Performance of Different Saccharomyces Strains on Secondary Fermentation during the Production of Beer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains, Media, and Culture Conditions
2.2. Cell Concentration and Viability Determination
2.3. Vitality Determination
2.4. Foam Stability
2.5. Apparent Extract (AE), Alcohol Concentration, and pH Measurement
2.6. Volatile Analysis
2.7. Sensory Evaluation of Beer
2.8. Sample Preparation for Proteomic Analysis
2.9. LC-MS/MS
2.10. Data Processing and Analysis
3. Results and Discussion
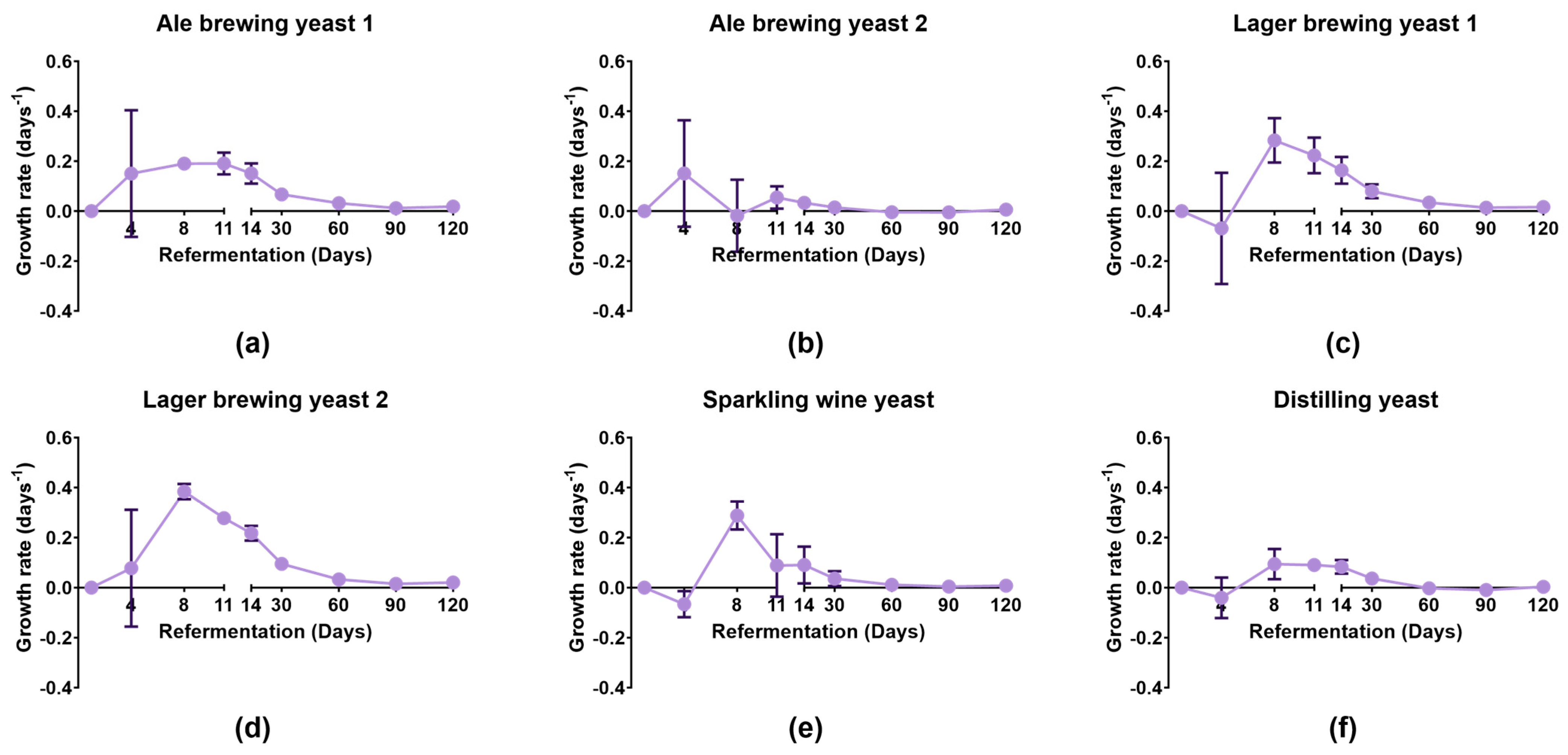
3.1. Growth of Yeast Strains during Bottle Conditioning
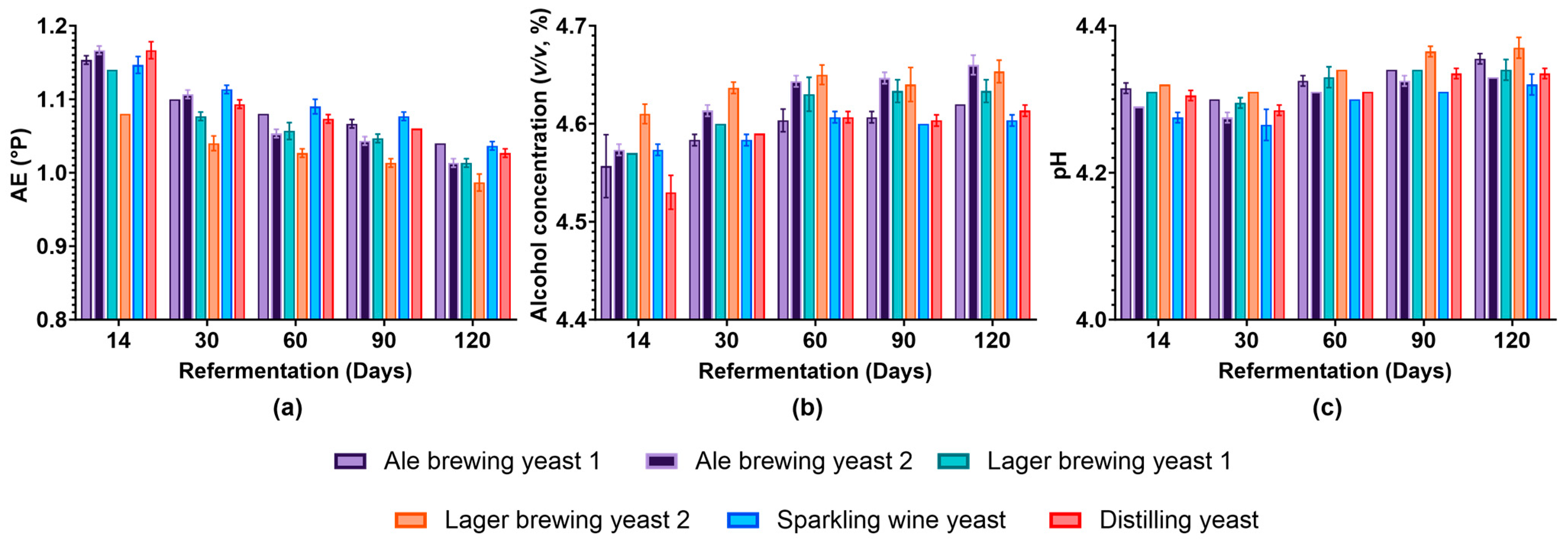
3.2. Impact of Yeast Strain on Beer Characteristics
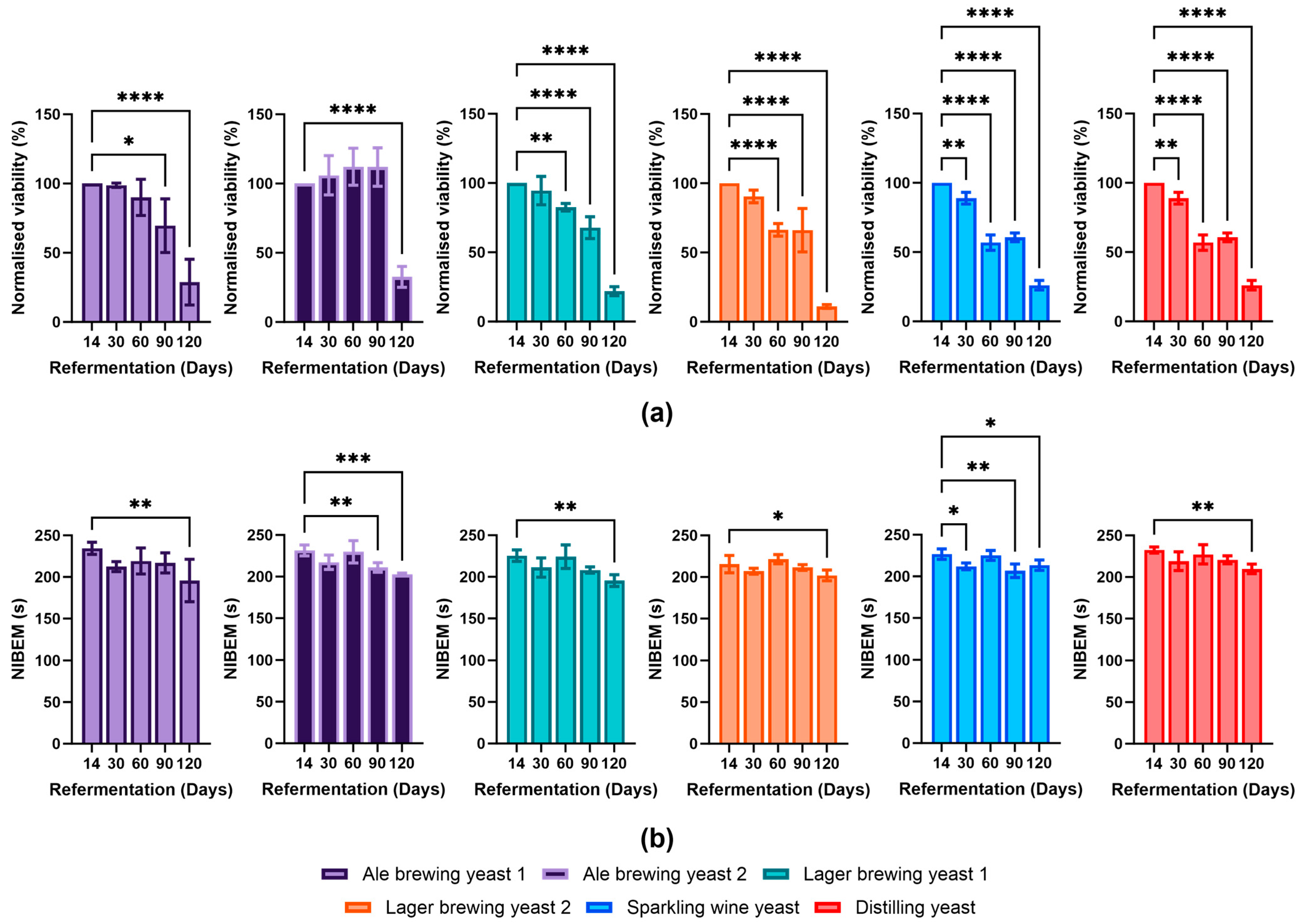
3.3. Yeast Viability and Its Impact on Foam Stability during Bottle Conditioning
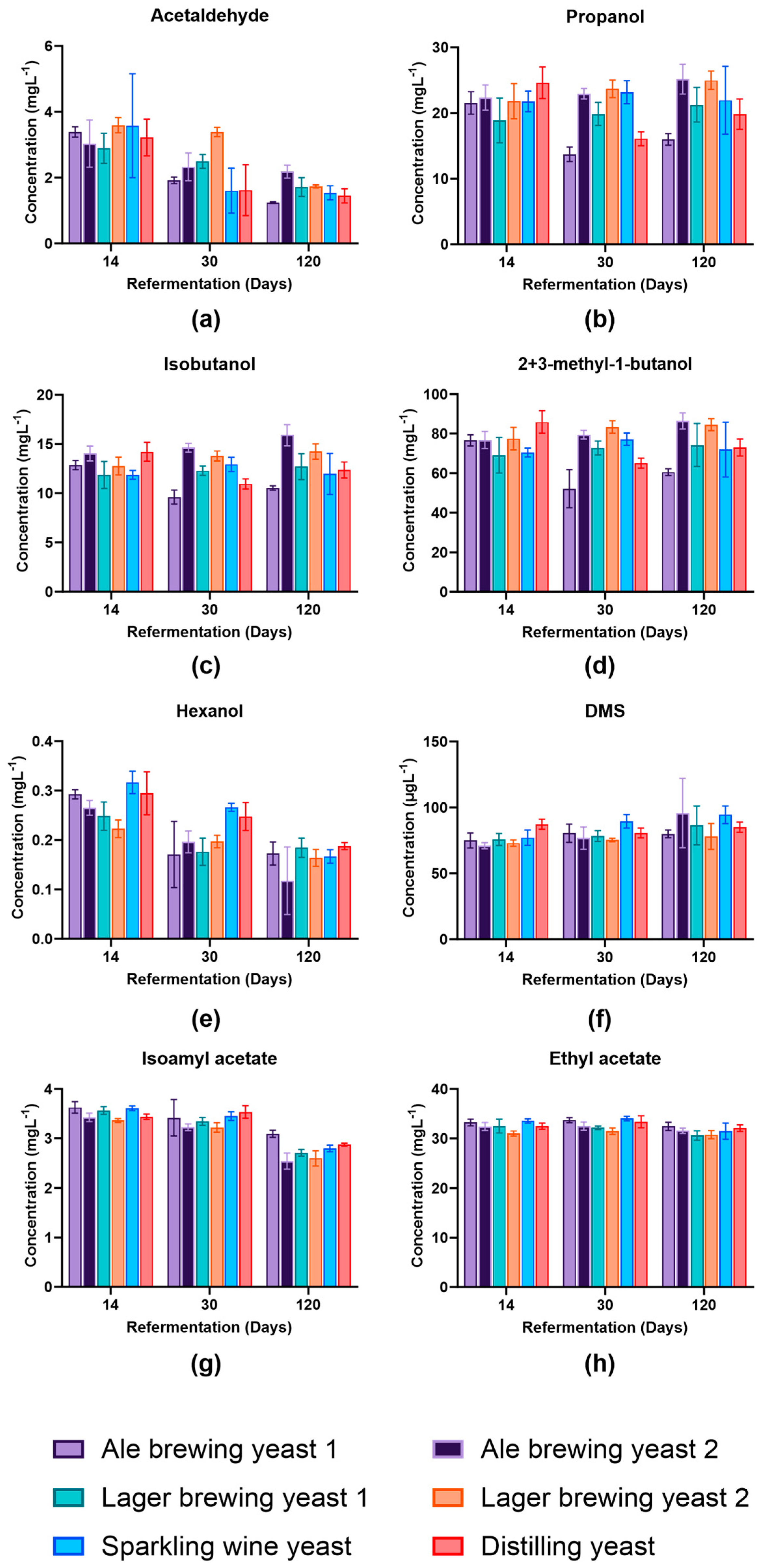
3.4. Impact of Yeast Strain on Volatile Profile
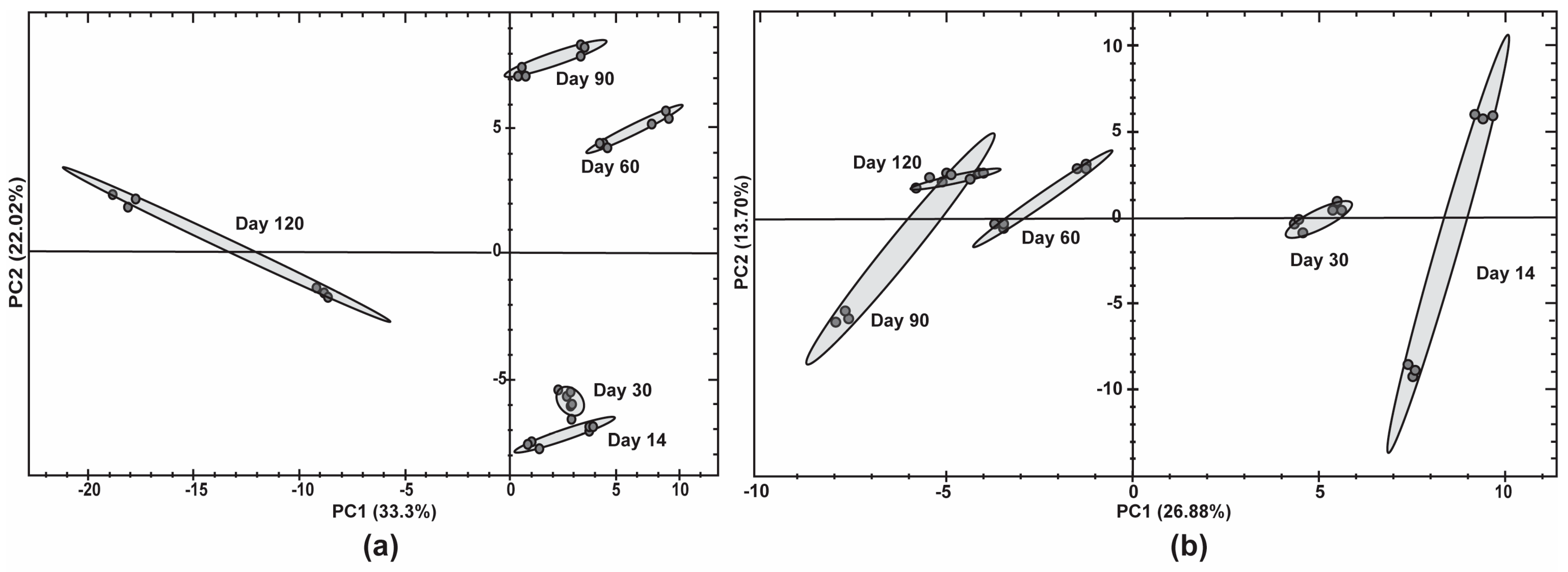
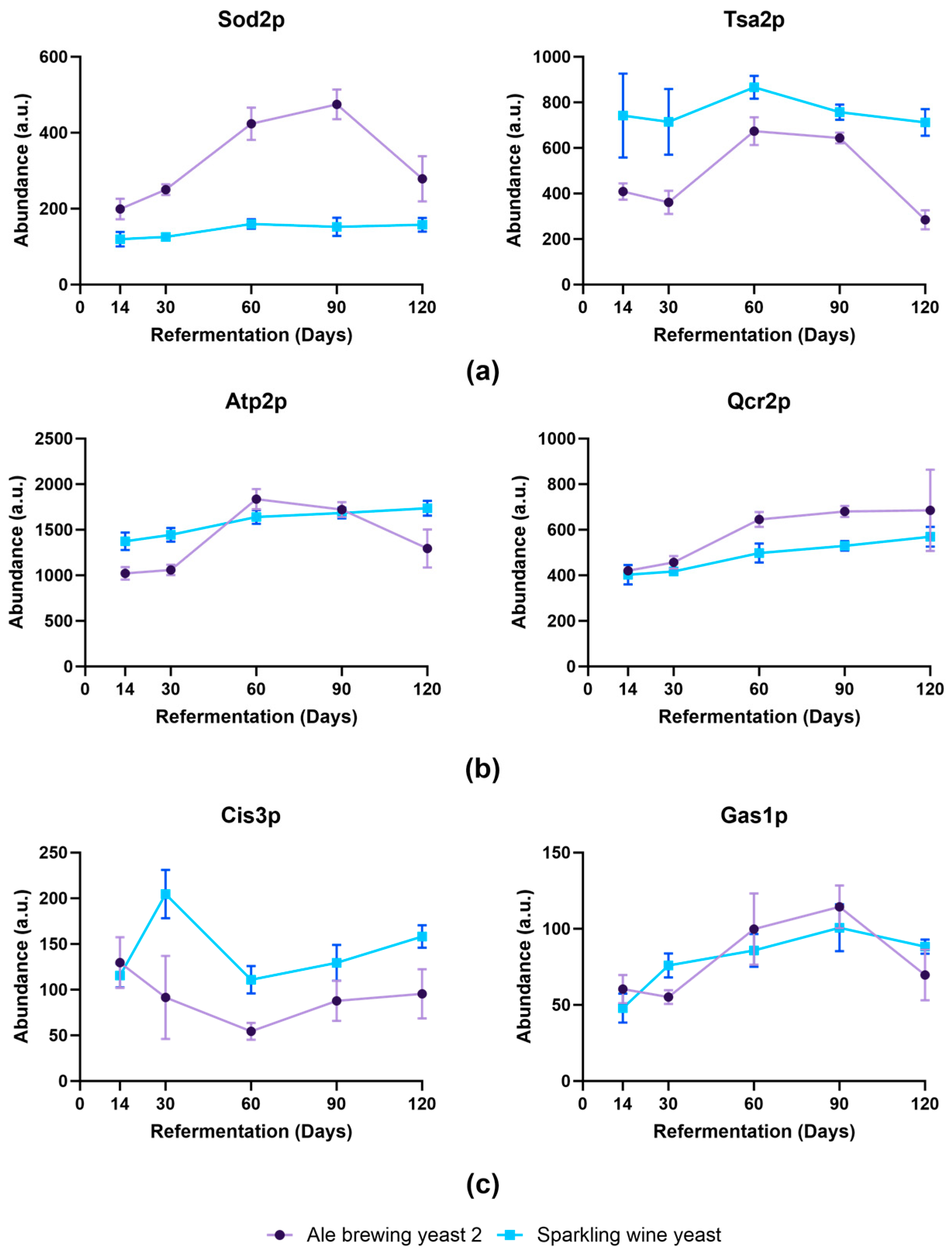
3.5. Proteomic Profiling to Investigate Key Protein Changes That Are Associated with Decreased Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekoninck, T.M.L.; Mertens, T.; Delvaux, F.; Delvaux, F.R. Influence of Beer Characteristics on Yeast Refermentation Performance during Bottle Conditioning of Belgian Beers. J. Am. Soc. Brew. Chem. 2013, 71, 23–34. [Google Scholar] [CrossRef]
- Marconi, O.; Rossi, S.; Galgano, F.; Sileoni, V.; Perretti, G. Influence of yeast strain, priming solution and temperature on beer bottle conditioning. J. Sci. Food Agric. 2016, 96, 4106–4115. [Google Scholar] [CrossRef] [PubMed]
- Derdelinckx, G.; Vanderhasselt, M.; Maudoux, M.; Dufour, M. Refermentation in bottles and kegs: A rigorous approach. Brauwelt Int. 1992, 2, 156–164. [Google Scholar]
