Industrial Production of Bioactive Nutrient-Enhanced Extra Virgin Olive Oil under Continuous-Flow Ultrasound and Pulsed Electric Field Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Olive Cultivar
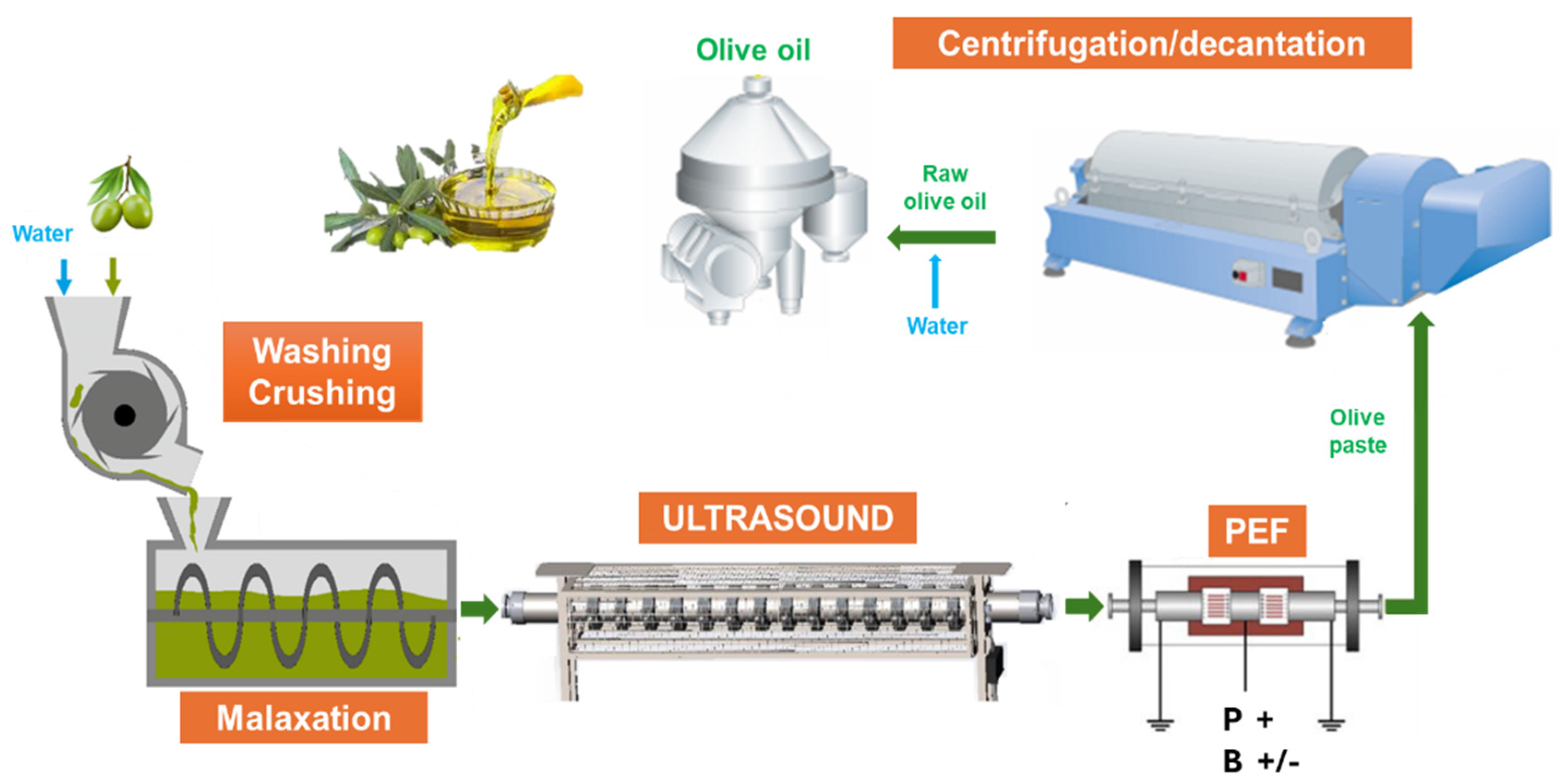
2.2. Sustainable Industrial Production of EVOO: Description of the Devices Used
2.2.1. Conventional Equipment
2.2.2. US Device
2.2.3. PEF Equipment
2.3. General Procedure for Industrial-Scale EVOO Production
2.3.1. Control Process (CTRL)
2.3.2. US-Assisted Process
2.3.3. US-PEF-Assisted Process
2.3.4. PEF-Assisted Process
2.4. General Analytical Procedures for EVOO Analysis
2.4.1. Quality Parameters of Coratina Oil Samples: FFA, PV, Insoluble Impurities, Moisture and Volatiles and Organoleptic Assessment
2.4.2. Quality Parameters of Coratina Oil Samples: UV-Specific Extinctions, Total Polyphenols, Tocotrienols and Tocopherols
2.4.3. Purity Parameters of Coratina Oil Samples
2.4.4. Statistical Analyses
2.4.5. Shelf-Life Evaluations on Green Coratina Oil Samples
3. Results and Discussion
3.1. Technology-Assisted EVOO Production
3.2. Quality Assessment of Oil Samples
3.3. Purity Evaluation of Oil Samples
3.4. Micronutrient Yield
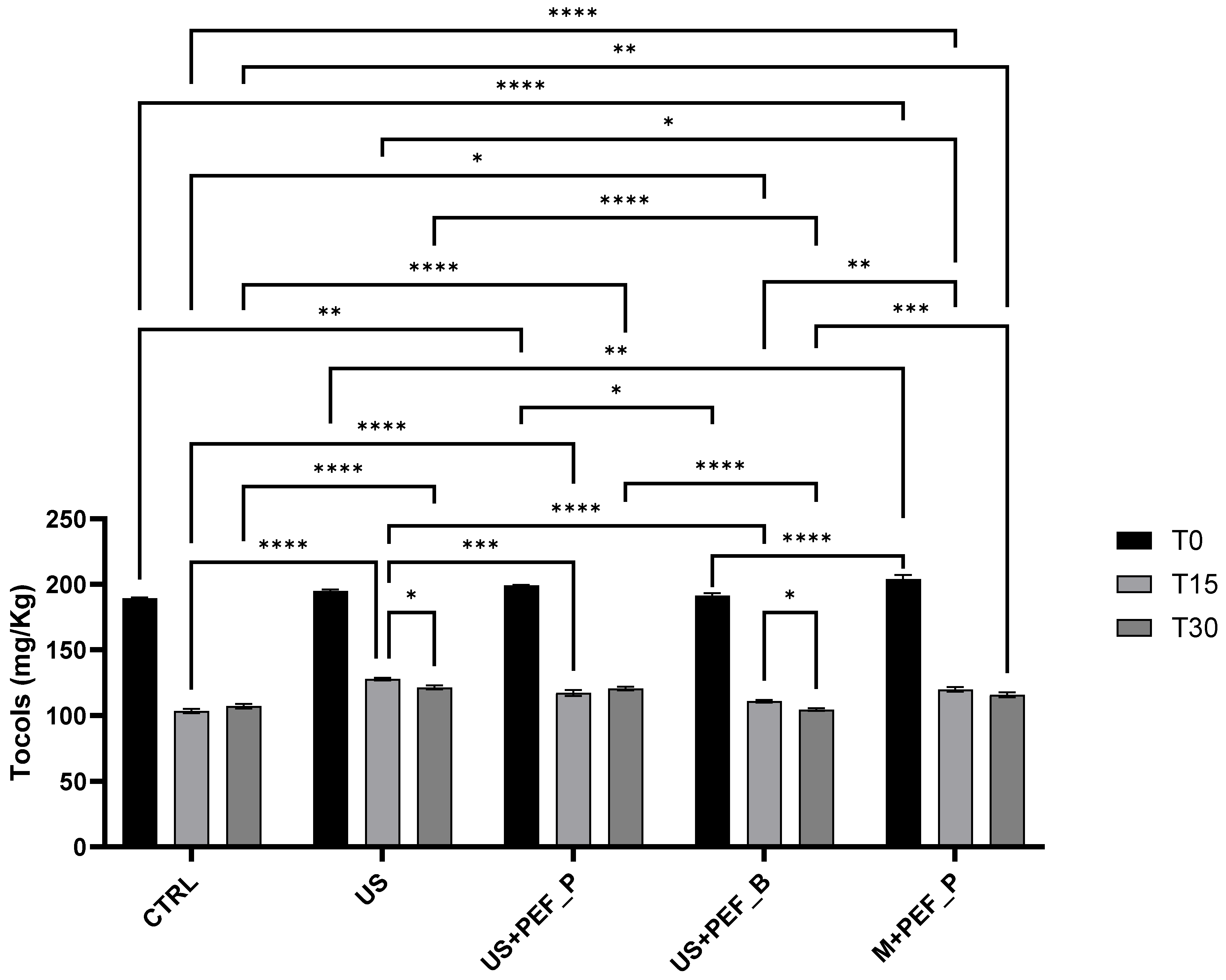
3.5. Preliminary Evaluation of Micronutrient Stability in EVOOs
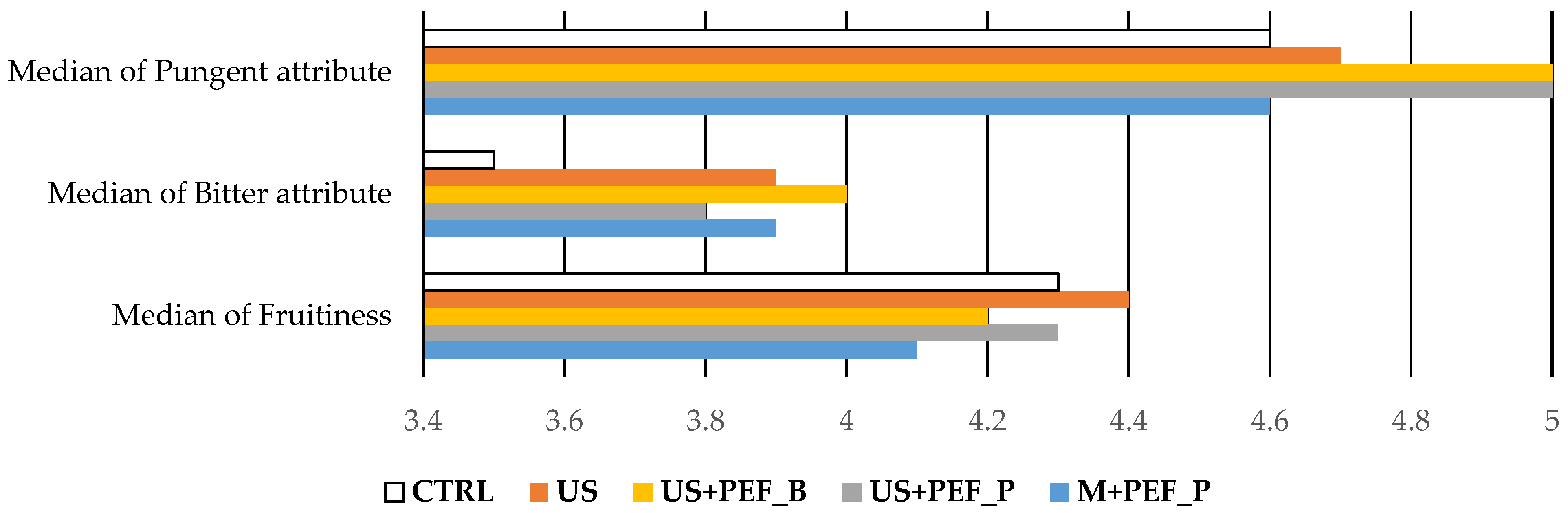
3.6. Preliminary Assessment of Stability of Organoleptic Characteristics of Obtained EVOO
3.7. Technical and Economic Feasibility
