Comparison of Different Deodorizing Treatments on the Flavor of Paddy Field Carp, Analyzed by the E-Nose, E-Tongue and Gas Chromatography–Ion Mobility Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electronic Nose Analysis
2.3. Electronic Tongue Analysis
2.4. GC-IMS Analysis
2.5. Free Amino Acid (FAA) Analysis
2.6. Taste Activity Value (TAV) Analysis
2.7. Taste Nucleotide Analysis
2.8. Equivalent Umami Concentration (EUC)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Electronic Nose Analysis
3.1.1. Flavor Profile
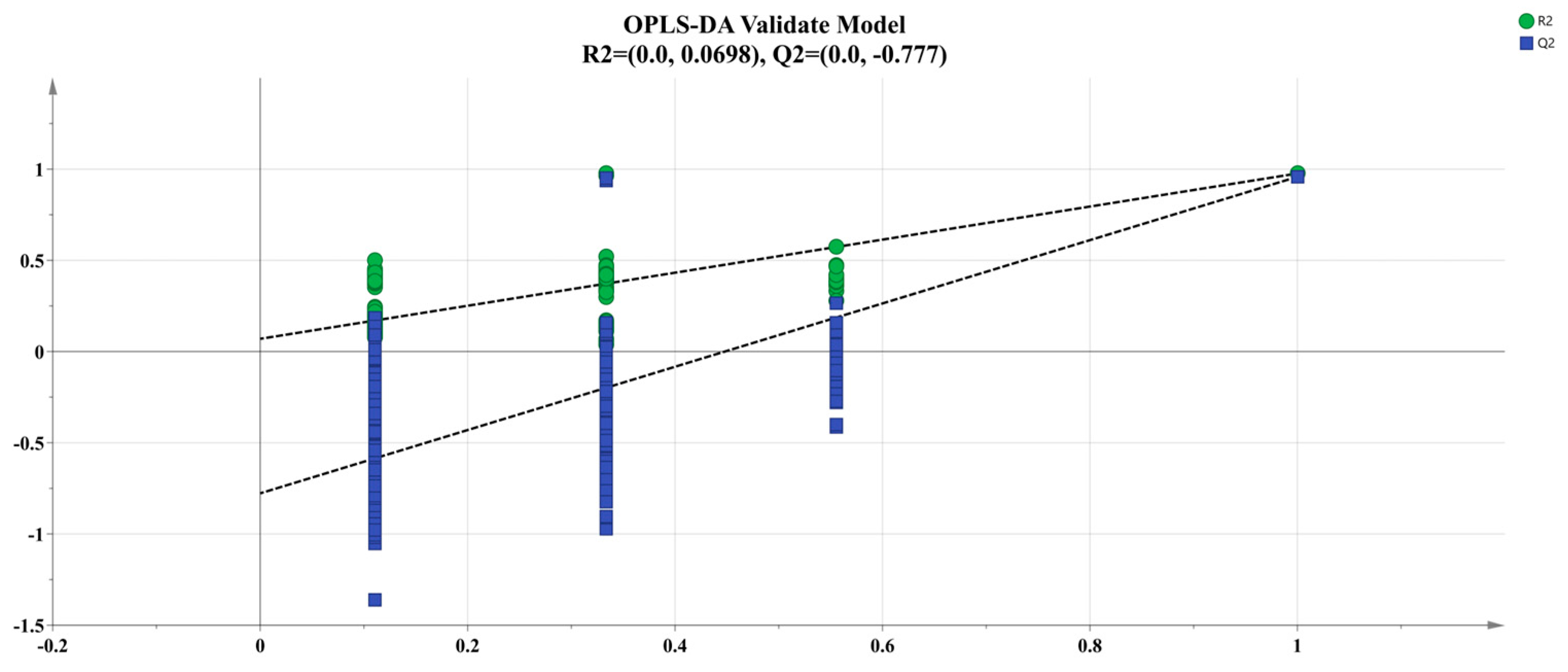
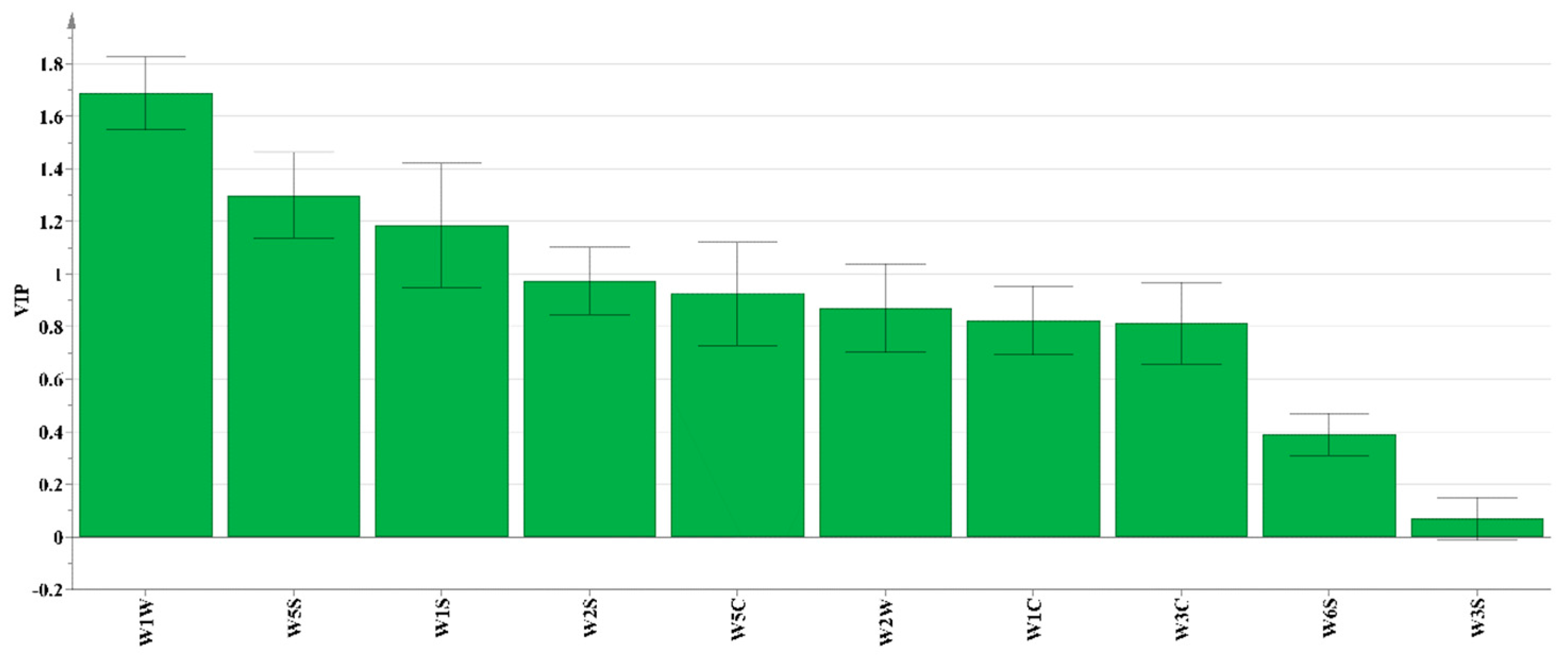
3.1.2. Orthogonal Partial Least Squares–Discriminant Analysis (OPLS-DA)
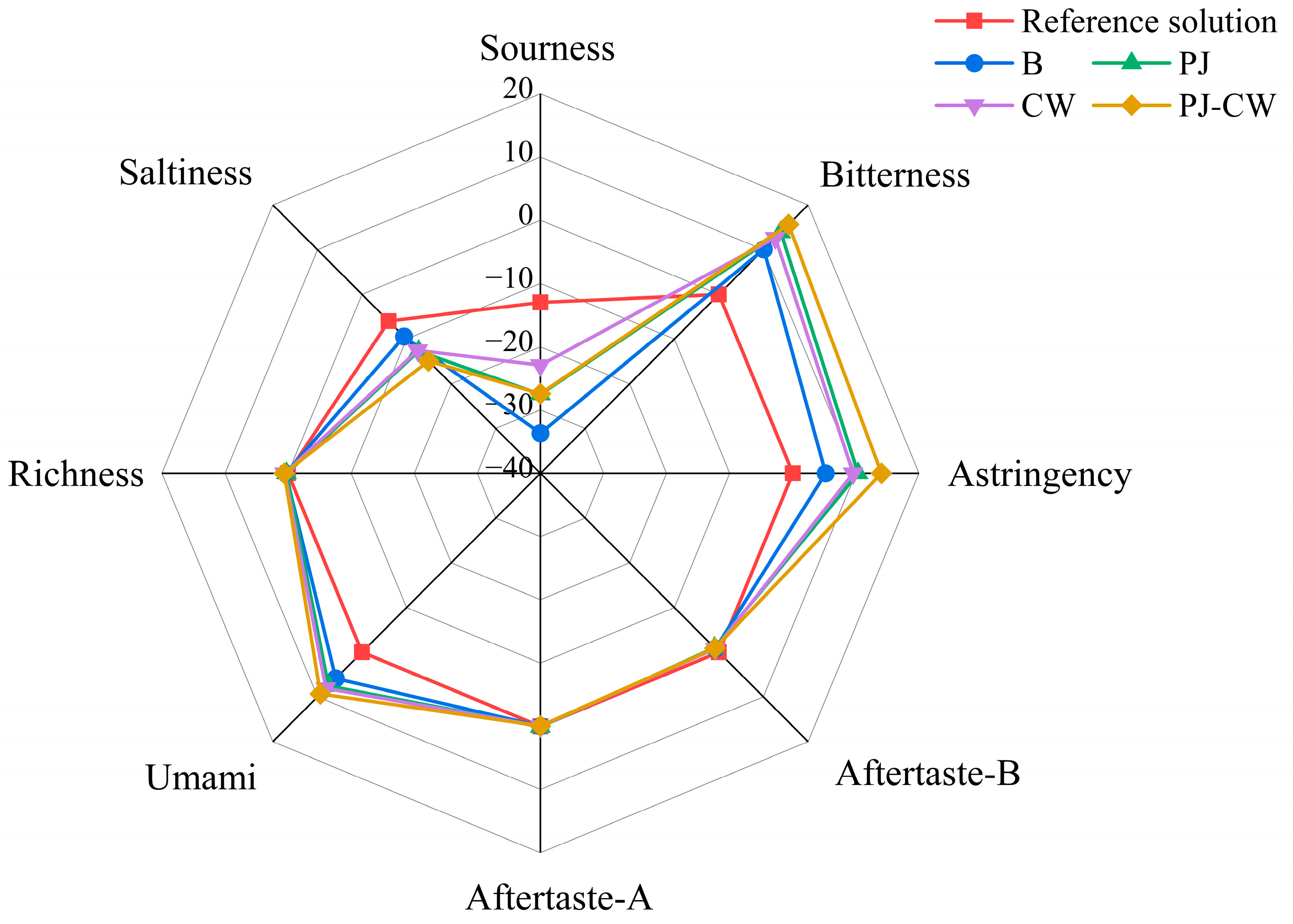
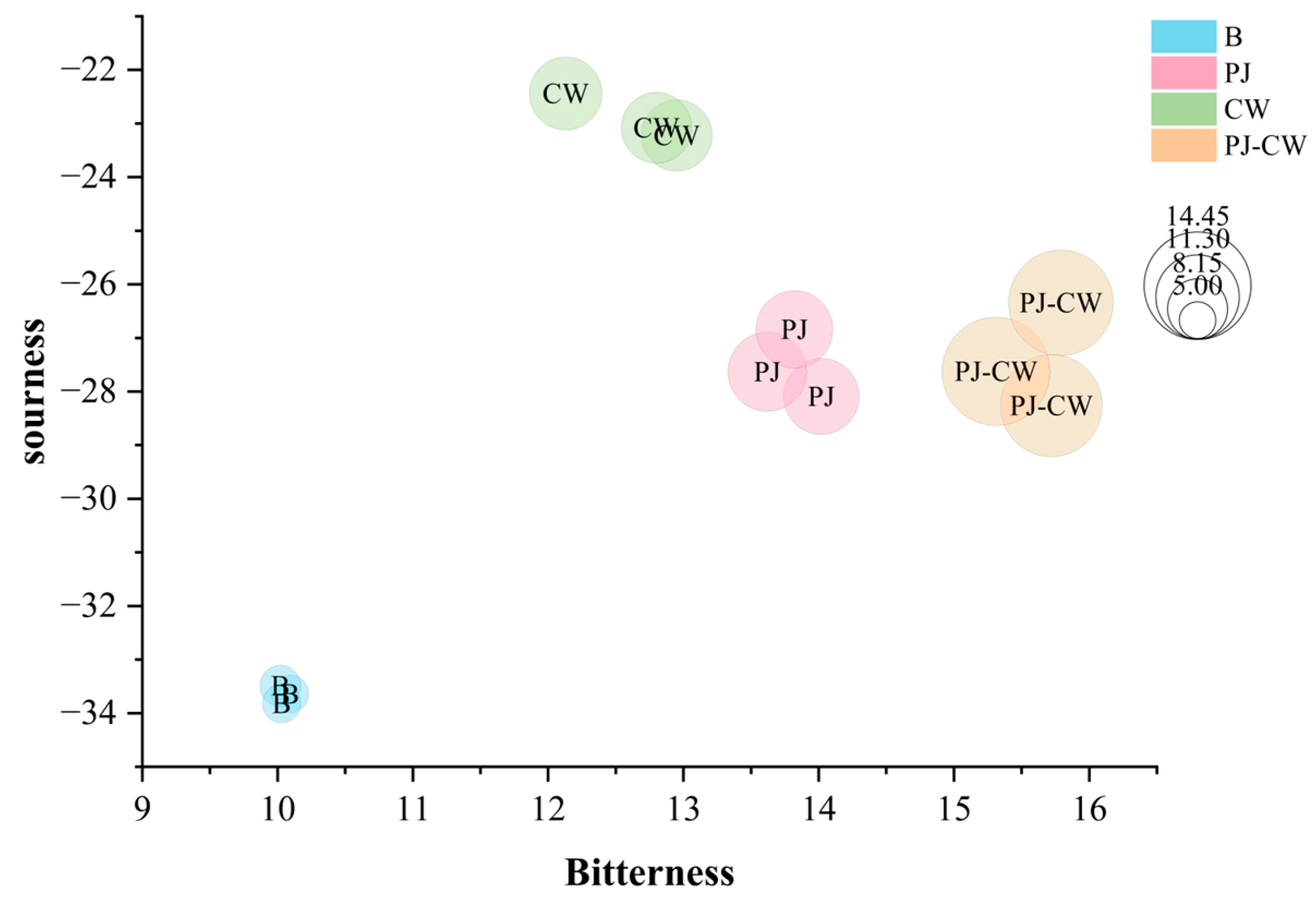
3.2. Electronic Tongue
3.2.1. Flavor Profile
3.2.2. Principal Component Analysis (PCA)
3.3. Amino Acid Content
3.4. Taste Nucleotide
3.5. Volatile Components Analysis
3.5.1. GC-IMS Two-Dimensional Analysis
3.5.2. Fingerprint Analysis of VOCs in Paddy Field Carp
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Guan, C.; Li, Z.; Luo, J.; Ren, B.; Chen, C.; Xu, Y.; Ding, J.; Huang, H. Review of Rice–Fish–Duck Symbiosis System in China—One of the Globally Important Ingenious Agricultural Heritage Systems (GIAHS). Sustainability 2023, 15, 1910. [Google Scholar] [CrossRef]
- Ren, W.; Hu, L.; Guo, L.; Zhang, J.; Tang, L.; Zhang, E.; Zhang, J.; Luo, S.; Tang, J.; Chen, X. Preservation of the Genetic Diversity of a Local Common Carp in the Agricultural Heritage Rice–Fish System. Proc. Natl. Acad. Sci. USA 2018, 115, E546–E554. [Google Scholar] [CrossRef]
- Luo, H.; Pan, X.; Huang, Y.; Li, Z.; Ye, H.; Zhou, K.; Wen, L.; Qin, J.; He, W.; Du, X.; et al. Genome-Wide Association Study Reveals Markers and Candidate Genes Associated with Growth in the Rice Flower Carp, an Economic Fish Species of Integrated Rice-Fish Culture in China. Front. Mar. Sci. 2023, 10, 1130667. [Google Scholar] [CrossRef]
- Wu, T.; Wang, M.; Wang, P.; Tian, H.; Zhan, P. Advances in the Formation and Control Methods of Undesirable Flavors in Fish. Foods 2022, 11, 2504. [Google Scholar] [CrossRef]
- Choi, H.Y.; Woo, H.E.; Go, E.S.; Kim, J.S.; Choi, J.H. Flavor Characteristics of Garlic Fish Cakes Using Electronic Nose and Tongue Analyses. Sci. Rep. 2024, 14, 6048. [Google Scholar] [CrossRef]