- Saison, D.; De Schutter, D.P.; Vanbeneden, N.; Daenen, L.; Delvaux, F.; Delvaux, F.R. Decrease of Aged Beer Aroma by the Reducing Activity of Brewing Yeast. J. Agric. Food Chem. 2010, 58, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Improved Flavor Stability by Aging Beer in the Presence of Yeast. J. Am. Soc. Brew. Chem. 2011, 69, 50–56. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Derdelinckx, G.; Verachtert, H. Bioflavoring and beer refermentation. Appl. Microbiol. Biotechnol. 2003, 62, 140–150. [Google Scholar] [CrossRef]
- Brey, S.E.; de Costa, S.; Rogers, P.J.; Bryce, J.H.; Morris, P.C.; Mitchell, W.J.; Stewart, G.G. The Effect of Proteinase A on Foam-Active Polypeptides During High and Low Gravity Fermentation. J. Inst. Brew. 2003, 109, 194–202. [Google Scholar] [CrossRef]
- Chen, E.C.H.; Jamieson, A.M.; Van Gheluwe, G. The Release of Fatty Acids as a Consequence of Yeast Autolysis. J. Am. Soc. Brew. Chem. 1980, 38, 13–18. [Google Scholar] [CrossRef]
- Penacho, V.; Valero, E.; Gonzalez, R. Transcription profiling of sparkling wine second fermentation. Int. J. Food Microbiol. 2012, 153, 176–182. [Google Scholar] [CrossRef]
- Martínez-García, R.; García-Martínez, T.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J. Changes in sparkling wine aroma during the second fermentation under CO2 pressure in sealed bottle. Food Chem. 2017, 237, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Martí-Raga, M.; Martín, V.; Gil, M.; Sancho, M.; Zamora, F.; Mas, A.; Beltran, G. Contribution of yeast and base wine supplementation to sparkling wine composition. J. Sci. Food Agric. 2016, 96, 4962–4972. [Google Scholar] [CrossRef] [PubMed]
- Dilmetz, B.A.; Desire, C.T.; Meneses, J.; Klingler-Hoffmann, M.; Young, C.; Hoffmann, P. Impact of propagation time on yeast physiology during bottle conditioning of beer on an industrial scale. Food Chem. 2024, 435, 137655. [Google Scholar] [CrossRef] [PubMed]
