Hydrogen Sulfide Improves Postharvest Quality of Okra (Abelmoschus esculentus (L.) Moench) Pods by Enhancing Antioxidant Capacity and Delaying Lignification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determination of Weight Loss Rate and Hardness
2.3. Determination of Ascorbic Acid, Soluble Protein, Protopectin, and Soluble Pectin Contents
2.4. Determination of Lignin and Cellulose Contents
2.5. Determination of Malondialdehyde (MDA), Superoxide Anion (O2·−), and Hydrogen Peroxide (H2O2) Contents
2.6. Determination of Catalase (CAT), Ascorbic Acid Peroxidase (APX), and Superoxide Dismutase (SOD) Activities
2.7. Determination of Phenylalanine Ammonialyase (PAL), Cinnamyl Alcohol Dehydrogenase (CAD), 4-Coumarate/CoA Ligase (4CL), Cinnamate 4-Hydroxylase (C4H), and Peroxidase (POD) Activities
2.8. RNA Extraction and Sequencing
2.9. Analysis of Sequencing Data
2.10. Statistical Analysis
3. Results
3.1. Effects of H2S on the Appearance of Okra Pods
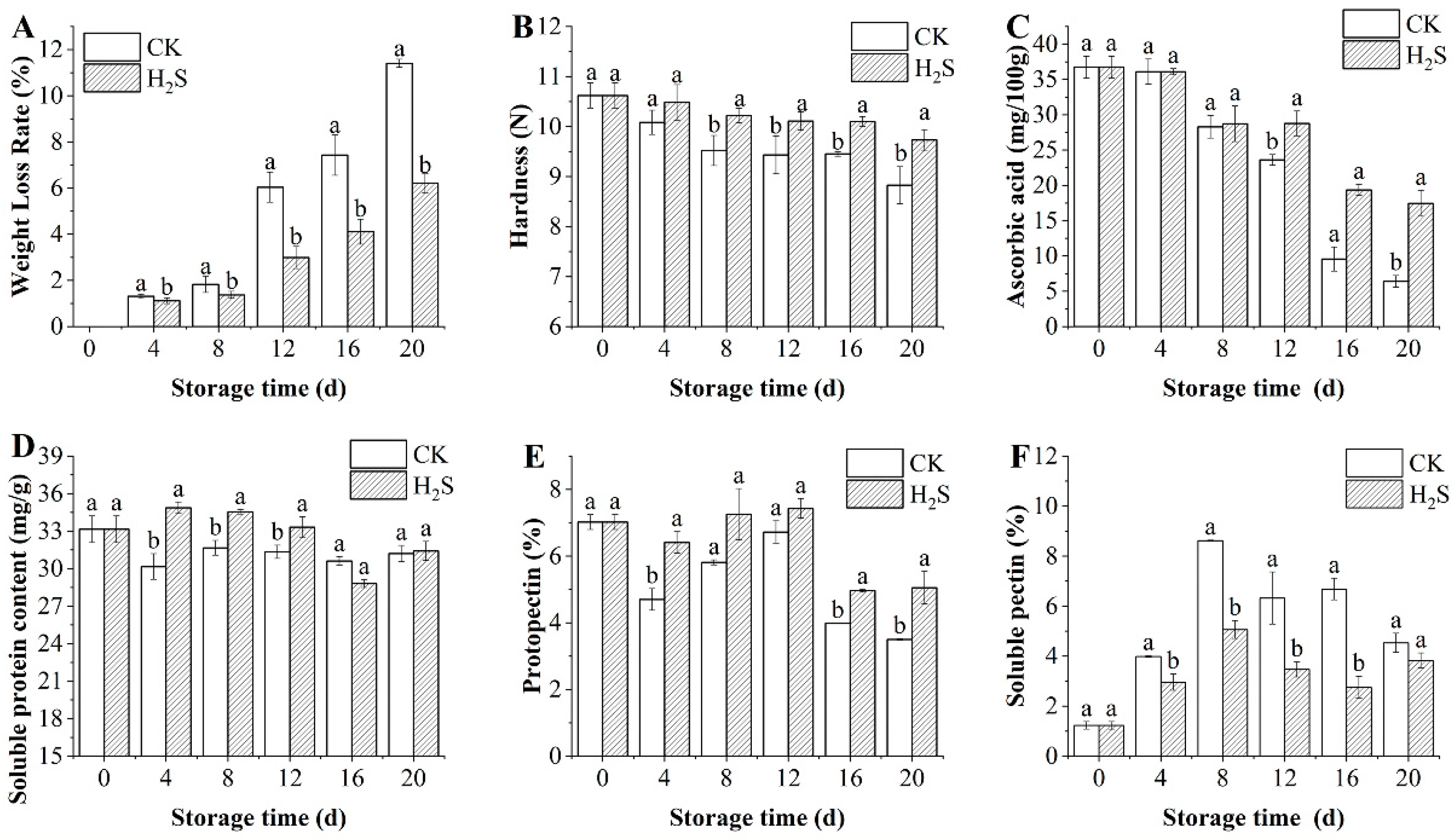
3.2. Effects of H2S on Weight Loss Rate and Hardness of Okra Pods
3.3. Effects of H2S on the Contents of Ascorbic Acid, Soluble Protein, Protopectin, and Soluble Pectin in Okra Pods
3.4. Effects of H2S on the Contents of Lignin and Cellulose Content in Okra Pods
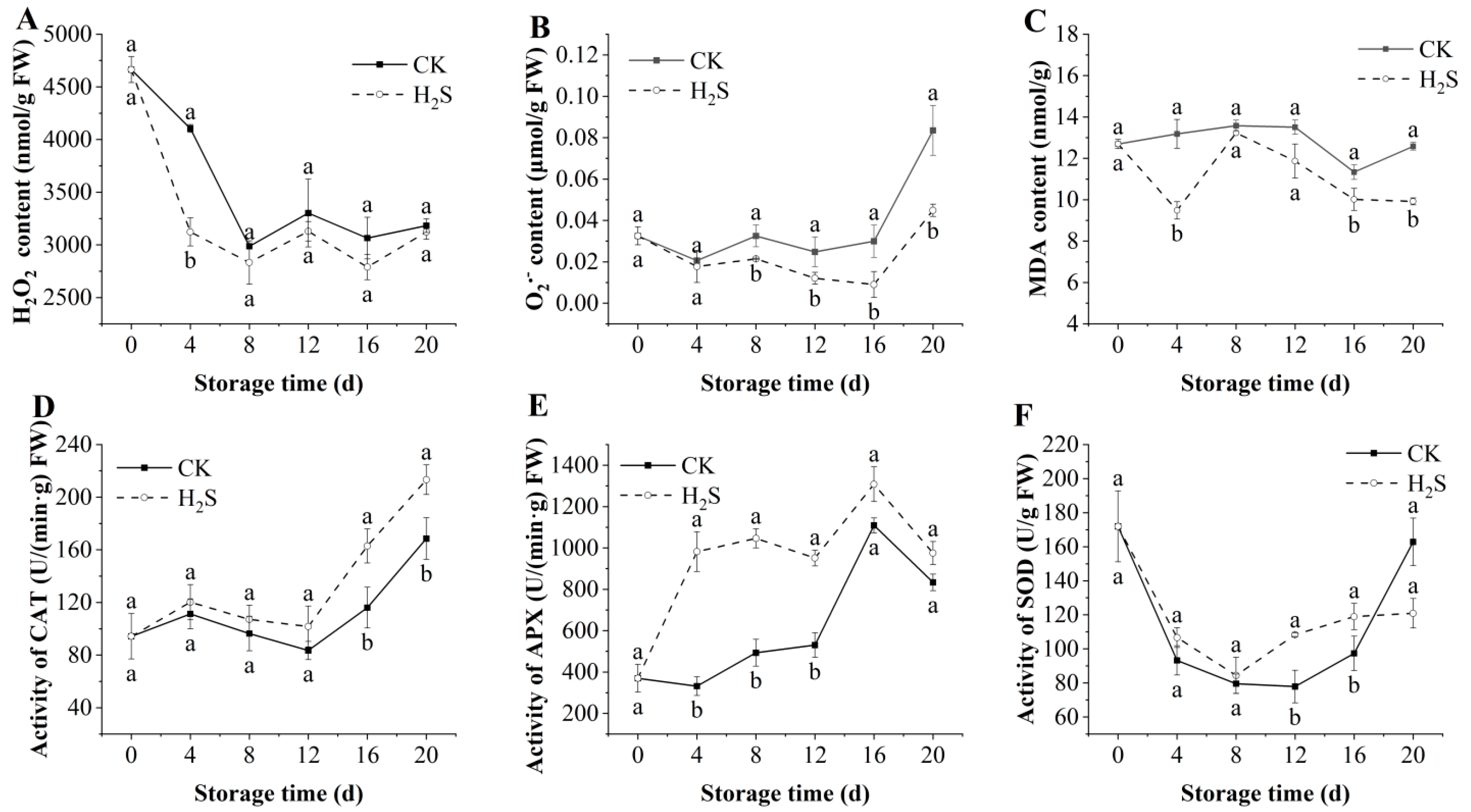
3.5. Effects of H2S on the Contents of H2O2, O2·−, and MDA in Okra Pods
3.6. Effects of H2S on the Activities of CAT, APX, SOD in Okra Pods
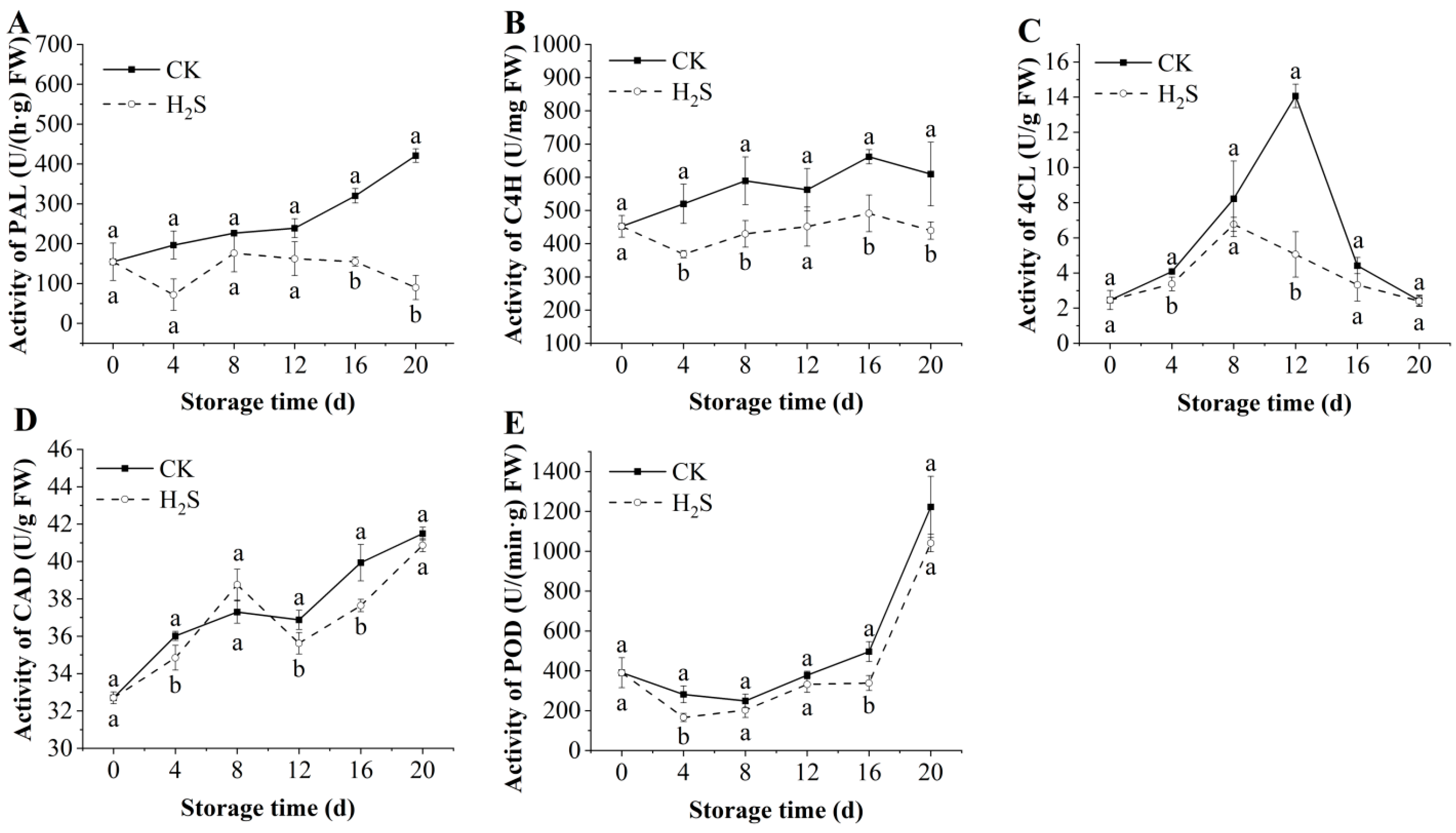
3.7. Effects of H2S on the Activities of PAL, CAD, 4CL, C4H, and POD in Okra Pods
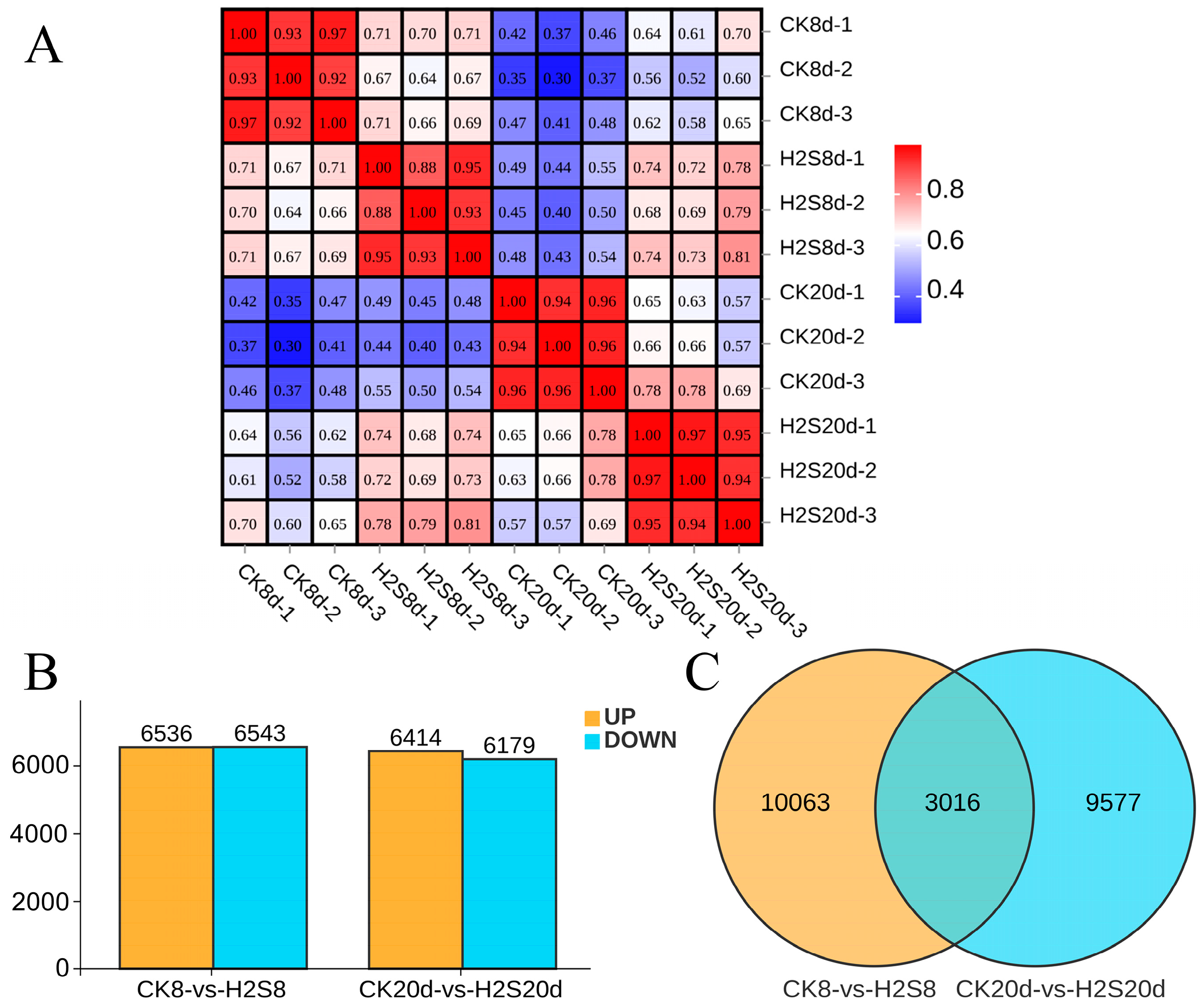
