Characterization of Fibers Prepared by Centrifugal Spinning from Biotechnologically Derived Chicken Gelatin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
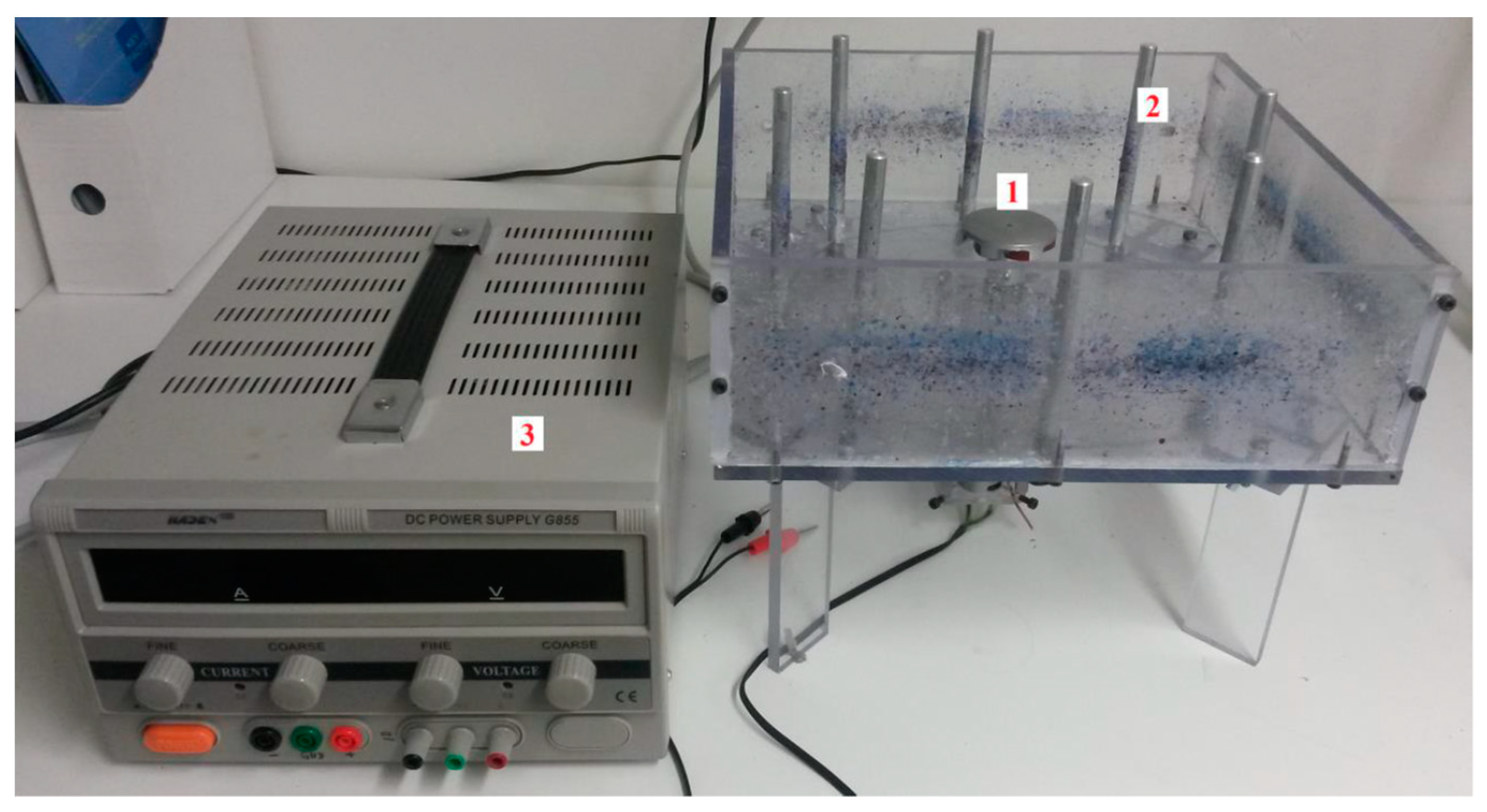
2.2. Appliances and Tools
2.3. Processing of Chicken Paws into Gelatin
2.4. Preparation of Fibres
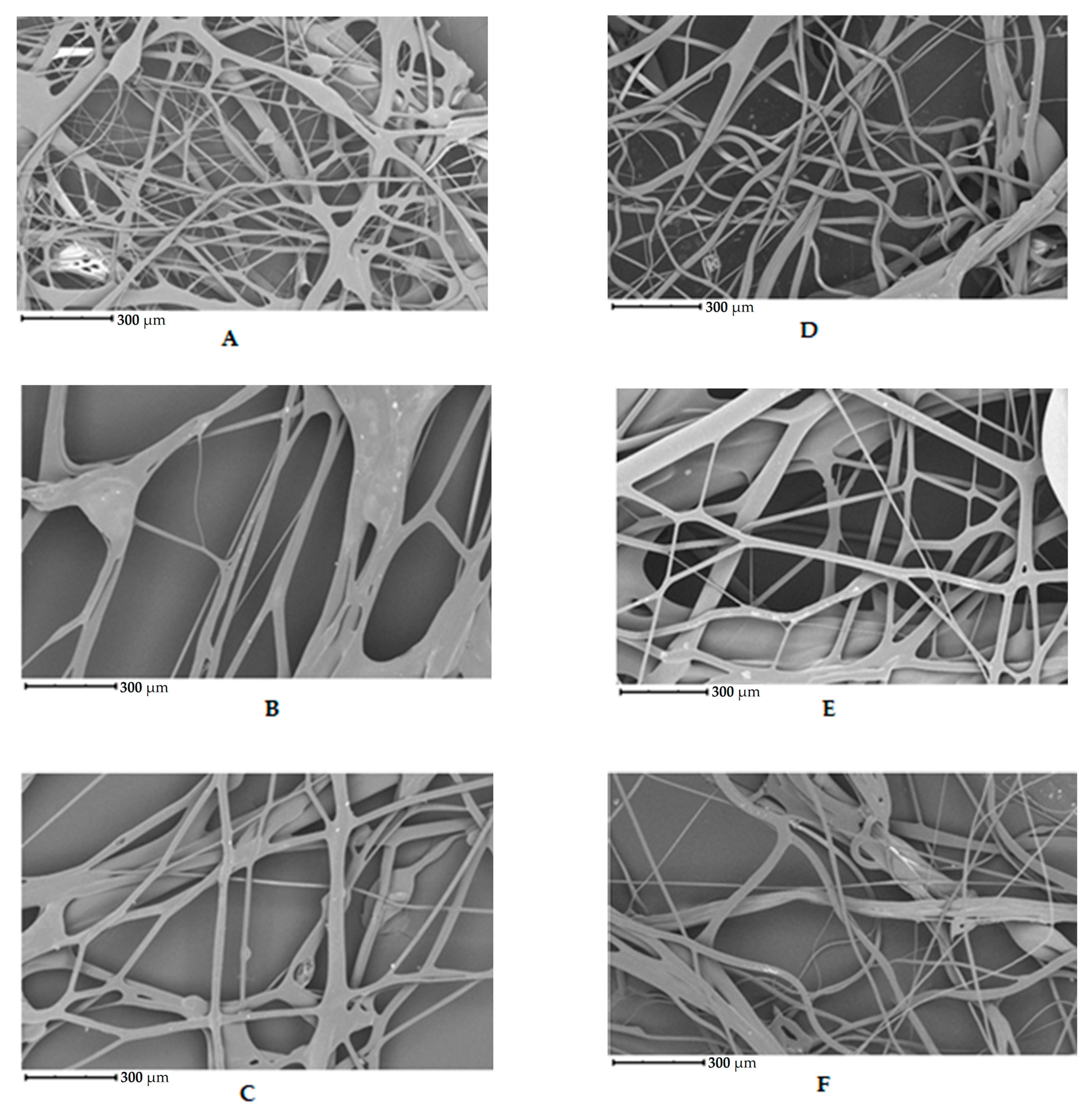
2.5. Morphology of Fibers
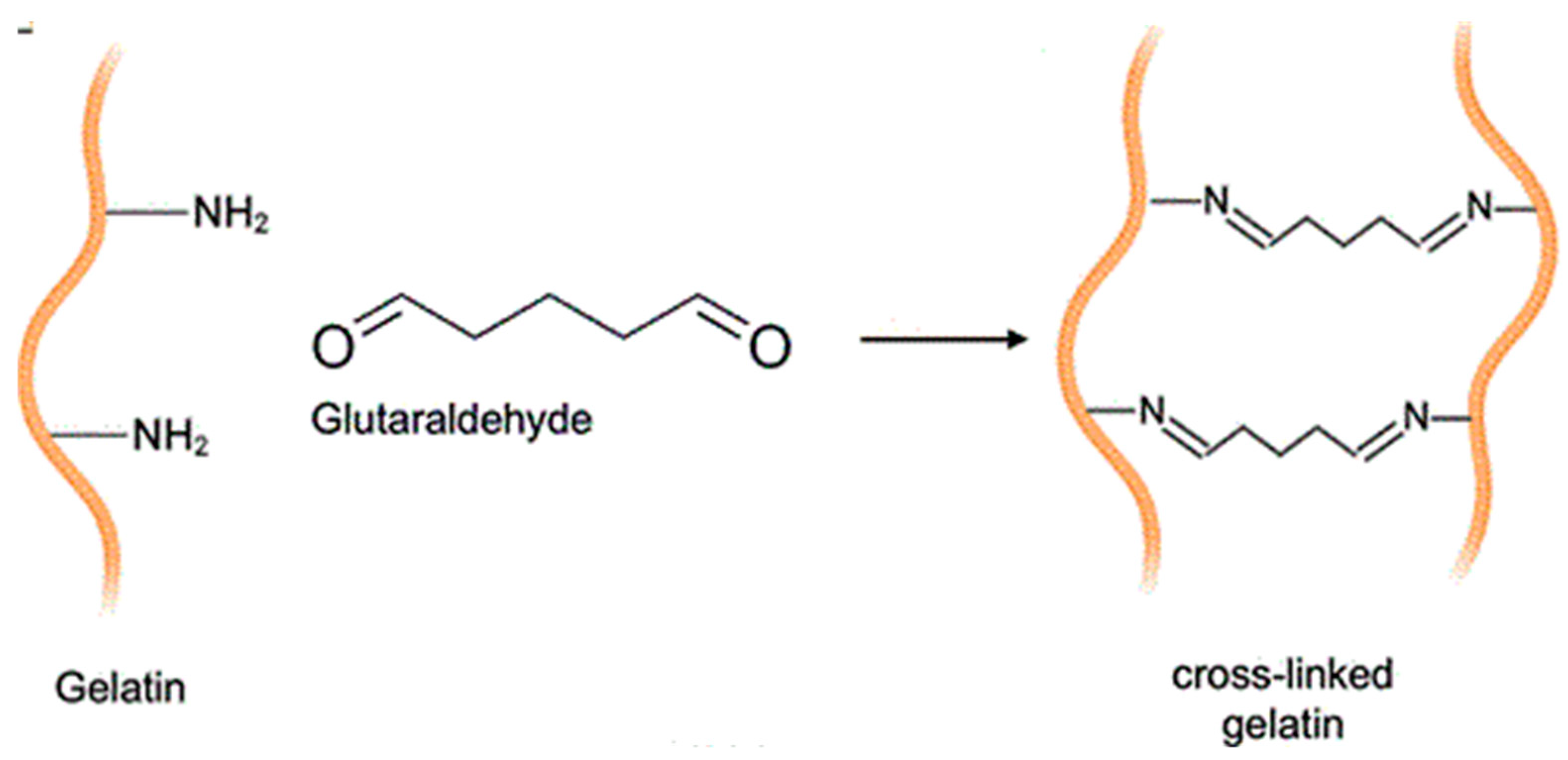
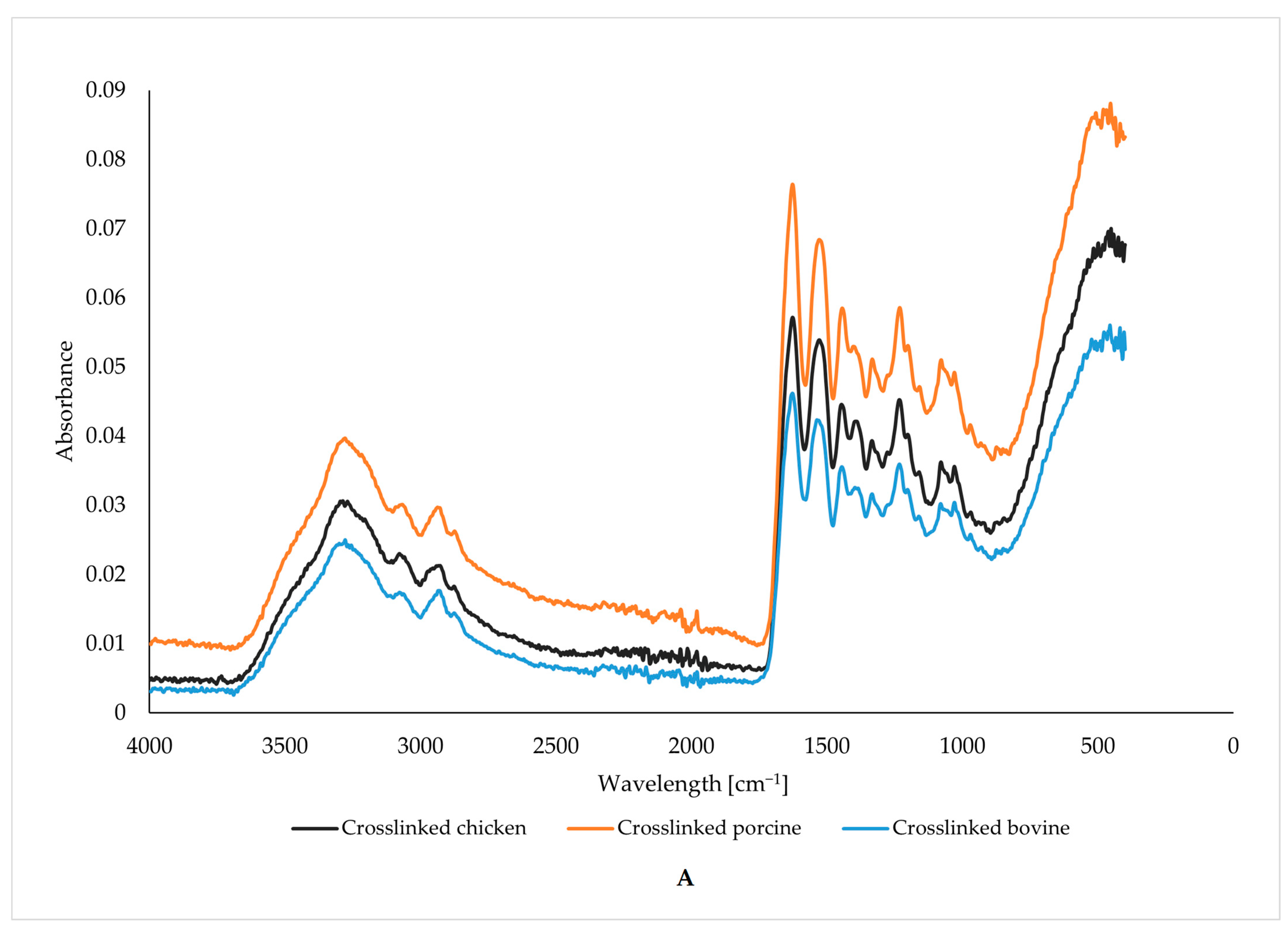
2.6. Vibrational Characterization of Functional Groups
2.7. Swelling and Solubility
2.8. Statistical Analysis
3. Results and Discussion
3.1. Morphology of Fibers
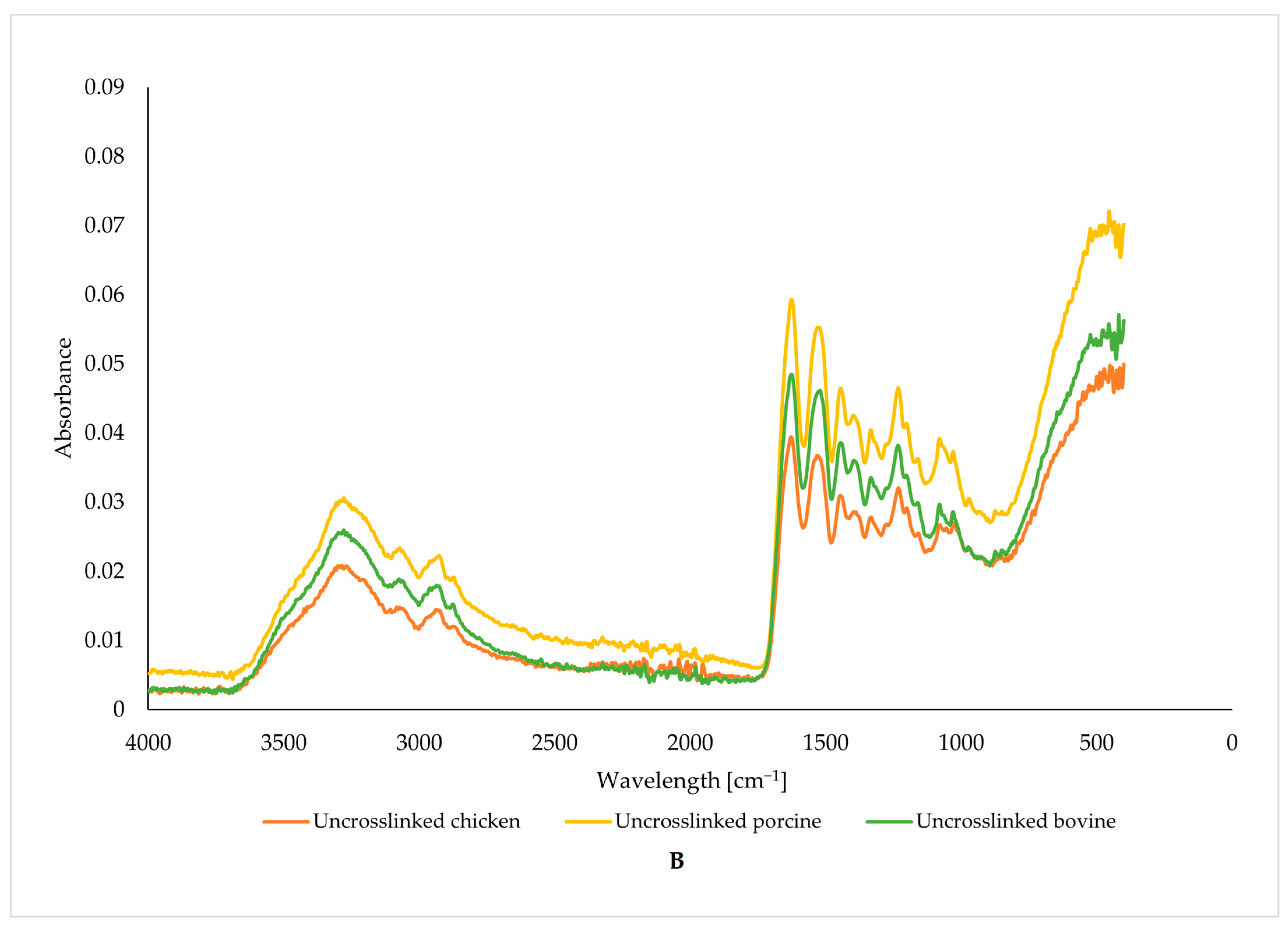
3.2. Vibrational Characterization of Functional Groups
3.3. Swelling and Solubility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhattarai, N.; Li, Z.; Edmondson, D.; Zhang, M. Alginate-based nanofibrous scaffolds: Structural, mechanical, and biological properties. Adv. Mater. 2006, 18, 1463–1467. [Google Scholar] [CrossRef]
- Moon, S.; Farris, R.J. Electrospinning of heated gelatin-sodium alginate-water solutions. Polym. Eng. Sci. 2009, 49, 1616–1620. [Google Scholar] [CrossRef]
- Songchotikunpan, P.; Tattiyakul, J.; Supaphol, P. Extraction and electrospinning of gelatin from fish skin. Int. J. Biol. Macromol. 2008, 42, 247–255. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.-M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Mokrejš, P.; Gál, R.; Mrázek, P. Biotechnology-Based Production of Food Gelatine from Poultry By-Products, Prague, Czech. Republic. Patent CZ 307665, 6 February 2019. [Google Scholar]
