Utilization of Germinated Seeds as Functional Food Ingredients: Optimization of Nutrient Composition and Antioxidant Activity Evolution Based on the Germination Characteristics of Chinese Chestnut (Castanea mollissima)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Germination and Sample Preparation
2.3. Length, Diameter, and Fresh Weight of Sprouts
2.4. Determination of Amylose and Amylopectin
2.5. Determination of Sugar Components
2.6. Determination of Vitamin C Content
2.7. Determination of GABA Content
2.8. Determination of Total Phenolic Content
2.9. Determination of Total Flavonoid Content
2.10. DPPH Radical Scavenging Activity
2.11. ABTS Radical Scavenging Activity
2.12. Statistical Analysis
3. Results
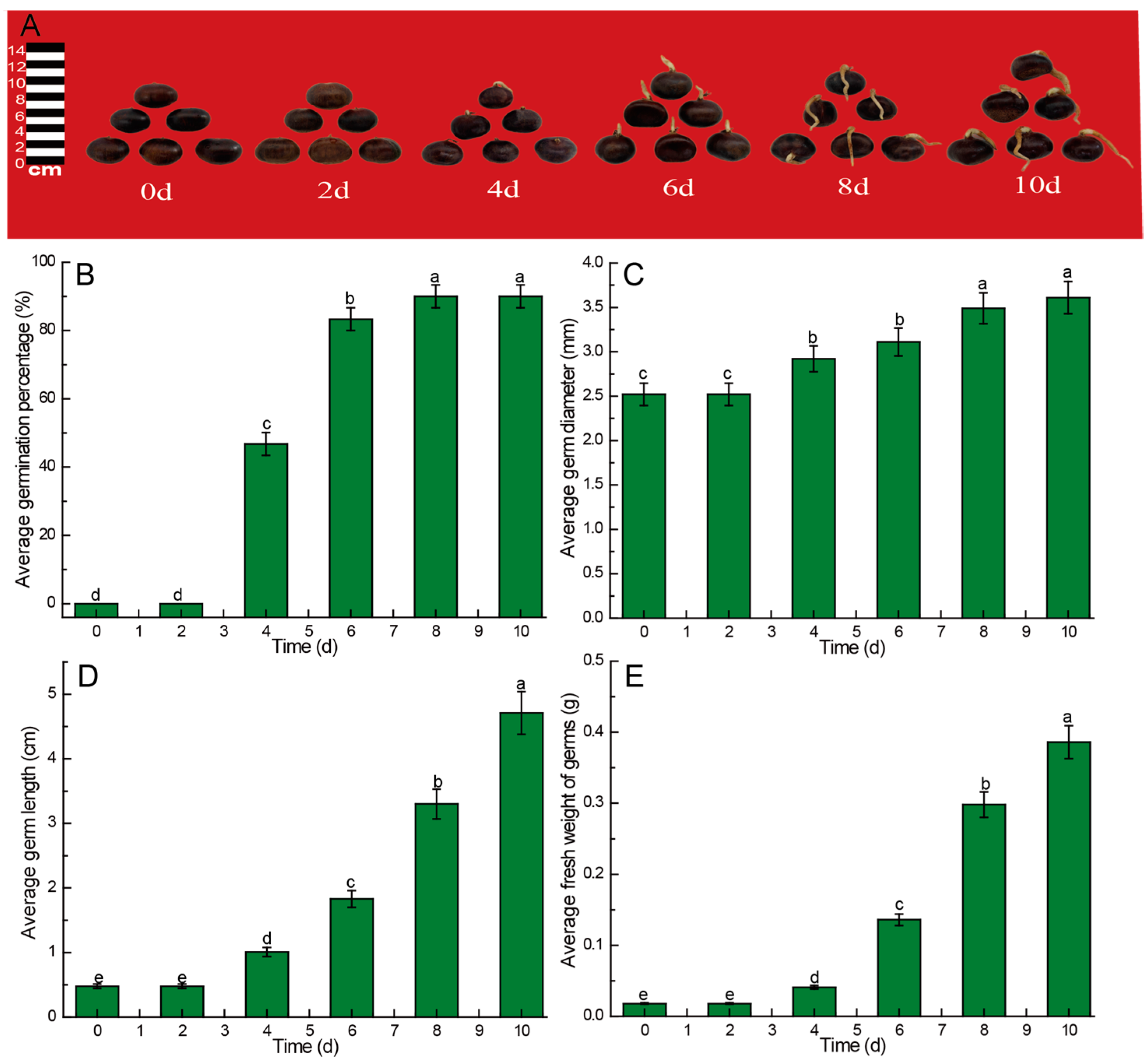
3.1. Effect of Germination Time on the Growth of Chinese Chestnut Sprouts
3.2. Effects of Germination Time on Nutrient Composition in Different Parts of Germinated Chinese Chestnuts
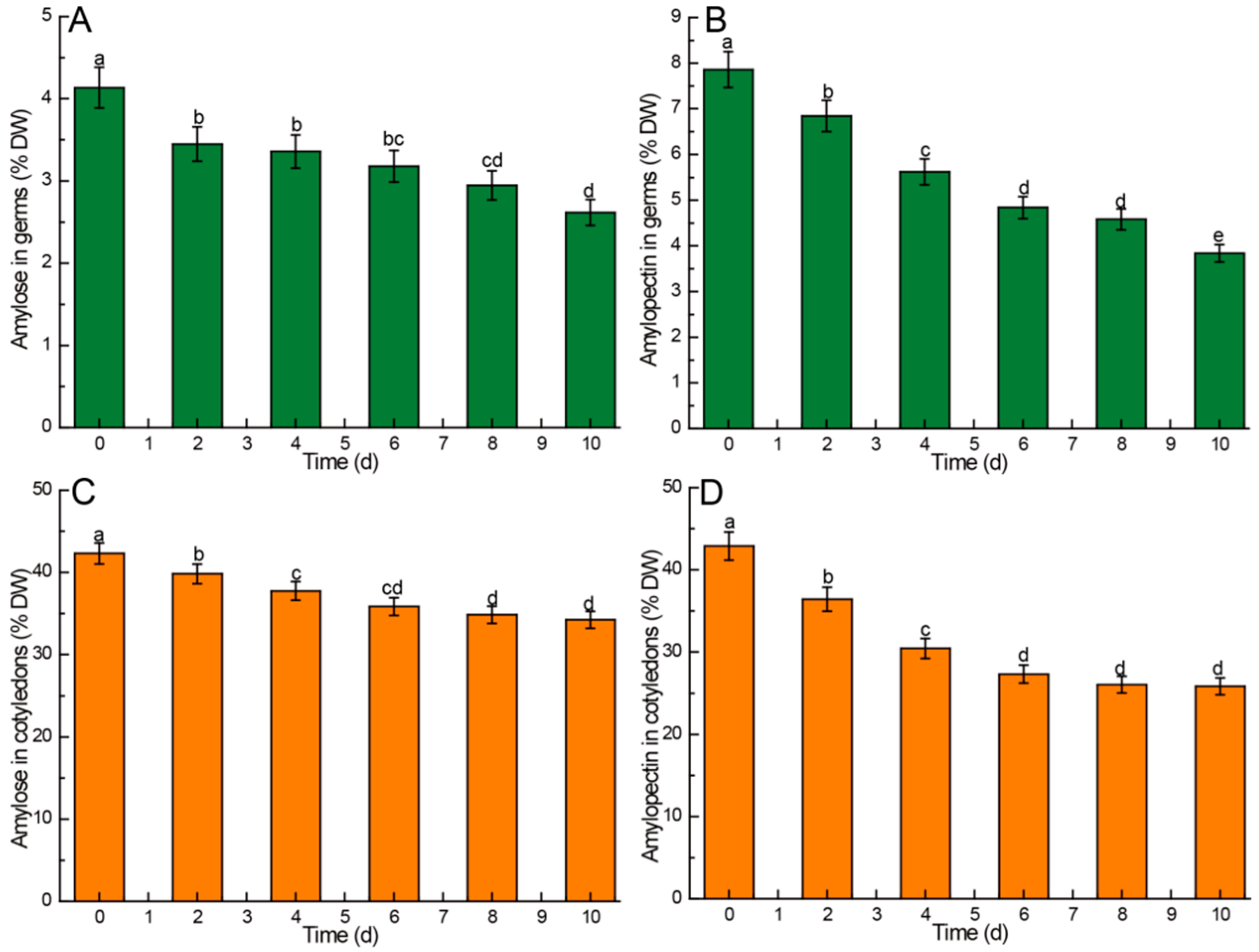
3.2.1. Amylose and Amylopectin
3.2.2. Sugar Components
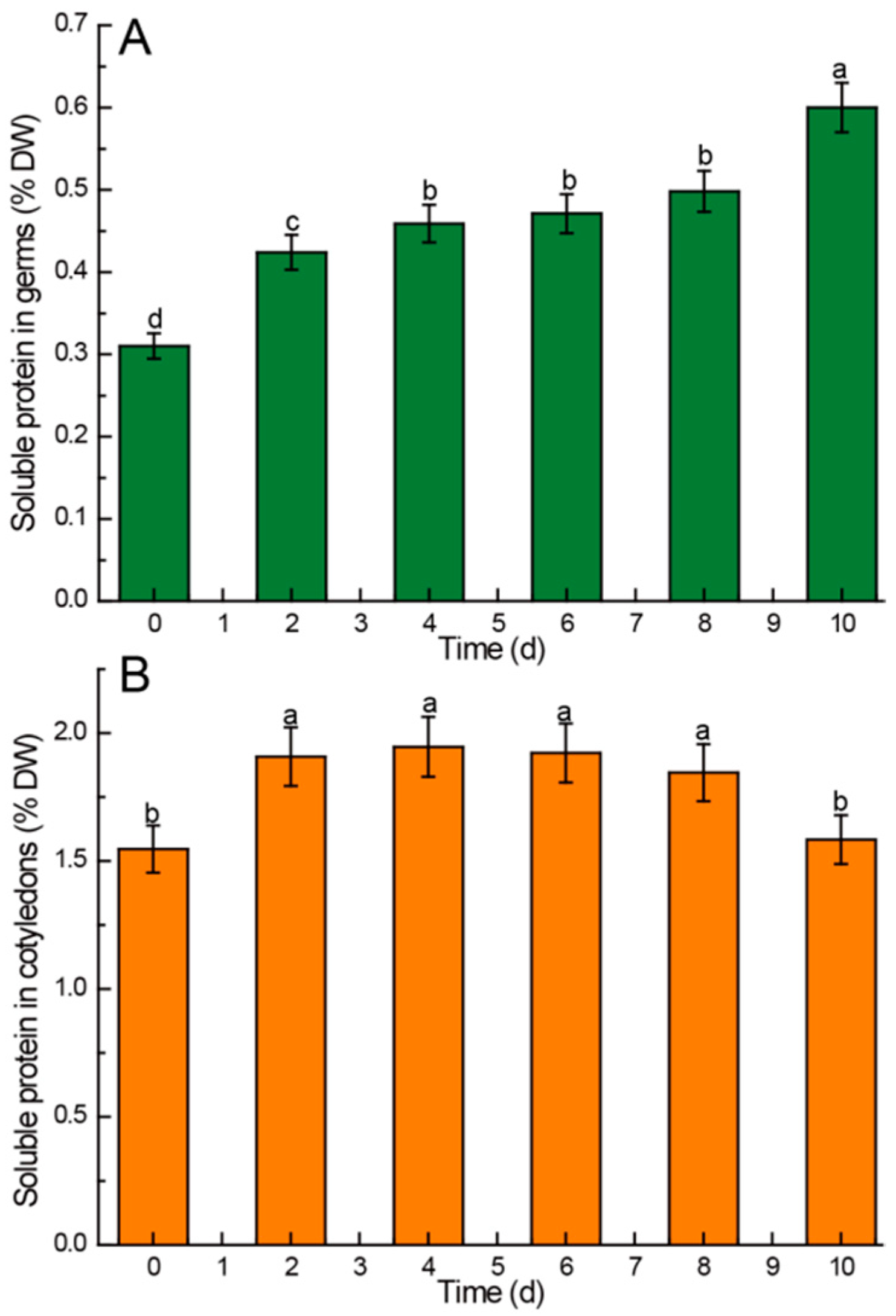
3.2.3. Soluble Protein
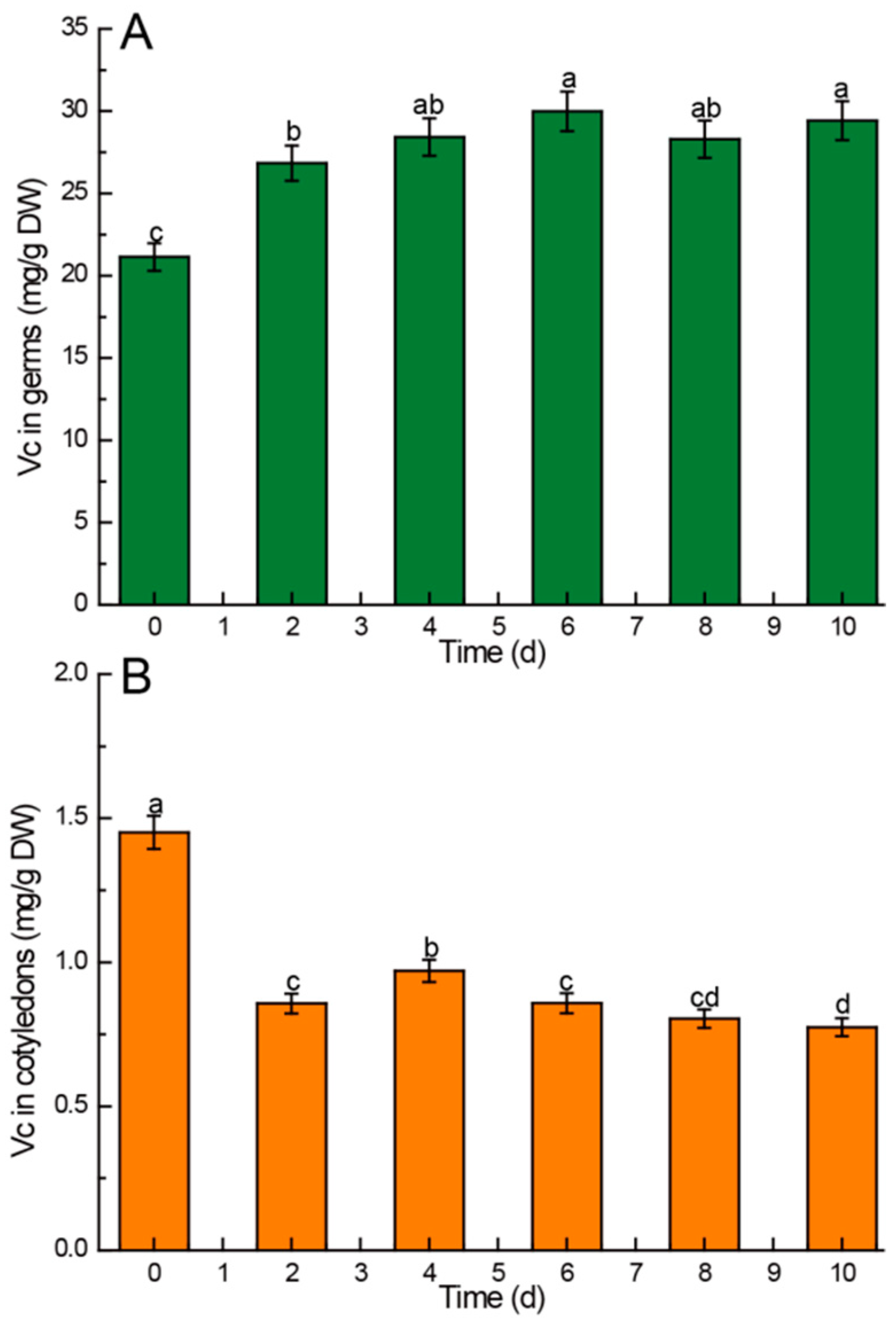
3.2.4. Vitamin C
3.3. Effects of Germination Time on Functional Components Content in Different Parts of Germinated Chinese Chestnuts
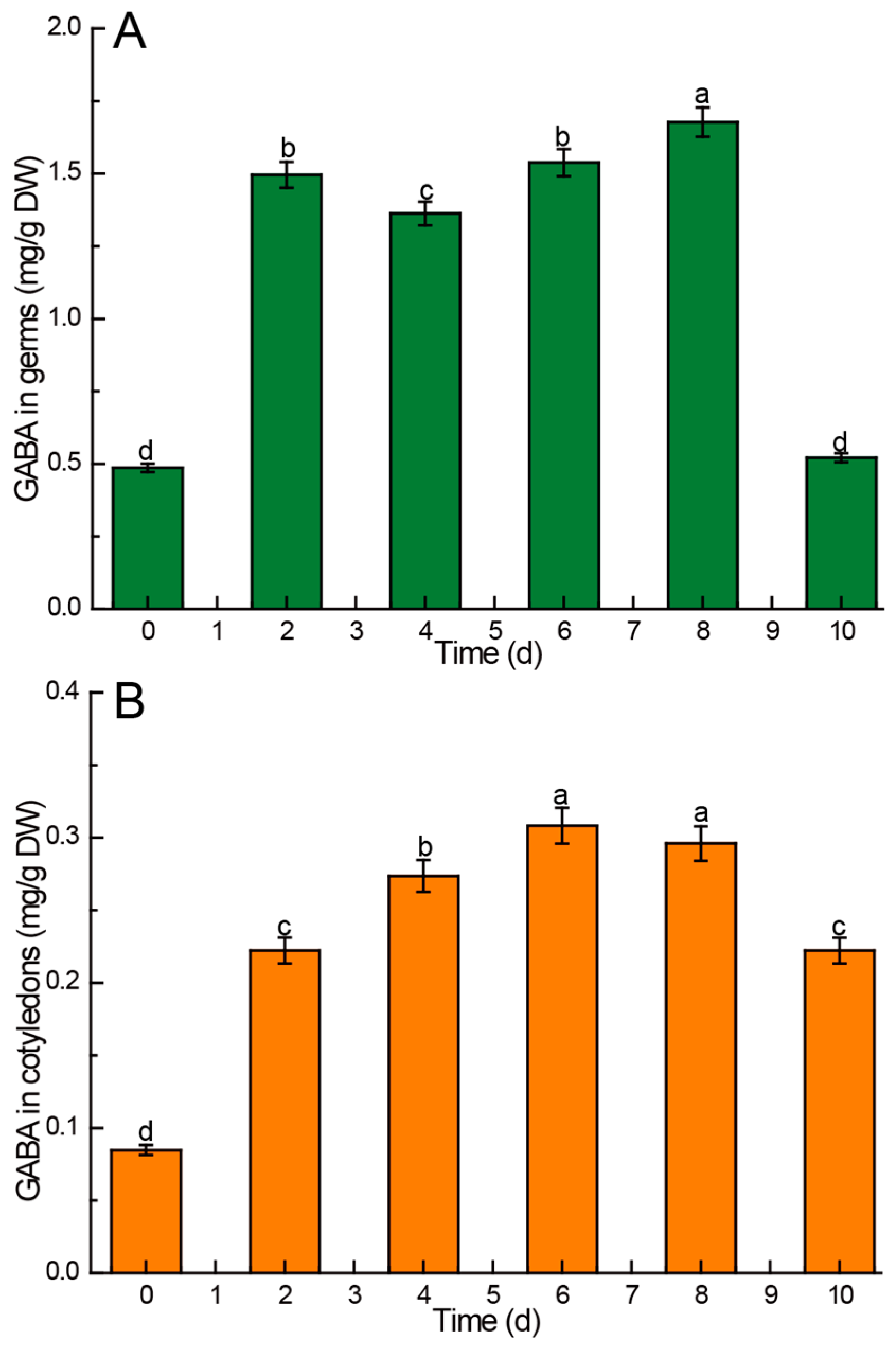
3.3.1. GABA
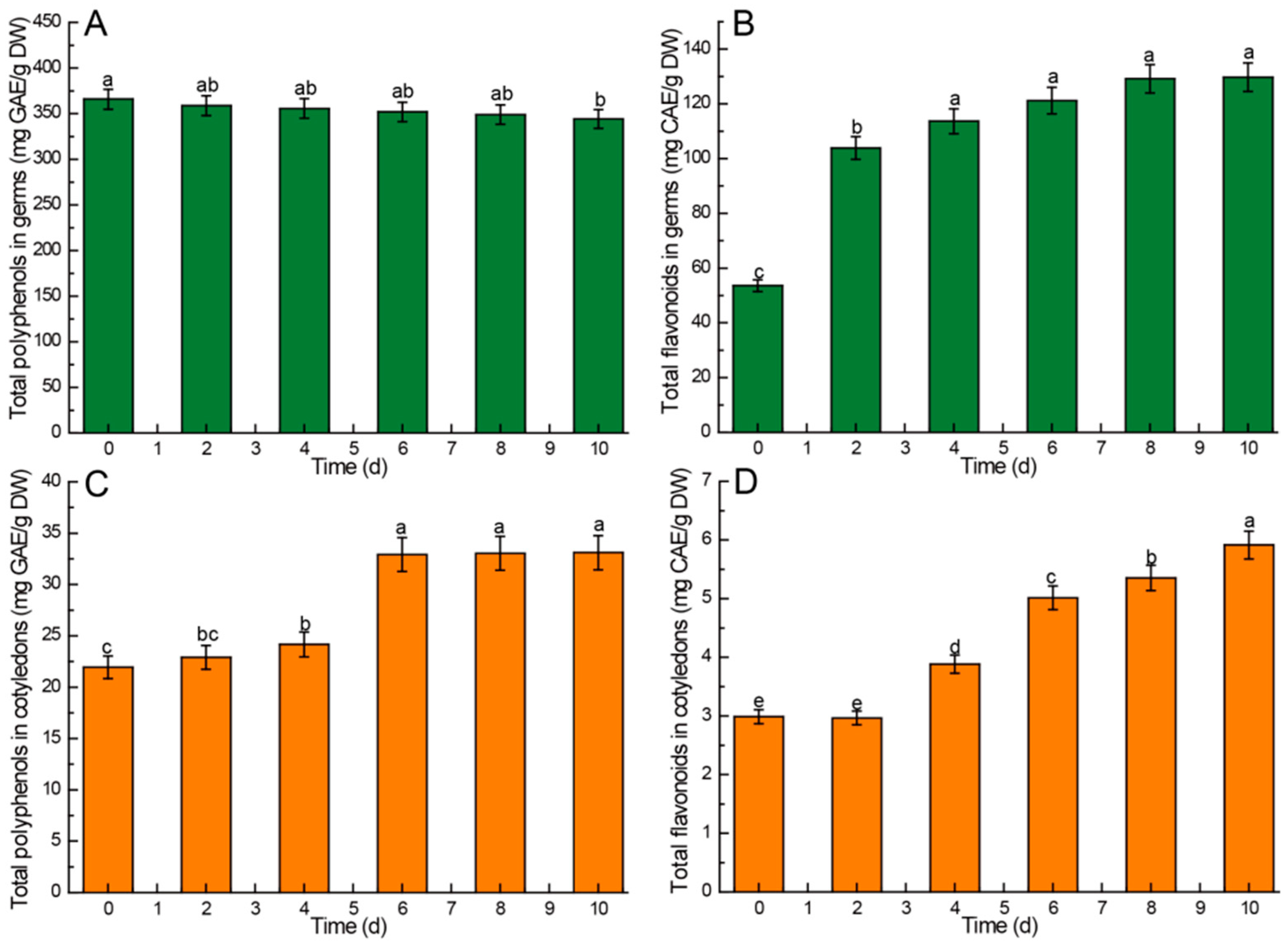
3.3.2. Total Phenols and Flavonoids
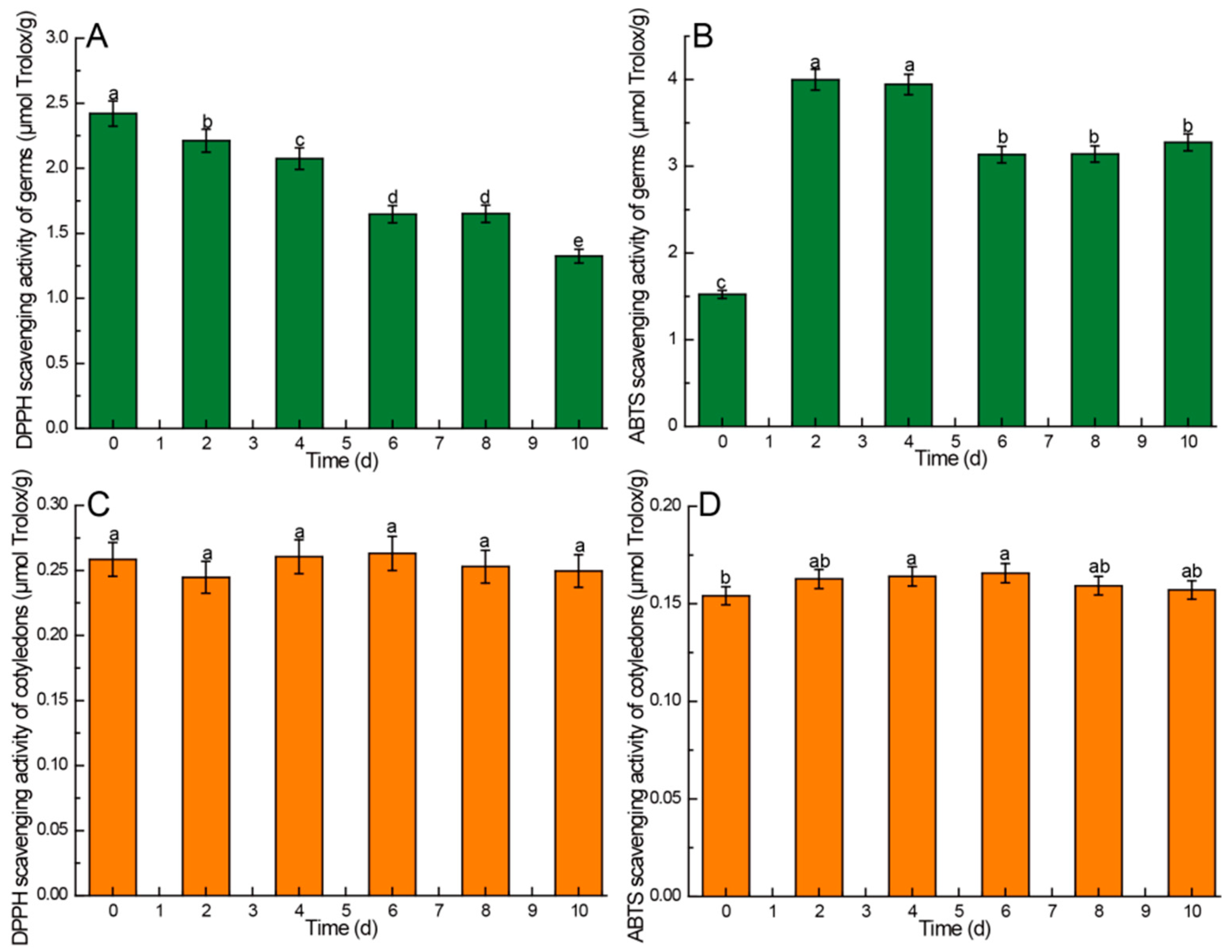
3.4. Effects of Germination Time on Antioxidant Activity of Different Parts of Germinated Chinese Chestnuts
4. Discussion
4.1. Kinetic Changes of Biomass Growth of Germ of Chinese Chestnut during Germination
4.2. Kinetic Changes of Amylose and Amylopectin in Chinese Chestnuts during Germination
4.3. Kinetic Changes of Sugar Components in Chinese Chestnuts during Germination
4.4. Kinetic Changes of Soluble Protein in Chinese Chestnuts during Germination
4.5. Kinetic Changes of Vitamin C in Chinese Chestnuts during Germination
4.6. Kinetic Changes of GABA in Chinese Chestnuts during Germination
4.7. Kinetic Changes of Total Polyphenols and Flavonoids in Chinese Chestnuts during Germination
4.8. Kinetic Changes of Antioxidant Activity in Chinese Chestnuts during Germination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, P.K.; Bhujel, P.; Kafle, N. Chestnut production and its prospects in Nepal. Sustain. Food. Agric. 2022, 3, 1–10. [Google Scholar] [CrossRef]
- Tian, G.; Li, Y. Lignocellulose mulch increases the economic benefit of Chinese chestnut by suppressing weed and ameliorating soil properties. Sci. Hortic. 2022, 291, 110576. [Google Scholar] [CrossRef]
- Cornejo, F.; Caceres, P.J.; Martínez-Villaluenga, C.; Rosell, C.M.; Frias, J. Effects of germination on the nutritive value and bioactive compounds of brown rice breads. Food Chem. 2015, 173, 298–304. [Google Scholar] [CrossRef]
- Abbas, Y.; Ahmad, A. Impact of processing on nutritional and antinutritional factors of legumes: A review. Food Sci. Technol. 2018, 19, 199–215. [Google Scholar]
- Ali, A.S.; Elozeiri, A.A. Metabolic processes during seed germination. In Advances in Seed Biology; In Tech Open: London, UK, 2017; pp. 141–166. [Google Scholar]
