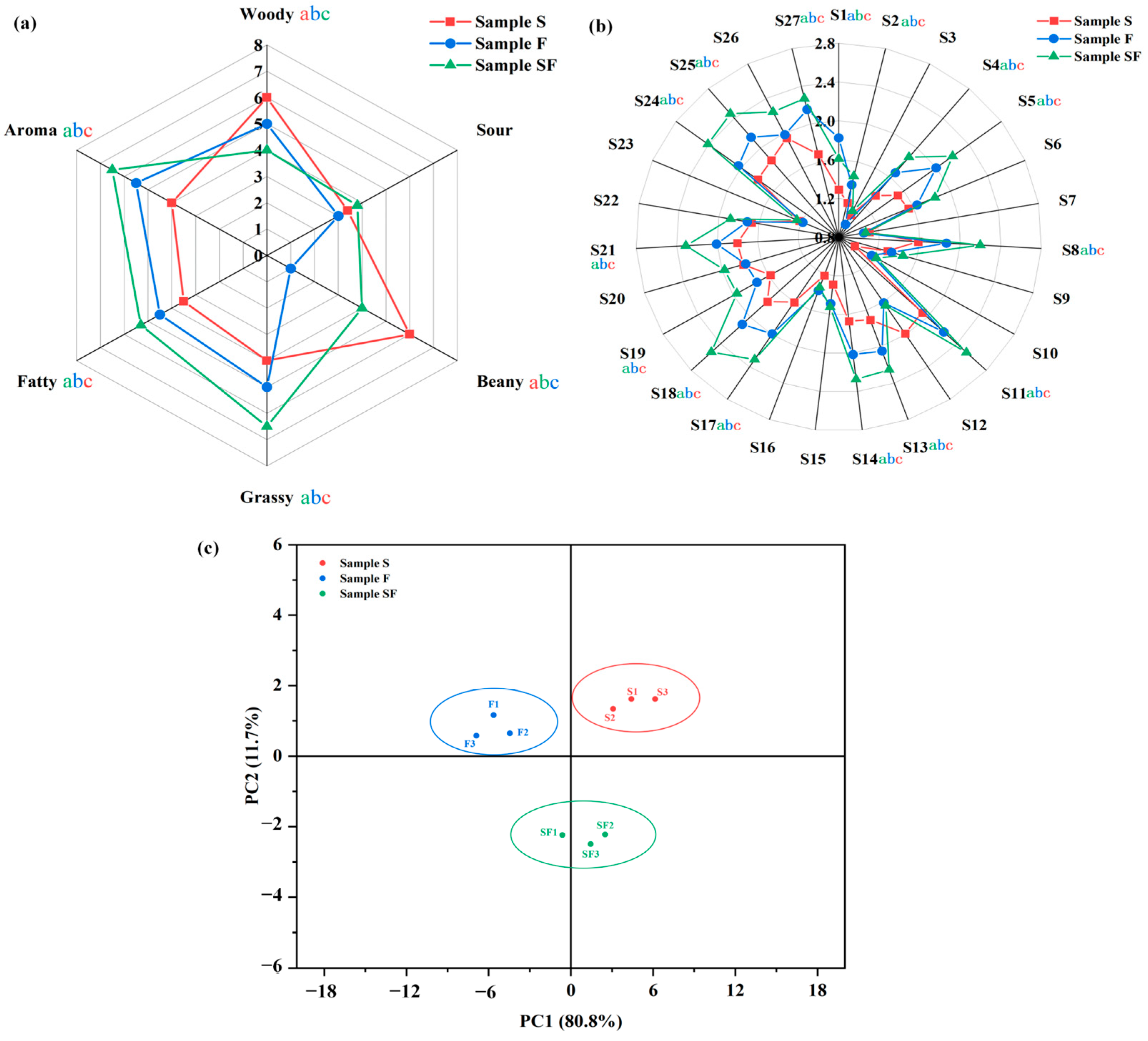
3.1. Sensory Evaluation of Seasoning Samples
3.2. E-Nose Analysis of Seasoning Samples
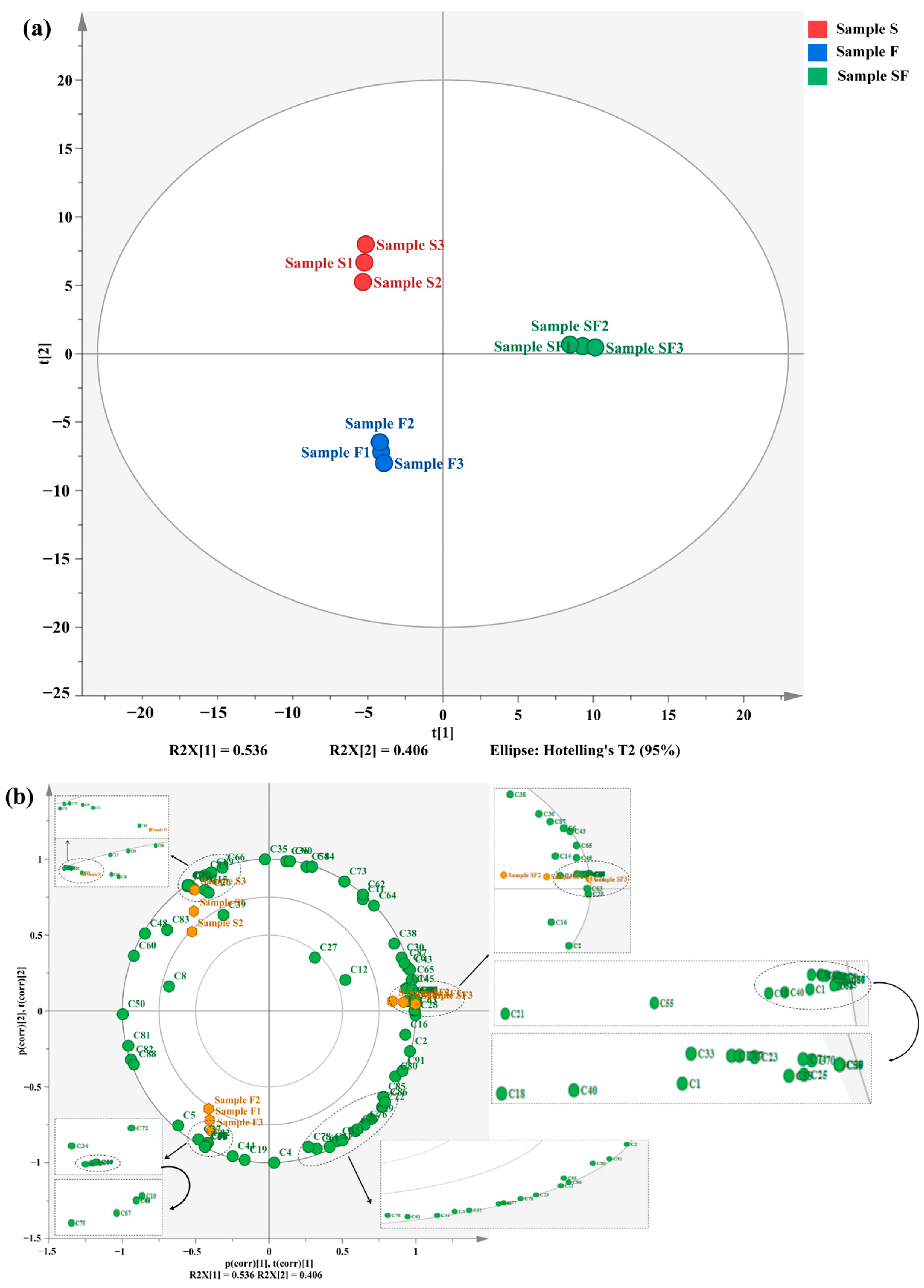
3.3. Volatile Flavoring Compounds in Seasoning Samples Detected via GC–MS
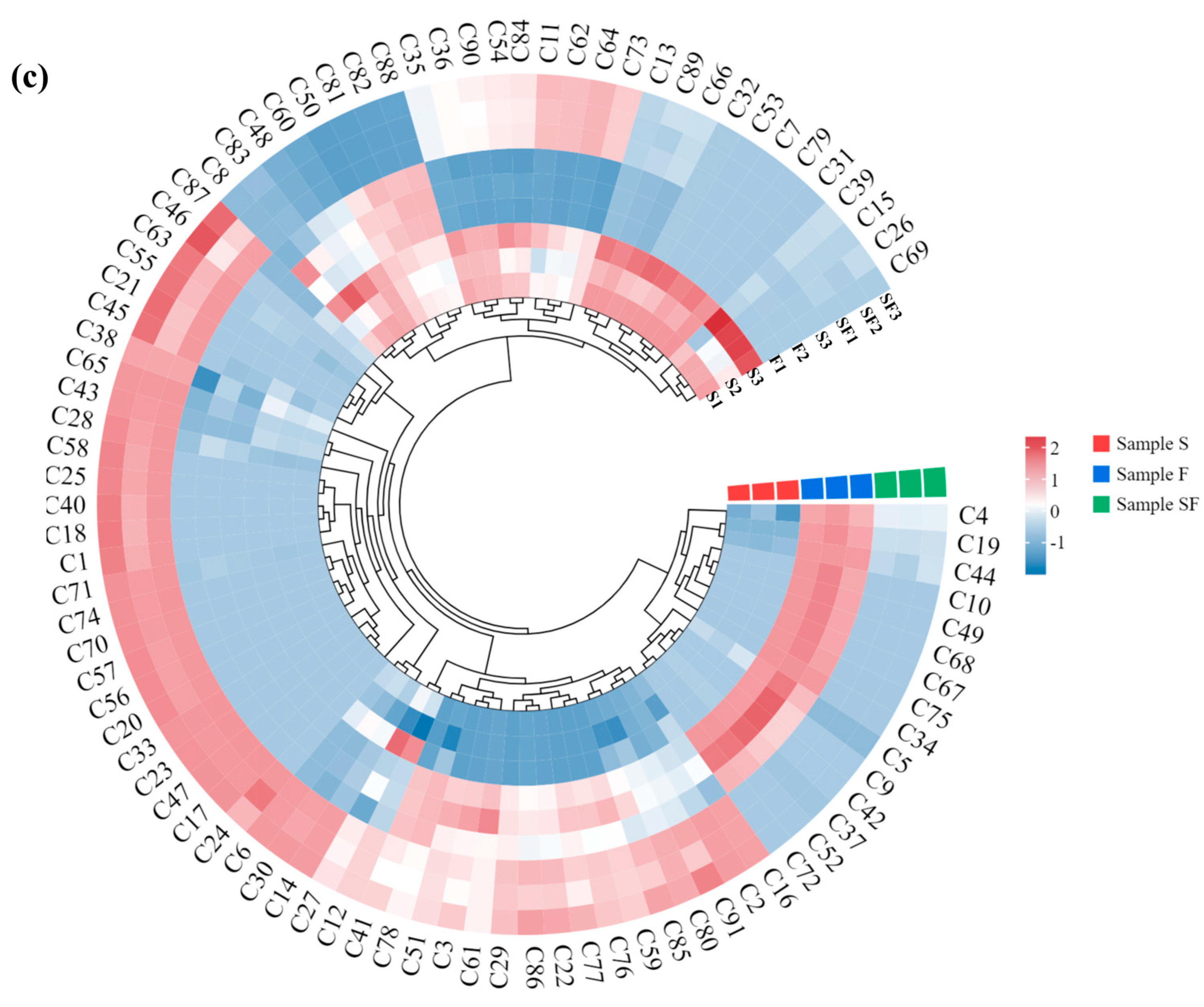
3.4. Correlation Analysis of Volatile Flavoring Compounds in Seasoning Samples
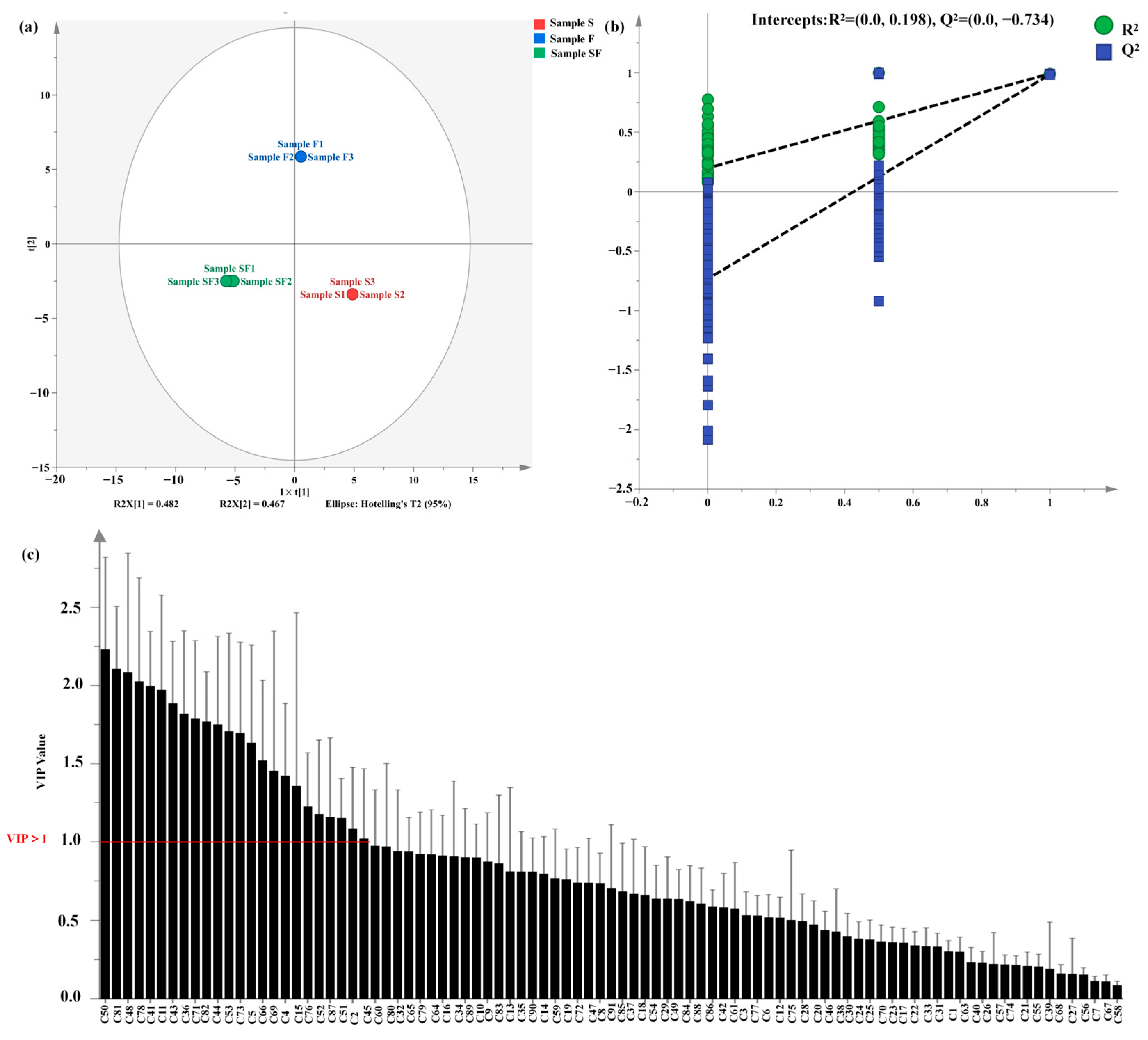
3.5. Analysis of the Relative Odor Activity Values
3.6. Correlation Analysis between E-Nose Data and Important Volatile Flavoring Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| No. | Sensor | Responding Compounds | Feature Descriptions |
|---|---|---|---|
| 1 | S1 | Short-chain alkanes | Propane, smoke, etc. |
| 2 | S2 | Carbonaceous materials, etc. | Alcohol, smoke, isobutane, formaldehyde, etc. |
| 3 | S3 | Hydrogen | Hydrogen, hydrogen-containing gas, etc. |
| 4 | S4 | P-sulfur compounds | Hydrogen sulfide, sulfide, etc. |
| 5 | S5 | Nitrogenous substances | Ammonia, amines, etc. |
| 6 | S6 | Aldehydes and ketones | Toluene, acetone, ethanol, hydrogen, etc. |
| 7 | S7 | Short-chain alkanes flammable gases, etc. | Methane, natural gas, biogas, etc. |
| 8 | S8 | Liquefied gas | Liquefied gas |
| 9 | S9 | Alkanes, alcohols, ketones, etc. | Toluene, formaldehyde, benzene, alcohol, acetone, etc. |
| 10 | S10 | Pair containing hydrogen, hydrogen, etc. | Hydrogen |
| 11 | S11 | P-alkanes, carbon monoxide, etc. | Liquefied gas, alkanes, etc. |
| 12 | S12 | For some organic solvents | Liquefied gas and methane |
| 13 | S13 | Short-chain alkanes | Methane |
| 14 | S14 | For short-chain alkanes | Methane, gas, smoke, etc. |
| 15 | S15 | Sensitive to nitrogenous substances | Ammonia, amines, etc. |
| 16 | S16 | Sulfide sensitive | Hydrogen sulfide, sulfide, etc. |
| 17 | S17 | Alcohols, some organic solvents, etc. | Aromatic hydrocarbons, aliphatic hydrocarbons, alicyclic hydrocarbons, halogenated hydrocarbons, ethers, esters, diol derivatives, acetonitrile, pyridine, phenol, etc. |
| 18 | S18 | Sensitive to alcohols, aldehydes, ketones, and benzenes | Alcohols, ketones, aldehydes, aromatic compounds, etc. |
| 19 | S19 | Sensitive to short-chain alkanes | Methane, biogas, natural gas, etc. |
| 20 | S20 | Flammable gas, etc. | Flammable gas, etc. |
| 21 | S21 | VOC | For example, phenols, ketones, ethyl acetate, cyclohexanone, chlorobenzene, methyl benzene, ether, etc. |
| 22 | S22 | Sensitive to alkanes, olefins, aromatics, etc. | Liquefied gas, natural gas, gas, propane, propylene, butane, butene, etc. |
| 23 | S23 | Sensitive to alkanes, olefins, hydrogen, etc. | Liquefied gas, flammable gas, etc. |
| 24 | S24 | Sensitive to alkanes, carbon monoxide, aldehydes, alcohols, nitrogen oxides, ketones, aldehydes, etc. | Alkanes, alcohol, gas fumes, etc. |
| 25 | S25 | Sensitive to some organic solvents | Aldehydes, ethers, esters, ketones, aromatic hydrocarbons, aliphatic hydrocarbons, alicyclic hydrocarbons, and halogenated hydrocarbons |
| 26 | S26 | Sulfides, nitrides, carbides, hydrocarbons and nitrogen hydrogen compounds, etc. | Smoke, cooking odor, etc. |
| 27 | S27 | Sensitive to short-chain alkanes | Methane, gas, etc. |
| No. | Compounds | CAS | Formula | Odor Perception | Relative Content (%) | ||
|---|---|---|---|---|---|---|---|
| S | F | SF | |||||
| Alcohols (13) | |||||||
| C1 | 1-Pentanol | 71-41-0 | C5H12O | grassy aroma | ND | ND | 0.0792 ± 0.0127 |
| C2 | 1-Adamantanol | 768-95-6 | C10H16O | — | ND | 0.4002 ± 0.1414 | 1.1873 ± 0.0099 |
| C3 | 1-Octanol | 111-87-5 | C8H18O | fruit aroma | ND | 0.2673 ± 0.0849 | 0.2359 ± 0.0495 |
| C4 | 3-Heptanol, 2,6-dimethyl- | 19549-73-6 | C9H20O | grassy aroma | 1.1082 ± 0.4132 c | 3.1254 ± 0.1697 a | 2.0569 ± 0.5632 b |
| C5 | 4-Ethylcyclohexanol | 4534-74-1 | C8H16O | — | 1.4172 ± 0.2828 b | 3.4030 ± 0.5657 a | 0.7901 ± 0.0011 c |
| C6 | Benzyl alcohol | 100-51-6 | C7H8O | cherry fruit, nutty, tropical fruit | 0.0555 ± 0.0566 | ND | 0.2609 ± 0.0035 |