- -
- 24 tons/day of olive paste with the conventional process with 4 malaxation tanks;
- -
- 36 tons/day of olive paste with the ultrasonic process;
- -
- 36 tons/day of olive paste with the US process combined with the PEF (both positive, “P”, and bipolar, “B”).
| Process | Malax. (Time) | Process Flow Rate * (ton/h) | Daily Processed Olive Paste (ton/Day) | Oil Yields (w/w %) | Oil Prod. (ton/Day) ** | Energy Cons. (kW/Day) | Energy Cons. (kW/ton Oil) |
|---|---|---|---|---|---|---|---|
| CTRL | 30 min | 1.0 | 24 | 14.6 | 3.5 | 1944 | 555 |
| US | - | 1.5 | 36 | 14.0 | 5.0 | 1905 | 381 |
| US + PEF_P | - | 1.5 | 36 | 14.2 | 5.1 | 1934 | 379 |
| US + PEF_B | - | 1.5 | 36 | 13.8 | 4.9 | 1934 | 395 |
| M + PEF_P | 30 min | 1.0 | 24 | 14.4 | 3.5 | 1980 | 566 |
| Process | Total Polyphenols (%) | Total Tocols (%) | Oil Prod. (%) | Energy Cons. per kg Oil (%) | Total Equipment Cost (%) |
|---|---|---|---|---|---|
| US | +12.6 | +3.2 | +42.9 | −34.6 | −17.1 |
| US + PEF_P | +7.9 | +5.3 | +45.7 | −34.8 | −1.7 |
| US + PEF_B | - | - | +40.0 | −32.1 | −1.7 |
| M + PEF_P | −6.9 | +7.9 | - | +1.8 | +11.9 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive Oil Intake and Risk of Cardiovascular Disease and Mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Infante, R.; Infante, M.; Pastore, D.; Pacifici, F.; Chiereghin, F.; Malatesta, G.; Donadel, G.; Tesauro, M.; Della-Morte, D. An Appraisal of the Oleocanthal-Rich Extra Virgin Olive Oil (EVOO) and Its Potential Anticancer and Neuroprotective Properties. Int. J. Mol. Sci. 2023, 24, 17323. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Tsolis, T.; Kyriakou, D.; Sifnaiou, E.; Thomos, D.; Glykos, D.; Tsiafoulis, C.G.; Garoufis, A. NMR Analysis of Extra Virgin Olive Oil of the Epirus Region of Greece with Emphasis on Selected Phenolic Compounds. Molecules 2024, 29, 1111. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL-Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), “Anti-Inflammatory Properties” (ID 1882), “Contributes to the Upper Respiratory Tract Health” (ID 3468), “Can Help to Maintain a Normal Function of Gastrointestinal Tract” (3779), and “Contributes to Body Defences against External Agents” (ID 3467) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar] [CrossRef]
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA Health Claim on Olive Oil Polyphenols: Acid Hydrolysis Validation and Total Hydroxytyrosol and Tyrosol Determination in Italian Virgin Olive Oils. Molecules 2019, 24, 2179. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I. Authorised EU Health Claims for Polyphenols in Olive Oil. In Foods, Nutrients and Food Ingredients with Authorised EU Health Claims; Elsevier: Amsterdam, The Netherlands, 2014; pp. 212–228. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 432/2012. 2012, pp. 1–40. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R0432&from=EN (accessed on 15 May 2024).
