Exploring the Multifaceted Biological Activities of Anthocyanins Isolated from Two Andean Berries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Plant Material
2.3. Anthocyanin Extraction and Characterization
2.4. Spectrophotometric Determination of Anthocyanin
2.5. HPLC-DAD Analysis of Anthocyanins
2.6. Antimicrobial Activity Assay
2.7. Antioxidant Activity
2.8. Antibiofilm Activity
2.9. Antitumoral Activity Assay
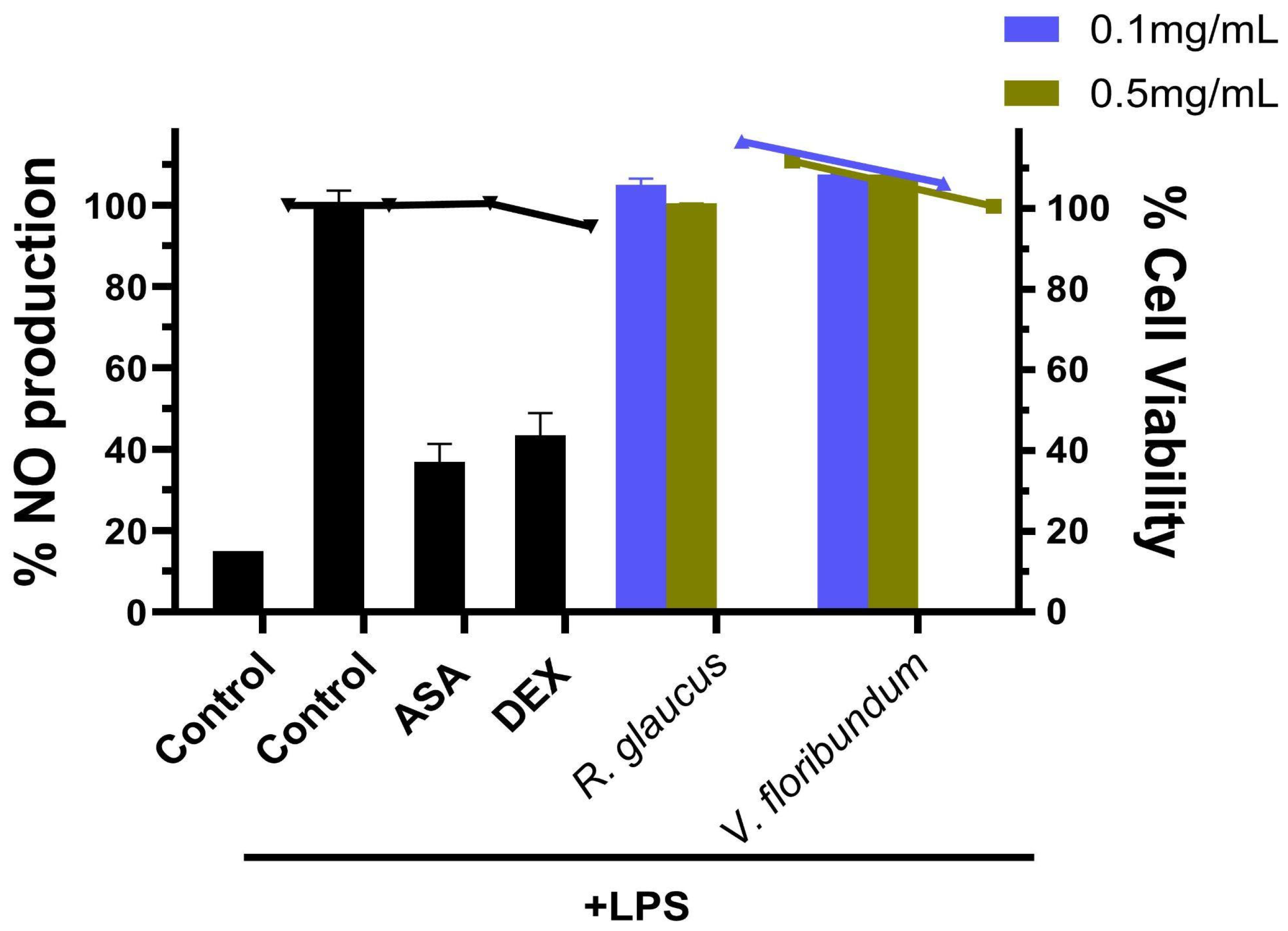
2.10. Anti-Inflammatory Activity
2.11. Hemolytic Activity Assay
3. Results
3.1. Chemical Characterization
| Peak ID | RT (min) | [M − H]− | MS/MS | [M + H]+ | MS/MS | Identification | V. floribundum | R. glaucus | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.18 | 191 | 191->111(100), 173(65), 127(20), 85(15) | 193 | 193->147(100), 157(90), 175(25), 165(15) | Quinic acid | 0.03 | 0.003 | [20] |
| 2 | 3.91 | 221 | 221->185(100), 203(30), 167(25), 95(5) 441->221(100), 185(10) | Quinic acid derivate | 0.63 | 0.004 | [17] | ||
| 3 | 7.80 | 219 | 219->111(100), 173(95), 157(10),87(5), 191(5) | 221 | 221->203(100), 175(30), 185(10) 203->157(100), 185(45), 175(15) | Quinic acid derivate isomer | 0.06 | 0.35 | [17] |
| 4 | 10.45 | 177 | 177->131(100), 145(90), 177(40), 117(35), 103(15) | N.I | 0.07 | 0.60 | |||
| 10.45 | 141 | 141->141(100) | N.I | 0.01 | 0.02 | ||||
| 10.93 | 465 | 465->303(100) | Delphinidin-3-pyranoside | 0.40 | 0.004 | [21] | |||
| 5 | 11.55 | 447 | 447->285(100), 245(25), 321(20) 179(10) | 449 | 449->287(100) | Cyanidin-3-pyranoside | 2.85 | 0.10 | [17] |
| 11.55 | 435 | 435->303(100) | Delphinidin-3-arabinoside | 0.32 | 0.002 | [17] | |||
| 11.97 | 611 | 611->287(100), 449(15) | Cyanidin-3-pyranoside hexoside | 0.001 | 0.02 | [17] | |||
| 6 | 12.28 | 417 | 417->285(100), 371(40), 339(15), 299(10) | 419 | 419->287(100) | Cyanidin-3-arabinoside | 2.05 | 0.000 | [17] |
| 12.27 | 593 | 593->285(100), 299(30), | 595 | 595->287(100), 449(20) | Derivate of cyanidin 3-O-sambubioside | 0.002 | 2.54 | [22] | |