- Lee, J.; Rai, P.K.; Jeon, Y.J.; Kim, K.-H.; Kwon, E.E. The Role of Algae and Cyanobacteria in the Production and Release of Odorants in Water. Environ. Pollut. 2017, 227, 252–262. [Google Scholar] [CrossRef]
- Li, X.; Xie, W.; Bai, F.; Wang, J.; Zhou, X.; Gao, R.; Xu, X.; Zhao, Y. Influence of Thermal Processing on Flavor and Sensory Profile of Sturgeon Meat. Food Chem. 2022, 374, 131689. [Google Scholar] [CrossRef]
- Zhao, G.; Yuan, Y.; Zhou, H.; Zhao, L.; Jiang, Y. Determination of Volatile Compounds in Different Parts of Grass Carp Using GC✕GC-MS Combined with Chemometrics. Food Biosci. 2023, 56, 103403. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Cao, Y.; Gong, X.; Li, J.; Lu, X.; Dai, Y. Analysis of Volatile Components of Tilapia Enzymolysis Solution after Different Deodorization Treatments. IOP Conf. Ser. Earth Environ. Sci. 2020, 571, 012121. [Google Scholar] [CrossRef]
- Xu, Y.-X.; Jiang, Z.-D.; Du, X.-P.; Zheng, M.-J.; Fan-Yang, Y.; Ni, H.; Chen, F. The Identification of Biotransformation Pathways for Removing Fishy Malodor from Bangia fusco-purpurea Using Fermentation with Saccharomyces cerevisiae. Food Chem. 2022, 380, 132103. [Google Scholar] [CrossRef]
- Gharby, S.; Oubannin, S.; Ait Bouzid, H.; Bijla, L.; Ibourki, M.; Gagour, J.; Koubachi, J.; Sakar, E.H.; Majourhat, K.; Lee, L.-H.; et al. An Overview on the Use of Extracts from Medicinal and Aromatic Plants to Improve Nutritional Value and Oxidative Stability of Vegetable Oils. Foods 2022, 11, 3258. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Al-Zubaidy, A.M.A. Exploring Natural Essential Oil Components and Antibacterial Activity of Solvent Extracts from Twelve Perilla Frutescens L. Genotypes. Arab. J. Chem. 2020, 13, 7390–7402. [Google Scholar] [CrossRef]
- Ahmed, H. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla Frutescens (L.) Britt. Molecules 2018, 24, 102. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Zhang, Z.; Ren, Q.; Ji, Z.; Xu, X.; Xu, Y.; Mao, J. Case Study on the Influence of Serving Temperature on the Aroma Release and Perception of Huangjiu, a Fermented Alcoholic Beverage. Food Res. Int. 2024, 178, 113948. [Google Scholar] [CrossRef]
- Wadehra, A.; Patil, P.S. Application of Electronic Tongues in Food Processing. Anal. Methods 2016, 8, 474–480. [Google Scholar] [CrossRef]