- Gál, R.; Mokrejš, P.; Mrázek, P.; Pavlačková, J.; Janáčová, D.; Orsavová, J. Chicken heads as a promising by-product for preparation of food gelatins. Molecules 2020, 25, 494. [Google Scholar] [CrossRef] [PubMed]
- Mrázek, P.; Gál, R.; Mokrejš, P.; Krejčí, O.; Orsavová, J. Thermal stability of prepared chicken feet gelatine gel in comparison with commercial gelatines. Potravin. Slovak J. Food Sci. 2020, 14, 535–543. [Google Scholar] [CrossRef]
- Prokopová, A.; Mokrejš, P.; Gál, R.; Pavlačková, J.; Hurajová, A. Characterization of poultry gelatins prepared by a biotechnological method for targeted changes at the molecular level. Int. J. Mol. Sci. 2024, 25, 916. [Google Scholar] [CrossRef]
- Mokrejš, P.; Mrázek, P.; Gál, R.; Pavlačková, J. Biotechnological preparation of gelatines from chicken feet. Polymers 2019, 11, 1060. [Google Scholar] [CrossRef]
- Jung, H. (Ed.) Edible films and coatings: A review. In Innovations in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2005; pp. 213–255. [Google Scholar]
- Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a Request from the Commission Related to Flavouring Group Evaluation 10: Aliphatic Primary and Secondary Saturated and Unsaturated Alcohols, Aldehydes, Acetals, Carboxylic Acids and Esters Containing an Additional Oxygenated Functional Group and Lactones from Chemical Groups 9, 13 and 30, Commission Regulation (EC) No. 1565/2000 of 18 July 2000. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2005.246 (accessed on 13 March 2022).