| C7 | (R,R)-2,3-Butanediol | 24347-58-8 | C4H10O2 | fruit, cream aroma | 0.0114 ± 0.0014 | ND | ND |
| C8 | 5-Indanol | 1470-94-6 | C9H10O | floral, fruity aroma | 1.7133 ± 0.4243 a | 1.5143 ± 0.7071 a | 1.0475 ± 0.2134 a |
| C9 | 2-Ethylhexanol | 104-76-7 | C8H18O | sweet, floral scent | ND | 0.6647 ± 0.2828 | ND |
| C10 | Citronellol | 106-22-9 | C10H20O | citrus, fruity aroma | ND | 0.6654 ± 0.0849 | ND |
| C11 | (S)-(+)-3-Methyl-2-butanol- | 1517-66-4 | C5H12O | — | 9.1506 ± 1.5556 a | 6.3878 ± 0.0000 b | 10.2621 ± 2.5431 a |
| C12 | 1-[1-Methyl-2-(2-propenyloxy)ethoxy]-2-propanolhoxy]- | 55956-25-7 | C9H18O3 | — | 3.6892 ± 0.9617 a | 3.6236 ± 0.0283 a | 4.0728 ± 0.9988 a |
| C13 | 4,5-Octanediol, 2,7-dimethyl- | 154468-18-5 | C10H22O2 | — | 0.6098 ± 0.1414 | ND | 0.0862 ± 0.0035 |
| Aldehydes (3) | |||||||
| C14 | 3-(4-Hydroxy-phenyl)-propionaldehyde | 20238-83-9 | C9H10O2 | — | 0.7552 ± 0.2828 b | 0.7207 ± 0.0283 b | 1.2925 ± 0.5637 a |
| C15 | 2,4-Dimethylbenzaldehyde | 15764-16-6 | C9H10O | almond, burnt sugar odor | 8.6542 ± 1.4142 a | 6.8828 ± 1.0283 b | 7.0034 ± 1.5423 b |
| C16 | Hexanal | 66-25-1 | C6H12O | fatty aroma, grass aroma | 2.0000 ± 0.2828 b | 2.2275 ± 0.2927 b | 2.8765 ± 0.8965 a |
| Ketones (19) | |||||||
| C17 | Acetone | 67-64-1 | C3H6O | mint-like aroma | ND | ND | 0.1013 ± 0.0045 |
| C18 | 2-Heptanone | 110-43-0 | C7H14O | fruit aroma | ND | ND | 0.3588 ± 0.0596 |
| C19 | 3-Ethylcyclopentanone | 10264-55-8 | C7H14O | pungent smell | ND | 0.5360 ± 0.0424 | 0.1547 ± 0.0014 |
| C20 | 2-Nonanone | 821-55-6 | C9H18O | as fruit, flower, oil and herbs like aroma | ND | ND | 0.1691 ± 0.0023 |
| C21 | 3-Octen-2-one | 1669-44-9 | C8H14O | fatty, nutty, spicy | ND | ND | 0.0390 ± 0.0000 |
| C22 | 3,5-Dimethyl-2-cyclohexen-1-one | 1123-09-7 | C8H12O | — | ND | 0.0797 ± 0.0127 | 0.1121 ± 0.0001 |
| C23 | 3,5-Diethyl-2,6-dimethylcyclohex-2-en-1-one | 65416-17-3 | C12H20O | — | ND | ND | 0.1121 ± 0.0261 |
| C24 | 2-Decanone | 693-54-9 | C10H20O | fatty, wheat aroma | ND | ND | 0.1281 ± 0.0000 |
| C25 | Bicycl [3.3.1]nonane-2,6-dione | 16473-11-3 | C9H12O2 | — | ND | ND | 0.1173 ± 0.0008 |
| C26 | 2-Tridecanone | 593-08-8 | C13H26O | fatty, earthy, dairy | 0.0485 ± 0.0424 | ND | 0.0036 ± 0.0002 |
| C27 | 3-Ethyl-4-methyl-pyrrole-2,5-dione- | 20189-42-8 | C7H9NO2 | — | 0.3765 ± 0.1414 a | 0.3418 ± 0.0566 a | 0.3966 ± 0.0056 a |
| C28 | 2,3-Pentanedione | 600-14-6 | C5H8O2 | — | 0.2031 ± 0.0042 b | 0.2239 ± 0.0284 b | 0.4114 ± 0.0156 a |
| C29 | 2,3-Hexanedione | 3848-24-6 | C6H10O2 | creamy, fruity, burnt sugar odor | ND | 0.3154 ± 0.0212 | 0.3875 ± 0.0099 |
| C30 | 2-Pentadecanone | 2345-28-0 | C15H30O | fatty, spicy flowers | 0.2473 ± 0.0566 b | 0.2057 ± 0.0071 b | 0.3551 ± 0.0001 a |
| C31 | Acetophenone | 98-86-2 | C8H8O | sweet, almond aroma | 0.0809 ± 0.0013 | ND | ND |
| C32 | 2-Hexanoylfuran | 14360-50-0 | C10H14O2 | — | 0.7719 ± 0.2828 | ND | ND |
| C33 | Acetoin | 513-86-0 | C4H8O2 | sweet, dairy aroma | ND | ND | 0.0975 ± 0.0036 |
| C34 | 3,5-Octadien-2-one | 30086-02-3 | C8H12O | fatty | ND | 0.7095 ± 0.0127 | ND |
| C35 | Acetylvaleryl | 96-04-8 | C7H12O2 | — | 0.6574 ± 0.0707 | ND | 0.3509 ± 0.0038 |
| Esters (6) | |||||||
| C36 | 2-Phenylethyl hexanoate | 6290-37-5 | C14H20O2 | fresh, light fatty, wine aroma | 4.4708 ± 0.0990 a | 1.1902 ± 0.1413 c | 3.3734 ± 0.7785 b |
| C37 | Phenethyl acetate | 103-45-7 | C10H12O2 | sweet honey, floral | 0.0302 ± 0.0003 | 0.4031 ± 0.0215 | ND |
| C38 | Gamma-octanoic lactone | 104-50-7 | C8H14O2 | strong sweet herb aroma | 0.3100 ± 0.0141 b | 0.2410 ± 0.0656 c | 0.4092 ± 0.0002 a |
| C39 | 2-Phenylethyl propionate | 122-70-3 | C11H14O2 | sweet red rose aromas, fruity undertones, strong sweet honey | 0.3583 ± 0.0707 a | 0.3149 ± 0.0057 a | 0.3186 ± 0.0007 a |
| C40 | 4-Hexanolide | 695-06-7 | C6H10O2 | Fruity aroma | ND | ND | 0.0461 ± 0.0023 |
| C41 | Methyl 2-hydroxy-4-methylvalerate | 40348-72-9 | C7H14O3 | — | 0.3037 ± 0.1414 c | 4.1476 ± 1.1123 a | 3.4326 ± 0.2103 b |
