Novel Carboxymethyl Cellulose/Gelatin-Based Film Incorporated with Zein-Stabilized Lemon Essential Oil Pickering Emulsion for the Preservation of Cherries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
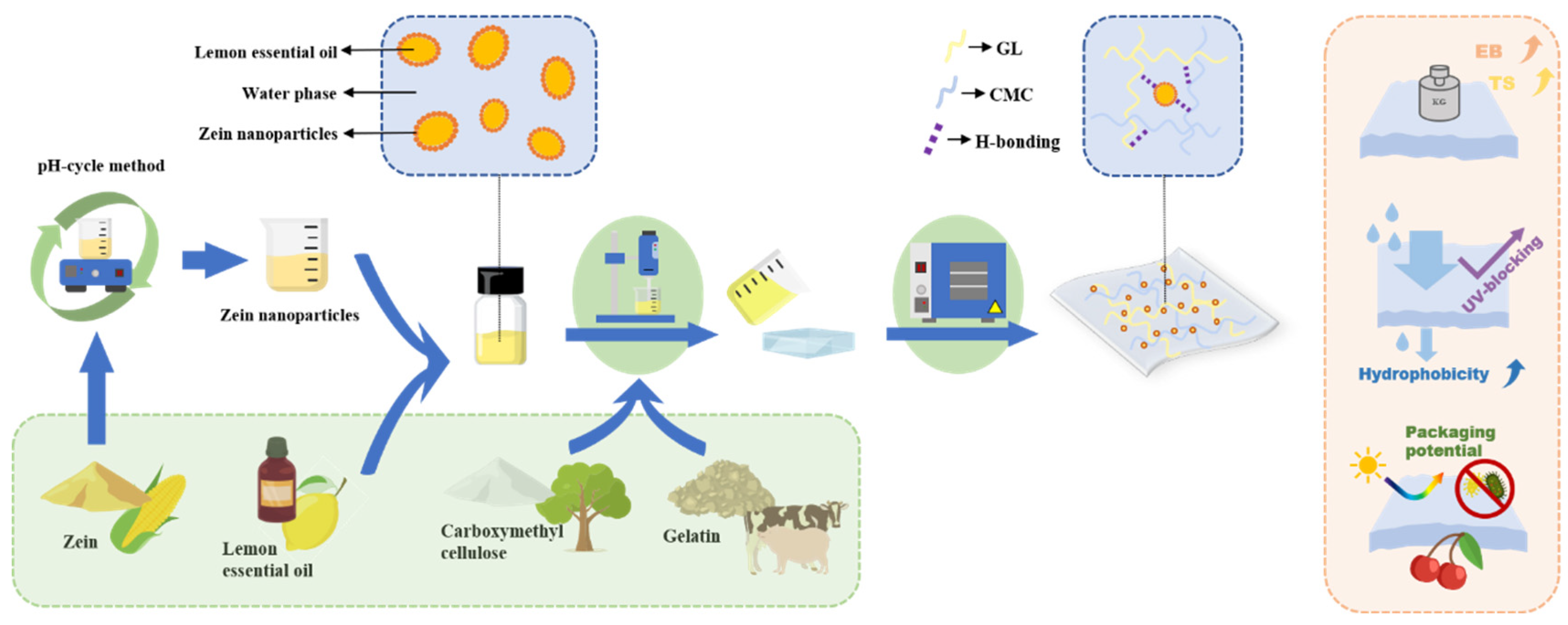
2.2. Preparation of ZLPE
2.3. Characterization of ZLPE
2.3.1. Droplet Size Distribution and Zeta Potential
2.3.2. Confocal Laser Scanning Microscopy (CLSM)
2.4. Fabrication of Composite Films
2.5. Rheological Properties
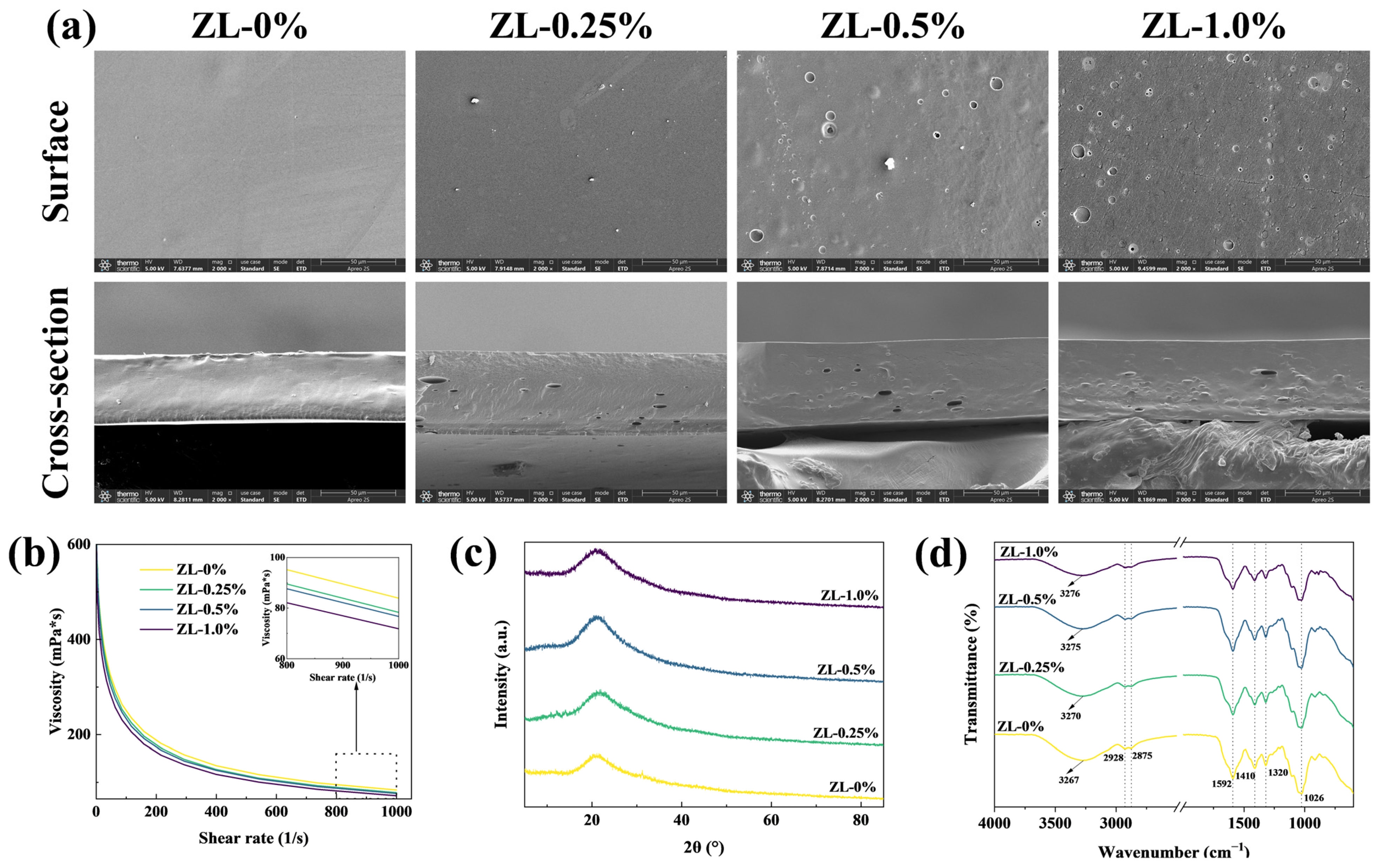
2.6. Structure and Morphology
2.6.1. Scanning Electron Microscope (SEM)
2.6.2. X-ray Diffraction (XRD)
2.6.3. Fourier Transform Infrared (FT-IR)
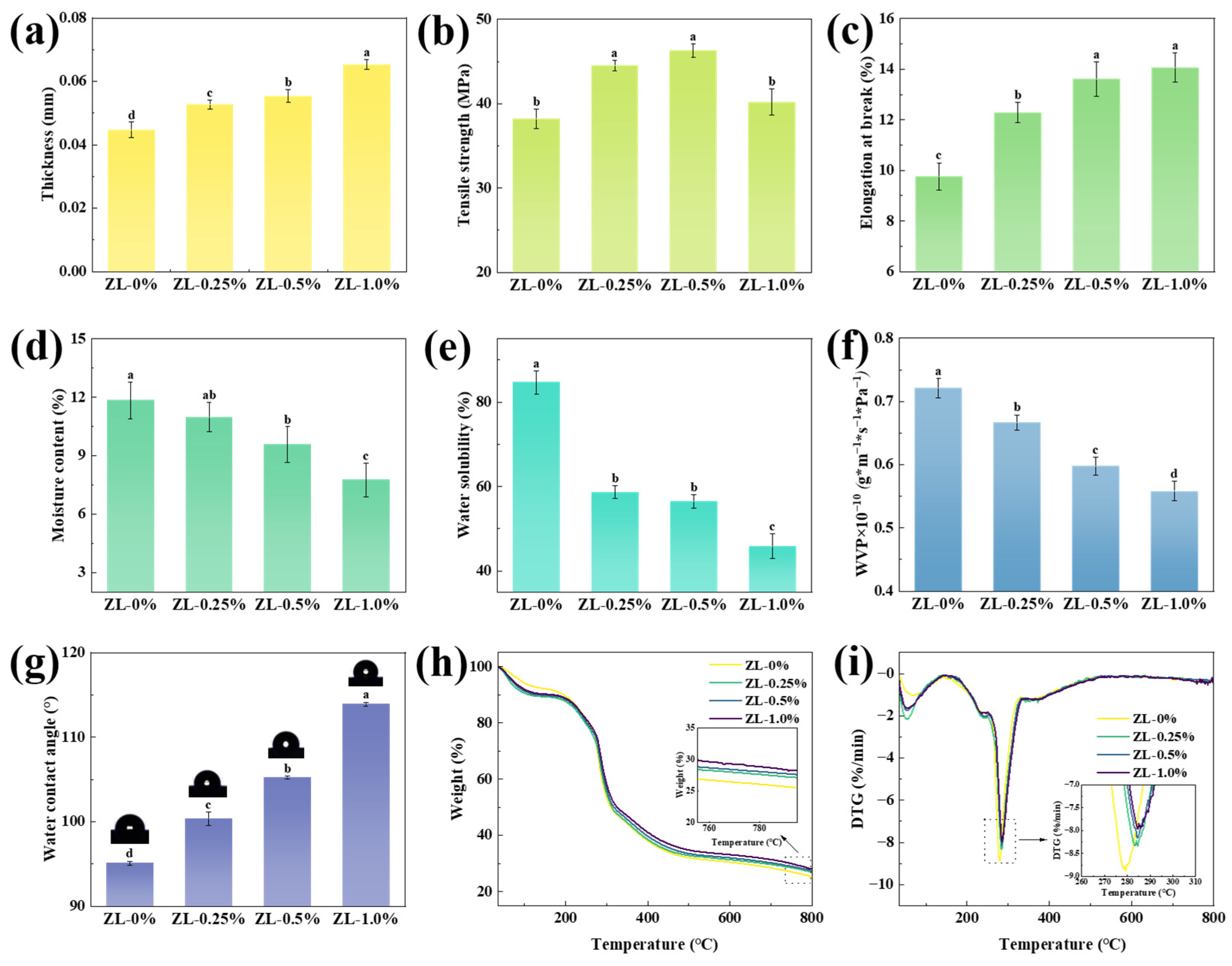
2.7. Mechanical Properties
2.7.1. Thickness
2.7.2. Tensile Strength (TS) and Elongation at Break (EB)
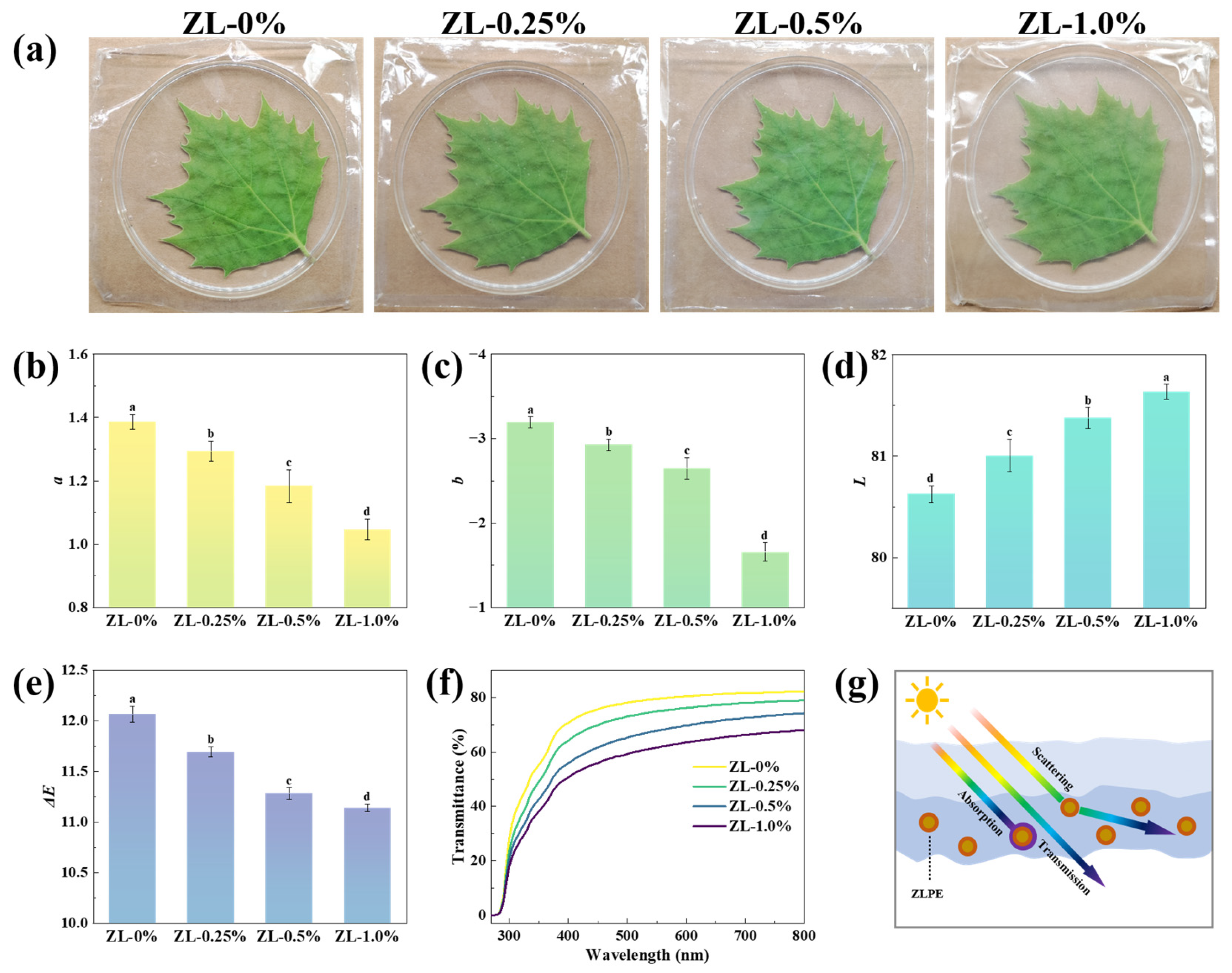
2.8. Optical Properties
2.8.1. Appearance and Color
2.8.2. Transmittance
2.9. Water-Resistance Properties
2.9.1. Moisture Content (MC) and Water Solubility (WS)
2.9.2. Water Vapor Permeability (WVP)
2.9.3. Water Contact Angle (WCA)
2.10. Thermogravimetric Analysis (TGA)
2.11. Antioxidant Activity
2.12. Antibacterial Activity
2.13. Application for Cherry Preservation
2.13.1. Appearance of Cherries
2.13.2. Weight Loss Rate of Cherries
2.13.3. Firmness of Cherries
2.13.4. Soluble Solids Content of Cherries
2.14. Statistical Analysis
3. Results and Discussion
3.1. Characterization of ZLPE
3.2. Characterization of the Composite Films
3.2.1. Rheological Properties of the Film-Forming Solutions
3.2.2. Morphology and Structure
3.2.3. Optical Properties
3.2.4. Mechanical Properties
3.2.5. Water-Resistance Properties
3.2.6. Thermal Stabilities
3.3. Antioxidant Activity and Antibacterial Activity
3.4. Preservative Ability
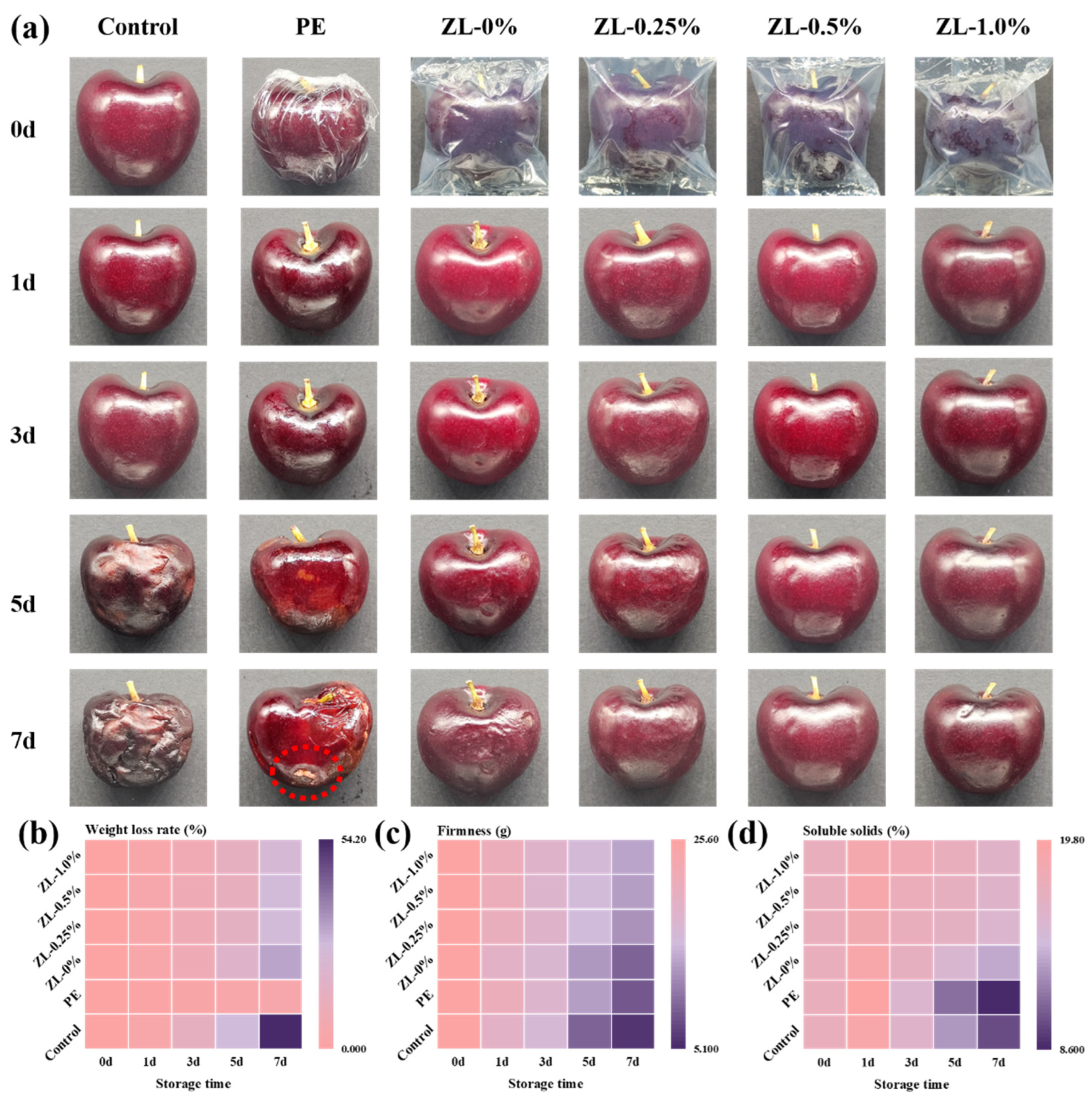
3.4.1. Appearance
3.4.2. Weight Loss Rate
3.4.3. Firmness