| 12.33 | 727 | 727->287(100), 581(30), 375(10) | Cy-3-xylosylrutinoside | 0.000 | 0.51 | [22] | |||
| 12.69 | 433 | 433->271(100), 387(15) | Pelargonidin 3-glucoside | 0.02 | 0.38 | [22] | |||
| 7 | 13.42 | 13.42 | 155 | 155->109(100), 127(5) | 2.79 | 0.64 | |||
| 8 | 13.53 | 345, 247 | 345->247(100), 157(10) 247->157(100), 201(20), 229(10), 129(10) | 249 | 249->203(100), 231(10), 175(10) 203->157(100), 185(60), 175(5) | N.I | 6.25 | 13.65 | |
| 9 | 14.67 | 287 | 287->241(100), 167(90), 185(70), 231(50), 213(45) | Cyanidin | 1.42 | 0.02 | |||
| 14.73 | 557 | 557->287(100), 243(10) | catechin-(4-8) cyanidin | 0.26 | 0.02 | ||||
| 10 | 20.48 | 575 | 575->299(100), 271(10) | N.I | 0.10 | 0.07 | |||
| 20.48 | 277 | 277->203(100), 231(55), 157(5) | N.I | 6.82 | 7.30 |
3.2. Minimum Inhibitory Concentration
3.3. Biofilm Inhibition Assay
3.4. Antioxidant Activity
3.5. Antitumoral Activity
3.6. Anti-Inflamatory Activity
3.7. Hemolytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ponder, A.; Hallmann, E.; Kwolek, M.; Średnicka-Tober, D.; Kazimierczak, R. Genetic Differentiation in Anthocyanin Content among Berry Fruits. Curr. Issues Mol. Biol. 2021, 43, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Giné Bordonaba, J.; Chope, G.A.; Terry, L.A. Maximising blackcurrant anthocyanins: Temporal changes during ripening and storage in different genotypes. J. Berry Res. 2010, 1, 73–80. [Google Scholar] [CrossRef]
- Pérez, B.P.; Endara, A.B.; Garrido, J.A.; Ramírez Cárdenas, L.d.l.Á. Extraction of anthocyanins from Mortiño (Vaccinium floribundum) and determination of their antioxidant capacity. Rev. Fac. Nac. Agron. Medellín 2021, 74, 9453–9460. [Google Scholar] [CrossRef]
- Schreckinger, M.E.; Wang, J.; Yousef, G.; Lila, M.A.; Gonzalez de Mejia, E. Antioxidant capacity and in vitro inhibition of adipogenesis and inflammation by phenolic extracts of Vaccinium floribundum and Aristotelia chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976. [Google Scholar] [CrossRef]