- Dilmetz, B.A.; Desire, C.T.; Donnellan, L.; Meneses, J.; Klingler-Hoffmann, M.; Young, C.; Hoffmann, P. Assessment of yeast physiology during industrial-scale brewing practices using the redox-sensitive dye resazurin. Yeast 2023, 40, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Gaugaz, F.Z. Fast and Sensitive Total Protein and Peptide Assays for Proteomic Analysis. Anal. Chem. 2015, 87, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Bruderer, R.; Bernhardt, O.M.; Gandhi, T.; Miladinović, S.M.; Cheng, L.-Y.; Messner, S.; Ehrenberger, T.; Zanotelli, V.; Butscheid, Y.; Escher, C.; et al. Extending the Limits of Quantitative Proteome Profiling with Data-Independent Acquisition and Application to Acetaminophen-Treated Three-Dimensional Liver Microtissues. Mol. Cell. Proteom. 2015, 14, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, E.W.; Bandeira, N.; Perez-Riverol, Y.; Sharma, V.; Carver, J.J.; Mendoza, L.; Kundu, D.J.; Wang, S.; Bandla, C.; Kamatchinathan, S.; et al. The ProteomeXchange consortium at 10 years: 2023 update. Nucleic Acids Res. 2022, 51, D1539–D1548. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2021, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.D.; Miyasato, S.R.; Aleksander, S.; Karra, K.; Nash, R.S.; Skrzypek, M.S.; Weng, S.; Engel, S.R.; Cherry, J.M. Saccharomyces genome database update: Server architecture, pan-genome nomenclature, and external resources. Genetics 2023, 224, iyac191. [Google Scholar] [CrossRef]
- Stewart, G.G. Yeast Nutrition. In Brewing and Distilling Yeasts; Springer International Publishing: Cham, Switzerland, 2017; pp. 109–146. [Google Scholar]
