Wild Mushrooms as a Source of Bioactive Compounds and Their Antioxidant Properties—Preliminary Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Characteristics of the Fungal Species Studied
2.3. Identification of Active Compounds in Ethanolic Extracts of Analysed Fungi
2.3.1. Extraction of Bioactive Compounds
2.3.2. Analysis of Extracts by GC-MS
2.4. Determination of Total Polyphenols with Folin-Ciocalteu Reagent
2.4.1. Determination of Standard Solutions of Gallic Acid
2.4.2. Determination of Total Polyphenols in the Test Extract
2.5. Determination of Antioxidant Activity
2.5.1. Determination of Antioxidant Activity by the DPPH Method, Determination of the IC50 Parameter
2.5.2. Determination of Antioxidant Activity Using ABTS
2.6. Determination of Selected Elements by ICP-OES Method
2.6.1. Mineralisation of Raw Materials
2.6.2. Determination of Elements by ICP-OES Method
2.7. Thermogravimetric Evaluation of Mushroom Fruiting Bodies
2.8. Statistical Analysis
3. Results
3.1. Identification of Active Compounds in the Ethanol Extracts of the Analysed Fungi
3.2. Total Polyphenols Content
3.3. Determination of Antioxidant Activity by the Method with DPPH
3.4. Determination of Antioxidant Activity by the Method with ABTS
3.5. Determination of Selected Minerals by ICP-OES
3.6. Determination of Aluminum and Heavy Metals by ICP-OES
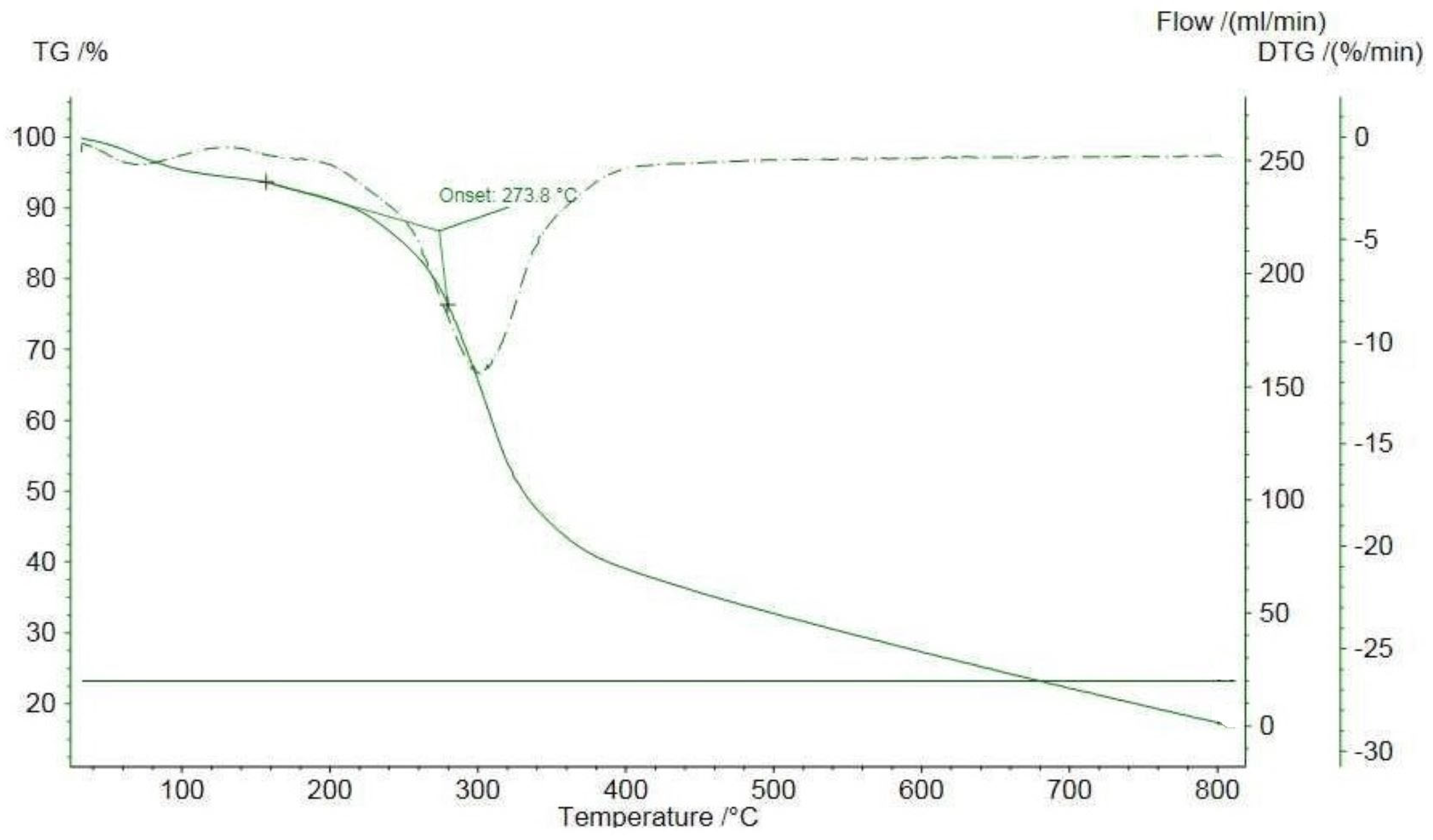
3.7. Thermogravimetric Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berry, E.M. Food Insecurity, Social Inequity, and Sustainability. World Rev. Nutr. Diet. 2020, 121, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Rahut, D.B.; Aryal, J.P.; Manchanda, N.; Sonobe, T. Chapter 6—Expectations for Household Food Security in the Coming Decades: A Global Scenario. In Future Foods; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 107–131. ISBN 978-0-323-91001-9. [Google Scholar]
- Petrescu, D.C.; Vermeir, I.; Petrescu-Mag, R.M. Consumer Understanding of Food Quality, Healthiness, and Environmental Impact: A Cross-National Perspective. Int. J. Environ. Res. Public Health 2020, 17, 169. [Google Scholar] [CrossRef]
- Gopal, J.; Sivanesan, I.; Muthu, M.; Oh, J.-W. Scrutinizing the Nutritional Aspects of Asian Mushrooms, Its Commercialization and Scope for Value-Added Products. Nutrients 2022, 14, 3700. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional Composition and Biological Properties of Sixteen Edible Mushroom Species. Appl. Sci. 2022, 12, 8074. [Google Scholar] [CrossRef]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef]
- Anusiya, G.; Gowthama Prabu, U.; Yamini, N.V.; Sivarajasekar, N.; Rambabu, K.; Bharath, G.; Banat, F. A Review of the Therapeutic and Biological Effects of Edible and Wild Mushrooms. Bioengineered 2021, 12, 11239–11268. [Google Scholar] [CrossRef]
- Hu, S.H.; Liang, Z.C.; Chia, Y.C.; Lien, J.L.; Chen, K.S.; Lee, M.Y.; Wang, J.C. Antihyperlipidemic and Antioxidant Effects of Extracts from Pleurotus citrinopileatus. J. Agric. Food Chem. 2006, 54, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Qiu, H.; Zheng, J. Physicochemical characteristics and anti-hyperlipidemic effect of polysaccharide from BaChu mushroom (Helvella leucopus). Food Chem. X 2022, 15, 100443. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M.; Oboh, G. Blood Glucose Lowering and Effect of Oyster (Pleurotus ostreatus)- and Shiitake (Lentinus subnudus)-Supplemented Diet on Key Enzymes Linked Diabetes and Hypertension in Streptozotocin-Induced Diabetic in Rats. Food Front. 2022, 3, 161–171. [Google Scholar] [CrossRef]