- Wesoły, M.; Przewodowski, W.; Ciosek-Skibińska, P. Electronic Noses and Electronic Tongues for the Agricultural Purposes. TrAC Trends Anal. Chem. 2023, 164, 117082. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. Electronic Noses and Tongues to Assess Food Authenticity and Adulteration. Trends Food Sci. Technol. 2016, 58, 40–54. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent Progress in Food Flavor Analysis Using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, X.; Hong, P.; Liu, M.; Li, Z.; Zhou, C.; Zhong, S.; Liu, S. GC-MS, GC-IMS, and E-Nose Analysis of Volatile Aroma Compounds in Wet-Marinated Fermented Golden Pomfret Prepared Using Different Cooking Methods. Foods 2024, 13, 390. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Pei, Z.; Wei, P.; Xiang, D.; Cao, X.; Shen, X.; Li, C. Volatile Flavour Components and the Mechanisms Underlying Their Production in Golden Pompano (Trachinotus blochii) Fillets Subjected to Different Drying Methods: A Comparative Study Using an Electronic Nose, an Electronic Tongue and SDE-GC-MS. Food Res. Int. 2019, 123, 217–225. [Google Scholar] [CrossRef]
- Luo, X.; Xiao, S.; Ruan, Q.; Gao, Q.; An, Y.; Hu, Y.; Xiong, S. Differences in Flavor Characteristics of Frozen Surimi Products Reheated by Microwave, Water Boiling, Steaming, and Frying. Food Chem. 2022, 372, 131260. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhang, J.; Li, X.; Wang, J.; Yi, S.; Zhu, W.; Xu, Y.; Li, J. Prediction of the Freshness of Horse Mackerel (Trachurus japonicus) Using E-Nose, E-Tongue, and Colorimeter Based on Biochemical Indexes Analyzed during Frozen Storage of Whole Fish. Food Chem. 2023, 402, 134325. [Google Scholar] [CrossRef]
- Lin, R.; Yuan, H.; Wang, C.; Yang, Q.; Guo, Z. Study on the Flavor Compounds of Fo Tiao Qiang under Different Thawing Methods Based on GC–IMS and Electronic Tongue Technology. Foods 2022, 11, 1330. [Google Scholar] [CrossRef]
- Xiao, N.; Xu, H.; Jiang, X.; Sun, T.; Luo, Y.; Shi, W. Evaluation of Aroma Characteristics in Grass Carp Mince as Affected by Different Washing Processes Using an E-Nose, HS-SPME-GC-MS, HS-GC-IMS, and Sensory Analysis. Food Res. Int. 2022, 158, 111584. [Google Scholar] [CrossRef]
- Zhou, P.; Zhao, F.; Chen, M.; Ye, N.; Lin, Q.; Ouyang, L.; Cai, X.; Meng, P.; Gong, X.; Wang, Y. Determination of 21 Free Amino Acids in 5 Types of Tea by Ultra-High Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry (UHPLC–MS/MS) Using a Modified 6-Aminoquinolyl- N -Hydroxysuccinimidyl Carbamate (AQC) Method. J. Food Compos. Anal. 2019, 81, 46–54. [Google Scholar] [CrossRef]