- Ohanenye, I.C.; Tsopmo, A.; Ejike, C.E.; Udenigwe, C.C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci. Technol. 2020, 101, 213–222. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.; Maurya, A.; Das, S.; Prasad, J.; Yadav, A.; Singh, V.K.; Singh, B.K.; Dubey, N.K.; Dwivedy, A.K. Nanoencapsulation strategies for improving nutritional functionality, safety and delivery of plant-based foods: Recent updates and future opportunities. Plant Nano Biol. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Dziki, D.; Gawlik-Dziki, U. Processing of germinated grains. In Sprouted Grains; AACC International Press: St Paul, MN, USA, 2019; pp. 69–90. [Google Scholar]
- Guzmán-Ortiz, F.A.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Mora-Escobedo, R.; Rojas-León, A.; Rodríguez-Marín, M.L.; Falfán-Cortés, R.N.; Román-Gutiérrez, A.D. Enzyme activity during germination of different cereals: A review. Food Rev. Int. 2019, 35, 177–200. [Google Scholar] [CrossRef]
- Wu, F.; Chen, H.; Yang, N.; Wang, J.; Duan, X.; Jin, Z.; Xu, X. Effect of germination time on physicochemical properties of brown rice flour and starch from different rice cultivars. J. Cereal Sci. 2013, 58, 263–271. [Google Scholar] [CrossRef]
- Atudorei, D.; Stroe, S.G.; Codină, G.G. Impact of germination on the microstructural and physicochemical properties of different legume types. Plants 2021, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Kaur, R.; Singh, N.; Kaur, S.; Rishi, V.; Bhunia, R.K. Mobilization of storage lipid reserve and expression analysis of lipase and lipoxygenase genes in rice (Oryza sativa var. Pusa Basmati1) bran during germination. Phytochemistry 2020, 180, 112538. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Gill, B.S. Comparative evaluation of physicochemical, nutritional and molecular interactions of flours from different cereals as affected by germination duration. J. Food Meas. Charact. 2020, 14, 1147–1157. [Google Scholar] [CrossRef]
- Takahashi, M.; Uematsu, Y.; Kashiwaba, K.; Yagasaki, K.; Hajika, M.; Matsunaga, R.; Komatsu, K.; Ishimoto, M. Accumulation of high levels of free amino acids in soybean seeds through integration of mutations conferring seed protein deficiency. Planta 2003, 217, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Wilckens, R.; Jara, J.; Aranda, M.; Valdivia, W.; Bustamante, L.; Graf, F.; Obal, I. Protein and antioxidant composition of quinoa (Chenopodium quinoa Willd.) sprout from seeds submitted to water stress, salinity and light conditions. Ind. Crop. Prod. 2017, 107, 558–564. [Google Scholar] [CrossRef]
- Kim, H.Y.; Hwang, I.G.; Kim, T.M.; Woo, K.S.; Park, D.S.; Kim, J.H.; Kim, D.J.; Lee, J.; Lee, Y.R.; Jeong, H.S. Chemical and functional components in different parts of rough rice (Oryza sativa L.) before and after germination. Food Chem. 2012, 134, 288–293. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Singh, B. Germination behaviour, physico-nutritional properties, and diastase activity of brown rice influenced by germination time and temperature. Acta Aliment. 2018, 47, 70–79. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, S.H.; Jang, G.Y.; Li, M.; Lee, Y.R.; Lee, J.; Jeong, H.S. Changes of phenolic acids and vitamin E profiles on germinated rough rice (Oryza sativa L.) treated by high hydrostatic pressure. Food Chem. 2017, 217, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Cai, W.; Xu, B. Improvement in beta-carotene, vitamin B2, GABA, free amino acids and isoflavones in yellow and black soybeans upon germination. LWT Food Sci. Technol. 2017, 75, 488–496. [Google Scholar] [CrossRef]