3.8. Transcriptome Profile of Postharvest Okra Pods RNA Libraries
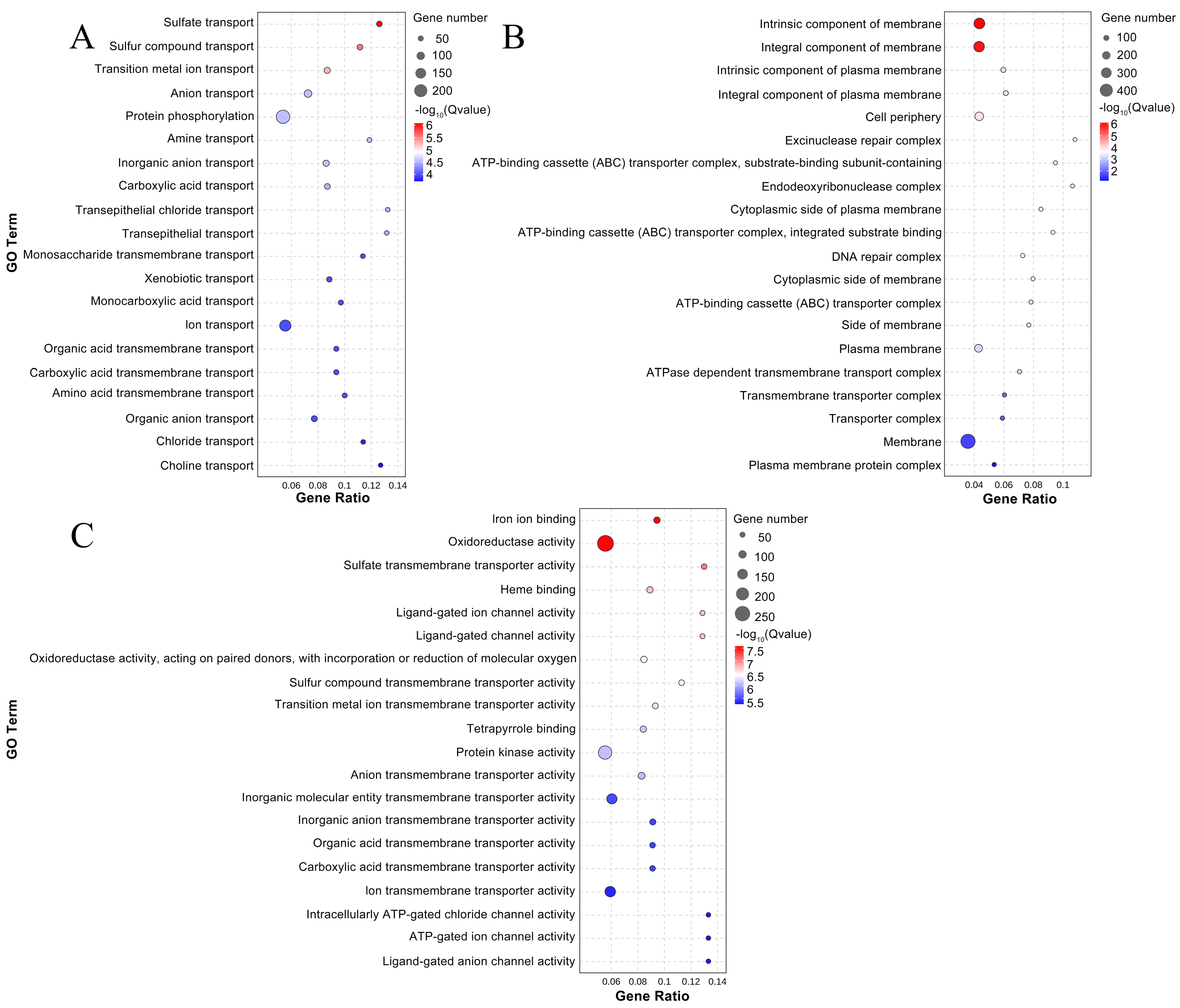
3.9. Functional Enrichment of DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, M.T. Phytochemical information and pharmacological activities of Okra (Abelmoschus esculentus): A literature-based review. Phytother. Res. 2019, 33, 72–80. [Google Scholar] [CrossRef]
- Cui, Y.T.; Wu, J.G.; Chen, Y.Y.; Ji, F.C.; Li, X.Y.; Yang, J.; Hong, S.B.; Zhu, Z.J.; Zang, Y.X. Optimization of near-infrared reflectance models in determining flavonoid composition of okra (Abelmoschus esculentus L.) pods. Food Chem. 2023, 418, 135953. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bao, Z.Y.; Chen, Y.N.; Lan, Q.Q.; Song, C.B.; Shi, L.Y.; Chen, W.; Cao, S.F.; Yang, Z.F.; Zheng, Q.B. Exogenous glutathione modulates redox homeostasis in okra (Abelmoschus esculentus) during storage. Postharvest Biol. Technol. 2023, 195, 112145. [Google Scholar] [CrossRef]
- Sun, M.; Yang, X.L.; Zhu, Z.P.; Xu, Q.Y.; Wu, K.X.; Kang, Y.J.; Wang, H.; Xiong, A.S. Comparative transcriptome analysis provides insight into nitric oxide suppressing lignin accumulation of postharvest okra (Abelmoschus esculentus L.) during cold storage. Plant Physiol. Biochem. 2021, 167, 49–67. [Google Scholar] [CrossRef]
- Corpas, F.J.; Palma, J.M. Functions of NO and H2S signal molecules against plant abiotic stress. Methods Mol. Biol. 2023, 2642, 97–109. [Google Scholar]
- Zimmermann, K.K.; Spassov, S.G.; Strosing, K.M.; Ihle, P.M.; Engelstaedter, H.; Hoetzel, A.; Faller, S. Hydrogen sulfide exerts anti-oxidative and anti-inflammatory effects in acute lung injury. Inflammation 2018, 41, 249–259. [Google Scholar] [CrossRef]