3.4.4. Soluble Solid Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bachmann, M.; Zibunas, C.; Hartmann, J.; Tulus, V.; Suh, S.; Guillen-Gosalbez, G.; Bardow, A. Towards circular plastics within planetary boundaries. Nat. Sustain. 2023, 6, 599–610. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernandez, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Yildirim-Yalcin, M.; Tornuk, F.; Toker, O.S. Recent advances in the improvement of carboxymethyl cellulose-based edible films. Trends Food Sci. Technol. 2022, 129, 179–193. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Zhang, J.; Yao, J. Molecular interactions in gelatin/chitosan composite films. Food Chem. 2017, 235, 45–50. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Szymanowska, U.; Skrzypek, T.; Basiura-Cembala, M.; Lupina, K.; Biendl, M. Edible films based on gelatin, carboxymethyl cellulose, and their blends as carriers of potassium salts of iso- α -acids: Structural, physicochemical and antioxidant properties. Food Hydrocoll. 2021, 115, 106574. [Google Scholar] [CrossRef]
- Khan, S.; Abdo, A.A.A.; Shu, Y.; Zhang, Z.; Liang, T. The Extraction and Impact of Essential Oils on Bioactive Films and Food Preservation, with Emphasis on Antioxidant and Antibacterial Activities—A Review. Foods 2023, 12, 4169. [Google Scholar] [CrossRef]
- Freche, E.; Gieng, J.; Pignotti, G.; Ibrahim, S.A.; Feng, X. Applications of lemon or cinnamon essential oils in strawberry fruit preservation: A review. J. Food Process. Preserv. 2022, 46, 16526. [Google Scholar] [CrossRef]
- Magalhaes, D.; Vilas-Boas, A.A.; Teixeira, P.; Pintado, M. Functional Ingredients and Additives from Lemon by-Products and Their Applications in Food Preservation: A Review. Foods 2023, 12, 1095. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Rhim, J.-W. Gelatin/agar-based multifunctional film integrated with copper-doped zinc oxide nanoparticles and clove essential oil Pickering emulsion for enhancing the shelf life of pork meat. Food Res. Int. 2022, 160, 111690. [Google Scholar] [CrossRef]