- Fakir, M.S.A.; Rahman, A.B.; Sarker, S.R.; Rahman, A.B.; Fakir, M.S.A. Light and temperature effects on sprout yield and its proximate composition and vitamin C content in Lignosus and Mung beans. J. Bangladesh Agric. Univ. 2017, 15, 248. [Google Scholar]
- Desai, A.D.; Kulkarni, S.S.; Sahoo, A.K.; Ranveer, R.C.; Dandge, P.B. Effect of supplementation of malted ragi flour on the nutritional and sensorial quality characteristics of cake. Adv. J. Food Sci. Technol. 2010, 2, 67–71. [Google Scholar]
- Lee, X.Y.; Tan, J.S.; Cheng, L.H. Gamma aminobutyric acid (GABA) enrichment in plant-based food—A mini review. Food Rev. Int. 2023, 39, 5864–5885. [Google Scholar] [CrossRef]
- Baranzelli, J.; Kringel, D.H.; Colussi, R.; Paiva, F.F.; Aranha, B.C.; de Miranda, M.Z.; Zavareze, E.R.; Dias, A.R.G. Changes in enzymatic activity, technological quality and gamma-aminobutyric acid (GABA) content of wheat flour as affected by germination. LWT Food Sci. Technol. 2018, 90, 483–490. [Google Scholar] [CrossRef]
- Kamjijam, B.; Bednarz, H.; Suwannaporn, P.; Jom, K.N.; Niehaus, K. Localization of amino acids in germinated rice grain: Gamma-aminobutyric acid and essential amino acids production approach. J. Cereal Sci. 2020, 93, 102958. [Google Scholar] [CrossRef]
- Komatsuzaki, N.; Tsukahara, K.; Toyoshima, H.; Suzuki, T.; Shimizu, N.; Kimura, T. Effect of soaking and gaseous treatment on GABA content in germinated brown rice. J. Food Eng. 2007, 78, 556–560. [Google Scholar] [CrossRef]
- Chu, C.; Yan, N.; Du, Y.; Liu, X.; Chu, M.; Shi, J.; Zhang, H.; Liu, Y.; Zhang, Z. iTRAQ-based proteomic analysis reveals the accumulation of bioactive compounds in Chinese wild rice (Zizania latifolia) during germination. Food Chem. 2019, 289, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Chan, C.L.; Dai, S.H.; Sui, Z.Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Wang, X.; Gu, Z.; Beta, T. Changes of phenolic profiles and antioxidant activity in canary seed (Phalaris canariensis L.) during germination. Food Chem. 2016, 194, 608–618. [Google Scholar] [CrossRef]
- Aziz, A.; Noreen, S.; Khalid, W.; Mubarik, F.; Niazi, M.K.; Koraqi, H.; Ali, A.; Lima, C.M.G.; Alansari, W.S.; Eskandrani, A.A.; et al. Extraction of bioactive compounds from different vegetable sprouts and their potential role in the formulation of functional foods against various disorders: A literature-based review. Molecules 2022, 27, 7320. [Google Scholar] [CrossRef]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Zhou, Z.; Fan, Z.; Meenu, M.; Xu, B. Impact of germination time on resveratrol, phenolic acids, and antioxidant capacities of different varieties of peanut (Arachis hypogaea Linn.) from China. Antioxidants 2021, 10, 1714. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Frías, J.; Gulewicz, P.; Gulewicz, K.; Vidal-Valverde, C. Food safety evaluation of broccoli and radish sprouts. Food Chem. Toxicol. 2008, 46, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Kigel, J. Seed germination in arid and semiarid regions. In Seed Development and Germination; Routledge: London, UK, 2017; pp. 645–699. [Google Scholar]