| Acids (6) | |||||||
| C42 | Valeric acid | 109-52-4 | C5H10O2 | cheese aroma | ND | 0.2964 ± 0.1273 | ND |
| C43 | Octanoic acid | 124-07-2 | C8H16O2 | bouquet, wine, fruit aroma | 1.3609 ± 0.0849 b | 0.6334 ± 0.0425 c | 4.0491 ± 1.0051 a |
| C44 | Hexanoic acid | 142-62-1 | C6H12O2 | Fatty aroma | 0.6793 ± 0.0990 c | 3.6271 ± 0.9130 a | 1.2895 ± 0.0999 b |
| C45 | Decanoic acid | 334-48-5 | C10H20O2 | soapy, waxy | 0.9065 ± 0.9899 b | 0.8339 ± 0.0464 b | 1.8000 ± 0.4561 a |
| C46 | Butanoic acid | 107-92-6 | C4H8O2 | sour, cheesy, creamy | ND | ND | 0.1701 ± 0.0027 |
| C47 | 2-Methylcaproic acid | 4536-23-6 | C7H14O2 | cheesy | ND | ND | 0.4775 ± 0.1458 |
| C48 | 3,4-Dimethoxycinnamic acid | 14737-89-4 | C11H12O4 | — | 0.9899 ± 0.1235 a | 0.2828 ± 0.0211 b | 0.0071 ± 0.0001 c |
| C49 | 2-Oxo-octanoic acid | 328-51-8 | C8H14O3 | — | ND | 0.0424 ± 0.0004 | ND |
| Amines (4) | |||||||
| C50 | 2-(Aziridin-1-yl)ethanamine | 4025-37-0 | C4H10N2 | — | 4.5000 ± 0.1414 | 4.2438 ± 0.8698 | ND |
| C51 | Acetamide | 60-35-5 | C2H5NO | — | ND | 1.2182 ± 0.2970 | 1.1371 ± 0.2356 |
| C52 | Aniline | 62-53-3 | C6H7N | pungent odor | ND | 1.2157 ± 0.2354 | ND |
| C53 | 2-Methyl-4-methoxybenzenamine | 102-50-1 | C8H11NO | — | 2.5419 ± 0.7651 | ND | ND |
| Aromatics (10) | |||||||
| C54 | 3-(Ethylamino)phenol | 621-31-8 | C8H11NO | — | 0.6035 ± 0.1414 a | 0.2147 ± 0.0198 b | 0.5262 ± 0.0056 a |
| C55 | Olivetol | 500-66-3 | C11H16O2 | — | ND | ND | 0.0375 ± 0.0021 |
| C56 | 4-Hydroxy-3-methoxystyrene | 7786-61-0 | C9H10O2 | smoky bacon, spicy, clove phenolic, woody notes | ND | ND | 0.0212 ± 0.0012 |
| C57 | Benzimidazole | 51-17-2 | C7H6N2 | nutty | ND | ND | 0.0422 ± 0.0001 |
| C58 | Naphthalene | 91-20-3 | C10H8 | floral and fruity aroma | ND | ND | 0.0067 ± 0.0000 |
| C59 | Benzonitrile | 100-47-0 | C7H5N | — | ND | 0.5005 ± 0.0245 | 0.5280 ± 0.3214 |
| C60 | 1-Ethylindan | 4830-99-3 | C11H14 | — | 5.0058 ± 0.0000 a | 4.5770 ± 1.0023 b | 4.0342 ± 0.7598 c |
| C61 | Phenylethyl Alcohol | 60-12-8 | C8H10O | rose scent | ND | 0.2858 ± 0.1131 | 0.2123 ± 0.0236 |
| C62 | o-Tolidine, N,N′-bis(trimethylsilyl)- | 149388-08-9 | C20H32N2Si2 | — | 0.6093 ± 0.1414 | ND | 0.8182 ± 0.1518 |
| C63 | Benzothiazole | 95-16-9 | C7H5NS | mushroom, smoke | 0.0082 ± 0.0028 b | 0.0120 ± 0.0028 b | 0.0582 ± 0.0000 a |
| Heterocyclics (28) | |||||||
| C64 | 2-Methylpyrazine | 109-08-0 | C5H6N2 | roasted, chocolate, nutty aromas | 0.7610 ± 0.0846 b | 0.2239 ± 0.0042 c | 1.0492 ± 0.04586 a |
| C65 | 2,5-Dimethylpyrazine | 123-32-0 | C6H8N2 | fried potatoes, dark chocolate aromas | 0.3881 ± 0.0113 b | 0.2623 ± 0.0849 c | 1.0932 ± 0.5623 a |
| C66 | Pyrazine, 2-Ethyl-6-methyl- | 13925-03-6 | C7H10N2 | roasted hazelnut aromas | 2.4744 ± 0.5657 a | 0.2339 ± 0.0044 c | 0.8331 ± 0.00114 b |
| C67 | 2-Ethyl-5-methylpyrazine- | 13360-64-0 | C7H10N2 | roasted and nutty aromas | ND | 0.0110 ± 0.0001 | ND |
| C68 | 3-Ethyl-2,5-dimethylpyrazine | 13360-65-1 | C8H12N2 | roasted, chocolate, nutty aroma | ND | 0.0226 ± 0.0013 | ND |
| C69 | Pyrazine, ethyltrimethyl- (8CI,9CI) | 17398-16-2 | C9H14N2 | peanut, hazelnut, cocoa | 1.8934 ± 1.1314 | ND | ND |
| C70 | 2-Butyl-3-methylpyrazine | 15987-00-5 | C9H14N2 | caramel, baked goods aroma | ND | ND | 0.1148 ± 0.0036 |
| C71 | N-pentylpyrzine | 6303-75-9 | C9H14N2 | — | 0.8319 ± 0.0424 b | 0.8986 ± 0.1273 b | 3.6800 ± 0.8895 a |
| C72 | 5-Ethyl-2,3-dimethylpyrazine | 15707-34-3 | C8H12N2 | — | ND | 0.4770 ± 0.0990 | ND |
| C73 | 2-Acetyl-3,5-dimethylpyrazine | 4300-08-25 | C8H10N2O | roasted, chocolate, nutty aroma | 2.5950 ± 0.0000 b | 0.2159 ± 0.0212 c | 2.8398 ± 0.0127 a |
| C74 | 2-Propylpyrazine | 18138-03-9 | C7H10N2 | soft nutty | ND | ND | 0.0413 ± 0.0023 |
| C75 | Pyrazine, 2,5-dimethyl-3-propyl- (8CI,9CI) | 18433-97-1 | C9H14N2 | nutty | ND | 0.2147 ± 0.0198 | ND |
| C76 | 2,6-Dimethylpyrazine | 108-50-9 | C6H8N2 | nutty, coffee, cocoa, musty bread, meaty | ND | 1.2300 ± 0.3111 | 1.4220 ± 0.0236 |