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. EFSA Health Claims-Based Virgin Olive Oil Shelf-Life. Antioxidants 2023, 12, 1563. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Romero, N.; Saavedra, J.; Tapia, F.; Sepúlveda, B.; Aparicio, R. Influence of Agroclimatic Parameters on Phenolic and Volatile Compounds of Chilean Virgin Olive Oils and Characterization Based on Geographical Origin, Cultivar and Ripening Stage. J. Sci. Food Agric. 2016, 96, 583–592. [Google Scholar] [CrossRef]
- International Olive Council. Trade Standard Applying to Olive Oils and Olive-Pomace Oils (COI/T.15/NC No 3/Rev.19/2022). 2022, pp. 1–16. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/12/Norme-comerciale-REV-19_ENK.pdf (accessed on 10 May 2024).
- Rossi, R. The EU Olive and Olive Oil Sector: Main Features, Challenges and Prospects; European Parliamentary Research Service EPRS_BRI(2017)608690_EN; European Parliament. 2017, pp. 1–12. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2017/608690/EPRS_BRI(2017)608690_EN.pdf (accessed on 10 May 2024).
- Juliano, P.; Gaber, M.A.F.M.; Romaniello, R.; Tamborrino, A.; Berardi, A.; Leone, A. Advances in Physical Technologies to Improve Virgin Olive Oil Extraction Efficiency in High-Throughput Production Plants. Food Eng. Rev. 2023, 15, 625–642. [Google Scholar] [CrossRef]
- Keceli, T.M. The Impact of Innovative Processing Technologies and Chemometric Methods on Virgin Olive Oil Quality—A Review. Riv. Ital. Sostanze Grasse 2023, 100, 217–226. [Google Scholar]
- Olmo-Cunillera, A.; Lozano-Castellón, J.; Pérez, M.; Miliarakis, E.; Tresserra-Rimbau, A.; Ninot, A.; Romero-Aroca, A.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Optimizing the Malaxation Conditions to Produce an Arbequina EVOO with High Content of Bioactive Compounds. Antioxidants 2021, 10, 1819. [Google Scholar] [CrossRef] [PubMed]
- Nardella, M.; Moscetti, R.; Bedini, G.; Bandiera, A.; Chakravartula, S.S.N.; Massantini, R. Impact of Traditional and Innovative Malaxation Techniques and Technologies on Nutritional and Sensory Quality of Virgin Olive Oil—A Review. Food Chem. Adv. 2023, 2, 100163. [Google Scholar] [CrossRef]
- Cappelli, A.; Lupori, L.; Cini, E. Should extra virgin olive oil production change the approach? A systematic review of challenges and opportunities to increase sustainability, productivity, and product quality. J. Agric. Eng. 2023, 54, 1479. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Hbaieb, R.H. Beyond the traditional virgin olive oil extraction systems: Searching innovative and sustainable plant engineering solutions. Food Res. Int. 2013, 54, 1926–1933. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Dipalmo, T.; Schiano, C.; La Notte, D.; Pati, S. What’s now, What’s new and what’s next in virgin olive oil elaboration systems? A perspective on current knowledge and future trends. J. Agric. Eng. 2014, 45, 49–59. [Google Scholar] [CrossRef]
- Roselli, L.; Cicia, G.; Del Giudice, T.; Cavallo, C.; Vecchio, R.; Carfora, V.; Caso, D.; Sardaro, R.; Carlucci, D.; De Gennaro, B. Testing Consumers’ Acceptance for an Extra-Virgin Olive Oil with a Naturally Increased Content in Polyphenols: The Case of Ultrasounds Extraction. J. Funct. Foods 2020, 69, 103940. [Google Scholar] [CrossRef]
- Andreou, V.; Kourmbeti, E.; Dimopoulos, G.; Psarianos, M.; Katsaros, G.; Taoukis, P. Optimization of Virgin Olive Oil Yield and Quality Applying Nonthermal Processing. Food Bioprocess. Technol. 2022, 15, 891–903. [Google Scholar] [CrossRef]
- Caponio, F.; Leone, A.; Squeo, G.; Tamborrino, A.; Summo, C. Innovative Technologies in Virgin Olive Oil Extraction Process: Influence on Volatile Compounds and Organoleptic Characteristics. J. Sci. Food Agric. 2019, 99, 5594–5600. [Google Scholar] [CrossRef]