- Chikane, A.; Rakshe, P.; Kumbhar, J.; Gadge, A.; More, S. A review on anthocyanins: Coloured pigments as food, pharmaceutical ingredients and the potential health benefits. Int. J. Sci. Res. Sci. Technol. 2022, 9, 547–550. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Jęcek, M.; Nowak, P.; Zajdel, R. Food Anthocyanins: Malvidin and Its Glycosides as Promising Antioxidant and Anti-Inflammatory Agents with Potential Health Benefits. Nutrients 2023, 15, 3016. [Google Scholar] [CrossRef]
- Marques-da-Silva, D.; Rodrigues, J.R.; Lagoa, R. Anthocyanins, effects in mitochondria and metabolism. In Mitochondrial Physiology and Vegetal Molecules; Elsevier: Amsterdam, The Netherlands, 2021; pp. 267–300. ISBN 9780128215623. [Google Scholar]
- Yildiz, E.; Guldas, M.; Ellergezen, P.; Acar, A.G.; Gurbuz, O. Obesity-associated Pathways of Anthocyanins. Food Sci. Technol. 2021, 41, 1–13. [Google Scholar] [CrossRef]
- Barani, Y.H.; Zhang, M.; Mujumdar, A.S.; Chang, L. Preservation of color and nutrients in anthocyanin-rich edible flowers: Progress of new extraction and processing techniques. J. Food Process. Preserv. 2022, 46, e16474. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, H.; Shao, S.; Sun, S.; Yang, D.; Lv, S. Anthocyanin: A review of plant sources, extraction, stability, content determination and modifications. Int. J. Food Sci. Technol. 2022, 57, 7573–7591. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Beltrán-Noboa, A.; Proaño-Ojeda, J.; Guevara, M.; Gallo, B.; Berrueta, L.A.; Giampieri, F.; Perez-Castillo, Y.; Battino, M.; Álvarez-Suarez, J.M.; Tejera, E. Metabolomic profile and computational analysis for the identification of the potential anti-inflammatory mechanisms of action of the traditional medicinal plants Ocimum basilicum and Ocimum tenuiflorum. Food Chem. Toxicol. 2022, 164, 113039. [Google Scholar] [CrossRef]
- CLSI. Dilution AST for Aerobically Grown Bacteria—CLSI. Available online: https://clsi.org/standards/products/microbiology/documents/m07/ (accessed on 26 May 2022).