- Shao, P.; Yu, J.; Chen, H.; Gao, H. Development of microcapsule bioactive paper loaded with cinnamon essential oil to improve the quality of edible fungi. Food Packag. Shelf 2021, 27, 100617. [Google Scholar] [CrossRef]
- Ming, L.; Wu, H.; Liu, A.; Naeem, A.; Dong, Z.; Fan, Q.; Zhang, G.; Liu, H.; Li, Z. Evolution and critical roles of particle properties in Pickering emulsion: A review. J. Mol. Liq. 2023, 388, 122775. [Google Scholar] [CrossRef]
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-Grade Pickering Emulsions: Preparation, Stabilization and Applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Wang, Y. Nanocellulose-stabilized Pickering emulsions: Fabrication, stabilization, and food applications. Adv. Colloid Interface Sci. 2023, 318, 102970. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Bio-nano-composites containing at least two components, chitosan and zein, for food packaging applications: A review of the nano-composites in comparison with the conventional counterparts. Carbohydr. Polym. 2022, 280, 119027. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chu, Y.; Feng, X.; Gao, C.; Wu, D.; Cheng, W.; Meng, L.; Zhang, Y.; Tang, X. Effects of zein stabilized clove essential oil Pickering emulsion on the structure and properties of chitosan-based edible films. Int. J. Biol. Macromol. 2020, 156, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, C.; Liu, Y.; He, Z.; Zhang, M.; Wang, C.; Yang, Z.; Li, P. Enhanced mechanical properties and antibacterial activities of chitosan films through incorporating zein-gallic acid conjugate stabilized cinnamon essential oil Pickering emulsion. Int. J. Biol. Macromol. 2024, 258, 128933. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Gao, Y.; Zhong, Q. Effects of acidification by glucono-delta-lactone or hydrochloric acid on structures of zein-caseinate nanocomplexes self-assembled during a pH cycle. Food Hydrocoll. 2018, 82, 173–185. [Google Scholar] [CrossRef]