- Wilkie, S.E.; Borland, G.; Carter, R.N.; Morton, N.M.; Selman, C. Hydrogen sulfide in ageing, longevity and disease. Biochem. J. 2021, 478, 3485–3504. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Wang, C.P.; Liu, Y.; Jiang, W.W.; Li, W.X.; Cheng, F.S.; Ma, C.G.; Nie, Y. Effects of hydrogen sulfide on the quality deterioration of button mushrooms and the interaction with ethylene. Food Bioprocess Technol. 2021, 14, 1983–1995. [Google Scholar] [CrossRef]
- Wang, W.; Ni, Z.J.; Song, C.B.; Ma, W.P.; Cao, S.Q.; Wei, Z.J. Hydrogen sulfide treatment improves quality attributes via regulating the antioxidant system in goji berry (Lycium barbarum L.). Food Chem. 2023, 405, 134858. [Google Scholar] [CrossRef]
- Zhang, W.L.; Pan, Y.G.; Jiang, Y.M.; Zhang, Z.K. Advances in gas fumigation technologies for postharvest fruit preservation. Crit. Rev. Food Sci. Nutr. 2023, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lata, D.; Homa, F.; Nayyer, M.A.; Kumar, A.; Aftab, M.A.; Siddiqui, M.W. Effect of postharvest hydrogen sulphide on lignification and biochemical markers of pointed gourd. Plant Biol. 2022, 24, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Q.; Zhu, D.; Zhao, L.Y.; Luo, Y.T.; Li, J.T.; Xie, B.; Liu, Y.; Bao, Y.Q.; Wu, Z.G.; Zheng, Y.H.; et al. Hydrogen sulfide retards fruit softening and prevents flesh browning in cold-stored peaches by regulating cell wall-modifying enzymes, phenolic, and proline metabolism. Postharvest Biol. Technol. 2024, 207, 112620. [Google Scholar] [CrossRef]