- Code of Federal Regulations. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172#p-172.230(a)(2) (accessed on 13 March 2022).
- Gungor, M.; Sagirli, M.N.; Calisir, M.D.; Selcuk, S.; Kilic, A. Developing centrifugal spun thermally cross-linked gelatin based fibrous biomats for antibacterial wound dressing applications. Polym. Eng. Sci. 2021, 61, 2311–2322. [Google Scholar] [CrossRef]
- Babczyk, P.; Conzendorf, C.; Klose, J.; Schulze, M.; Harre, K.; Tobiasch, E. Stem cells on biomaterials for synthetic grafts to promote vascular healing. J. Clin. Med. 2014, 3, 39–87. [Google Scholar] [CrossRef] [PubMed]
- Suput, D.Z.; Lazic, V.L.; Popovic, S.Z.; Hromis, N.M. Edible films and coatings: Sources, properties and application. Food Feed Res. 2015, 42, 11–22. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.d.R. Antimicrobial efficiency of chitosan coating enriched with bioactive compounds to improve the safety of fresh cut broccoli. LWT 2013, 50, 78–87. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Wang, Y.; Yu, L. Active and robust composite films based on gelatin and gallic acid integrated with microfibrillated cellulose. Foods 2021, 10, 2831. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioğlu, A. Incorporating phenolic compounds opens a new perspective to use zein films as flexible bioactive packaging materials. Food Res. Int. 2010, 44, 550–556. [Google Scholar] [CrossRef]