- Liu, F.; Chan, W.; Chen, M.; Xu, F.; Ma, J.; Zhong, F. Tailoring physicochemical properties of chitosan films and their protective effects on meat by varying drying temperature. Carbohydr. Polym. 2019, 212, 150–159. [Google Scholar] [CrossRef]
- Yang, Z.; Li, M.; Li, Y.; Li, Z.; Huang, X.; Wang, X.; Shi, J.; Zou, X.; Zhai, X.; Povey, M.; et al. Improving properties of Litsea cubeba oil Pickering emulsion-loaded gelatin-based bio-nanocomposite film via optimizing blending ratio: Application for mango preservation. Food Hydrocoll. 2023, 145, 109052. [Google Scholar] [CrossRef]
- Gao, J.; Liang, H.; Li, S.; Zhou, B. Development of zein/soluble soybean polysaccharide nanoparticle-stabilized Pickering emulsions. J. Food Sci. 2021, 86, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ni, X.; Wen, M.; Lou, S.; Xiao, W.; Gao, Z. Preparation of antioxidant konjac glucomannan-based films enriched with Ocimum gratissimum L. essential oil Pickering emulsion and its effect on walnuts preservation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 665, 131220. [Google Scholar] [CrossRef]
- Xu, J.; He, M.; Wei, C.; Duan, M.; Yu, S.; Li, D.; Zhong, W.; Tong, C.; Pang, J.; Wu, C. Konjac glucomannan films with Pickering emulsion stabilized by TEMPO-oxidized chitin nanocrystal for active food packaging. Food Hydrocoll. 2023, 139, 108539. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, S.; Hu, Y.; Fu, Q.; Cheng, X.; Li, Y.; Wu, P.; Li, H.; Ai, S. Pectin-based film activated with carboxylated cellulose nanocrystals-stabilized oregano essential oil Pickering emulsion. Food Hydrocoll. 2024, 151, 109781. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, M.; Zhang, Z.; Li, C.; Xia, G.; Shi, H.; Liu, Z. Chitosan-based edible film incorporated with wampee (Clausena lansium) seed essential oil: Preparation, characterization and biological activities. Int. J. Biol. Macromol. 2023, 253, 127683. [Google Scholar] [CrossRef]