- Duan, W.; Huang, Y.; Xiao, J.; Zhang, Y.; Tang, Y. Determination of Free Amino Acids, Organic Acids, and Nucleotides in 29 Elegant Spices. Food Sci. Nutr. 2020, 8, 3777–3792. [Google Scholar] [CrossRef]
- China National Standard GB 5413.40-2016; Food Safety National Standard of the People’s Republic of China—Determination of Nucleotides in Infant Food and Dairy Products. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016. (In Chinese)
- Moerdijk-Poortvliet, T.C.W.; de Jong, D.L.C.; Fremouw, R.; de Reu, S.; de Winter, J.M.; Timmermans, K.; Mol, G.; Reuter, N.; Derksen, G.C.H. Extraction and Analysis of Free Amino Acids and 5′-Nucleotides, the Key Contributors to the Umami Taste of Seaweed. Food Chem. 2022, 370, 131352. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Finardi, S.; De Souza, C.K.; Meinert, C.; Pateiro, M.; Hoffmann, T.G.; Domínguez, R.; Bertoli, S.L.; Kumar, M.; Lorenzo, J.M. Applications of Electronic Nose, Electronic Eye and Electronic Tongue in Quality, Safety and Shelf Life of Meat and Meat Products: A Review. Sensors 2023, 23, 672. [Google Scholar] [CrossRef]
- Mao, J.; Fu, J.; Zhu, Z.; Cao, Z.; Zhang, M.; Yuan, Y.; Chai, T.; Chen, Y. Flavor Characteristics of Semi-Dried Yellow Croaker (Pseudosciaena crocea) with KCl and Ultrasound under Sodium-Reduced Conditions before and after Low Temperature Vacuum Heating. Food Chem. 2023, 426, 136574. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, F.; Bao, J. Volatile Components of American Silver Carp Analyzed by Electronic Nose and MMSE-GC-MS-O. J. Food Biochem. 2019, 43, 11. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Nieuwoudt, H.; Opara, U.L. Evaluation of Biochemical Markers Associated with the Development of Husk Scald and the Use of Diffuse Reflectance NIR Spectroscopy to Predict Husk Scald in Pomegranate Fruit. Sci. Hortic. 2018, 232, 240–249. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhang, M.; Qi, J.; Zhao, W.; Gu, J.; Guo, W.; Li, Y. Screening of Specific Quantitative Peptides of Beef by LC–MS/MS Coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Q.; Liu, S.; Hong, P.; Zhou, C.; Zhong, S. Characterization of the Effect of Different Cooking Methods on Volatile Compounds in Fish Cakes Using a Combination of GC–MS and GC-IMS. Food Chem. X 2024, 22, 101291. [Google Scholar] [CrossRef]