- Afiati, F.; Firza, S.F.; Kusmiati; Aliya, L.S. The Effectiveness β-Glucan of Shiitake Mushrooms and Saccharomyces cerevisiae as Antidiabetic and Antioxidant in Mice Sprague Dawley Induced Alloxan. AIP Conf. Proc. 2019, 2120, 070006. [Google Scholar] [CrossRef]
- Nguyen, T.M.N.; Le, H.S.; Le, B.V.; Kim, Y.H.; Hwang, I. Anti-Allergic Effect of Inotodiol, a Lanostane Triterpenoid from Chaga Mushroom, via Selective Inhibition of Mast Cell Function. Int. Immunopharmacol. 2020, 81, 106244. [Google Scholar] [CrossRef]
- Sadi, G.; Emsen, B.; Kaya, A.; Kocabaş, A.; Çınar, S.; Kartal, D.İ. Cytotoxicity of Some Edible Mushrooms Extracts over Liver Hepatocellular Carcinoma Cells in Conjunction with Their Antioxidant and Antibacterial Properties. Pharmacogn. Mag. 2015, 11, S6–S18. [Google Scholar] [CrossRef]
- Osman, A.; Toliba, A.O. Hepatoprotective Effects of Crude Phenolic-rich Extract from Oyster Mushroom (Pleurotus ostreatus). Egypt. J. Food Sci. 2019, 47, 157–164. [Google Scholar] [CrossRef][Green Version]
- Schreiber, D.; Marx, L.; Felix, S.; Clasohm, J.; Weyland, M.; Schäfer, M.; Klotz, M.; Lilischkis, R.; Erkel, G.; Schäfer, K.-H. Anti-Inflammatory Effects of Fungal Metabolites in Mouse Intestine as Revealed by In Vitro Models. Front. Physiol. 2017, 8, 566. [Google Scholar] [CrossRef]
- Kou, R.-W.; Xia, B.; Han, R.; Li, Z.-Q.; Yang, J.-R.; Yin, X.; Gao, Y.-Q.; Gao, J.-M. Neuroprotective Effects of a New Triterpenoid from Edible Mushroom on Oxidative Stress and Apoptosis through the BDNF/TrkB/ERK/CREB and Nrf2 Signaling Pathway in Vitro and in Vivo. Food Funct. 2022, 13, 12121–12134. [Google Scholar] [CrossRef] [PubMed]
- Ellan, K.; Thayan, R.; Raman, J.; Hidari, K.I.P.J.; Ismail, N.; Sabaratnam, V. Anti-Viral Activity of Culinary and Medicinal Mushroom Extracts against Dengue Virus Serotype 2: An in-Vitro Study. BMC Complement. Altern. Med. 2019, 19, 260. [Google Scholar] [CrossRef]
- Moazzem Hossen, S.M.; Hossain, M.S.; Akbar, S.; Tahmida, U.; Mawa, J.; Uddin Emon, N. Wild Mushrooms Showed Analgesic and Cytotoxic Properties along with Phytoconstituent’s Binding Affinity to COX-1, COX-2 and Cytochrome P450 2C. Heliyon 2021, 7, e07997. [Google Scholar] [CrossRef]
- Guggenheim, A.G.; Wright, K.M.; Zwickey, H.L. Immune Modulation From Five Major Mushrooms: Application to Integrative Oncology. Integr. Med. 2014, 13, 32–44. [Google Scholar]
- Goodwin, G.M.; Croal, M.; Feifel, D.; Kelly, J.R.; Marwood, L.; Mistry, S.; O’Keane, V.; Peck, S.K.; Simmons, H.; Sisa, C.; et al. Psilocybin for Treatment Resistant Depression in Patients Taking a Concomitant SSRI Medication. Neuropsychopharmacology 2023, 48, 1492–1499. [Google Scholar] [CrossRef]
- Hakami Zanjani, A.A.; Nguyen, T.Q.T.; Jacobsen, L.; Khandelia, H. The Molecular Basis of the Antidepressant Action of the Magic Mushroom Extract, Psilocin. Biochim. Biophys. Acta Proteins Proteom. 2023, 1871, 140914. [Google Scholar] [CrossRef]
- Sinaeve, S.; Husson, C.; Antoine, M.-H.; Welti, S.; Stévigny, C.; Nortier, J. Nephroprotective Effects of Two Ganoderma Species Methanolic Extracts in an In Vitro Model of Cisplatin Induced Tubulotoxicity. J. Fungi. 2022, 8, 1002. [Google Scholar] [CrossRef]
- Park, H.-J. Current Uses of Mushrooms in Cancer Treatment and Their Anticancer Mechanisms. Int. J. Mol. Sci. 2022, 23, 10502. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Dalvi, Y.B.; Lamrood, P.Y.; Shinde, B.P.; Nair, C.K.K. Historical and Current Perspectives on Therapeutic Potential of Higher Basidiomycetes: An Overview. 3 Biotech 2019, 9, 362. [Google Scholar] [CrossRef]