- Yi, B.H.; Liu, Y.; Wu, Z.G.; Zheng, Y.H.; Chen, H.J.; Jin, P. Hydrogen sulfide alleviates chilling injury of zucchini fruit by regulating antioxidant capacity, endogenous hydrogen sulfide, proline, and polyamine metabolism. Postharvest Biol. Technol. 2024, 208, 112638. [Google Scholar] [CrossRef]
- Lv, Y.M.; Elnur, E.; Wang, W.; Thakur, K.; Du, J.; Li, H.N.; Ma, W.P.; Liu, Y.Q.; Ni, Z.J.; Wei, Z.J. Hydrogen sulfide treatment increases the antioxidant capacity of fresh Lingwu Long Jujube (Ziziphus jujuba cv. Mill) fruit during storage. Curr. Res. Food Sci. 2022, 5, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ni, Z.J.; Thakur, K.; Cao, S.Q.; Wei, Z.J. Recent update on the mechanism of hydrogen sulfide improving the preservation of postharvest fruits and vegetables. Curr. Opin. Food Sci. 2022, 47, 100906. [Google Scholar] [CrossRef]
- Zhang, C.H.; Dong, W.Q.; Gen, W.; Xu, B.Y.; Shen, C.J.; Yu, C.L. De Novo Transcriptome Assembly and Characterization of the Synthesis Genes of Bioactive Constituents in Abelmoschus esculentus (L.) Moench. Genes. 2018, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.H.; Wu, T.T.; Zhao, X.; Wang, Z.Q.; Chen, Y. Comparative physiological and full-length transcriptome analyses reveal the molecular mechanism of melatonin-mediated salt tolerance in okra (Abelmoschus esculentus L.). BMC Plant Biol. 2021, 21, 180. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Ma, K.; Yao, Y.L.; Song, Z.Y.; Zhou, Y.W.; Si, Z.W.; Lu, H.Y.; Chen, W.X.; Li, X.P. Benzothiadiazole induced changes in the transcriptome and regulation of banana fruit ripening and disease resistance. Postharvest Biol. Technol. 2023, 196, 112161. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, Y.F.; Liu, Y.; Chen, Q.M.; Yin, F.L.; Song, M.B.; Cai, W.; Shuai, L. Methyl jasmonate treatment alleviates chilling injury and improves antioxidant system of okra pod during cold storage. Food Sci. Nutr. 2023, 11, 2049–2060. [Google Scholar] [CrossRef]