- Vanbeneden, N.; Vanderputten, D.; Vanderhaegen, B.; Derdelinckx, G.; Van Landschoot, A. Influence of the Sugar Composition of the Added Extract on the Refermentation of Beer in Bottles. J. Am. Soc. Brew. Chem. 2006, 64, 206–213. [Google Scholar] [CrossRef]
- Werner-Washburne, M.; Braun, E.L.; Crawford, M.E.; Peck, V.M. Stationary phase in Saccharomyces cerevisiae. Mol. Microbiol. 1996, 19, 1159–1166. [Google Scholar] [CrossRef]
- Li, G.; Liu, F. Changes in Organic Acids during Beer Fermentation. J. Am. Soc. Brew. Chem. 2015, 73, 275–279. [Google Scholar] [CrossRef]
- Van Landschoot, A.; Vanbeneden, N.; Vanderputten, D.; Derdelinckx, G. Effect of pitching yeast preparation on the refermentation of beer in bottles. Cerevisia 2004, 29, 140–145. [Google Scholar]
- Song, L.; Chen, Y.; Guo, Q.; Huang, S.; Guo, X.; Xiao, D. Regulating the Golgi apparatus sorting of proteinase A to decrease its excretion in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2019, 46, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Leisegang, R.; Stahl, U. Degradation of a Foam-Promoting Barley Protein by a Proteinase from Brewing Yeast. J. Inst. Brew. 2005, 111, 112–117. [Google Scholar] [CrossRef]
- Michel, M.; Meier-Dörnberg, T.; Jacob, F.; Methner, F.-J.; Wagner, R.S.; Hutzler, M. Review: Pure non-Saccharomyces starter cultures for beer fermentation with a focus on secondary metabolites and practical applications. J. Inst. Brew. 2016, 122, 569–587. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- González-Jiménez, M.d.C.; Mauricio, J.C.; Moreno-García, J.; Puig-Pujol, A.; Moreno, J.; García-Martínez, T. Endogenous CO2 Overpressure Effect on Higher Alcohols Metabolism during Sparkling Wine Production. Microorganisms 2023, 11, 1630. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaegen, B.; Delvaux, F.; Daenen, L.; Verachtert, H.; Delvaux, F.R. Aging characteristics of different beer types. Food Chem. 2007, 103, 404–412. [Google Scholar] [CrossRef]