- Ragucci, S.; Castaldi, S.; Landi, N.; Isticato, R.; Di Maro, A. Antifungal Activity of Ageritin, a Ribotoxin-like Protein from Cyclocybe aegerita Edible Mushroom, against Phytopathogenic Fungi. Toxins 2023, 15, 578. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Ragucci, S.; Russo, R.; Valletta, M.; Pizzo, E.; Ferreras, M.; Di Maro, A. The ribotoxin-like protein Ostreatin from Pleurotus ostreatus fruiting bodies: Confirmation of a novel ribonuclease family expressed in basidiomycetes. Int. J. Biol. Macromol. 2020, 161, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Ragucci, S.; Culurciello, R.; Russo, R.; Valletta, M.; Pedone, P.V.; Pizzo, E.; Di Maro, A. Ribotoxin-like Proteins from Boletus Edulis: Structural Properties, Cytotoxicity and in Vitro Digestibility. Food Chem. 2021, 359, 129931. [Google Scholar] [CrossRef] [PubMed]
- Ragucci, S.; Landi, N.; Russo, R.; Valletta, M.; Pedone, P.V.; Chambery, A.; Di Maro, A. Ageritin from Pioppino Mushroom: The Prototype of Ribotoxin-Like Proteins, a Novel Family of Specific Ribonucleases in Edible Mushrooms. Toxins 2021, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Ragucci, S.; Landi, N.; Citores, L.; Iglesias, R.; Russo, R.; Clemente, A.; Saviano, M.; Pedone, P.V.; Chambery, A.; Ferreras, J.M.; et al. The Biological Action and Structural Characterization of Eryngitin 3 and 4, Ribotoxin-like Proteins from Pleurotus Eryngii Fruiting Bodies. Int. J. Mol. Sci. 2023, 24, 14435. [Google Scholar] [CrossRef] [PubMed]
- Rampolli, F.I.; Kamler, P.; Carnevale Carlino, C.; Bedussi, F. The Deceptive Mushroom: Accidental Amanita Muscaria Poisoning. Eur. J. Case Rep. Intern. Med. 2021, 8, 002212. [Google Scholar] [CrossRef] [PubMed]
- Okhovat, A.; Cruces, W.; Docampo-Palacios, M.L.; Ray, K.P.; Ramirez, G.A. Psychoactive Isoxazoles, Muscimol, and Isoxazole Derivatives from the Amanita (Agaricomycetes) Species: Review of New Trends in Synthesis, Dosage, and Biological Properties. Int. J. Med. Mushrooms 2023, 25, 1–10. [Google Scholar] [CrossRef]
- Dushkov, A.; Vosáhlová, Z.; Tzintzarov, A.; Kalíková, K.; Krízek, T.; Ugrinova, I. Analysis of the Ibotenic Acid, Muscimol, and Ergosterol Content of an Amanita Muscaria Hydroalcoholic Extract with an Evaluation of Its Cytotoxic Effect against a Panel of Lung Cell Lines In Vitro. Molecules 2023, 28, 6824. [Google Scholar] [CrossRef] [PubMed]
- Ramawad, H.A.; Paridari, P.; Jabermoradi, S.; Gharin, P.; Toloui, A.; Safari, S.; Yousefifard, M. Muscimol as a Treatment for Nerve Injury-Related Neuropathic Pain: A Systematic Review and Meta-Analysis of Preclinical Studies. Korean J. Pain 2023, 36, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Hsu, D.Z.; Liu, C.T.; Chandrasekaran, V.R.M.; Liu, M.Y. The protective effect of muscimol against systemic inflammatory response in endotoxemic mice is independent of GABAergic and cholinergic receptors. Can. J. Physiol. Pharmacol. 2022, 100, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Mago, P.; Sharma, R.; Hafeez, I.; Nawaz, I.; Joshi, M.; Mehrotra, R. Mushroom based Cosmeceuticals: An Upcoming Biotechnology Sector. Biosci. Biotechnol. Res. Asia 2023, 20, 381–394. [Google Scholar] [CrossRef]
- Burt, T.; Young, G.; Lee, W.; Kusuhara, H.; Langer, O.; Rowland, M.; Sugiyama, Y. Phase 0/Microdosing Approaches: Time for Mainstream Application in Drug Development? Nat. Rev. Drug Discov. 2020, 19, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Sevindik, M.; Akgül, H.; Akata, I.; Selamoglu, Z. Geastrum pectinatum as an Alternative Antioxidant Source with Some Biochemical Analysis. Med. Mycol. Open Access 2017, 3, 1–4. [Google Scholar] [CrossRef][Green Version]
- Map of Life. Available online: https://scistarter.org/map-of-life (accessed on 6 May 2024).