- Tumpa, K.; Vidaković, A.; Drvodelić, D.; Šango, M.; Idžojtić, M.; Perković, I.; Poljak, I. The effect of seed size on germination and seedling growth in sweet chestnut (Castanea sativa mill.). Forests 2021, 12, 858. [Google Scholar] [CrossRef]
- Cruz, B.R.; Abraão, A.S.; Lemos, A.M.; Nunes, F.M. Chemical composition and functional properties of native chestnut starch (Castanea sativa Mill). Carbohyd. Polym. 2013, 94, 594–602. [Google Scholar] [CrossRef]
- Sun, S.H.; Wang, H.; Xie, J.P.; Su, Y. Simultaneous determination of rhamnose, xylitol, arabitol, fructose, glucose, inositol, sucrose, maltose, injujube (Zizyphus jujube Mill.) extract: Comparison of HPLC-ELSD, LC-ESI-MS/MS and GC-MS. Chem. Cent. J. 2016, 10, 25. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, G.; Liu, Y.; Zhang, A.; Li, Y.; Liu, R.; Shi, L.; Jia, L.; Zhang, J. Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds. J. Exp. Bot. 2012, 63, 1809–1822. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, Y.; Wei, Z.Z.; Yuan, W.X.; Li, Y.L.; Zhang, C.H.; Xue, X.T.; Zhou, H.J. Determination and comparison of γ-aminobutyric acid (GABA) content in pu-erh and other types of Chinese tea. J. Agric. Food Chem. 2011, 59, 3641–3648. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Campa Negrillo, A.; Suárez Valles, B.; Ferreira Fernández, J. Phenolic content and antioxidant activity in seeds of common bean (Phaseolus vulgaris L.). Foods 2021, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, N.; Babaei, K.; Farsaraei, S.; Pirbalouti, A.G. Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind. Crop. Prod. 2020, 152, 112570. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Chang, H.Y.; Ho, Y.L.; Sheu, M.J.; Lin, Y.H.; Tseng, M.C.; Wu, S.H.; Huang, G.J.; Chang, Y.S. Antioxidant and free radical scavenging activities of Phellinus merrillii extracts. Bot. Stud. 2007, 48, 407–417. [Google Scholar]
- Liu, C.; Wang, S.; Chang, X.; Wang, S. Structural and functional properties of starches from Chinese chestnuts. Food Hydrocolloid. 2015, 43, 568–576. [Google Scholar] [CrossRef]
- Gabriele, M.; Parri, E.; Felicioli, A.; Sagona, S.; Pozzo, L.; Biondi, C.; Domenici, V.; Pucci, L. Phytochemical Composition and Antioxidant Activity of Tuscan Bee Pollen of Different Botanic Origins. Ital. J. Food Sci. 2015, 27, 248–259. [Google Scholar]
- Zhang, Z.P.; Liang, G.J.; Zhang, X.F.; Zhang, G.L.; Chao, H.H.; Li, L.; Shen, W. Growth of mouse oocytes to maturity from premeiotic germ cells in vitro. PLoS ONE 2012, 7, e41771. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, S.C.; Lal, M.A. Seed dormancy and germination. In Plant Physiology, Development and Metabolism; Springer Nature: Singapore, 2023; pp. 625–640. [Google Scholar]