- Treu, L.; Campanaro, S.; Nadai, C.; Toniolo, C.; Nardi, T.; Giacomini, A.; Valle, G.; Blondin, B.; Corich, V. Oxidative stress response and nitrogen utilization are strongly variable in Saccharomyces cerevisiae wine strains with different fermentation performances. Appl. Microbiol. Biotechnol. 2014, 98, 4119–4135. [Google Scholar] [CrossRef]
- Gibson, B.R.; Lawrence, S.J.; Boulton, C.A.; Box, W.G.; Graham, N.S.; Linforth, R.S.T.; Smart, K.A. The oxidative stress response of a lager brewing yeast strain during industrial propagation and fermentation. FEMS Yeast Res. 2008, 8, 574–585. [Google Scholar] [CrossRef]
- Porras–Agüera, J.A.; Moreno–García, J.; García–Martínez, T.; Moreno, J.; Mauricio, J.C. Impact of CO2 overpressure on yeast mitochondrial associated proteome during the “prise de mousse” of sparkling wine production. Int. J. Food Microbiol. 2021, 348, 109226. [Google Scholar] [CrossRef]
- Stanley, D.; Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar] [CrossRef]
- Cabib, E.; Blanco, N.; Grau, C.; Rodríguez-Peña, J.M.; Arroyo, J. Crh1p and Crh2p are required for the cross-linking of chitin to β(1-6)glucan in the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 2007, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Udom, N.; Chansongkrow, P.; Charoensawan, V.; Auesukaree, C. Coordination of the Cell Wall Integrity and High-Osmolarity Glycerol Pathways in Response to Ethanol Stress in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2019, 85, e00551-19. [Google Scholar] [CrossRef]
- Bermejo, C.; Rodríguez, E.; García, R.; Rodríguez-Peña, J.M.; Concepción, M.L.R.d.l.; Rivas, C.; Arias, P.; Nombela, C.; Posas, F.; Arroyo, J. The Sequential Activation of the Yeast HOG and SLT2 Pathways Is Required for Cell Survival to Cell Wall Stress. Mol. Biol. Cell 2008, 19, 1113–1124. [Google Scholar] [CrossRef]