| C77 | Pyrazine, 2,3-dimethyl- | 5910-89-4 | C6H8N2 | nutty, almond aroma | ND | 0.2324 ± 0.0325 | 0.2480 ± 0.0365 |
| C78 | Tetramethylpyrazine | 1124-11-4 | C8H12N2 | nutty, weedy earth, cocoa, peanut, coffee, asparagus | 8.1366 ± 1.4142 c | 12.5390 ± 2.356 b | 11.1259 ± 3.2658 a |
| C79 | 2-n-Butyl furan | 4466-24-4 | C8H12O | — | 0.7429 ± 0.1414 | ND | ND |
| C80 | 1-(2-Furyl)pentan-1-one | 3194-17-0 | C9H12O2 | — | 4.8815 ± 0.2828 c | 5.3408 ± 1.2832 a | 5.8141 ± 0.9865 b |
| C81 | Benzofuran, 2,3-dihydro-2-methyl- | 1746-11-8 | C9H10O | — | 3.4637 ± 0.5657 b | 4.2814 ± 0.9685 c | 0.0126 ± 0.0001 a |
| C82 | 2,3-Dihydrobenzofuran | 496-16-2 | C8H8O | sweet and nutty | 2.2616 ± 0.2828 b | 3.0904 ± 0.3625 a | 0.0251 ± 0.0005 c |
| C83 | 2-n-Heptylfuran | 3777-71-7 | C11H18O | green, fatty, greasy, lactone, pork | 8.7422 ± 0.4243 a | 8.2710 ± 1.4786 b | 8.0197 ± 1.3664 b |
| C84 | 3-Cyano-4,6-dimethyl-2-hydroxypyridine | 769-28-8 | C8H8N2O | astringent, burnt taste | 0.5630 ± 0.0849 a | 0.2193 ± 0.0023 b | 0.5067 ± 0.0269 b |
| C85 | Pyridine | 110-86-1 | C5H5N | spicy | 0.7783 ± 0.0990 c | 1.0955 ± 0.2369 b | 1.2557 ± 0.0085 a |
| C86 | 2-Acetyl pyrrole | 1072-83-9 | C6H7NO | walnuts, licorice, toast, fried hazelnuts and fish-like aroma | ND | 0.2303 ± 0.2589 | 0.3402 ± 0.0566 |
| C87 | 3-Tert-butyl-1H-1,2,4-triazole | 96440-78-7 | C6H11N3 | nutty | 0.3582 ± 0.0707 | ND | 1.3425 ± 0.4808 |
| C88 | 5-Methyl-[1,2,4]triazolo [1,5-a]pyrimidin-7-ol | 2503-56-2 | C6H6N4O | — | 0.3302 ± 0.00424 b | 0.4351 ± 0.0071 a | 0.0911 ± 0.0016 c |
| C89 | Cyclotetrasiloxane, octamethyl- | 556-67-2 | C8H24O4Si4 | — | 0.7738 ± 0.0990 | ND | 0.1501 ± 0.0707 |
| C90 | Dodecamethylcyclohexasiloxane | 540-97-6 | C12H36O6Si6 | — | 2.0215 ± 0.283 a | 1.3961 ± 0.0085 b | 1.8237 ± 0.0325 c |
| C91 | 2,3,5-Trimethyl-6-propylpyrazine | 92233-82-4 | C10H16N2 | — | ND | 0.2237 ± 0.0386 | 0.4810 ± 0.1131 |
References
- Meng, J.; Wang, J.; Hao, Y.; Zhu, M.; Wang, J. Effects of Lactobacillus fermentum GD01 fermentation on the nutritional components and flavor substances of three kinds of bean milk. Food Sci. Technol. 2023, 184, 115006. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (Poly)phenols, Volatile Compounds from Vegetables, Medicinal and Aromatic Plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef]
- Cao, C.; Waterhouse, G.; Sun, W.; Zhao, M.; Sun-Waterhouse, D.; Su, G. Effects of Fermentation with Tetragenococcus halophilus and Zygosaccharomyces rouxii on the Volatile Profiles of Soybean Protein Hydrolysates. Foods 2023, 12, 4513. [Google Scholar] [CrossRef]
- Seo, W.H.; Lee, H.G.; Baek, H.H. Evaluation of Bitterness in Enzymatic Hydrolysates of Soy Protein Isolate by Taste Dilution Analysis. J. Food Sci. 2008, 73, S41–S46. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Applications of soy protein hydrolysates in the emerging functional foods: A review. Int. J. Food Sci. Technol. 2020, 55, 421–428. [Google Scholar] [CrossRef]
- Liang, Z.; Yi, M.; Sun, J.; Zhang, T.; Wen, R.; Li, C.; Reshetnik, E.I.; Gribanova, S.L.; Liu, L.; Zhang, G. Physicochemical properties and volatile profile of mung bean flour fermented by Lacticaseibacillus casei and Lactococcus lactis. LWT 2022, 163, 113565. [Google Scholar] [CrossRef]
- Bai, J.; He, L.; Zhang, J.; Gu, X.; Wu, B.; Wang, A.; Zhu, Y.; Zhang, J.; Zhao, Y.; Yuan, J.; et al. Influences of Lactiplantibacillus plantarum and Saccharomyces cerevisiae fermentation on the nutritional components, flavor property and lipid-lowering effect of highland barley. J. Future Foods 2024, 4, 258–266. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Fasmon, D.M.; Kouba, M.; Lopez-Alonso, M.; Lopez, P.S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of monosodium L-glutamate produced by fermentation with Corynebacterium glutamicum KCCM 80187 for all animal species (CJ Europe GmbH). EFSA J. 2021, 19, e6982. [Google Scholar]