- Giminder, A. Mushroom Atlas: How to Identify 340 Species of Central European Fungi Without Error; Weltbild Media: Augsburg, Germany, 2011; ISBN 978-83-258-0588-3. [Google Scholar]
- Michael Wood & Fred Stevens. California Fungi: Panus conchatus. Available online: http://www.mykoweb.com/CAF/species/Panus_conchatus.html (accessed on 6 May 2024).
- Species Fungorum Home Page. Available online: https://www.speciesfungorum.org/ (accessed on 6 May 2024).
- Mirek, Z. Red List of Plants and Fungi in Poland; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2006; ISBN 978-83-89648-38-9. [Google Scholar]
- Ordinance of the Minister for the Environment of 9 October 2014 on the Protection of Mushroom Species. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20140001408 (accessed on 6 May 2024).
- Phallus Hadriani, Dune Stinkhorn Fungus. Available online: https://www.first-nature.com/fungi/phallus-hadriani.php# (accessed on 14 May 2024).
- Geastrum pectinatum, Beaked Earthstar Fungus. Available online: https://www.first-nature.com/fungi/geastrum-pectinatum.php (accessed on 14 May 2024).
- Gumińska, B.; Wojewoda, W. Fungi and Their Determination; Państwowe Wydaw Rolnicze i Leśne: Warszawa, Poland, 1985. [Google Scholar]
- Moreno, G.; Khalid, A.N.; Alvarado, P.; Kreisel, H. Phallus hadriani and P. roseus from Pakistan. Mycotaxon 2013, 125, 45–51. [Google Scholar] [CrossRef]
- Wang, X.; Bau, T. Seven New Species of the Genus Geastrum (Geastrales, Geastraceae) in China. J. Fungi 2023, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Weinstein, S.A.; De Haro, L.; Bédry, R.; Schaper, A.; Rumack, B.H.; Zilker, T. Mushroom Poisoning: A Proposed New Clinical Classification. Toxicon 2019, 157, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Niksic, M.; Klaus, A.; Argyropoulos, D. Chapter 22—Safety of Foods Based on Mushrooms. In Regulating Safety of Traditional and Ethnic Foods; Prakash, V., Martín-Belloso, O., Keener, L., Astley, S., Braun, S., McMahon, H., Lelieveld, H., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 421–439. ISBN 978-0-12-800605-4. [Google Scholar]
- Alam, S.M.N. Chapter 6—Safety in the Shrimp Supply Chain. In Regulating Safety of Traditional and Ethnic Foods; Prakash, V., Martín-Belloso, O., Keener, L., Astley, S., Braun, S., McMahon, H., Lelieveld, H., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 99–123. ISBN 978-0-12-800605-4. [Google Scholar]
- van den Brandhof, J.G.; Wösten, H.A.B. Risk Assessment of Fungal Materials. Fungal Biol. Biotechnol. 2022, 9, 1–20. [Google Scholar] [CrossRef]
- Till, R. Lohmeyer. Mushrooms. Identifying and Collecting; Parragon: Warszawa, Poland, 2006; ISBN 978-1-40547-937-0. [Google Scholar]
- Wojewoda, W. (1932–2010) Critical List of the Large-Flowered Basal Fungi of Poland; Władysław Szafer Institute of Botany of the Polish Academy of Sciences: Kraków, Poland, 2003; ISBN 83-89648-09-1. [Google Scholar]
- Index Fungorum—Search Page. Available online: https://www.indexfungorum.org/names/Names.asp (accessed on 6 May 2024).
- Mycobank. Available online: https://www.mycobank.org/ (accessed on 6 May 2024).
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of Vitamins, Mineral Elements, and Some Phenolic Compounds in Cultivated Mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef]
- Škubla, P. The Great Atlas of Mushrooms; Elipsa: Warszawa, Poland, 2007; ISBN 978-83-245-9550-1. [Google Scholar]
- Thorn, R.G.; Moncalvo, J.-M.; Reddy, C.; Vilgalys, R. Phylogenetic Analyses and the Distribution of Nematophagy Support a Monophyletic Pleurotaceae within the Polyphyletic Pleurotoid-Lentinoid Fungi. Mycologia 2000, 92, 241. [Google Scholar] [CrossRef]
- Global Mapper—Discover Life. Available online: https://www.discoverlife.org/mp/20m?act=make_map (accessed on 6 May 2024).