- Lammi, C.; Mulinacci, N.; Cecchi, L.; Bellumori, M.; Bollati, C.; Bartolomei, M.; Franchini, C.; Clodoveo, M.L.; Corbo, F.; Arnoldi, A. Virgin Olive Oil Extracts Reduce Oxidative Stress and Modulate Cholesterol Metabolism: Comparison between Oils Obtained with Traditional and Innovative Processes. Antioxidants 2020, 9, 798. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Beamonte, R.; Ripalda, M.; Herrero-Continente, T.; Barranquero, C.; Dávalos, A.; López De Las Hazas, M.C.; Álvarez-Lanzarote, I.; Sánchez-Gimeno, A.C.; Raso, J.; Arnal, C.; et al. Pulsed Electric Field Increases the Extraction Yield of Extra Virgin Olive Oil without Loss of Its Biological Properties. Front. Nutr. 2022, 9, 1065543. [Google Scholar] [CrossRef]
- Cecchi, L.; Bellumori, M.; Corbo, F.; Milani, G.; Clodoveo, M.L.; Mulinacci, N. Implementation of the Sono-Heat-Exchanger in the Extra Virgin Olive Oil Extraction Process: End-User Validation and Analytical Evaluation. Molecules 2019, 24, 2379. [Google Scholar] [CrossRef]
- Servili, M.; Veneziani, G.; Taticchi, A.; Romaniello, R.; Tamborrino, A.; Leone, A. Low-Frequency, High-Power Ultrasound Treatment at Different Pressures for Olive Paste: Effects on Olive Oil Yield and Quality. Ultras. Sonochem. 2019, 59, 104747. [Google Scholar] [CrossRef] [PubMed]
- Regulation, H. Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. OJ L 1991, 248, 1–83. [Google Scholar]
- Nardella, M.; Moscetti, R.; Nallan Chakravartula, S.S.; Bedini, G.; Massantini, R. A Review on High-Power Ultrasound-Assisted Extraction of Olive Oils: Effect on Oil Yield, Quality, Chemical Composition and Consumer Perception. Foods 2021, 10, 2743. [Google Scholar] [CrossRef]
- Clodoveo. Industrial Ultrasound Applications in The Extra-Virgin Olive Oil Extraction Process: History, Approaches, and Key Questions. Foods 2019, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Taticchi, A.; Selvaggini, R.; Esposto, S.; Sordini, B.; Veneziani, G.; Servili, M. Physicochemical Characterization of Virgin Olive Oil Obtained Using an Ultrasound-Assisted Extraction at an Industrial Scale: Influence of Olive Maturity Index and Malaxation Time. Food Chem. 2019, 289, 7–15. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Sordini, B.; Lorefice, A.; Daidone, L.; Pagano, M.; Tomasone, R.; Servili, M. Extra-Virgin Olive Oil Extracted Using Pulsed Electric Field Technology: Cultivar Impact on Oil Yield and Quality. Front. Nutr. 2019, 6, 134. [Google Scholar] [CrossRef]
- Puértolas, E.; Martínez De Marañón, I. Olive Oil Pilot-Production Assisted by Pulsed Electric Field: Impact on Extraction Yield, Chemical Parameters and Sensory Properties. Food Chem. 2015, 167, 497–502. [Google Scholar] [CrossRef]
- Leone, A.; Tamborrino, A.; Esposto, S.; Berardi, A.; Servili, M. Investigation on the Effects of a Pulsed Electric Field (PEF) Continuous System Implemented in an Industrial Olive Oil Plant. Foods 2022, 11, 2758. [Google Scholar] [CrossRef]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; García-González, D.L.; Moreda, W.; et al. Olive Oil Quality and Authenticity: A Review of Current EU Legislation, Standards, Relevant Methods of Analyses, Their Drawbacks and Recommendations for the Future. Trends Food Sci. Technol. 2020, 105, 483–493. [Google Scholar] [CrossRef]
- Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil, and Repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012. OJ L 2022, 284, 1–22. Available online: http://data.europa.eu/eli/reg_del/2022/2104/oj (accessed on 7 August 2024).
- Commission Delegated Regulation (EU) 2024/1401 of 7 March 2024 Amending Delegated Regulation (EU) 2022/2104 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil. OJ L 2024. Available online: http://data.europa.eu/eli/reg_del/2024/1401/oj (accessed on 7 August 2024).
- Codex Alimentarius—Standard for Olive Oils and Olive Pomace Oils CXS 33-1981, Adopted in 1981. Revised in 1989, 2003, 2015, 2017. Amended in 2009, 2013, 2021; 2017. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B33-1981%252FCXS_033e.pdf (accessed on 6 May 2024).
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Modelling Virgin Olive Oil Potential Shelf-Life from Antioxidants and Lipid Oxidation Progress. Antioxidants 2022, 11, 539. [Google Scholar] [CrossRef]
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. OJ L 2006, 404, 9–25. Available online: http://data.europa.eu/eli/reg/2006/1924/oj (accessed on 7 August 2024).