- Mahieu, B.; Qannari, E.M.; Jaillais, B. Extension and Significance Testing of Variable Importance in Projection (VIP) Indices in Partial Least Squares Regression and Principal Components Analysis. Chemom. Intell. Lab. Syst. 2023, 242, 104986. [Google Scholar] [CrossRef]
- Bai, J.; Fan, Y.; Zhu, L.; Wang, Y.; Hou, H. Characteristic Flavor of Antarctic Krill (Euphausia superba) and White Shrimp (Penaeus vannamei) Induced by Thermal Treatment. Food Chem. 2022, 378, 132074. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Yang, X.; Fan, X.; Pan, J.; Dong, X. Nutrient Composition and Flavor Profile of Crucian Carp Soup Utilizing Fish Residues through Comminution and Pressure-Conduction Treatment. Foods 2024, 13, 800. [Google Scholar] [CrossRef]
- Wang, M.; Zhan, P.; Geng, J.; Wang, P.; He, W.; Tian, H. Identification and Inhibition of Key Off-Odorants in Boiled Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Subjected to Perilla Polyphenols Extract. LWT-Food Sci. Technol. 2024, 199, 116120. [Google Scholar] [CrossRef]
- Zaukuu, J.-L.Z.; Soós, J.; Bodor, Z.; Felföldi, J.; Magyar, I.; Kovacs, Z. Authentication of Tokaj Wine (Hungaricum) with the Electronic Tongue and Near Infrared Spectroscopy. J. Food Sci. 2019, 84, 3437–3444. [Google Scholar] [CrossRef]
- Fu, C.; Wu, D.; Jin, Z.; Xie, G.; Lu, J. Development of a Novel Cooking Wine with High-Efficiency Deodorizing Capability via a Rapid Fermentation Strategy. LWT-Food Sci. Technol. 2022, 153, 112431. [Google Scholar] [CrossRef]
- Cao, J.; Feng, A.; He, Y.; Wang, J.; Liu, Z.; Xia, G.; Lin, X.; Shen, X.; Zhou, D.; Li, C. The Effect and Mechanism of Four Drying Methods on the Quality of Tilapia Fillet Products. Food Front. 2022, 3, 316–327. [Google Scholar] [CrossRef]
- Liu, T.-T.; Xia, N.; Wang, Q.-Z.; Chen, Q.-W. Identification of the Non-Volatile Taste-Active Components in Crab Sauce. Foods 2019, 8, 324. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. MEASUREMENT OF THE RELATIVE TASTE INTENSITY OF SOME L-?-AMINO ACIDS AND 5’-NUCLEOTIDES. J. Food Sci. 1971, 36, 846–849. [Google Scholar] [CrossRef]