- Ucak, I.; Khalily, R.; Carrillo, C.; Tomasevic, I.; Barba, F.J. Potential of propolis extract as a natural antioxidant and antimicrobial in gelatin films applied to rainbow trout (Oncorhynchus mykiss) fillets. Foods 2020, 9, 1584. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Z.; Huang, Y.; Niu, L.; Miao, J.; Lai, K. Effects of acidulants on the rheological properties of gelatin extracted from the skin of tilapia (Oreochromis mossambicus). Foods 2022, 11, 2812. [Google Scholar] [CrossRef]
- Olatunji, O. (Ed.) Processing and characterization of natural polymers. In Natural Polymers: Industry Techniques and Applications; Springer: London, UK, 2016; pp. 19–61. [Google Scholar]
- Gennadios, A. (Ed.) Soft gelatin capsules. In Protein Based Films and Coatings; CRC Press: Boca Raton, FL, USA, 2002; pp. 393–443. [Google Scholar]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific: Hackensack, NJ, USA, 2008; pp. 22–154. [Google Scholar]
- Drabek, J.; Zatloukal, M. Meltblown technology for production of polymeric microfibers/nanofibers: A review. Phys. Fluids 2019, 31, 091301. [Google Scholar] [CrossRef]
- Sarkar, K.; Gomez, C.; Zambrano, S.; Ramirez, M.; de Hoyos, E.; Vasquez, H.; Lozano, K. Electrospinning to Forcespinning™. Mater. Today 2010, 13, 12–14. [Google Scholar] [CrossRef]