- Fauzi, A.; Hameed, I.; Kadhim, M. A Review: Uses of Gas Chromatography-Mass Spectrometry (GC-MS) Technique for Analysis of Bioactive Natural Compounds of Some Plants. Int. J. Toxicol. Pharmacol. Res. 2017, 9, 81–85. [Google Scholar] [CrossRef]
- Sharmila, M.; Rajeswari, M.; Dh, G. Physicochemical Analysis of the Entire Plant Powder Ludwigia Perennis L. by Using Different Solvents. Int. J. Pharmacol. Res. 2017, 7, 192–195. [Google Scholar]
- Chemdata.nist.gov. Mass Spectrometry Data Center. NIST Method 23 GC/retention Index Library. Available online: https://chemdata.nist.gov/dokuwiki/doku.php?id=chemdata:ridatabase (accessed on 15 March 2024).
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content. Analyt. Methods 2013, 5, 5990. [Google Scholar]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation, First Action 2017. J. AOAC Int. 2018, 101, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicrylhydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Thermal Analysis of Foods. Available online: https://people.umass.edu/~mcclemen/581Thermal.html (accessed on 15 May 2024).
- Akanmu, M.; Adeosun, S.; Ilesanmi, O. Neuropharmacological Effects of Oleamide in Male and Female Mice. Behav. Brain Res. 2007, 182, 88–94. [Google Scholar] [CrossRef]
- Mendelson, W.B.; Basile, A.S. The Hypnotic Actions of the Fatty Acid Amide, Oleamide. Neuropsychopharmacol 2001, 25, S36–S39. [Google Scholar] [CrossRef]
- Yerlikaya, S.; Djamgoz, M.B.A. Oleamide, a Sleep-Inducing Compound: Effects on Ion Channels and Cancer. Bioelectricity 2022, 4, 136–144. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Watanabe, N.; Kitakaze, T.; Sugimoto, K.; Izawa, T.; Kai, K.; Harada, N.; Yamaji, R. Oleamide rescues tibialis anterior muscle atrophy of mice housed in small cages. Br. J. Nutr. 2021, 126, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, W.; Li, M.; Li, F.; Gong, R.; Wu, Y. Relationship between Dietary Decanoic Acid and Coronary Artery Disease: A Population-Based Cross-Sectional Study. Nutrients 2023, 15, 4308. [Google Scholar] [CrossRef]
- Diacylglycerol in food industry: Synthesis methods, functionalities, health benefits, potential risks and drawbacks. Trends Food Sci. Technol. 2020, 97, 114–125. [CrossRef]
- Pain, E.; Shinhmar, S.; Williams, R.S.B. Using Dictyostelium to Advance Our Understanding of the Role of Medium Chain Fatty Acids in Health and Disease. Front. Cell Dev. Biol. 2021, 9, 722066. [Google Scholar] [CrossRef]
- Ameen, A.O.; Freude, K.; Aldana, B.I. Fats, Friends or Foes: Investigating the Role of Short- and Medium-Chain Fatty Acids in Alzheimer’s Disease. Biomedicines 2022, 10, 2778. [Google Scholar] [CrossRef]
- Choi, H.S.; Park, S.J.; Lee, Z.H.; Lim, S.-K. The Effects of a High Fat Diet Containing Diacylglycerol on Bone in C57BL/6J Mice. Yonsei Med. J. 2015, 56, 951–960. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Tang, T.-K.; Phuah, E.-T.; Tan, C.-P.; Wang, Y.; Li, Y.; Cheong, L.-Z.; Lai, O.-M. Production, Safety, Health Effects and Applications of Diacylglycerol Functional Oil in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2509–2525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lee, Y.-Y.; Mao, Y.; Wang, Y.; Zhang, Z. Future of Structured Lipids: Enzymatic Synthesis and Their New Applications in Food Systems. Foods 2022, 11, 2400. [Google Scholar] [CrossRef]
- Mercola, J.; D’Adamo, C.R. Linoleic Acid: A Narrative Review of the Effects of Increased Intake in the Standard American Diet and Associations with Chronic Disease. Nutrients 2023, 15, 3129. [Google Scholar] [CrossRef] [PubMed]
- Jandacek, R.J. Linoleic Acid: A Nutritional Quandary. Healthcare 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Kumar, G.S. Potential Role of Mycosterols in Hyperlipidemia—A Review. Steroids 2021, 166, 108775. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Olszewski, R.; Banach, M.; Gluba-Brzozka, A. The Use of Plant Sterols and Stanols as Lipid-Lowering Agents in Cardiovascular Disease. Curr. Pharm. Des. 2017, 23, 2488–2495. [Google Scholar] [CrossRef]
- Barkas, F.; Bathrellou, E.; Nomikos, T.; Panagiotakos, D.; Liberopoulos, E.; Kontogianni, M.D. Plant Sterols and Plant Stanols in Cholesterol Management and Cardiovascular Prevention. Nutrients 2023, 15, 2845. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From Preclinical Evidence to Potential Clinical Applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar]