- Guillaume, C.; Ravetti, L. Shelf-Life Prediction of Extra Virgin Olive Oils Using an Empirical Model Based on Standard Quality Tests. J. Chem. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Roca, M.; Gandul-Rojas, B. Mathematical Model to Predict the Formation of Pyropheophytin a in Virgin Olive Oil during Storage. J. Agric. Food Chem. 2012, 60, 7040–7049. [Google Scholar] [CrossRef]
- Psomiadou, E.; Karakostas, K.X.; Blekas, G.; Tsimidou, M.Z.; Boskou, D. Proposed Parameters for Monitoring Quality of Virgin Olive Oil (Koroneiki cv). Eur. J. Lipid Sci. Technol. 2003, 105, 403–409. [Google Scholar] [CrossRef]
- Pagliarini, E.; Zanoni, B.; Giovanelli, G. Predictive Study on Tuscan Extra Virgin Olive Oil Stability under Several Commercial Conditions. J. Agric. Food Chem. 2000, 48, 1345–1351. [Google Scholar] [CrossRef]
- Mancebo-Campos, V.; Fregapane, G.; Desamparados Salvador, M. Kinetic Study for the Development of an Accelerated Oxidative Stability Test to Estimate Virgin Olive Oil Potential Shelf Life. Eur. J. Lipid Sci. Technol. 2008, 110, 969–976. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Evolution of Major and Minor Components and Oxidation Indices of Virgin Olive Oil during 21 Months Storage at Room Temperature. Food Chem. 2007, 100, 36–42. [Google Scholar] [CrossRef]
- Krichene, D.; Allalout, A.; Mancebo-Campos, V.; Salvador, M.D.; Zarrouk, M.; Fregapane, G. Stability of Virgin Olive Oil and Behaviour of Its Natural Antioxidants under Medium Temperature Accelerated Storage Conditions. Food Chem. 2010, 121, 171–177. [Google Scholar] [CrossRef]
- Krichene, D.; Salvador, M.D.; Fregapane, G. Stability of Virgin Olive Oil Phenolic Compounds during Long-Term Storage (18 Months) at Temperatures of 5–50 °C. J. Agric. Food Chem. 2015, 63, 6779–6786. [Google Scholar] [CrossRef]
- Calligaris, S.; Manzocco, L.; Anese, M.; Nicoli, M.C. Accelerated Shelf Life Testing. In Food Quality and Shelf Life; Elsevier: Amsterdam, The Netherlands, 2019; pp. 359–392. [Google Scholar] [CrossRef]
- Nicoli, M.C. (Ed.) Shelf Life Assessment of Food; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Mizrahi, S. Accelerated Shelf Life Testing of Foods. In Food and Beverage Stability and Shelf Life; Elsevier: Amsterdam, The Netherlands, 2011; pp. 482–506. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Calcio Gaudino, E.; Binello, A.; Rego, D.; Pereira, M.; Martínez, M.; Cravotto, G. Combined Ultrasound and Pulsed Electric Fields in Continuous-Flow Industrial Olive-Oil Production. Foods 2022, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- ISO/IEC 17025:2017; General requirements for the competence of testing and calibration laboratories; Edition 3. ISO: Geneva, Switzerland, 2017.
- International Olive Council. Sensory Analysis of Olive Oil—Method for The Organoleptic Assessment of Virgin Olive Oil (COI-T20-Doc.-15-REV-10-2018-Eng). 2018. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T20-Doc.-15-REV-10-2018-Eng.pdf (accessed on 2 February 2024).
- International Olive Council. Determination of Free Fatty Acids, Cold Method (COI/T.20/Doc. n°34/Rev.1 2017). 2017. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T.20-Doc.-No-34-Rev.-1-2017.pdf (accessed on 2 February 2024).
- International Olive Council. Determination of Peroxide Value (Method-COI-T.20-Doc.-No-35-Rev.-1-2017). 2017. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/Method-COI-T.20-Doc.-No-35-Rev.-1-2017.pdf (accessed on 2 February 2024).
- ISO 663:2017; Animal and Vegetable Fats and Oils—Determination of Insoluble Impurities Content; Edition 5. ISO: Geneva, Switzerland, 2017.
- ISO 662:2016; Animal and Vegetable Fats and Oils—Determination of Moisture and Volatile Matter Content; Edition 3. ISO: Geneva, Switzerland, 2016.
- International Olive Council. Spectrophotometric Investigation in The Ultraviolet (Method-COI-T.20-Doc.-No-19-Rev.-5-2019-2). 2019. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/Method-COI-T.20-Doc.-No-19-Rev.-5-2019-2.pdf (accessed on 2 February 2024).
- International Olive Council. Determination of Biophenols in Olive Oils by Hplc (COI-T.20-Doc.-No-29-Rev-1-2017). 2017. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T.20-Doc.-No-29-Rev-1-2017.pdf (accessed on 2 February 2024).
- Dieffenbacher, A.; Pocklington, W.D. Determination of tocopherols and tocotrienols in vegetable oils and fats by high performance liquid chromatography. In Standard Methods for the Analysis of Oils, Fats, and Derivatives: Supplement to the 7th Revised and Enlarged Edition; Blackwell Scientific Publications: Oxford UK; Boston, MA, USA, 1992; Volume 2.432. [Google Scholar]
- International Olive Council. Determination of Fatty Acid Methyl Esters by Gas Chromatography (COI-T.20-Doc.-No-33-Rev.-1-2017). 2017. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T.20-Doc.-No-33-Rev.-1-2017.pdf (accessed on 2 February 2024).
- International Olive Council. Determination of The Composition and Content of Sterols, Triterpenic Dialcohols and Aliphatic Alcohols by Capillary Column Gas Chromatography (COI-T20-Doc-26-Rev5-EN). 2020. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2020/07/COI-T20-Doc-26-Rev5-EN-.pdf (accessed on 2 February 2024).