- Xie, P.D.; Yang, Y.Y.; Oyom, W.; Su, T.T.; Tang, Y.B.; Wang, Y.; Li, Y.C.; Prusky, D.; Bi, Y. Chitooligosaccharide accelerated wound healing in potato tubers by promoting the deposition of suberin polyphenols and lignin at wounds. Plant Physiol. Biochem. 2023, 199, 107714. [Google Scholar] [CrossRef] [PubMed]
- Oomen, R.J.F.J.; Tzitzikas, E.N.; Bakx, E.J.; Straatman, E.I.; Bush, M.S.; McCann, M.C.; Schols, H.A.; Visser, R.G.F.; Vincken, J.P. Modulation of the cellulose content of tuber cell walls by antisense expression of different potato (Solanum tuberosum L.) CesA clones. Phytochemistry 2004, 65, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Y.L.; He, C.C.; Zhu, S.J. Postharvest exogenous application of abscisic acid reduces internal browning in pineapple. J. Agric. Food Chem. 2015, 63, 5313–5320. [Google Scholar] [CrossRef]
- Xu, H.S.; Ding, S.H.; Zhou, H.; Yi, Y.J.; Deng, F.M.; Wang, R.R. Quality attributes and related enzyme activities in peppers during storage: Effect of hydrothermal and calcium chloride treatment. Int. J. Food Prop. 2019, 22, 1475–1491. [Google Scholar] [CrossRef]
- Xue, Y.Y.; Shen, Z.Y.; Tao, F.; Zhou, J.J.; Xu, B.L. Transcriptomic Analysis Reveal the Molecular Mechanisms of Seed Coat Development in Cucurbita pepo L. Front. Plant Sci. 2022, 13, 772685. [Google Scholar] [CrossRef] [PubMed]