- Kopylov, A.T.; Malsagova, K.A.; Stepanov, A.A.; Kaysheva, A.L. Diversity of Plant Sterols Metabolism: The Impact on Human Health, Sport, and Accumulation of Contaminating Sterols. Nutrients 2021, 13, 1623. [Google Scholar] [CrossRef]
- Bao, L.; Sun, H.; Zhao, Y.; Feng, L.; Wu, K.; Shang, S.; Xu, J.; Shan, R.; Duan, S.; Qiu, M.; et al. Hexadecanamide Alleviates Staphylococcus Aureus-Induced Mastitis in Mice by Inhibiting Inflammatory Responses and Restoring Blood-Milk Barrier Integrity. PLoS Pathog. 2023, 19, e1011764. [Google Scholar] [CrossRef]
- Poochi, S.P.; Easwaran, M.; Balasubramanian, B.; Anbuselvam, M.; Meyyazhagan, A.; Park, S.; Bhotla, H.K.; Anbuselvam, J.; Arumugam, V.A.; Keshavarao, S.; et al. Employing Bioactive Compounds Derived from Ipomoea obscura (L.) to Evaluate Potential Inhibitor for SARS-CoV-2 Main Protease and ACE2 Protein. Food Front. 2020, 1, 168–179. [Google Scholar] [CrossRef]
- Arsana, I.N.; Juliasih, N.K.A.; Ayu Sauca Sunia Widyantari, A.A.; Suriani, N.L.; Manto, A. GC-MS Analysis of the Active Compound in Ethanol Extracts of White Pepper (Piper nigrum L.) and Pharmacological Effects. Cell. Mol. Biomed. Rep. 2022, 2, 151–161. [Google Scholar] [CrossRef]
- Stender, S.; Dyerberg, J. Influence of Trans Fatty Acids on Health. Ann. Nutr. Metab. 2004, 48, 61–66. [Google Scholar] [CrossRef]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns. Foods 2021, 10, 2452. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Destaillats, F.; Dionisi, F.; Krauss, R.M.; Baer, D.J. Vaccenic Acid and Trans Fatty Acid Isomers from Partially Hydrogenated Oil Both Adversely Affect LDL Cholesterol: A Double-Blind, Randomized Controlled Trial. Am. J. Clin. Nutr. 2015, 102, 1339–1346. [Google Scholar] [CrossRef]
- Agostoni, C.; Moreno, L.; Shamir, R. Palmitic Acid and Health: Introduction. Crit. Rev. Food Sci. Nutr. 2016, 56, 1941–1942. [Google Scholar] [CrossRef] [PubMed]
- Waehler, R. Fatty acids: Facts vs. fiction. Int. J. Vitam. Nutr. Res. 2023, 93, 268–288. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Valle, R.G.D.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial Fatty Acids: An Update of Possible Mechanisms of Action and Implications in the Development of the next-Generation of Antibacterial Agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- PubChem Caproic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8892 (accessed on 26 March 2024).
- Roopashree, P.G.; Shetty, S.S.; Kumari, N.S. Effect of medium chain fatty acid in human health and disease. J. Funct. Foods 2021, 87, 104724. [Google Scholar] [CrossRef]
- Lorenz, H.-M.; Schmitt, W.H.; Tesar, V.; Müller-Ladner, U.; Tarner, I.; Hauser, I.A.; Hiepe, F.; Alexander, T.; Woehling, H.; Nemoto, K.; et al. Treatment of Active Lupus Nephritis with the Novel Immunosuppressant 15-Deoxyspergualin: An Open-Label Dose Escalation Study. Arthritis Res. Ther. 2011, 13, 1–12. [Google Scholar] [CrossRef]
- Bułło, B.; Rutkowski, B. ANCA-associated systemic small-vessel vasculitides—Update on therapeutic aspects. Forum Nefrol. 2012, 5, 5–18. [Google Scholar]
- Jakubczyk, D.; Dussart, F. Selected Fungal Natural Products with Antimicrobial Properties. Molecules 2020, 25, 911. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Skóra, B.; Pomianek, T.; Gmiński, J. Inonotus obliquus—From Folk Medicine to Clinical Use. J. Tradit. Complement. Med. 2021, 11, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Szymański, M.; Staniszewska, J. Qualitative and quantitative studies of Inonotus obliquus extracts. Postępy Fitoter. 2022, 1, 3–9. [Google Scholar] [CrossRef]
- Djenidi, H.; Khennouf, S.; Bouaziz, A. Antioxidant Activity and Phenolic Content of Commonly Consumed Fruits and Vegetables in Algeria. Progr. Nutr. 2020, 22, 224–235. [Google Scholar] [CrossRef]
- Smoliborska, J. Qualitative and Quantitative Studies of Extracts from Fungal Fruiting Bodies of Betula L. Master’s Thesis, Karol Marcinkowski Medical University, Poznań, Poland, 2015. [Google Scholar]
- Szajkowska, D. Antioxidant Activity of Species of the Genus Melilotus Mill. Master’s Thesis, Karol Marcinkowski Medical University, Poznań, Poland, 2014. [Google Scholar]