- García, R.; Bermejo, C.; Grau, C.; Pérez, R.; Rodríguez-Peña, J.M.; Francois, J.; Nombela, C.; Arroyo, J. The Global Transcriptional Response to Transient Cell Wall Damage in Saccharomyces cerevisiae and Its Regulation by the Cell Integrity Signaling Pathway. J. Biol. Chem. 2004, 279, 15183–15195. [Google Scholar] [CrossRef]
- Orlean, P. Architecture and Biosynthesis of the Saccharomyces cerevisiae Cell Wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Vitorino, M.V.; Godinho, C.P.; Bourbon-Melo, N.; Robalo, T.T.; Fernandes, F.; Rodrigues, M.S.; Sá-Correia, I. Yeast adaptive response to acetic acid stress involves structural alterations and increased stiffness of the cell wall. Sci. Rep. 2021, 11, 12652. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, J.; Li, Q. Microarray studies on lager brewer’s yeasts reveal cell status in the process of autolysis. FEMS Yeast Res. 2014, 14, 714–728. [Google Scholar] [CrossRef]
- Rossignol, T.; Dulau, L.; Julien, A.; Blondin, B. Genome-wide monitoring of wine yeast gene expression during alcoholic fermentation. Yeast 2003, 20, 1369–1385. [Google Scholar] [CrossRef]
| Name | Strain | Taxonomic Name | Commercial Use | Supplied Format | Source |
|---|---|---|---|---|---|
| Ale brewing yeast 1 | NA | S. cerevisiae | Primary ale fermentation and secondary fermentation of beer | Provided by agar slant | Coopers Brewery Ltd. (Regency Park, Australia) |
| Ale brewing yeast 2 | NA | S. cerevisiae | Secondary fermentation of beer | Isolated by filtration from a commercial beer | Dan Murphy’s (Adelaide, Australia) |
| Lager brewing yeast 1 | Nebulosa-TUM 66/70 | S. pastorianus | Primary and secondary fermentation of beer | Provided by agar slant | Weihenstephan for Brewing and Food Quality, (Munich, Germany) |