- Zhang, S.; He, Z.; Xu, F.; Cheng, Y.; Waterhouse, G.I.N.; Sun-Waterhouse, D.; Wu, P. Enhancing the performance of konjac glucomannan films through incorporating zein-pectin nanoparticle-stabilized oregano essential oil Pickering emulsions. Food Hydrocoll. 2022, 124, 107222. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, L.; Huang, H.; Xu, J.; Li, Y.; Yu, W.; Peng, S.; Zou, L.; Liu, W. The improvement of water barrier property in gelatin/carboxymethyl cellulose composite film by electrostatic interaction regulation and its application in strawberry preservation. Food Chem. 2024, 450, 139352. [Google Scholar] [CrossRef]
- Zhang, K.; Ren, T.; Harper, D.; Li, M. Development of antimicrobial films with cinnamaldehyde stabilized by ethyl lauroyl arginate and cellulose nanocrystals. Food Packag. Shelf 2022, 33, 100886. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Dunno, K.D.; Cavender, G.A.; Dawson, P. Fabrication and characterization of active nanocomposite films loaded with cellulose nanocrystals stabilized Pickering emulsion of clove bud oil. Int. J. Biol. Macromol. 2023, 224, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yan, X.; Cheng, M.; Wang, Y.; Wang, Y.; Wang, K.; Wang, X.; Wang, J. Effect of Pickering emulsion on the physical properties, microstructure and bioactivity of corn starch/cassia gum composite films. Food Hydrocoll. 2023, 141, 108713. [Google Scholar] [CrossRef]
- Zhao, F.; Huang, J.; Qi, J.; Li, Q.; Wu, H.; Ju, J. Proteomic analysis of antifungal mechanism of star anise essential oil against Aspergillus niger and its application potential in prolonging bread shelf life. LWT Food Sci. 2022, 169, 114023. [Google Scholar] [CrossRef]