- Shangguan, L.; Zhang, H.; Liu, Z.; An, F.; Yang, Q.; Zhang, X.; Yao, L.; Yang, S.; Dai, J.; Chen, X. Improved Glutamic Acid Production Capacity of Corynebacterium glutamicum by the ARTP Mutagenesis Method. Fermentation 2023, 9, 599. [Google Scholar] [CrossRef]
- Shangguan, L.; Liu, Z.; Zhang, H.; Yang, Q.; Zhang, X.; Yao, L.; Li, P.; Chen, X.; Dai, J. Improved umami taste of the enzymatic hydrolysate of soybean protein isolate by Corynebacterium glutamicum P-45 fermentation. Food Biosci. 2024, 58, 103565. [Google Scholar] [CrossRef]
- Fadel, H.H.M.; Lotfy, S.N.; Asker, M.M.S.; Mahmoud, M.G.; Al-Okbi, S.Y. Nutty-like flavor production by Corynbacterium glutamicum 1220T from enzymatic soybean hydrolysate. Effect of encapsulation and storage on the nutty flavoring quality. J. Adv. Res. 2018, 10, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Lv, Y.; Wen, R.; Wang, Y.; Chen, Q.; Kong, B. Characterization of selected Harbin red sausages on the basis of their flavour profiles using HS-SPME-GC/MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108345. [Google Scholar] [CrossRef]
- Yu, J.; Lu, K.; Zi, J.; Yang, X.; Xie, W. Characterization of aroma profiles and aroma—Active compounds in high-salt and low-salt shrimp paste by molecular sensory science. Food Biosci. 2022, 45, 101470. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Wang, Z.; Huang, P.; Kan, J. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, L.; Zhang, H.; Wang, W.; Li, P.; Chen, X.; Dai, J. Optimization of soybean isolated protein enzymatic process and its application in fermented seasonings. Sci. Technol. Food Ind. 2023, 44, 272–280. [Google Scholar]
- Putri, S.L.E.; Suantika, G.; Situmorang, M.L.; Christina, J.; Nikijuluw, C.; Putri, S.P.; Fukusaki, E. Shrimp count size: GC/MS-based metabolomics approach and quantitative descriptive analysis (QDA) reveal the importance of size in white leg shrimp (Litopenaeus vannamei). Metabolomics 2021, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, L.; Huang, H.; Wang, H.; Zhang, T.; Chen, J.; Du, G.; Kang, Z. Eliminating the capsule-like layer to promote glucose uptake for hyaluronan production by engineered Corynebacterium glutamicum. Nat. Commun. 2020, 11, 3120. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xue, Y.A.; Wang, Y.; Wang, W.; Shu, N.; Zhao, H.; Tang, F.; Yang, X.; Guo, Z.; Shan, C. The Fungal Communities and Flavor Profiles in Different Types of High-Temperature Daqu as Revealed by High-Throughput Sequencing and Electronic Senses. Front. Microbiol. 2021, 12, 784651. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Dong, S.; Dong, Y.; Gao, Q. Geographical origin identification of two salmonid species via flavor compound analysis using headspace-gas chromatography-ion mobility spectrometry combined with electronic nose and tongue. Food Res. Int. 2021, 145, 110385. [Google Scholar] [CrossRef]
- Tian, D.; Huang, G.; Ren, L.; Li, Y.; Yu, J.; Lu, Q.; Yang, Y.; Deng, X.; Li, Y.; Zhou, H. Effects of Monascus purpureus on ripe Pu-erh tea in different fermentation methods and identification of characteristic volatile compounds. Food Chem. 2024, 440, 138249. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qi, D.; Wang, W.; Miao, A.; Ma, C. GC-MS analysis combined with sensory analysis revealed the various aroma characteristics of black tea resulted from different grafting rootstocks. J. Food Sci. 2021, 86, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Y.; Liu, Y.; Wang, D.W. Analyzing Volatile Compounds of Young and Mature Docynia delavayi Fruit by HS-SPMEGC-MS and rOAV. Foods. 2022, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Peng, Z.; Hardie, W.J.; Huang, T.; Liu, Z.; Zhang, Y.; Xie, M.; Xiong, T. Exploring the typical flavours formation by combined with metatranscriptomics and metabolomics during Chinese Sichuan paocai fermentation. LWT 2022, 153, 112474. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Y.; Chen, Y.; Zhu, M.; He, C.; Li, Z.; Wang, Y.; Liu, Z. Characteristic fingerprints and change of volatile organic compounds of dark teas during solid-state fermentation with Eurotium cristatum by using HS-GC-IMS, HS-SPME-GC-MS, E-nose and sensory evaluation. LWT—Food Sci. Technol. 2022, 169, 113925. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, R.; Chen, Y.; Ho, C.; Hou, A.; Zhang, X.; Zhu, M.; Zhang, C.; Wang, Y.; Liu, Z.; et al. Dynamics changes in volatile profile, non-volatile metabolites and antioxidant activities of dark tea infusion during submerged fermentation with Eurotium cristatum. Food Biosci. 2023, 55, 102966. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Song, H. Variation of aroma components during frozen storage of cooked beef balls by SPME and SAFE coupled with GC-O-MS. J. Food Process Pres. 2021, 45, e15036. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Q.; Zhang, N.; Bak, K.H.; Soladoye, O.P.; Aluko, R.E.; Fu, Y.; Zhang, Y. Insights into formation, detection and removal of the beany flavor in soybean protein. Trends Food Sci. Tech. 2021, 112, 336–347. [Google Scholar] [CrossRef]
- Mu, H.; Dai, T.; Huang, S.; Wu, K.; Wang, M.; Tan, C.; Zhang, F.; Sheng, J.; Zhao, C. Physical and Chemical Properties, Flavor and Organoleptic Characteristics of a Walnut and Purple Rice Fermented Plant Drink. Foods 2024, 13, 400. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Surmounting the off-flavor challenge in plant-based foods. Crit. Rev. Food Sci. Nutr. 2023, 63, 10585–10606. [Google Scholar] [CrossRef]
- Yu, J.; Lu, K.; Zi, J.; Yang, X.; Zheng, Z.; Xie, W. Halophilic bacteria as starter cultures: A new strategy to accelerate fermentation and enhance flavor of shrimp paste. Food Chem. 2022, 393, 133393. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Sun, H.; Song, X.; Zhao, M.; Lin, W.; Sun, W.; Lin, L.; Li, W.; Su, G. Effect of fermentation with Tetragenococcus halophilus and Zygosaccharomyces rouxii on selected non-volatile taste compounds in soybean protein hydrolysates. Food Sci. Technol. 2023, 184, 115053. [Google Scholar] [CrossRef]
- Fadel, H.; Saad, R.; Asker, M.; Mahmoud, M.; Lotfy, S.; Abd El-Aleem, F.; Ahmed, M.; Elmansy, E. Production of enzymatically hydrolyzed soybean protein isolate by a novel neutral protease from Enterobacter sp. NRCM1 to be used as a base precursor of thermal process flavours. Egypt. J. Chem. 2021, 64, 2127–2140. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, L.; Liu, H.; Liu, H.; Zhou, Y.; Li, M.; Gao, R. Analysis of the changes of volatile flavor compounds in a traditional Chinese shrimp paste during fermentation based on electronic nose, SPME-GC-MS and HS-GC-IMS. Food Sci. Hum. Wellness 2023, 12, 173–182. [Google Scholar] [CrossRef]
- Cai, W.; Wang, Y.; Wang, W.; Shu, N.; Hou, Q.; Tang, F.; Shan, C.; Yang, X.; Guo, Z. Insights into the Aroma Profile of Sauce-Flavor Baijiu by GC-IMS Combined with Multivariate Statistical Analysis. J. Anal. Methods Chem. 2022, 2022, 4614330. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Xia, W.; Li, X.; Liu, S. Use of Wine and Dairy Yeasts as Single Starter Cultures for Flavor Compound Modification in Fish Sauce Fermentation. Front. Microbiol. 2019, 10, 2300. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Tang, Q.; Fan, L.; Xu, X.; Song, Z.; Hayat, K.; Feng, T.; Wang, Y. Identification of pork flavour precursors from enzyme-treated lard using Maillard model system assessed by GC–MS and partial least squares regression. Meat Sci. 2017, 124, 15–24. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, S.; Peng, Y.; Jin, Y.; Xu, D.; Xu, X. Effect of lactic acid bacteria on mackerel (Pneumatophorus japonicus) seasoning quality and flavor during fermentation. Food Biosci. 2021, 41, 100971. [Google Scholar] [CrossRef]
- Hu, M.; Wang, S.; Liu, Q.; Cao, R.; Xue, Y. Flavor profile of dried shrimp at different processing stages. LWT 2021, 146, 111403. [Google Scholar] [CrossRef]