- Quek, W.P.; Yu, W.; Tao, K.; Fox, G.P.; Gilbert, R.G. Starch structure-property relations as a function of barley germination times. Int. J. Biol. Macromol. 2019, 136, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, D.; Wang, T.; Li, Z.; Zhao, Y.; Jiang, Y.; Zhang, Q.; Cao, Q.; Fang, K.; Xing, Y.; et al. Identification and expression analysis of starch branching enzymes involved in starch synthesis during the development of chestnut (Castanea mollissima Blume) cotyledons. PLoS ONE 2017, 12, e0177792. [Google Scholar] [CrossRef]
- Mazur, A.K.; Nakatani, H. Multiple attack mechanism in the porcine pancreatic α-amylase hydrolysis of amylose and amylopectin. Arch. Biochem. Biophys. 1993, 306, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lara-Núñez, A.; García-Ayala, B.B.; Garza-Aguilar, S.M.; Flores-Sánchez, J.; Sánchez-Camargo, V.B.; Bravo-Alberto, C.E.; Vázquez-Santana, S.; Vázquez-Ramos, J.M. Glucose and sucrose differentially modify cell proliferation in maize during germination. Plant Physiol. Biochem. 2017, 113, 20–31. [Google Scholar] [CrossRef]
- Eveland, A.L.; Jackson, D.P. Sugars, signalling, and plant development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, P. Carbohydrate degradation during germination. In Seed Development and Germination; Routledge: London, UK, 2017; pp. 447–474. [Google Scholar]
- Dekkers, B.J.W.; Schuurmans, J.A.M.J.; Smeekens, S.C.M. Glucose delays seed germination in Arabidopsis thaliana. Planta 2004, 218, 579–588. [Google Scholar] [CrossRef]
- Ghosh, S.; Pal, A. Identification of differential proteins of mungbean cotyledons during seed germination: A proteomic approach. Acta Physiol. Plant. 2012, 34, 2379–2391. [Google Scholar] [CrossRef]
- Gallie, D.R. Increasing vitamin C content in plant foods to improve their nutritional value—Successes and challenges. Nutrients 2013, 5, 3424–3446. [Google Scholar] [CrossRef]
- Hacısevki, A. An overview of ascorbic acid biochemistry. J. Fac. Pharm. Ankara Univ. 2009, 38, 233–255. [Google Scholar]
- Agaj, A.; Peršurić, Ž.; Pavelić, S.K. Mediterranean Food Industry By-Products as a Novel Source of Phytochemicals with a Promising Role in Cancer Prevention. Molecules 2022, 27, 8655. [Google Scholar] [CrossRef]
- Vann, K.; Techaparin, A.; Apiraksakorn, J. Beans germination as a potential tool for GABA-enriched tofu production. J. Food Sci. Technol. 2020, 57, 3947–3954. [Google Scholar] [CrossRef] [PubMed]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res 2019, 10, 1567–1574. [Google Scholar]
- Wang, H.; Wang, J.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Chen, G.; Liu, R.H. Effect of germination on lignan biosynthesis, and antioxidant and antiproliferative activities in flaxseed (Linum usitatissimum L.). Food Chem. 2016, 205, 170–177. [Google Scholar] [CrossRef]
- Riganti, C.; Gazzano, E.; Polimeni, M.; Aldieri, E.; Ghigo, D. The pentose phosphate pathway: An antioxidant defense and a crossroad in tumor cell fate. Free Radical Biol. Med. 2012, 53, 421–436. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, K.; Liu, W.; Du, H.W. Regulation and expression of the pal in plant and its outlook. J. Fruit Sci. 2003, 20, 351–357. [Google Scholar]
- Kim, S.L.; Kim, S.K.; Park, C.H. Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Res. Int. 2004, 37, 319–327. [Google Scholar] [CrossRef]