- Cadena-Cruz, J.E.; Guamán-Ortiz, L.M.; Romero-Benavides, J.C.; Bailon-Moscoso, N.; Murillo-Sotomayor, K.E.; Ortiz-Guamán, N.V.; Heredia-Moya, J. Synthesis of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) and evaluation of their antioxidant and anticancer activities. BMC Chem. 2021, 15, 38. [Google Scholar] [CrossRef]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the hemolysis assay for the assessment of cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef] [PubMed]
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of anthocyanin content and profile throughout fruit development and ripening of highbush blueberry cultivars grown at two different altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Guevara-Terán, M.; Padilla-Arias, K.; Beltrán-Novoa, A.; González-Paramás, A.M.; Giampieri, F.; Battino, M.; Vásquez-Castillo, W.; Fernandez-Soto, P.; Tejera, E.; Alvarez-Suarez, J.M. Influence of Altitudes and Development Stages on the Chemical Composition, Antioxidant, and Antimicrobial Capacity of the Wild Andean Blueberry (Vaccinium floribundum Kunth). Molecules 2022, 27, 7525. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Di, Q.-R.; Zhong, L.; Zhou, J.-Z.; Shan, C.-J.; Liu, X.-L.; Ma, A.-M. Enhancement on antioxidant, anti-hyperglycemic and antibacterial activities of blackberry anthocyanins by processes optimization involving extraction and purification. Front. Nutr. 2022, 9, 1007691. [Google Scholar] [CrossRef]
- Liu, H.; Wu, H.; Wang, Y.; Wang, F.; Liu, X.; Zhou, J. Enhancement on antioxidant and antibacterial activities of Brightwell blueberry by extraction and purification. Appl. Biol. Chem. 2021, 64, 78. [Google Scholar] [CrossRef]
- Aita, S.; Capriotti, A.; Cavaliere, C.; Cerrato, A.; Giannelli Moneta, B.; Montone, C.; Piovesana, S.; Laganà, A. Andean Blueberry of the Genus Disterigma: A High-Resolution Mass Spectrometric Approach for the Comprehensive Characterization of Phenolic Compounds. Separations 2021, 8, 58. [Google Scholar] [CrossRef]
- Stein-Chisholm, R.; Beaulieu, J.; Grimm, C.; Lloyd, S. LC–MS/MS and UPLC–UV Evaluation of Anthocyanins and Anthocyanidins during Rabbiteye Blueberry Juice Processing. Beverages 2017, 3, 56. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; García-Estévez, I.; Rivas-Gonzalo, J.C.; Rodríguez de la Cruz, D.; Escribano-Bailón, M.T. Anthocyanins of the anthers as chemotaxonomic markers in the genus Populus L. Differentiation between Populus nigra, Populus alba and Populus tremula. Phytochemistry 2016, 128, 35–49. [Google Scholar] [CrossRef]