- He, B.; Wang, Y.; Jiang, Z.; Liu, S.; Zhu, J. Physical properties and antibacterial activity of the composited films based on carboxymethyl cellulose and gelatin functionalized with ε-polylysine. Int. J. Biol. Macromol. 2021, 191, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, Y.; Liu, Y.; Zhang, J.; Hossen, M.A.; Sameen, D.E.; Dai, J.; Li, S.; Qin, W. Fabrication and characterization of pH-responsive intelligent films based on carboxymethyl cellulose and gelatin/curcumin/chitosan hybrid microcapsules for pork quality monitoring. Food Hydrocoll. 2022, 124, 107224. [Google Scholar] [CrossRef]
- Fernanda Vargas-Torrico, M.; von Borries-Medrano, E.; Aguilar-Mendez, M.A. Development of gelatin/carboxymethylcellulose active films containing Hass avocado peel extract and their application as a packaging for the preservation of berries. Int. J. Biol. Macromol. 2022, 206, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, Q.; Xiao, J.; You, L.; Zhu, S.; Li, C.; Fu, X. Physicochemical and functional properties of chitosan-based edible film incorporated with Sargassum pallidum polysaccharide nanoparticles. Food Hydrocoll. 2023, 138, 108476. [Google Scholar] [CrossRef]
- Ahmad, H.N.; Yong, Y.; Wang, S.; Munawar, N.; Zhu, J. Development of novel carboxymethyl cellulose/gelatin-based edible films with pomegranate peel extract as antibacterial/antioxidant agents for beef preservation. Food Chem. 2024, 443, 138511. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Wang, D.; Wen, X.; Li, X.; Fang, F.; Richel, A.; Xiao, N.; Fauconnier, M.-L.; Hou, C.; Zhang, D. Incorporation of cinnamon essential oil-loaded Pickering emulsion for improving antimicrobial properties and control release of chitosan/ gelatin films. Food Hydrocoll. 2023, 138, 108438. [Google Scholar] [CrossRef]