- Weitz, R.T.; Harnau, L.; Rauschenbach, S.; Burghard, M.; Kern, K. Polymer Nanofibers via Nozzle-Free Centrifugal Spinning. Nano Lett. 2008, 8, 1187–1191. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; De la Garza, D.; Gallegos, L.; Pokhrel, M.; Alcoutlabi, M. Forcespinning: A new method for the mass production of Sn/C composite nanofiber anodes for lithium ion batteries. Solid State Ion. 2016, 286, 72–82. [Google Scholar] [CrossRef]
- Kavoosi, G.; Dadfar, S.M.M.; Purfard, A.M. Mechanical, physical, antioxidant, and antimicrobial Properties of gelatin films incorporated with thymol for potential use as nano wound dressing. J. Food Sci. 2013, 78, E244–E250. [Google Scholar] [CrossRef] [PubMed]
- Mohiti-Asli, M.; Loboa, E.G. (Eds.) Nanofibrous smart bandages for wound care. In Wound Healing Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 483–499. [Google Scholar]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Characterization of gelatin/zein films fabricated by electrospinning vs solvent casting. Food Hydrocoll. 2018, 74, 324–332. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D bioprinting methods and techniques: Applications on artificial blood vessel fabrication. Acta Cardiol. Sin. 2019, 35, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Mondal, A.; Gebeyehu, A.; Miranda, M.; Bahadur, D.; Patel, N.; Ramakrishnan, S.; Rishi, A.K.; Singh, M. Characterization and printability of sodium alginate-gelatin hydrogel for bioprinting NSCLC co-culture. Sci. Rep. 2019, 9, 19914. [Google Scholar] [CrossRef]