- Wang, W.; Cha, Y. Volatile Compounds in Seasoning Sauce Produced from Soy Sauce Residue by Reaction Flavor Technology. Prev. Nutr. Food Sci. 2018, 23, 356–363. [Google Scholar] [CrossRef]
- Prihanto, A.A.; Nurdiani, R.; Jatmiko, Y.D.; Firdaus, M.; Kusuma, T.S. Physicochemical and sensory properties of terasi (an Indonesian fermented shrimp paste) produced using Lactobacillus plantarum and Bacillus amyloliquefaciens. Microbiol. Res. 2021, 242, 126619. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Oh, J.; Hurh, B.S.; Jeong, G.H.; Shin, Y.K.; Kim, Y.S. Volatile Compounds Produced by Lactobacillus paracasei during Oat Fermentation. J. Food Sci. 2016, 81, C2915–C2922. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Y.; Shu, L.; He, Y. Study on metabolites of Bacillus producing soy sauce-like aroma in Jiang-flavor Chinese spirits. Food Sci. Nutr. 2020, 8, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.L.; Yang, W.J.; Kimatu, B.M.; Zhao, L.Y.; An, X.X.; Hu, Q.H. Comparison of flavour qualities of mushrooms (Flammulina velutipes) packed with different packaging materials. Food Chem. 2017, 232, 1–9. [Google Scholar]
- Reyes-Díaz, R.; González-Córdova, A.F.; Del Carmen Estrada-Montoya, M.; Méndez-Romero, J.I.; Mazorra-Manzano, M.A.; Soto-Valdez, H.; Vallejo-Cordoba, B. Volatile and sensory evaluation of Mexican Fresco cheese as affected by specific wild Lactococcus lactis strains. J. Dairy. Sci. 2020, 103, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, C.; Zhang, M.; He, J.; Shen, Z.; Geng, L.; Yuan, L.; Wang, S.; Wang, C. Study on the factors affecting the sauce-flavor compounds of soybean paste in fermentation. Food Biosci. 2024, 59, 103872. [Google Scholar] [CrossRef]
- Inoue, Y.; Kato, S.; Saikusa, M.; Suzuki, C.; Otsubo, Y.; Tanaka, Y.; Watanabe, H.; Hayase, F. Analysis of the cooked aroma and odorants that contribute to umami aftertaste of soy miso (Japanese soybean paste). Food Chem. 2016, 213, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Kleekayai, T.; Pinitklang, S.; Laohakunjit, N.; Suntornsuk, W. Volatile components and sensory characteristics of Thai traditional fermented shrimp pastes during fermentation periods. J. Food Sci. Technol.-Mysore 2016, 53, 1399–1410. [Google Scholar] [CrossRef]
- Xi, J.; Xu, D.; Wu, F.; Jin, Z.; Yin, Y.; Xu, X. The aroma compounds of Chinese steamed bread fermented with sourdough and instant dry yeast. Food Biosci. 2020, 38, 100775. [Google Scholar] [CrossRef]
- Kawtharani, H.; Beaufort, S.; Anson, P.; Taillandier, P.; Mathieu, F.; Snini, S.P. Impact of the Inoculation Method of Geotrichum candidum, Used as Biocontrol Agent, on T-2 Toxin Produced by Fusarium sporotrichioides and F. langsethiae during the Malting Process. Toxins 2022, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.; Liu, X.; Liu, Y.; Hou, A.; Wang, Y.; Li, L.; Peng, X.; Xiao, Y. Discrimination and characterization of volatile organic compounds and nutritional values of three varieties of chopped pepper seeds. Food Chem. X 2024, 21, 101150. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Ju, Y.; Zhan, C.; Hou, L. Improved umami flavor of soy sauce by adding enzymatic hydrolysate of low-value fish in the natural brewing process. LWT 2022, 155, 112911. [Google Scholar] [CrossRef]
- Xia, D.; Tan, X.; Wang, L.; Li, Z.; Hou, A.; Zhu, Y.; Lai, L.; Wang, Y. GC-MS Coupled with Rate-All-That-Apply (RATA) to Analyse the Volatile Flavor Substances of Yellow Wine during Fermentation. Foods 2023, 12, 1992. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, J.; Chen, J.; Jing, B.; Zhang, L.; Yu, X. Characterization of Differences in Flavor in Virgin Rapeseed Oils by Using Gas Chromatography-Mass Spectrometry, Electronic Nose, and Sensory Analysis. Eur. J. Lipid Sci. Tech. 2020, 122, 1900205. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Xiao, Q.; Liu, C.; Deng, F.; Zhou, H. SPME/GC-MS characterization of volatile compounds of Chinese traditional-chopped pepper during fermentation. Int. J. Food Prop. 2019, 22, 1863–1872. [Google Scholar] [CrossRef]