| Lager brewing yeast 2 | SafLager 34/70 | S. pastorianus | Primary lager fermentation | Dried yeast | Coopers Brewery Ltd., (Regency Park, Australia) |
| Sparkling wine yeast | Lalvin EC-1118™ | Saccharomyces cerevisiae bayanus | Secondary fermentation of sparkling wine | Dried yeast | Wine Quip, (Reservoir, Australia) |
| Distilling yeast | DistilaMax® HT | S. cerevisiae | Neutral spirit fermentation | Dried yeast | Wine Quip, (Reservoir, Australia) |
| Condition | Upregulated | Downregulated | ||
|---|---|---|---|---|
| Ale Brewing Yeast 2 | Sparkling Wine Yeast | Ale Brewing Yeast 2 | Sparkling Wine Yeast | |
| D30/D14 | 34 | 85 | 143 | 113 |
| D60/D14 | 230 | 179 | 316 | 183 |
| D90/D14 | 370 | 230 | 290 | 213 |
| D120/D14 | 660 | 223 | 234 | 298 |
| Ale Brewing Yeast 2 | Sparkling Wine Yeast | ||||||
|---|---|---|---|---|---|---|---|
| Term | p-Value * | Number of Proteins | Fold Enriched (%) | Term | p-Value * | Number of Proteins | Fold Enriched (%) |
| D30/D14 | |||||||
| No significant terms | Structural constituent of cell wall | 2.98 | 4 | 4.7 | |||
| D60/D14 | |||||||
| Catalytic activity | 7.02 | 110 | 47.8 | Glucosidase activity | 2.93 | 7 | 3.9 |
| Oxidoreductase activity | 6.22 | 31 | 13.5 | Hydrolase activity, hydrolyzing O-glycosyl compounds | 2.74 | 9 | 5.0 |
| Melatonin binding | 4.81 | 6 | 2.6 | ||||
| Electron transfer activity | 3.33 | 8 | 3.5 | ||||
| Proton transmembrane transporter activity | 2.63 | 11 | 4.8 | ||||
| Translation factor activity, RNA binding | 2.13 | 8 | 3.5 | ||||
| Day 90/Day14 | |||||||
| Catalytic activity | 9.11 | 168 | 45.4 | Catalytic activity | 3.43 | 99 | 43.0 |
| Oxidoreductase activity | 7.12 | 43 | 11.6 | Primary active transmembrane transporter activity | 2.45 | 11 | 4.8 |