- Švachová, V.; Khunová, V.; Pavliňák, D.; Fohlerová, Z.; Vojtová, L. The Effect of halloysite on structure and properties of polycaprolactone/gelatin nanofibers. Polym. Eng. Sci. 2017, 57, 506–512. [Google Scholar] [CrossRef]
- Tejiram, S.; Kavalukas, S.L.; Shupp, J.W.; Barbul, A. Wound Healing Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–39. [Google Scholar]
- Hlavatá, J. Spinnability of Polymer Solution Mixtures by Centrifugal Spinning. Master’s Thesis, Technical University in Liberec, Liberec, Czech Republic, 2015. (In Czech). [Google Scholar]
- Du, L.; Khiari, Z.; Pietrasik, Z.; Betti, M. Physicochemical and functional properties of gelatins extracted from turkey and chicken heads. Poult. Sci. 2013, 92, 2463–2474. [Google Scholar] [CrossRef]
- Mugnaini, G.; Gelli, R.; Mori, L.; Bonini, M. How to Cross-Link Gelatin: The Effect of Glutaraldehyde and Glyceraldehyde on the Hydrogel Properties. ACS Appl. Polym. Mater. 2023, 5, 9192–9202. [Google Scholar] [CrossRef]
- Padrão, J.; Silva, J.P.; Rodrigues, L.R.; Dourado, F.; Lanceros-Méndez, S.; Sencadas, V. Modifying fish gelatin electrospun membranes for biomedical applications: Cross-linking and swelling behavior. Soft Matter 2014, 12, 247–252. [Google Scholar] [CrossRef]
- Mîndru, T.B.; Ignat, L.; Mîndru, I.B.; Pinteala, M. Morphological aspects of polymer fiber mats obtained by air flow rotary-jet spinning. Fibers Polym. 2013, 14, 1526–1534. [Google Scholar] [CrossRef]