- Hassani, M.; Soleimani, M.; Esmaeilzadeh, E.; Zare-Abdollahi, D.; Khorram Khorshid, H.R. Healing Influence of Melilotus Officinalis Herbal Extract on Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice. Iran J. Pharm. Res. 2020, 19, 321–329. [Google Scholar] [CrossRef]
- Sowiński, J.; Adamczewska-Sowińska, K. Chapter 11—Forage Legumes for Human, Animals, and Environment. In Advances in Legumes for Sustainable Intensification; Meena, R.S., Kumar, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 205–226. ISBN 978-0-323-85797-0. [Google Scholar]
- Chu, M.; Khan, R.D.; Zhou, Y.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterization of Phenolic Compounds in Common Commercial Mushrooms and Their Potential Antioxidant Activities. Processes 2023, 11, 1711. [Google Scholar] [CrossRef]
- Orywal, K.; Socha, K.; Nowakowski, P.; Zoń, W.; Mroczko, B.; Perkowski, M. Dried Wild-Grown Mushrooms Can Be Considered a Source of Selected Minerals. Nutrients 2022, 14, 2750. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Shi, L.-E.; Jiang, Z.-B.; Bai, X.-L.; Ying, R.-F. Calcium Enrichment in Edible Mushrooms: A Review. J. Fungi 2023, 9, 338. [Google Scholar] [CrossRef]
- Kasuya, M.C.M.; da Luz, J.M.R.; Nunes, M.D.; da Silva, M.C.S.; de Carvalho, D.R.; de Assunção, L.S.; de Almeida Paula, T.; Moura, C.; Pereira Bento, C.B. Production of Selenium-Enriched Mushrooms in Coffee Husks and Use of This Colonized Residue. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015; pp. 301–309. [Google Scholar]
- Kumar Sharma, S.; Gautam, N. Chemical and Bioactive Profiling, and Biological Activities of Coral Fungi from Northwestern Himalayas. Sci. Rep. 2017, 7, 46570. [Google Scholar] [CrossRef]
- Malinowski, R.; Sotek, Z.; Stasińska, M.; Malinowska, K.; Radke, P.; Malinowska, A. Bioaccumulation of Macronutrients in Edible Mushrooms in Various Habitat Conditions of NW Poland—Role in the Human Diet. Int. J. Environ. Res. Public Health 2021, 18, 8881. [Google Scholar] [CrossRef]
- Florczak, J.; Chudy, J.; Barasińska, M.; Karwowski, B. Selected nutrients of wild mushrooms lilac earwig (Hirneola auricula judae), oyster mushroom (Pleurotus ostreatus) and velvetleaf winterberry (Flammulina velutipes). Bromatol. Chem. Toksykol. 2014, 47, 4–876. [Google Scholar]
- Ordinance of the Minister for Health of 30 April 2004 on the Maximum Levels of Chemical and Biological Contaminants Which May Be Present in Foods, Food Ingredients, Permitted Additives, Processing Aids or on the Surface of Foods. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20041201257 (accessed on 25 March 2024).
- Nickel in Food: European Commission Intends to Set Limits. Available online: https://www.eposrl.com/en/magazine_pt/nickel-in-food-european-commission-intends-to-set-limits (accessed on 26 March 2024).
- Wen, W.; Chen, B.Q.; Li, D. Research on aluminium content of the diet in haerbin and daily aluminium intake of the local people. Zhonghua Yu Fang Yi Xue Za Zhi 1993, 27, 32–36. [Google Scholar]
- Grzelczyk, J.; Fiurasek, P.; Kakkar, A.; Budryn, G. Evaluation of the Thermal Stability of Bioactive Compounds in Coffee Beans and Their Fractions Modified in the Roasting Process. Food Chem. 2022, 387, 132888. [Google Scholar] [CrossRef]
- Wieczorek, D.; Wybieralska, K.; Babś, A. Thermal stability of selected spices. Probl. Hig. Epidemiol. 2011, 92, 894–897. [Google Scholar]


| Sample Symbol | Name | Date of Collection | Forest Type | Method of Drying the Fruiting Bodies | Suitability for Consumption |
|---|---|---|---|---|---|
| AA | Aleuria aurantia | October 2018 | Mixed | Freeze-dried | Edible |
| PH | Phallus hadriani | September 2018 | Leafy | Freeze-dried | Only the young fruiting body is edible; the mature fruiting body is inedible (gives off a faecal odour). |
| PC | Panus conchatus | October 2019 | Mixed | Freeze-dried | Edible |
| GP | Geastrum pectinatum | September 2019 | Coniferous | Dried at room temperature | Inedible |
| Power [W] | Rise Time [min] | Pressure [PSI] | Maximum Temperature [°C] | Temperature Holding Time [min] |
|---|---|---|---|---|
| 600 | 20 | 195 | 210 | 10 |
| Name | n | X ± SD [mg GAE/100 g s.m] | V% [%] |
|---|---|---|---|
| Aleuria aurantia | 5 | 0.906 ± 0.025 | 2.8 |
| Phallus hadriani | 5 | 1.501 ± 0.025 | 1.7 |
| Panus conchatus | 5 | 0.900 ± 0.024 | 2.7 |
| Geastrum pectinatum | 5 | 0.617 ± 0.016 | 2.7 |