- Goffner, D.; Joffroy, I.; Grima, P.J.; Halpin, C.; Knight, M.E.; Schuch, W.; Boudet, A.M. Purification and characterization of isoforms of cinnamyl alcohol dehydrogenase from Eucalyptus xylem. Planta 1992, 188, 48–53. [Google Scholar] [CrossRef]
- Hu, L.Y.; Hu, S.L.; Wu, J.; Li, Y.H.; Zheng, J.L.; Wei, Z.J.; Liu, J.; Wang, H.L.; Liu, Y.S.; Zhang, H. Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. J. Agric. Food Chem. 2012, 60, 8684–8693. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unificationof biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic. Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, M.J.; Dong, W.Q.; Zhou, C.J.; Shi, L.Y.; Chen, W.; Cao, S.F.; Yang, Z.F.; Li, S.S. Gibberellic acid inhibited chlorophyll degradation in post-harvest okras. Postharvest Biol. Technol. 2024, 190, 111951. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, L.Q.; Jiang, D.M.; Wang, M.; Yu, H.; Yao, W.R. Combined metabolome and transcriptome analysis reveal the mechanism of eugenol inhibition of Aspergillus carbonarius growth in table grapes (Vitis vinifera L.). Food Res. Int. 2023, 170, 112934. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.T.; Zhang, H.Y.; Qi, Q.S.; Yan, S.J.; Chen, C.K.; Liang, L.Y. Transcriptome analysis of the preservation effect of three essential oil microcapsules on okra. Horticulturae 2024, 10, 193. [Google Scholar] [CrossRef]
- Zhao, Q. Lignification: Flexibility, biosynthesis and regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Morteza, S.A.; Roghayeh, M.; Farhang, R.; Vali, R.; Ali, S. Hydrogen sulfide treatment confers chilling tolerance in hawthorn fruit during cold storage by triggering endogenous H2S accumulation, enhancing antioxidant enzymes activity and promoting phenols accumulation. Sci. Hortic.-Amsterdam. 2018, 238, 264–271. [Google Scholar]