- Ran, R.; Zheng, T.; Tang, P.; Xiong, Y.; Yang, C.; Gu, M.; Li, G. Antioxidant and antimicrobial collagen films incorporating Pickering emulsions of cinnamon essential oil for pork preservation. Food Chem. 2023, 420, 136108. [Google Scholar] [CrossRef]
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-based films enriched with nanocellulose-stabilized Pickering emulsions containing different essential oils for possible applications in food packaging. Food Packag. Shelf 2021, 27, 100615. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Shen, R.; Yang, X. Characterizations of novel konjac glucomannan emulsion films incorporated with high internal phase Pickering emulsions. Food Hydrocoll. 2020, 109, 106088. [Google Scholar] [CrossRef]
- Chen, J.; Luo, L.; Cen, C.; Liu, Y.; Li, H.; Wang, Y. The nano antibacterial composite film carboxymethyl chitosan/gelatin/ nano ZnO improves the mechanical strength of food packaging. Int. J. Biol. Macromol. 2022, 220, 462–471. [Google Scholar] [CrossRef]
- Yang, Z.; Li, M.; Li, Y.; Huang, X.; Li, Z.; Zhai, X.; Shi, J.; Zou, X.; Xiao, J.; Sun, Y.; et al. Sodium alginate/guar gum based nanocomposite film incorporating β-Cyclodextrin/persimmon pectin-stabilized baobab seed oil Pickering emulsion for mushroom preservation. Food Chem. 2024, 437, 137891. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Gimenez, B.; Gomez-Estaca, J.; Aleman, A.; Gomez-Guillen, M.C.; Montero, M.P. Improvement of the antioxidant properties of squid skin gelatin films by the addition of hydrolysates from squid gelatin. Food Hydrocoll. 2009, 23, 1322–1327. [Google Scholar] [CrossRef]
- Liu, T.; Gao, Z.; Zhong, W.; Fu, F.; Li, G.; Guo, J.; Shan, Y. Preparation, Characterization, and Antioxidant Activity of Nanoemulsions Incorporating Lemon Essential Oil. Antioxidants 2022, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Meng, F.-B.; Lv, H.-J.; Gou, Z.-Z.; Qiu, J.; Li, Y.-C. Study on the bacteriostasis of lemon essential oil and the application of lemon essential oil nanoemulsion on fresh-cut kiwifruit. Front. Sustain. Food Syst. 2024, 8, 1394831. [Google Scholar] [CrossRef]