- Llivisaca, S.; Manzano, P.; Ruales, J.; Flores, J.; Mendoza, J.; Peralta, E.; Cevallos-Cevallos, J.M. Chemical, antimicrobial, and molecular characterization of mortiño (Vaccinium floribundum Kunth) fruits and leaves. Food Sci. Nutr. 2018, 6, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A.; Soto, C.Y.; López-R, M.; Riedl, K.M.; Browmiller, C.R.; Howard, L. Phenolic profile, in vitro antimicrobial activity and antioxidant capacity of Vaccinium meridionale swartz pomace. Heliyon 2020, 6, e03845. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Urbonaviciene, D.; Bobinaite, R.; Viskelis, P.; Bobinas, C.; Petruskevicius, A.; Klavins, L.; Viskelis, J. Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.). Antioxidants 2022, 11, 588. [Google Scholar] [CrossRef]
- Vg, M.K.; Murukan, G.; Aswathy, J.; Lawarence, B.; Murugan, K. Microbicidal potentiality of purified anthocyanin from in vitro culture of clerodendron infortunatum L. against selected pathogens. Int. J. Pharm. Pharm. Sci. 2018, 10, 68. [Google Scholar] [CrossRef]
- Sharma, N.; Tiwari, V.; Vats, S.; Kumari, A.; Chunduri, V.; Kaur, S.; Kapoor, P.; Garg, M. Evaluation of Anthocyanin Content, Antioxidant Potential and Antimicrobial Activity of Black, Purple and Blue Colored Wheat Flour and Wheat-Grass Juice against Common Human Pathogens. Molecules 2020, 25, 5785. [Google Scholar] [CrossRef] [PubMed]
- LaPlante, K.L.; Sarkisian, S.A.; Woodmansee, S.; Rowley, D.C.; Seeram, N.P. Effects of cranberry extracts on growth and biofilm production of Escherichia coli and Staphylococcus species. Phytother. Res. 2012, 26, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Mendes, M.; Morais, R.M.; Calhau, C.; Pintado, M.M. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J. Appl. Microbiol. 2016, 121, 693–703. [Google Scholar] [CrossRef]
- Gao, Z. Extraction, separation, and purification of blueberry anthocyanin using ethyl alcohol. Kem. Ind. 2017, 66, 655–659. [Google Scholar] [CrossRef]
- Cairone, F.; Simonetti, G.; Orekhova, A.; Casadei, M.A.; Zengin, G.; Cesa, S. Health Potential of Clery Strawberries: Enzymatic Inhibition and Anti-Candida Activity Evaluation. Molecules 2021, 26, 1731. [Google Scholar] [CrossRef]
- Salaheen, S.; Peng, M.; Joo, J.; Teramoto, H.; Biswas, D. Eradication and Sensitization of Methicillin Resistant Staphylococcus aureus to Methicillin with Bioactive Extracts of Berry Pomace. Front. Microbiol. 2017, 8, 253. [Google Scholar] [CrossRef]
- Quave, C.L.; Estévez-Carmona, M.; Compadre, C.M.; Hobby, G.; Hendrickson, H.; Beenken, K.E.; Smeltzer, M.S. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE 2012, 7, e28737. [Google Scholar] [CrossRef]
- Alarcón-Barrera, K.S.; Armijos-Montesinos, D.S.; García-Tenesaca, M.; Iturralde, G.; Jaramilo-Vivanco, T.; Granda-Albuja, M.G.; Giampieri, F.; Alvarez-Suarez, J.M. Wild Andean blackberry (Rubus glaucus Benth) and Andean blueberry (Vaccinium floribundum Kunth) from the Highlands of Ecuador: Nutritional composition and protective effect on human dermal fibroblasts against cytotoxic oxidative damage. J. Berry Res. 2018, 8, 223–236. [Google Scholar] [CrossRef]