- Donkor, O.N.; Stojanovska, L.; Ginn, P.; Ashton, J.; Vasiljevic, T. Germinated Grains-Sources of Bioactive Compounds. Food Chem. 2012, 135, 950–959. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Singh, B.; Kaur, G. In Vitro Nutrient Digestibility and Antioxidative Properties of Flour Prepared from Sorghum Germinated at Different Conditions. J. Food Sci. Technol. 2019, 56, 3077–3089. [Google Scholar] [CrossRef] [PubMed]
- Ljevar, A.; Curko, N.; Tomaševi´c, M.; Radoševi’c, K.; Gaurina Sřcek, V.; Kovǎcevi’c Gani’c, K. Phenolic Composition, Antioxidant Capacity and in Vitro Cytotoxicity Assessment of Fruit Wines. Food Technol. Biotechnol. 2016, 54, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.L.; Awika, J.M. Sorghum Polyphenols and Other Bioactive Components as Functional and Health Promoting Food Ingredients. J. Cereal Sci. 2018, 84, 112–124. [Google Scholar] [CrossRef]
- Arouna, N.; Gabriele, M.; Pucci, L. The impact of germination on sorghum nutraceutical properties. Foods 2020, 9, 1218. [Google Scholar] [CrossRef] [PubMed]
| Parts | Sugar Compounds | Time (d) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | ||
| Germ | Phenylglucoside | 0.0094 ± 0.0004 b | 0.0085 ± 0.0003 c | 0.0079 ± 0.0003 c | 0.0065 ± 0.0003 e | 0.0072 ± 0.0003 d | 0.0126 ± 0.0005 a |
| Cellobiose | 0.1034 ± 0.0041 a | 0.0948 ± 0.0038 b | 0.0914 ± 0.0037 b | 0.0656 ± 0.0026 d | 0.1059 ± 0.0042 a | 0.0749 ± 0.0030 c | |
| Sucrose | 92.5543 ± 3.7022 a | 89.5666 ± 3.5826 a | 83.3691 ± 3.3347 b | 68.2953 ± 2.7318 c | 58.1718 ± 2.3268 d | 52.4874 ± 2.0995 e | |
| Maltose | 0.3990 ± 0.0159 b | 0.3625 ± 0.0144 c | 0.3391 ± 0.0135 c | 0.3059 ± 0.0122 d | 0.4302 ± 0.0172 a | 0.1706 ± 0.0068 e | |
| Trehalose | 0.2011 ± 0.0080 a | 0.1916 ± 0.0076 ab | 0.1817 ± 0.0072 bc | 0.1566 ± 0.0062 d | 0.1740 ± 0.0069 c | 0.1314 ± 0.0053 e | |
| 2-Acetamido-2-deoxy-D-glucopyranose | 0.0385 ± 0.0015 a | 0.0355 ± 0.0014 b | 0.0352 ± 0.0014 b | 0.0358 ± 0.0014 b | 0.0302 ± 0.0012 c | 0.0206 ± 0.0008 d | |
| D-Sorbitol | 0.1722 ± 0.0068 e | 0.2682 ± 0.0107 d | 0.2892 ± 0.0115 c | 0.4199 ± 0.0167 a | 0.3229 ± 0.0129 b | 0.1520 ± 0.0061 f | |
| Xylitol | 0.0095 ± 0.0003 b | 0.0087 ± 0.0003 c | 0.0082 ± 0.0003 c | 0.0073 ± 0.0002 d | 0.0083 ± 0.0003 c | 0.0102 ± 0.0004 a | |
| D-Xylose | 0.2419 ± 0.0096 a | 0.2172 ± 0.0086 b | 0.1954 ± 0.0078 c | 0.1653 ± 0.0066 d | 0.2561 ± 0.0102 a | 0.0997 ± 0.0039 e | |
| D-Xylulose | 0.0041 ± 0.0001 e | 0.0047 ± 0.0001 d | 0.0056 ± 0.0002 c | 0.0065 ± 0.0002 b | 0.0078 ± 0.0003 a | 0.0029 ± 0.0001 f | |
| D-Galacturonic acid | 0.0159 ± 0.0006 a | 0.0143 ± 0.0005 b | 0.0136 ± 0.0005 bc | 0.0128 ± 0.0005 cd | 0.0124 ± 0.0004 d | 0.0078 ± 0.0003 e | |
| D-Ribose | 0.0417 ± 0.0016 e | 0.0465 ± 0.0018 d | 0.0500 ± 0.0020 d | 0.0545 ± 0.0021 c | 0.0696 ± 0.0027 a | 0.0595 ± 0.0023 b | |
| Barium D-ribose-5-phosphate | 0.0624 ± 0.0024 bc | 0.0644 ± 0.0024 bc | 0.0648 ± 0.0025 ab | 0.0602 ± 0.0024 c | 0.0689 ± 0.0027 a | 0.0276 ± 0.0011 d | |
| L-Rhamnose | 0.0176 ± 0.0007 a | 0.0179 ± 0.0007 a | 0.0178 ± 0.0007 a | 0.0172 ± 0.0006 a | 0.0167 ± 0.0006 a | 0.0125 ± 0.0005 b | |
| D-Mannose-6-phosphate sodium salt | 0.1485 ± 0.0059 b | 0.1366 ± 0.0054 c | 0.1240 ± 0.0049 d | 0.1324 ± 0.0052 cd | 0.1577 ± 0.0063 a | 0.0401 ± 0.0016 e | |