- Kasaai, M.R. Zein and zein -based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial activity and mechanism of limonene against Staphylococcus aureus. J. Food Saf. 2021, 41, 12918. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 2014, 106, 190–199. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Tang, J.; Zhang, H.; Dai, J.; Li, S.; Yan, J.; Qin, W.; Liu, Y. Preparation of sodium alginate/konjac glucomannan active films containing lycopene microcapsules and the effects of these films on sweet cherry preservation. Int. J. Biol. Macromol. 2022, 215, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Zhao, B.; Zhang, P.; Li, P.; Wei, X.; Song, K. Edible composite films: Enhancing the postharvest preservation of blueberry. Hortic. Environ. Biotechnol. 2024, 65, 355–373. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.; Sheng, W.; Yang, L.; Yang, Y.; Tang, T.; Wang, C.; Jiang, G.; Tian, Y. Novel Carboxymethyl Cellulose/Gelatin-Based Film Incorporated with Zein-Stabilized Lemon Essential Oil Pickering Emulsion for the Preservation of Cherries. Foods 2024, 13, 2602. https://doi.org/10.3390/foods13162602
He K, Sheng W, Yang L, Yang Y, Tang T, Wang C, Jiang G, Tian Y. Novel Carboxymethyl Cellulose/Gelatin-Based Film Incorporated with Zein-Stabilized Lemon Essential Oil Pickering Emulsion for the Preservation of Cherries. Foods. 2024; 13(16):2602. https://doi.org/10.3390/foods13162602
Chicago/Turabian StyleHe, Kaiwen, Wenyang Sheng, Li Yang, Yicheng Yang, Tingting Tang, Chenzhi Wang, Guangyang Jiang, and Yongqiang Tian. 2024. "Novel Carboxymethyl Cellulose/Gelatin-Based Film Incorporated with Zein-Stabilized Lemon Essential Oil Pickering Emulsion for the Preservation of Cherries" Foods 13, no. 16: 2602. https://doi.org/10.3390/foods13162602