| Structural constituent of ribosome | 3.72 | 31 | 8.4 | ||||
| Electron transfer activity | 2.59 | 9 | 2.4 | ||||
| Day 120/Day 14 | |||||||
| Catalytic activity | 5.87 | 104 | 46.6 | Catalytic activity | 5.87 | 104 | 46.6 |
| Ale Brewing Yeast 2 | Sparkling Wine Yeast | ||||||
|---|---|---|---|---|---|---|---|
| Term | p-Value * | Number of Proteins | Fold Enriched (%) | Term | p-Value * | Number of Proteins | Fold Enriched (%) |
| D30/D14 | |||||||
| No significant terms | No significant terms | ||||||
| D60/D14 | |||||||
| RNA polymerase III activity | 4.29 | 8 | 2.5 | No significant terms | |||
| Day 90/Day 14 | |||||||
| 5′-3′ RNA polymerase activity | 2.91 | 9 | 3.1 | No significant terms | |||
| Day 120/Day 14 | |||||||
| Structural molecule activity | 5.63 | 32 | 13.7 | Structural molecule activity | 9.61 | 44 | 14.8 |
| Endopeptidase inhibitor activity | 2.15 | 3 | 1.3 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dilmetz, B.A.; Brar, G.; Desire, C.T.; Meneses, J.; Klingler-Hoffmann, M.; Young, C.; Hoffmann, P. Performance of Different Saccharomyces Strains on Secondary Fermentation during the Production of Beer. Foods 2024, 13, 2593. https://doi.org/10.3390/foods13162593
Dilmetz BA, Brar G, Desire CT, Meneses J, Klingler-Hoffmann M, Young C, Hoffmann P. Performance of Different Saccharomyces Strains on Secondary Fermentation during the Production of Beer. Foods. 2024; 13(16):2593. https://doi.org/10.3390/foods13162593
Chicago/Turabian StyleDilmetz, Brooke A., Gurpreet Brar, Christopher T. Desire, Jon Meneses, Manuela Klingler-Hoffmann, Clifford Young, and Peter Hoffmann. 2024. "Performance of Different Saccharomyces Strains on Secondary Fermentation during the Production of Beer" Foods 13, no. 16: 2593. https://doi.org/10.3390/foods13162593