- Dhall, R.K.; Sharma, S.R.; Mahajan, B.V.C. Development of post-harvest protocol of okra for export marketing. J. Food Sci. Technol. 2014, 51, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.P.; Yu, J.X.; Qiao, X.H.; Yu, Z.F.; Xiong, A.S.; Sun, M. Hydrogen sulfide delays yellowing and softening, inhibits nutrient loss in postharvest celery. Sci. Hortic. 2023, 315, 111991. [Google Scholar] [CrossRef]
- Dong, W.Q.; Shi, L.Y.; Li, S.S.; Xu, F.; Yang, Z.F.; Cao, S.F. Hydrogen-rich water delays fruit softening and prolongs shelf life of postharvest okras. Food chem. 2022, 399, 133997. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.Y.; Yao, G.F.; Wang, S.S.; Li, T.T.; Sun, K.K.; Tang, J.; Huang, Z.Q.; Yang, F.; Li, Y.H.; Chen, X.Y.; et al. Hydrogen sulfide maintains good nutrition and delays postharvest denescence in postharvest tomato fruits by regulating antioxidative metabolism. J. Plant Growth Regul. 2021, 40, 2548–2559. [Google Scholar] [CrossRef]
- Claudia, L.; Helena, M.C. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Molecular Cell 2021, 81, 3691–3707. [Google Scholar]
- Deshi, V.; Siddiqui, M.W.; Homa, F.; Singh, J.P. Postharvest hydrogen sulfide infiltration modulates antioxidative metabolism and increases shelf life of litchi. Acta. Physiol. Plant 2020, 42, 264–271. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Masud, A.A.; Khan, M.I.; Islam, M.N.; Uddin, S.; Hossain, M.K. Role of reactive oxygen species in aging and age-related diseases: A review. ACS Appl. Bio Mater. 