- Wei, R.; Jiang, B.; Chen, J.; Xiang, L.; Liu, X. Removal of Fishy Flavor in Kelp (Laminaria japonica) by Natural Antioxidant Soaking Combined with Microbial Fermentation. Food Biosci. 2024, 60, 104212. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.-H.; Wang, X.; Tian, H.; Wang, L.; Zhou, D.-Y.; Qin, L. Compensatory Effect of Lipase on the Flavor of Lightly-Salted Large Yellow Croaker: Integration of Flavoromics and Lipidomics. Food Biosci. 2024, 59, 103907. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, Z.; Liu, Y.; Xiong, S. Flavor and Sensory Profile of Chinese Traditional Fish Noodles Produced by Different Silver Carp (Hypophthalmichthys molitrix) Mince Ingredients. Food Chem. X 2023, 20, 100977. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Lu, W.; Tian, T.; Shu, N.; Yang, Y.; Fan, S.; Han, X.; Ge, Y.; Xu, P. Analysis of Volatile Components in Dried Fruits and Branch Exudates of Schisandra Chinensis with Different Fruit Colors Using GC-IMS Technology. Molecules 2023, 28, 6865. [Google Scholar] [CrossRef] [PubMed]
- Podduturi, R.; Petersen, M.A.; Mahmud, S.; Rahman, M.d.M.; Jørgensen, N.O.G. Potential Contribution of Fish Feed and Phytoplankton to the Content of Volatile Terpenes in Cultured Pangasius (Pangasianodon hypophthalmus) and Tilapia (Oreochromis niloticus). J. Agric. Food Chem. 2017, 65, 3730–3736. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, J.; Xie, J. Progress on Odor Deterioration of Aquatic Products: Characteristic Volatile Compounds, Analysis Methods, and Formation Mechanisms. Food Biosci. 2023, 53, 102666. [Google Scholar] [CrossRef]
- Qiu, J.; Cai, L.; Xiong, S.; You, J.; Yin, T.; An, Y. Characterization of Aroma Profiles and Volatile Organic Compounds in Silver Carp Surimi Gel by GC-IMS, SPME-GC-MS, and Sensory Evaluation: Affected by Okara Insoluble Dietary Fiber with Varied Particle Sizes. Food Chem. Adv. 2023, 2, 100224. [Google Scholar] [CrossRef]
- Zhu, W.; Luan, H.; Bu, Y.; Li, X.; Li, J.; Ji, G. Flavor Characteristics of Shrimp Sauces with Different Fermentation and Storage Time. LWT-Food Sci. Technol. 2019, 110, 142–151. [Google Scholar] [CrossRef]
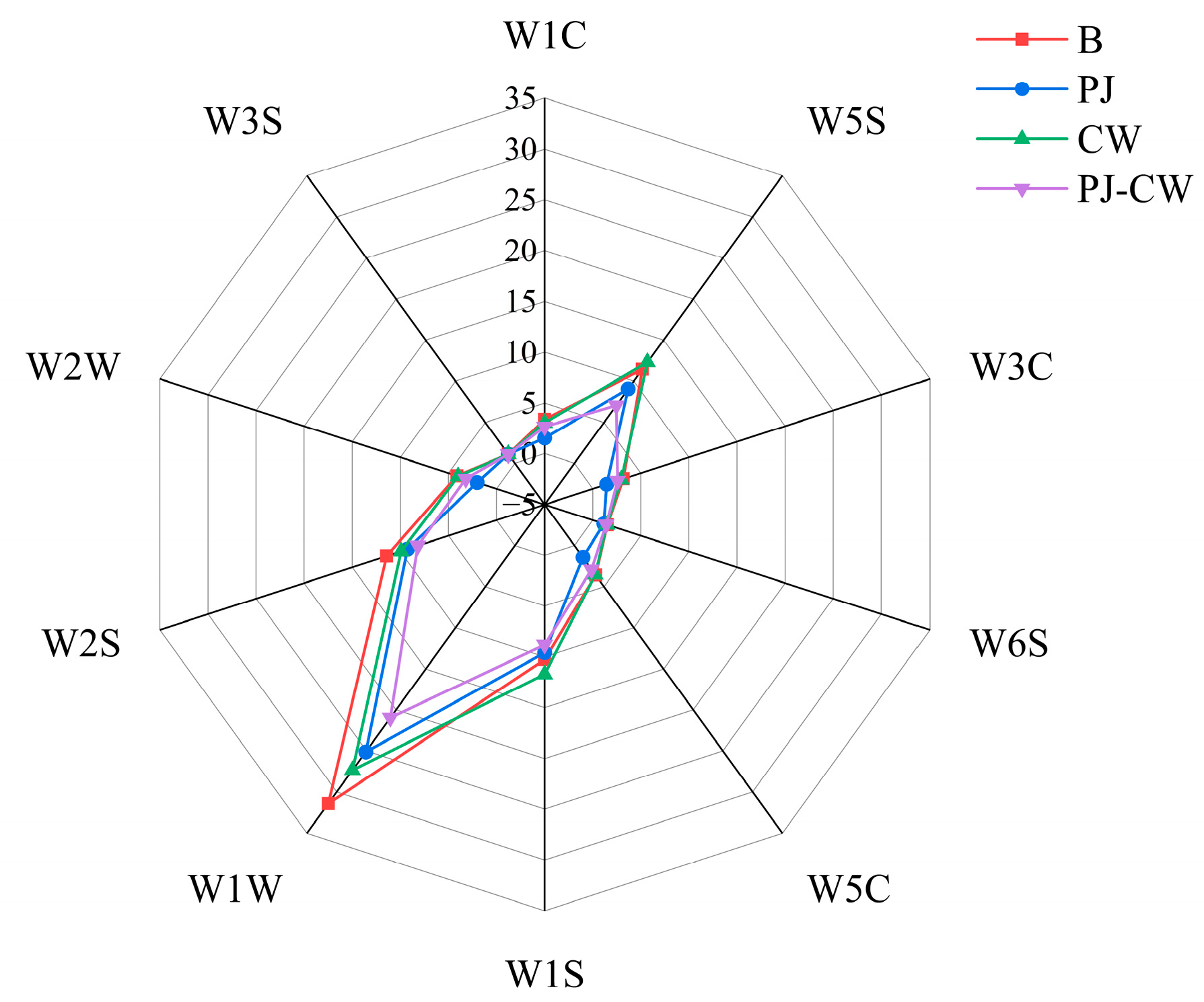







| Sensor Serial Number | Sensor Name | Sensitive Substances |
|---|---|---|
| 1 | W1C | Aromatic constituents, benzene |
| 2 | W5S | Nitrogen oxides |
| 3 | W3C | Aromatic constituents, ammonia |
| 4 | W6S | Hydrides |
| 5 | W5C | Short-chain alkane aromatic component |
| 6 | W1S | Methyl |
| 7 | W1W | Sulfides |
| 8 | W2S | Alcohols, aldehydes and ketones |
| 9 | W2W | Aromatic ingredients, organic sulfides |
| 10 | W3S | Long-chain alkanes |