- Prencipe, F.P.; Bruni, R.; Guerrini, A.; Rossi, D.; Benvenuti, S.; Pellati, F. Metabolite profiling of polyphenols in Vaccinium berries and determination of their chemopreventive properties. J. Pharm. Biomed. Anal. 2014, 89, 257–267. [Google Scholar] [CrossRef]
- Samaniego, I.; Brito, B.; Viera, W.; Cabrera, A.; Llerena, W.; Kannangara, T.; Vilcacundo, R.; Angós, I.; Carrillo, W. Influence of the Maturity Stage on the Phytochemical Composition and the Antioxidant Activity of Four Andean Blackberry Cultivars (Rubus glaucus Benth) from Ecuador. Plants 2020, 9, 1027. [Google Scholar] [CrossRef]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Chemical composition and phenolic compound profile of mortiño (Vaccinium floribundum Kunth). J. Agric. Food Chem. 2009, 57, 8274–8281. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martínez, L.; Mut-Salud, N.; Ruiz-García, J.A.; Falcón-Piñeiro, A.; Maijó-Ferré, M.; Baños, A.; De la Torre-Ramírez, J.M.; Guillamón, E.; Verardo, V.; Gómez-Caravaca, A.M. Phytochemicals Determination, and Antioxidant, Antimicrobial, Anti-Inflammatory and Anticancer Activities of Blackberry Fruits. Foods 2023, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- Lamdan, H.; Garcia-Lazaro, R.S.; Lorenzo, N.; Caligiuri, L.G.; Alonso, D.F.; Farina, H.G. Anti-proliferative effects of a blueberry extract on a panel of tumor cell lines of different origin. Exp. Oncol. 2020, 42, 101–108. [Google Scholar] [CrossRef]
- Tatar, M.; Varedi, M.; Naghibalhossaini, F. Epigenetic effects of blackberry extract on human colorectal cancer cells. Nutr. Cancer 2022, 74, 1446–1456. [Google Scholar] [CrossRef]
- Jazić, M.; Kukrić, Z.; Vulić, J.; Četojević-Simin, D. Polyphenolic composition, antioxidant and antiproliferative effects of wild and cultivated blackberries (Rubus fruticosus L.) pomace. Int. J. Food Sci. Technol. 2019, 54, 194–201. [Google Scholar] [CrossRef]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, Y.; Tao, X.; Zhang, M.; Sun, A. Protective effect of blueberry anthocyanins in a CCL4-induced liver cell model. LWT—Food Sci. Technol. 2015, 60, 1105–1112. [Google Scholar] [CrossRef]
- Gu, I.; Brownmiller, C.; Stebbins, N.B.; Mauromoustakos, A.; Howard, L.; Lee, S.-O. Berry Phenolic and Volatile Extracts Inhibit Pro-Inflammatory Cytokine Secretion in LPS-Stimulated RAW264.7 Cells through Suppression of NF-κB Signaling Pathway. Antioxidants 2020, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and Anti-Inflammatory Effects of Blueberry Anthocyanins on High Glucose-Induced Human Retinal Capillary Endothelial Cells. Oxid. Med. Cell. Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.S.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Blackberries and Mulberries: Berries with Significant Health-Promoting Properties. Int. J. Mol. Sci. 2023, 24, 12024. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P. Berry fruits for cancer prevention: Current status and future prospects. J. Agric. Food Chem. 2008, 56, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chu, Y.-F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