2022, 5, 4028–4054. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, J.R.; Gao, M.Y.; Qin, L.; Wang, Y. Decreased cellulose-degrading enzyme activity causes pod hardening of okra (Abelmoschus esculentus L. Moench). Plant Physiol. Biochem. 2021, 162, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Jaime, B.; Richard, A.D. Plant phenylalanine/tyrosine ammonia-lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar]
- Huang, T.H.; Li, Y.C.; Luo, J.; Wang, J.; Cai, Z.P.; Shen, Y.G.; Li, Y.X.; Zhang, W.; Chen, J.Y.; Zhu, L.Q. Hydrogen sulfide enhances resistance to Penicillium italicum by activating phenylpropanoid metabolism in postharvest navel orange fruit. Postharvest Biol. Technol. 2023, 198, 112259. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, W.; Chen, T.; Gong, X.; Chen, J.; Zhou, W.; Li, J. Hydrogen Sulfide Improves Postharvest Quality of Okra (Abelmoschus esculentus (L.) Moench) Pods by Enhancing Antioxidant Capacity and Delaying Lignification. Foods 2024, 13, 2617. https://doi.org/10.3390/foods13162617
Luo W, Chen T, Gong X, Chen J, Zhou W, Li J. Hydrogen Sulfide Improves Postharvest Quality of Okra (Abelmoschus esculentus (L.) Moench) Pods by Enhancing Antioxidant Capacity and Delaying Lignification. Foods. 2024; 13(16):2617. https://doi.org/10.3390/foods13162617
Chicago/Turabian StyleLuo, Weihua, Tinghui Chen, Xiao Gong, Jingjing Chen, Wei Zhou, and Jihua Li. 2024. "Hydrogen Sulfide Improves Postharvest Quality of Okra (Abelmoschus esculentus (L.) Moench) Pods by Enhancing Antioxidant Capacity and Delaying Lignification" Foods 13, no. 16: 2617. https://doi.org/10.3390/foods13162617