| Amino Acid | Taste Contribution | Thres-hold | B | PJ | CW | PJ-CW | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Content mg/100 g | TAV | Content mg/100 g | TAV | Content mg/100 g | TAV | Content mg/100 g | TAV | |||
| Methionine (Met *) | bitter/sweet | 30 | 245.73 ± 4.23 c | 8.19 | 284.36 ± 4.93 b | 9.48 | 282.66 ± 2.12 b | 9.42 | 411.58 ± 6.00 a | 13.72 |
| Proline (Pro) | bitter/sweet | 300 | 487.18 ± 10.20 c | 1.62 | 632.89 ± 26.58 ab | 2.11 | 600.97 ± 23.28 b | 2.00 | 662.85 ± 33.19 a | 2.21 |
| Glycine (Gly) | sweet | 130 | 714.40 ± 25.62 d | 5.50 | 941.21 ± 4.51 b | 7.24 | 810.93 ± 11.61 c | 6.24 | 980.45 ± 17.04 a | 7.54 |
| Cysteine (Cys) | bitter/sweet | - | 61.04 ± 1.62 c | / | 55.02 ± 0.25 d | / | 66.77 ± 0.58 b | / | 86.55 ± 1.95 a | / |
| Glutamate (Glu) | umami /sour | 30 | 78.21 ± 1.17 c | 2.61 | 66.97 ± 1.67 d | 2.23 | 85.66 ± 1.80 b | 2.86 | 95.36 ± 1.51 a | 3.18 |
| Glutamine (Gln) | umami | - | 50.03 ± 1.39 b | / | 42.22 ± 1.14 c | / | 49.59 ± 1.69 b | / | 55.00 ± 2.88 a | / |
| Arginine (Arg) | bitter/sweet | 50 | 1353.27 ± 21.61 c | 27.06 | 1491.16 ± 36.78 b | 29.82 | 1457.82 ± 21.61 b | 29.16 | 1642.33 ± 17.92 a | 32.85 |
| 1-Methyl-L-histidine (1-M-His) | - | - | 9.25 ± 0.08 d | / | 10.08 ± 0.24 c | / | 11.57 ± 0.33 b | / | 12.48 ± 0.29 a | / |
| 3-Methyl-L-histidine (3-M-His) | - | - | 10.69 ± 0.38 b | / | 11.75 ± 0.46 c | / | 12.42 ± 0.44 b | / | 15.05 ± 0.31 a | / |
| Lysine (Lys *) | sweet/bitter | 50 | 1480.14 ± 24.28 c | 29.60 | 1791.36 ± 26.00 b | 35.83 | 1737.50 ± 33.99 b | 34.75 | 1967.42 ± 34.61 a | 39.35 |
| Tyrosine (Tyr) | bitter | - | 285.57 ± 5.54 d | / | 330.87 ± 5.60 b | / | 316.68 ± 4.59 c | / | 410.23 ± 9.09 a | / |
| Leucine (Leu *) | bitter | 190 | 1120.53 ± 20.13 c | 5.90 | 995.71 ± 32.01 d | 5.24 | 1228.68 ± 22.11 b | 6.47 | 1367.03 ± 5.64 a | 7.19 |
| hydroxyproline (Hy-Pro) | sweet | - | 18.59 ± 0.50 d | / | 53.56 ± 1.65 a | / | 25.03 ± 0.96 c | / | 31.39 ± 0.84 b | / |
| Threonine (Thr *) | sweet | 260 | 740.87 ± 19.44 b | 2.85 | 746.55 ± 10.96 b | 2.87 | 760.99 ± 26.89 b | 2.93 | 899.33 ± 12.91 a | 3.46 |
| Serine (Ser) | sweet | 150 | 538.58 ± 19.08 b | 3.59 | 559.45 ± 12.93 b | 3.73 | 614.20 ± 21.74 a | 4.09 | 573.96 ± 22.11 b | 3.83 |
| Valine (Val *) | sweet/bitter | 40 | 573.61 ± 18.85 b | 14.34 | 555.41 ± 17.84 b | 13.88 | 552.15 ± 17.36 b | 13.80 | 657.49 ± 16.26 a | 16.44 |