- Chaochai, T.; Imai, Y.; Furuike, T.; Tamura, H. Preparation and properties of gelatin fibers fabricated by dry spinning. Fibers 2016, 4, 2. [Google Scholar] [CrossRef]
- Arican, F.; Uzuner-Demir, A.; Polat, O.; Sancakli, A.; Ismar, E. Fabrication of gelatin nanofiber webs via centrifugal spinning for N95 respiratory filters. Bull. Mater. Sci. 2022, 45, 93. [Google Scholar] [CrossRef]
- Peña-Rodriguez, C.; Martucci, J.F.; Neira, L.M.; Arbelaiz, A.; Eceiza, A.; A Ruseckaite, R. Functional properties and in vitro antioxidant and antibacterial effectiveness of pigskin gelatin films incorporated with hydrolysable chestnut tannin. Food Sci. Technol. Int. 2014, 21, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Nor, M.H.M.; Nazmi, N.N.M.; Sarbon, N.M. Effects of plasticizer concentrations on functional properties of chicken skin gelatin films. Int. Food Res. J. 2017, 24, 1910–1918. [Google Scholar]
- Sabantina, L.; Wehlage, D.; Klöcker, M.; Mamun, A.; Grothe, T.; García-Mateos, F.J.; Rodríguez-Mirasol, J.; Cordero, T.; Finsterbusch, K.; Ehrmann, A. Stabilization of electrospun PAN/gelatin nanofiber mats for carbonization. J. Nanomater. 2018, 2018, 6131085. [Google Scholar] [CrossRef]
- Kuppan, P.; Sethuraman, S.; Krishnan, U.M. PCL and PCL-gelatin nanofibers as esophageal tissue scaffolds: Optimization, characterization and cell-matrix interactions. J. Biomed. Nanotechnol. 2013, 9, 1540–1555. [Google Scholar] [CrossRef]