| D-Mannose | 0.0644 ± 0.0025 e | 0.0760 ± 0.0030 d | 0.0851 ± 0.0034 c | 0.1146 ± 0.0045 b | 0.1259 ± 0.0050 a | 0.1300 ± 0.0052 a | |
| Levoglucosan | 0.5264 ± 0.0210 a | 0.4905 ± 0.0196 b | 0.4595 ± 0.0183 c | 0.3218 ± 0.0128 d | 0.3469 ± 0.0138 d | 0.1648 ± 0.0065 e | |
| Inositol | 9.6263 ± 0.3850 a | 9.1316 ± 0.3652 ab | 8.8565 ± 0.3542 b | 7.8061 ± 0.3122 c | 7.7569 ± 0.3102 c | 5.7558 ± 0.2302 d | |
| D-Glucuronic acid | 0.0618 ± 0.0024 e | 0.0861 ± 0.0034 d | 0.1142 ± 0.0045 c | 0.1784 ± 0.0071 a | 0.1704 ± 0.0068 a | 0.1430 ± 0.0057 b | |
| Glucose | 81.6451 ± 3.2658 a | 78.0234 ± 3.1209 ab | 75.5457 ± 3.0218 bc | 72.3872 ± 2.8954 cd | 67.5278 ± 2.7011 d | 41.9263 ± 1.6770 e | |
| D-Galactose | 0.2651 ± 0.0106 c | 0.2867 ± 0.0114 bc | 0.2965 ± 0.0118 b | 0.3052 ± 0.0122 b | 0.3145 ± 0.0125 b | 0.6600 ± 0.0264 a | |
| L-Fucose | 0.0261 ± 0.0010 e | 0.0289 ± 0.0011 d | 0.0308 ± 0.0012 cd | 0.0330 ± 0.0013 bc | 0.0352 ± 0.0014 b | 0.0433 ± 0.0017 a | |
| D-Fructose | 54.2171 ± 2.1686 a | 51.1165 ± 2.0446 ab | 49.4387 ± 1.9775 bc | 48.2138 ± 1.9285 bc | 46.3311 ± 1.8532 c | 35.1437 ± 1.4057 d | |
| D-Arabinose | 0.0840 ± 0.0033 a | 0.0729 ± 0.0029 b | 0.0605 ± 0.0024 c | 0.0567 ± 0.0022 cd | 0.0550 ± 0.0022 d | 0.0270 ± 0.0010 e | |
| D-Arabinitol | 0.0158 ± 0.0006 b | 0.0163 ± 0.0006 ab | 0.0167 ± 0.0006 ab | 0.0175 ± 0.0007 a | 0.0170 ± 0.0006 ab | 0.0123 ± 0.0004 c | |
| D-Ribono-1,4-lactone | 0.0073 ± 0.0002 d | 0.0086 ± 0.0003 c | 0.0097 ± 0.0003 b | 0.0108 ± 0.0004 a | 0.0108 ± 0.0004 a | 0.0083 ± 0.0003 c | |
| Raffinose | 0.4615 ± 0.0184 a | 0.3886 ± 0.0155 b | 0.3639 ± 0.0145 c | 0.3056 ± 0.0122 d | 0.2424 ± 0.0096 e | 0.1741 ± 0.0069 f | |
| Cotyledon | Phenylglucoside | 0.0064 ± 0.0002 a | 0.0056 ± 0.0002 b | 0.0057 ± 0.0002 b | 0.0046 ± 0.0001 d | 0.0050 ± 0.0002 c | 0.0041 ± 0.0002 e |
| Cellobiose | 0.1134 ± 0.0045 b | 0.1345 ± 0.0053 a | 0.1159 ± 0.0046 b | 0.0723 ± 0.0028 d | 0.0623 ± 0.0024 e | 0.0796 ± 0.0031 c | |
| Sucrose | 119.2855 ± 4.7714 a | 118.9857 ± 4.7594 a | 118.9038 ± 4.7561 a | 117.6958 ± 4.7078 a | 120.1981 ± 4.8079 a | 107.3171 ± 4.2926 b | |
| Maltose | 1.4680 ± 0.0587 b | 1.6245 ± 0.0649 a | 1.2986 ± 0.0519 c | 0.8965 ± 0.0358 d | 0.8491 ± 0.0339 d | 0.8999 ± 0.0359 d | |
| Trehalose | 0.0114 ± 0.0004 d | 0.0202 ± 0.0008 a | 0.0124 ± 0.0004 d | 0.0144 ± 0.0005 c | 0.0158 ± 0.0006 b | 0.0138 ± 0.0005 c | |
| 2-Acetamido-2-deoxy-D-glucopyranose | 0.0401 ± 0.0016 a | 0.0355 ± 0.0014 b | 0.0331 ± 0.0013 c | 0.0245 ± 0.0009 d | 0.0336 ± 0.0013 bc | 0.0210 ± 0.0008 e | |
| D-Sorbitol | 0.4769 ± 0.0190 c | 0.6675 ± 0.0267 b | 0.4251 ± 0.0170 d | 0.3189 ± 0.0127 e | 0.8170 ± 0.0326 a | 0.4191 ± 0.0168 d | |
| Xylitol | 0.0069 ± 0.0002 b | 0.0091 ± 0.0003 a | 0.0066 ± 0.0002 bc | 0.0063 ± 0.0002 cd | 0.0064 ± 0.0002 bc | 0.0058 ± 0.0002 d | |
| D-Xylose | 0.0060 ± 0.0002 c | 0.0072 ± 0.0002 ab | 0.0070 ± 0.0002 b | 0.0076 ± 0.0003 a | 0.0068 ± 0.0002 b | 0.0073 ± 0.0003 ab | |
| D-Xylulose | - | - | - | - | - | - | |
| D-Galacturonic acid | - | - | - | - | - | - | |
| D-Ribose | - | - | - | - | - | - | |