- Shahidi, F.; Oh, W.Y. Lipid-derived flavor and off-flavor of traditional and functional foods: An overview. J. Food Bioact. 2020, 10, 20–31. [Google Scholar] [CrossRef]


| No. | Compounds | CAS | Threshold Value (mg/kg) | ROAV Values | ||
|---|---|---|---|---|---|---|
| S | F | SF | ||||
| Alcohols (7) | ||||||
| C1 | 1-Pentanol | 71-41-0 | 0.1502 | — | 3.73 | 13.14 |
| C2 | 1-Adamantanol | 768-95-6 | 2.0000 | — | 39.62 | 14.80 |
| C3 | 1-Octanol | 111-87-5 | 0.1258 | — | 46.77 | |
| C6 | Benzyl alcohol | 100-51-6 | 5.1000 | 0.34 | — | 1.28 |
| C7 | (R,R)-2,3-Butanediol | 24347-58-8 | 0.0951 | 3.73 | — | — |
| C9 | 2-Ethylhexanol | 104-76-7 | 25.4822 | — | 0.49 | — |
| C10 | Citronellol | 106-22-9 | 2.2000 | — | 5.64 | — |
| Ketones (6) | ||||||
| C17 | Acetone | 67-64-1 | 0.8320 | — | — | 3.04 |
| C18 | 2-Heptanone | 110-43-0 | 0.1400 | — | — | 63.91 |
| C20 | 2-Nonanone | 821-55-6 | 0.0820 | — | — | 51.42 |
| C21 | 3-Octen-2-one | 1669-44-9 | 0.2500 | — | — | 33.82 |
| C31 | Acetophenone | 98-86-2 | 1.6000 | 1.57 | ||
| C34 | 3,5-Octadien-2-one | 30086-02-3 | 0.1500 | — | 88.21 | — |
| Esters (4) | ||||||
| C37 | Acetic acid, phenethyl acetate | 103-45-7 | 0.2496 | 3.75 | 30.12 | — |
| C38 | Gamma-octanoic lactone | 104-50-7 | 0.2000 | 48.10 | 22.47 | 51.01 |
| C39 | 2-Phenylethyl propionate | 122-70-3 | 18.0000 | 0.62 | 0.33 | 0.44 |
| C40 | 4-Hexanolide | 695-06-7 | 12.5000 | — | — | 0.09 |
| Acids (4) | ||||||
| C43 | Octanoic acid | 124-07-2 | 3.0000 | 14.08 | 3.94 | 33.66 |
| C45 | Decanoic acid | 334-48-5 | 10.0000 | 2.81 | 1.56 | 4.49 |
| Amines (2) | ||||||
| C51 | Acetamide | 60-35-5 | 160.0000 | — | 0.14 | 0.18 |
| C52 | Aniline | 62-53-3 | 70.1000 | — | 0.32 | — |
| Aromatics (3) | ||||||
| C56 | 4-Hydroxy-3-methoxystyrene | 7786-61-0 | 0.0120 | — | — | 44.04 |
| C58 | Naphthalene | 91-20-3 | 0.0060 | — | — | 27.71 |
| C63 | Benzothiazole | 90-05-1 | 0.0800 | 3.16 | 2.81 | 18.14 |
| Heterocyclics (15) | ||||||
| C64 | 2-Methylpyrazine | 109-08-0 | 30.0000 | 0.79 | 0.14 | 0.87 |
| C65 | 2,5-Dimethylpyrazine | 123-32-0 | 1.7500 | 6.88 | 2.80 | 26.98 |
| C67 | 2-Ethyl-5-methylpyrazine- | 13360-64-0 | 0.0160 | — | 12.79 | — |
| C68 | 3-Ethyl-2,5-dimethylpyrazine | 13360-65-1 | 0.0086 | — | 49.05 | — |
| C70 | 2-Butyl-3-methylpyrazine | 15987-00-5 | 0.2600 | — | — | 11.00 |
| C71 | N-pentylpyrzine | 6303-75-9 | 9.0000 | 2.87 | 1.86 | 10.20 |
| C72 | 5-Ethyl-2,3-dimethylpyrazine | 15707-34-3 | 0.5300 | — | 16.78 | — |
| C74 | 2-Propylpyrazine | 18138-03-9 | 0.1596 | — | — | 6.46 |
| C75 | Pyrazine, 2,5-dimethyl-3-propyl- (8CI,9CI) | 18433-97-1 | 2.0000 | — | 2.00 | — |
| C76 | 2,6-Dimethylpyrazine | 108-50-9 | 0.7180 | — | 5.97 | 49.38 |
| C77 | Pyrazine, 2,3-dimethyl | 5910-89-4 | 0.8000 | — | 5.42 | 7.73 |
| C78 | Tetramethylpyrazine | 1124-11-4 | 2.5250 | 100.00 | 100.00 | 100.00 |
| C79 | 2-n-Butyl furan | 4466-24-4 | 10.0000 | 2.31 | — | — |
| C85 | Pyridine | 110-86-1 | 2.0000 | 12.08 | 10.22 | 15.66 |
| C86 | 2-Acetyl pyrrole | 1072-83-9 | 58.5853 | — | 0.07 | 0.145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shangguan, L.; Liu, Z.; Xu, L.; Yang, Q.; Zhang, X.; Yao, L.; Li, P.; Chen, X.; Dai, J. Effect of Corynebacterium glutamicum Fermentation on the Volatile Flavors of the Enzymatic Hydrolysate of Soybean Protein Isolate. Foods 2024, 13, 2591. https://doi.org/10.3390/foods13162591
Shangguan L, Liu Z, Xu L, Yang Q, Zhang X, Yao L, Li P, Chen X, Dai J. Effect of Corynebacterium glutamicum Fermentation on the Volatile Flavors of the Enzymatic Hydrolysate of Soybean Protein Isolate. Foods. 2024; 13(16):2591. https://doi.org/10.3390/foods13162591
Chicago/Turabian StyleShangguan, Lingling, Zixiong Liu, Linglong Xu, Qiao Yang, Xiaoling Zhang, Lan Yao, Pei Li, Xiong Chen, and Jun Dai. 2024. "Effect of Corynebacterium glutamicum Fermentation on the Volatile Flavors of the Enzymatic Hydrolysate of Soybean Protein Isolate" Foods 13, no. 16: 2591. https://doi.org/10.3390/foods13162591