- Esti, M.; Contini, M.; Moneta, E.; Sinesio, F. Phenolics Compounds and Temporal Perception of Bitterness and Pungency in Extra-Virgin Olive Oils: Changes Occurring throughout Storage. Food Chem. 2009, 113, 1095–1100. [Google Scholar] [CrossRef]
- Shahidi, F.; De Camargo, A. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Gallina Toschi, T.; Biguzzi, B.; Cerretani, L.; Bendini, A.; Rotondi, A.; Lercker, G. Effect of Crushing Time and Temperature of Malaxation on the Oxidative Stability of a Monovarietal Extra-Virgin Olive Oil, Obtained by Different Industrial Processing Systems. Progr. Nutr. 2004, 6, 132–138. [Google Scholar]
- Morelló, J.-R.; Motilva, M.-J.; Tovar, M.-J.; Romero, M.-P. Changes in Commercial Virgin Olive Oil (Cv Arbequina) during Storage, with Special Emphasis on the Phenolic Fraction. Food Chem. 2004, 85, 357–364. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Vieira, T.M.F.D.S.; Regitano-D’Arce, M.A.B.; De Alencar, S.M.; Calori-Domingues, M.A.; Canniatti-Brazaca, S.G. Gamma Radiation Induced Oxidation and Tocopherols Decrease in In-Shell, Peeled and Blanched Peanuts. Int. J. Mol. Sci. 2012, 13, 2827–2845. [Google Scholar] [CrossRef]
- Rossi, M.; Alamprese, C.; Ratti, S. Tocopherols and Tocotrienols as Free Radical-Scavengers in Refined Vegetable Oils and Their Stability during Deep-Fat Frying. Food Chem. 2007, 102, 812–817. [Google Scholar] [CrossRef]
- Fregapane, G.; Gómez-Rico, A.; Inarejos, A.M.; Salvador, M.D. Relevance of Minor Components Stability in Commercial Olive Oil Quality during the Market Period. Eur. J. Lipid Sci. Technol. 2013, 115, 541–548. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Chamorro, F.; Pereira, A.G.; Carrera-Casais, A.; Fraga-Corral, M.; Carpena, M.; Simal-Gandara, J.; Prieto, M.A. Evolution of Flavors in Extra Virgin Olive Oil Shelf-Life. Antioxidants 2021, 10, 368. [Google Scholar] [CrossRef]
- Dias, S.; Pino-Hernández, E.; Gonçalves, D.; Rego, D.; Redondo, L.; Alves, M. Challenges and Opportunities for Pilot Scaling-Up Extraction of Olive Oil Assisted by Pulsed Electric Fields: Process, Product, and Economic Evaluation. Appl. Sci. 2024, 14, 3638. [Google Scholar] [CrossRef]




| Parameters | US + PEF_P (3) | US + PEF_B (4) | M_PEF_P (5) |
|---|---|---|---|
| Voltage; Pulse; Frequency | [10 kV; 30 µs; 12 Hz] | [10 kV; 30 µs; 5 Hz] | [10 kV; 30 µs; 17 Hz] |
| Electric Field | 2 kV/cm | 2.0 kV/cm | 2 kV/cm |
| Total Energy | 5.1 kJ/kg | 5.1 kJ/kg | 5.1 kJ/kg |
| Power | 1.5 kW | 1.5 kW | 1.5 kW |
| Analysis | Method | Parameter (Meas. Unit) | CTRL | US | US + PEF_P | US + PEF_B | M + PEF_P | EVOO Spec. # |
|---|---|---|---|---|---|---|---|---|
| FFA (expressed as oleic acid) | [56] | % ± U § | 0.20 ± 0.07 | 0.15 ± 0.07 | 0.22 ± 0.07 | 0.17 ± 0.07 | 0.17 ± 0.07 | ≤0.80 |
| PV | [57] | meq O2/kg ± U § | 6.2 ± 1.1 | 6.0 ± 1.1 | 5.9 ± 1.1 | 6.2 ± 1.1 | 5.7 ± 1.1 | ≤20.0 |
| Insoluble impurities | [58] | w/w % ± U § | <0.01 ± 0.01 | 0.01 ± 0.01 | <0.01 ± 0.01 | <0.01 ± 0.01 | <0.01 ± 0.01 | ≤0.10 |
| Moisture and volatile matter (B) | [59] | w/w % ± U § | 0.15 ± 0.04 | 0.15 ± 0.05 | 0.16 ± 0.05 | 0.14 ± 0.05 | 0.14 ± 0.05 | ≤0.20 |
| UV spectrophotometric analysis | [60] | K232 (±SD) * | 1.34 ± 0.03 | 1.97 ± 0.03 | 1.87 ± 0.01 | 1.82 ± 0.09 | 1.83 ± 0.07 | ≤2.50 |
| K268 (±SD) * | 0.18 ± 0.03 | 0.19 ± 0.02 | 0.20 ± 0.01 | 0.19 ± 0.005 | 0.17 ± 0.01 | ≤0.22 | ||
| ΔK (±SD) * | −0.002 ± 0.000 | −0.001 ± 0.003 | 0.000 ± 0.001 | −0.001 ± 0.003 | −0.001 ± 0.000 | ≤0.01 | ||
| Organoleptic assessment | [55] | Category | EVOO | EVOO | EVOO | EVOO | EVOO | |