| Name | n | IC50 ± SD [mg/mL] | V [%] |
|---|---|---|---|
| Methanolic extract | |||
| Aleuria aurantia | 5 | 40.86 ± 0.98 | 2.4 |
| Phallus hadriani | 5 | 18.19 ± 0.88 | 4.8 |
| Panus conchatus | 5 | 35.21 ± 1.30 | 3.7 |
| Geastrum pectinatum | 5 | 84.13 ± 2.10 | 2.5 |
| Aqueous extracts | |||
| Aleuria aurantia | 5 | 17.06 ± 0.88 | 5.2 |
| Phallus hadriani | 5 | 4.74 ± 0.32 | 6.8 |
| Panus conchatus | 5 | 21.26 ± 1.24 | 5.8 |
| Geastrum pectinatum | 5 | 37.92 ± 2.60 | 6.9 |
| Name | n | IC50 ± SD [mg/mL] | V [%] |
|---|---|---|---|
| Methanolic extract | |||
| Aleuria aurantia | 5 | 73.38 ± 6.21 | 8.4 |
| Phallus hadriani | 5 | 31.71 ± 3.01 | 9.5 |
| Panus conchatus | 5 | 72.83 ± 5.44 | 7.5 |
| Geastrum pectinatum | 5 | 86.20 ± 4.12 | 4.8 |
| Aqueous extracts | |||
| Aleuria aurantia | 5 | 53.43 ± 4.63 | 8.7 |
| Phallus hadriani | 5 | 10.21 ± 1.01 | 9.9 |
| Panus conchatus | 5 | 47.16 ± 2.06 | 4.4 |
| Geastrum pectinatum | 5 | 67.55 ± 5.21 | 7.7 |
| Sample | Mineral Content [mg/g Dry Weight] | ||||
|---|---|---|---|---|---|
| Calcium X ± SD | Copper X ± SD | Iron X ± SD | Sodium X ± SD | Magnesium X ± SD | |
| Aleuria aurantia | 0.238 ± 0.005 | 0.022± 0.001 | 0.067 ± 0.002 | 0.089 ± 0.002 | 0.988 ± 0.033 |
| Phallus hadriani | 8.977 ± 1.350 | 0.008 ± 0.000 | 0.14 ± 0.003 | 0.041 ± 0.001 | 3.402 ± 0.062 |
| Panus conchatus | 0.126 ± 0.002 | 0.005 ± 0.000 | 0.021 ± 0.002 | 0.066 ± 0.001 | 0.950 ± 0.011 |
| Geastrum pectinatum | 6.328 ± 0.170 | 0.037 ± 0.001 | 0.135 ± 0.012 | 0.040 ± 0.003 | 1.524 ± 0.040 |
| Zinc X ± SD | Silicon X ± SD | Potassium X ± SD | Phosphorus X ± SD | Manganese X ± SD | |
| Aleuria aurantia | 0.137 ± 0.002 | <0 | 27.296 ± 0.311 | 13.970 ± 0.507 | 0.018 ± 0.000 |
| Phallus hadriani | 0.192 ± 0.002 | 0.064 ± 0.004 | 26.070 ± 0.843 | 4.687 ± 0.103 | 0.038 ± 0.000 |
| Panus conchatus | 0.021 ± 0.000 | 0.015 ± 0.002 | 11.350 ± 0.222 | 5.680 ± 0.218 | 0.015 ± 0.000 |
| Geastrum pectinatum | 0.087 ± 0.001 | 0.077 ± 0.003 | 18.900 ± 1.100 | 0.436 ± 0.025 | 0.133 ± 0.002 |
| Heavy Metal and Aluminium Content [mg/g Dry Weight] | |||||
|---|---|---|---|---|---|
| Sample | Cadmium X ± SD | Lead X ± SD | Aluminum X ± SD | Nickel X ± SD | Cobalt X ± SD |
| Aleuria aurantia | 0.0005 ± 0.0000 | <0 | 0.0515 ± 0.0030 | <0 | <0 |
| Phallus hadriani | 0.0002 ± 0.0000 | 0.0005 ± 0.0002 | 0.1042 ± 0.0057 | 0.0007 ± 0.0001 | |
| Panus conchatus | <0 | <0 | 0.0075 ± 0.0003 | <0 | |
| Geastrum pectinatum | 0.0003 ± 0.0000 | 0.0007 ± 0.0001 | 0.0833 ± 0.0048 | 0.0034 ± 0.0005 | |
| Sample | Thermal Stability Temperature [°C] | Temperature at Maximum Rate of Mass Change [°C] | Weight Loss [%] | |||
|---|---|---|---|---|---|---|
| First Changes Stage 1 | Layout Stage 2 | First Changes Stage 1 | Layout Stage 2 | First Changes Stage 1 | Layout Stage 2 | |
| PH | 51.2 ± 1.03 | 228.7 ± 1.11 | 67.9 ± 2.13 | 319.7 ± 4.11 | 6.56 ± 2.02 | 67.91 ± 2.28 |
| PC | 51.7 ± 2.01 | 256.8 ± 2.73 | 68.2 ± 3.01 | 311.6 ± 3.04 | 6.75 ± 1.01 | 71.40 ± 4.08 |
| GP | 57.4 ± 3.19 | 246.3 ± 5.10 | 67.9 ± 4.15 | 319.7 ± 4.23 | 6.59 ± 2.12 | 67.88 ± 9.02 |
| AA | 51.1 ± 0.08 | 259.4 ± 1.27 | 68.8 ± 2.13 | 301.7 ± 3.11 | 4.93 ± 0.16 | 67.60 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolesławska, I.; Górna, I.; Sobota, M.; Bolesławska-Król, N.; Przysławski, J.; Szymański, M. Wild Mushrooms as a Source of Bioactive Compounds and Their Antioxidant Properties—Preliminary Studies. Foods 2024, 13, 2612. https://doi.org/10.3390/foods13162612
Bolesławska I, Górna I, Sobota M, Bolesławska-Król N, Przysławski J, Szymański M. Wild Mushrooms as a Source of Bioactive Compounds and Their Antioxidant Properties—Preliminary Studies. Foods. 2024; 13(16):2612. https://doi.org/10.3390/foods13162612
Chicago/Turabian StyleBolesławska, Izabela, Ilona Górna, Marta Sobota, Natasza Bolesławska-Król, Juliusz Przysławski, and Marcin Szymański. 2024. "Wild Mushrooms as a Source of Bioactive Compounds and Their Antioxidant Properties—Preliminary Studies" Foods 13, no. 16: 2612. https://doi.org/10.3390/foods13162612
APA StyleBolesławska, I., Górna, I., Sobota, M., Bolesławska-Król, N., Przysławski, J., & Szymański, M. (2024). Wild Mushrooms as a Source of Bioactive Compounds and Their Antioxidant Properties—Preliminary Studies. Foods, 13(16), 2612. https://doi.org/10.3390/foods13162612