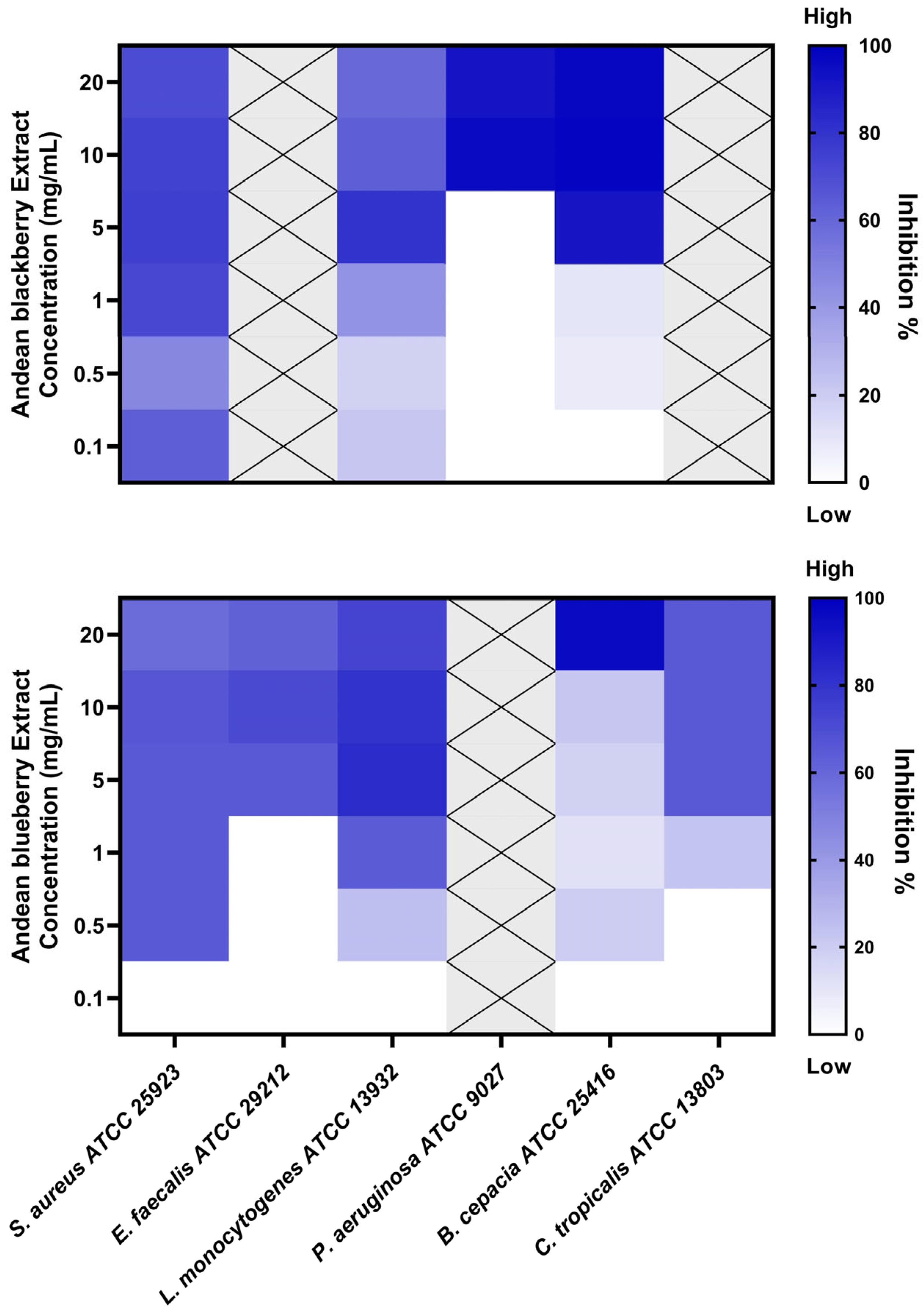
| Strain | R. glaucus | V. floribundum | |
|---|---|---|---|
| MIC mg/mL | MIC mg/mL | ||
| Gram-positive | Staphylococcus aureus ATCC 25923 | 1.2 | 2.1 |
| Enterococcus faecalis ATCC 29212 | 1.0 | 2.5 | |
| Listeria monocytogenes ATCC 13932 | 1.2 | 2.2 | |
| Gram-negative | Pseudomonas aeruginosa ATCC 27853 | 8 | 12 |
| Salmonella typhimurium ATCC 14028 | 10 | 16 | |
| Burkholderia cepacea ATCC 25416 | 10 | 14 | |
| Escherichia coli ATCC 25922 | 12 | 18 | |
| Yeast | Candida krusei ATCC 14243 | 110 | 150 |
| Candida glabrata ATCC 66032 | 150 | 180 | |
| Candida tropicalis ATCC 1369 | 120 | 100 | |
| Candida albicans ATCC 10231 | 100 | 100 |
| Strains | R. glaucus Extract | V. floribundum Extract | ||
|---|---|---|---|---|
| MBIC50 (mg/mL) | Inhibition Percentage | MBIC50 (mg/mL) | Inhibition Percentage | |
| Staphylococcus aureus ATCC 25923 | 1 | 72 ± 6.6% | 0.5 | 64 ± 7.1% |
| Enterococcus faecalis ATCC 29212 | NA | NA | 5 | 63 ± 12.2% |
| Listeria monocytogenes ATCC 13932 | 5 | 80 ± 16.7% | 1 | 63 ± 5.7% |
| Pseudomonas aeruginosa ATCC 9027 | 10 | 96 ± 2.0% | NA | NA |
| Burkholderia cepacia ATCC 25416 | 5 | 91 ± 9.1% | 20 | 96 ± 3.5% |
| Candida tropicalis ATCC 13803 | NA | NA | 5 | 64 ± 12.9% |
| IC50 | Rubus glaucus | Vaccinium floribundum | Control |
|---|---|---|---|
| µg/mL | 24.13 ± 3.73 | 21.77 ± 3.15 | 5.47 ± 0.30 |
| Cell Lines | R. glaucus (mg/mL) | V. floribundum (mg/mL) |
|---|---|---|
| MDA-MB-231 | 3.69 ± 0.60 | 2.31 ± 0.23 |
| MCF-7 | 3.33 ± 0.76 | >5.00 |
| HeLa | 1.40 ± 0.31 | >5.00 |
| THJ29T | 2.38 ± 0.75 | >5.00 |
| NIH3T3 | 2.22 ± 0.20 | 2.60 ± 0.90 |
| % Hemolysis | |
|---|---|
| C− | 0 ± 0.3 |
| C+ | 100.0 ± 1.4 |
| Rg 10 mg/mL | 6.3 ± 0.5 |
| Rg 50 mg/mL | 10.2 ± 3 |
| Vf 10 mg/mL | 0 ± 1.3 |
| Vf 50 mg/mL | 1.3 ± 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba-Ostria, C.; Carrera-Pacheco, S.E.; Gonzalez-Pastor, R.; Zuñiga-Miranda, J.; Mayorga-Ramos, A.; Tejera, E.; Guamán, L.P. Exploring the Multifaceted Biological Activities of Anthocyanins Isolated from Two Andean Berries. Foods 2024, 13, 2625. https://doi.org/10.3390/foods13162625
Barba-Ostria C, Carrera-Pacheco SE, Gonzalez-Pastor R, Zuñiga-Miranda J, Mayorga-Ramos A, Tejera E, Guamán LP. Exploring the Multifaceted Biological Activities of Anthocyanins Isolated from Two Andean Berries. Foods. 2024; 13(16):2625. https://doi.org/10.3390/foods13162625
Chicago/Turabian StyleBarba-Ostria, Carlos, Saskya E. Carrera-Pacheco, Rebeca Gonzalez-Pastor, Johana Zuñiga-Miranda, Arianna Mayorga-Ramos, Eduardo Tejera, and Linda P. Guamán. 2024. "Exploring the Multifaceted Biological Activities of Anthocyanins Isolated from Two Andean Berries" Foods 13, no. 16: 2625. https://doi.org/10.3390/foods13162625