| Median of fruitiness (Mf) | 4.3 | 4.4 | 4.3 | 4.2 | 4.1 | >0.0 | ||
| Median of bitter attribute | 3.5 | 3.9 | 3.8 | 4.0 | 3.9 | - | ||
| Median of pungent attribute | 4.6 | 4.7 | 5.0 | 5.0 | 4.6 | - | ||
| Median of the negative attribute with the highest intensity (Md) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | =0 | ||
| Notes perceived with the highest intensity: | Almond, green, floral | Almond, green, floral | Almond, green, floral | Almond, green, floral | Almond, green, floral | - |
| Analysis | Method | Compound (Meas. Unit) | CTRL | US | US + PEF_P | US + PEF_B | M + PEF_P |
|---|---|---|---|---|---|---|---|
| Tocopherols and tocotrienols | [61] | α-tocopherol (the only detectable) (mg/kg) (CL 0.95, SE 1.74) * | 189 c (185 ÷ 193) | 195 bc (191 ÷ 199) | 199 ab (195 ÷ 203) | 191 bc (188 ÷ 195) | 204 a (200 ÷ 208) |
| Polyphenols | [60] | mg/kg (CL 0.95, SE 19.6) * (RRF 4.95) | 980 bc (936 ÷ 1023) | 1103 a (1059 ÷ 1146) | 1057 ab (1014 ÷ 1101) | 989 bc (946 ÷ 1033) | 912 c (868 ÷ 955) |
| Technique | T0 (mg/kg) * | T15 (mg/kg) * | T30 (mg/kg) * | Loss % T15 | Loss % T30 |
|---|---|---|---|---|---|
| CTRL | 980 cd, 1 (943 ÷ 1017) | 841 b, 2 (805 ÷ 878) | 836 a, 2 (799 ÷ 873) | −14.1 | −14.7 |
| US | 1103 a, 1 (1066 ÷ 1140) | 895 b, 2 (858 ÷ 932) | 905 a, 2 (868 ÷ 942) | −18.8 | −17.9 |
| US + PEF_P | 1057 ab, 1 (1021 ÷ 1094) | 978 a, 2 (941 ÷ 1015) | 901 a, 3 (865 ÷ 938) | −7.5 | −14.7 |
| US + PEF_B | 989 bc, 1 (953 ÷ 1026) | 910 ab, 2 (874 ÷ 947) | 873 a, 2 (836 ÷ 910) | −8.0 | −11.8 |
| M + PEF_P | 912 d, 1 (875 ÷ 948) | 862 b, 1 (825 ÷ 899) | 856 a, 1 (819 ÷ 892) | −5.4 | −6.1 |
| Technique | T0 (mg/kg) * | T15 (mg/kg) * | T30 (mg/kg) * | Loss % T15 | Loss % T30 |
|---|---|---|---|---|---|
| CTRL | 189 c, 1 (185÷194) | 103 d, 2 (99÷108) | 107 b, 2 (103÷112) | −45.5 | −43.4 |
| US | 195 b,c 1 (190÷199) | 128 a, 2 (123÷132) | 121 a, 3 (117÷126) | −34.4 | −37.9 |
| US + PEF_P | 199 a,b, 1 (195÷204) | 117 b,c, 2 (113÷122) | 121 a, 2 (116÷125) | −41.2 | −39.2 |
| US + PEF_B | 191 c, 1 (187÷196) | 111 c, 2 (106÷115) | 105 b, 3 (100÷109) | −41.9 | −45.0 |
| M + PEF_P | 204 a, 1 (199÷209) | 120 b, 2 (125÷124) | 116 a, 2 (111÷120) | −41.2 | −43.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boffa, L.; Calcio Gaudino, E.; Grillo, G.; Binello, A.; Capaldi, G.; Rego, D.; Pereira, M.; Cravotto, G. Industrial Production of Bioactive Nutrient-Enhanced Extra Virgin Olive Oil under Continuous-Flow Ultrasound and Pulsed Electric Field Treatment. Foods 2024, 13, 2613. https://doi.org/10.3390/foods13162613
Boffa L, Calcio Gaudino E, Grillo G, Binello A, Capaldi G, Rego D, Pereira M, Cravotto G. Industrial Production of Bioactive Nutrient-Enhanced Extra Virgin Olive Oil under Continuous-Flow Ultrasound and Pulsed Electric Field Treatment. Foods. 2024; 13(16):2613. https://doi.org/10.3390/foods13162613
Chicago/Turabian StyleBoffa, Luisa, Emanuela Calcio Gaudino, Giorgio Grillo, Arianna Binello, Giorgio Capaldi, Duarte Rego, Marcos Pereira, and Giancarlo Cravotto. 2024. "Industrial Production of Bioactive Nutrient-Enhanced Extra Virgin Olive Oil under Continuous-Flow Ultrasound and Pulsed Electric Field Treatment" Foods 13, no. 16: 2613. https://doi.org/10.3390/foods13162613
APA StyleBoffa, L., Calcio Gaudino, E., Grillo, G., Binello, A., Capaldi, G., Rego, D., Pereira, M., & Cravotto, G. (2024). Industrial Production of Bioactive Nutrient-Enhanced Extra Virgin Olive Oil under Continuous-Flow Ultrasound and Pulsed Electric Field Treatment. Foods, 13(16), 2613. https://doi.org/10.3390/foods13162613