| Isoleucine (Ile *) | bitter | 90 | 628.23 ± 19.74 c | 6.98 | 603.72 ± 19.84 c | 6.71 | 694.56 ± 10.73 b | 7.72 | 753.31 ± 24.48 a | 8.37 |
| Histidine (His) | bitter/sour | 20 | 381.74 ± 17.59 b | 19.09 | 373.21 ± 14.28 b | 18.66 | 356.01 ± 4.77 b | 17.80 | 504.17 ± 13.52 a | 25.21 |
| SWAA | 6213.41 ± 44.68 d | 7110.97 ± 99.30 b | 6909.02 ± 69.18 c | 7913.34 ± 55.86 a | ||||||
| BIAA | 6617.04 ± 33.32 d | 7113.72 ± 116.96 c | 7293.80 ± 45.00 b | 8462.95 ± 92.17 a | ||||||
| UMAA | 128.24 ± 2.21 c | 109.20 ± 1.22 d | 135.25 ± 2.47 b | 150.37 ± 3.87 a | ||||||
| SOAA | 459.95 ± 17.21 b | 440.18 ± 15.18 b | 441.67 ± 6.42 b | 599.53 ± 14.56 a | ||||||
| EAA | 4789.11 ± 35.40 d | 4977.11 ± 83.51 c | 5256.55 ± 40.40 b | 6056.16 ± 40.99 a | ||||||
| TFAA | 8777.67 ± 41.94 c | 9545.51 ± 125.65 b | 9664.19 ± 91.11 b | 11,125.98 ± 92.68 a | ||||||
| Amino Acid | Threshold (mg/100 g) | B | PJ | CW | PJ-CW | ||||
|---|---|---|---|---|---|---|---|---|---|
| Content (mg/100 g) | TAV | Content (mg/100 g) | TAV | Content (mg/100 g) | TAV | Content (mg/100 g) | TAV | ||
| CMP | - | 36.89 ± 0.53 d | / | 59.00 ± 0.46 c | / | 84.89 ± 0.86 a | / | 81.90 ± 1.23 b | / |
| AMP | 50 | 4.21 ± 0.03 d | 0.08 | 4.46 ± 0.04 c | 0.09 | 4.96 ± 0.05 b | 0.10 | 5.87 ± 0.03 a | 0.12 |
| IMP | 25 | 1.35 ± 0.03 c | 0.05 | 1.05 ± 0.01 d | 0.04 | 1.56 ± 0.04 a | 0.06 | 1.45 ± 0.03 b | 0.06 |
| EUC (gMSG/100 g) | 2.64 | 2.32 | 3.07 | 3.15 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Zou, Y.; Zhang, Y.; Liao, M.; Chen, D.; Guo, Z. Comparison of Different Deodorizing Treatments on the Flavor of Paddy Field Carp, Analyzed by the E-Nose, E-Tongue and Gas Chromatography–Ion Mobility Spectrometry. Foods 2024, 13, 2623. https://doi.org/10.3390/foods13162623
Fu C, Zou Y, Zhang Y, Liao M, Chen D, Guo Z. Comparison of Different Deodorizing Treatments on the Flavor of Paddy Field Carp, Analyzed by the E-Nose, E-Tongue and Gas Chromatography–Ion Mobility Spectrometry. Foods. 2024; 13(16):2623. https://doi.org/10.3390/foods13162623
Chicago/Turabian StyleFu, Chenying, Yiming Zou, Yixiang Zhang, Mengxiang Liao, Duhuang Chen, and Zebin Guo. 2024. "Comparison of Different Deodorizing Treatments on the Flavor of Paddy Field Carp, Analyzed by the E-Nose, E-Tongue and Gas Chromatography–Ion Mobility Spectrometry" Foods 13, no. 16: 2623. https://doi.org/10.3390/foods13162623