| Barium D-ribose-5-phosphate | 0.0482 ± 0.0019 a | 0.0479 ± 0.0019 a | 0.0438 ± 0.0017 b | 0.0416 ± 0.0016 b | 0.0420 ± 0.0016 b | 0.0356 ± 0.0014 c | |
| L-Rhamnose | 0.0099 ± 0.0003 a | 0.0089 ± 0.0003 bc | 0.0089 ± 0.0003 bc | 0.0093 ± 0.0003 ab | 0.0096 ± 0.0003 ab | 0.0084 ± 0.0003 c | |
| D-Mannose-6-phosphate sodium salt | 0.0215 ± 0.0008 a | 0.0196 ± 0.0007 b | 0.0164 ± 0.0006 c | 0.0159 ± 0.0006 cd | 0.0165 ± 0.0006 c | 0.0148 ± 0.0005 d | |
| D-Mannose | 0.0275 ± 0.0011 d | 0.0370 ± 0.0014 c | 0.0287 ± 0.0011 d | 0.0540 ± 0.0021 a | 0.0347 ± 0.0013 c | 0.0435 ± 0.0017 b | |
| Levoglucosan | 0.0824 ± 0.0033 a | 0.0842 ± 0.0033 a | 0.0706 ± 0.0028 b | 0.0843 ± 0.0033 a | 0.0804 ± 0.0032 a | 0.0691 ± 0.0027 b | |
| Inositol | 1.4963 ± 0.0598 b | 1.7381 ± 0.0695 a | 0.9445 ± 0.0377 c | 1.4921 ± 0.0596 b | 1.7602 ± 0.0704 a | 0.9322 ± 0.0372 c | |
| D-Glucuronic acid | 0.1003 ± 0.0040 c | 0.1479 ± 0.0059 b | 0.1542 ± 0.0061 b | 0.1710 ± 0.0068 a | 0.1649 ± 0.0065 a | 0.0885 ± 0.0035 d | |
| Glucose | 0.7112 ± 0.0284 e | 1.3680 ± 0.0547 d | 1.7088 ± 0.0683 c | 2.7176 ± 0.1087 a | 2.1952 ± 0.0878 b | 1.7671 ± 0.0706 c | |
| D-Galactose | 0.0544 ± 0.0021 d | 0.0416 ± 0.0016 e | 0.0665 ± 0.0026 c | 0.1137 ± 0.0045 a | 0.0942 ± 0.0033 b | 0.0893 ± 0.0035 b | |
| L-Fucose | 0.0139 ± 0.0005 bc | 0.0149 ± 0.0005 ab | 0.0131 ± 0.0005 c | 0.0157 ± 0.0006 a | 0.0144 ± 0.0005 b | 0.0145 ± 0.0005 b | |
| D-Fructose | 1.5422 ± 0.0616 c | 1.6012 ± 0.0556 bc | 1.6178 ± 0.0647 bc | 3.2509 ± 0.1300 a | 3.2262 ± 0.1290 a | 1.7295 ± 0.0692 b | |
| D-Arabinose | 0.0084 ± 0.0003 bc | 0.0073 ± 0.0003 d | 0.0085 ± 0.0003 bc | 0.0097 ± 0.0004 a | 0.0088 ± 0.0004 b | 0.0080 ± 0.0003 c | |
| D-Arabinitol | 0.0069 ± 0.0002 a | 0.0069 ± 0.0003 a | 0.0068 ± 0.0003 a | 0.0069 ± 0.0003 a | 0.0068 ± 0.0003 a | 0.0067 ± 0.0003 a | |
| D-Ribono-1,4-lactone | 0.0071 ± 0.0003 bc | 0.0066 ± 0.0003 c | 0.0076 ± 0.0003 ab | 0.0073 ± 0.0003 ab | 0.0078 ± 0.0003 a | 0.0078 ± 0.0003 a | |
| Raffinose | 0.8876 ± 0.0355 b | 0.7389 ± 0.0295 c | 0.9823 ± 0.0392 a | 0.7802 ± 0.0312 c | 0.9798 ± 0.0392 a | 0.2049 ± 0.0082 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Wang, H.; Jiang, Y.; Jiang, Y.; Tang, Y.; Li, X.; Zhao, Y. Utilization of Germinated Seeds as Functional Food Ingredients: Optimization of Nutrient Composition and Antioxidant Activity Evolution Based on the Germination Characteristics of Chinese Chestnut (Castanea mollissima). Foods 2024, 13, 2605. https://doi.org/10.3390/foods13162605
Yuan J, Wang H, Jiang Y, Jiang Y, Tang Y, Li X, Zhao Y. Utilization of Germinated Seeds as Functional Food Ingredients: Optimization of Nutrient Composition and Antioxidant Activity Evolution Based on the Germination Characteristics of Chinese Chestnut (Castanea mollissima). Foods. 2024; 13(16):2605. https://doi.org/10.3390/foods13162605
Chicago/Turabian StyleYuan, Junwei, Haifen Wang, Yunbin Jiang, Yuqian Jiang, Yao Tang, Xihong Li, and Yuhua Zhao. 2024. "Utilization of Germinated Seeds as Functional Food Ingredients: Optimization of Nutrient Composition and Antioxidant Activity Evolution Based on the Germination Characteristics of Chinese Chestnut (Castanea mollissima)" Foods 13, no. 16: 2605. https://doi.org/10.3390/foods13162605