- Kim, S.E.; Heo, D.N.; Lee, J.B.; Kim, J.R.; Park, S.H.; Jeon, S.H.; Kwon, I.K. Electrospun gelatin/polyurethane blended nanofibers for wound healing. Biomed. Mater. 2009, 4, 044106. [Google Scholar] [CrossRef]
- Sun, C.; Yin, H.; He, J.; Zou, L.; Xu, Y. Fabrication and characterization of nanofibrous gelatin/chitosan-poly (ethylene oxide) membranes by electrospinning with acetic acid as solvent. J. Polym. Res. 2021, 28, 482. [Google Scholar] [CrossRef]
- Riyajan, S.-A.; Teprak, A. A Novel Environmentally Friendly Biopolymer Product from Gelatin and Natural Rubber: Effect of Bagasse Fiber and Urea. J. Polym. Environ. 2018, 27, 225–233. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Liu, Y.; Zhang, C.-J.; Zhu, P. Alginate/gelatin blended hydrogel fibers cross-linked by Ca2+ and oxidized starch: Preparation and properties. Mater. Sci. Eng. C 2019, 99, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Etxabide, A.; Akbarinejad, A.; Chan, E.W.; Guerrero, P.; de la Caba, K.; Travas-Sejdic, J.; Kilmartin, P.A. Effect of gelatin concentration, ribose and glycerol additions on the electrospinning process and physicochemical properties of gelatin nanofibers. Eur. Polym. J. 2022, 180, 111597. [Google Scholar] [CrossRef]
| Amino Acid | Content [g/kg] | SD | Amino Acid | Content [g/kg] | SD |
|---|---|---|---|---|---|
| Hyp | 130.36 | 6.51 | Ile | 12.45 | 0.62 |
| Asp | 45.03 | 2.12 | Leu | 27.45 | 1.23 |
| Thr | 18.06 | 0.89 | Tyr | 1.27 | 0.06 |
| Ser | 18.75 | 1.76 | Phe | 18.99 | 0.78 |
| Glu | 108.11 | 5.33 | His | 6.33 | 0.32 |
| Pro | 93.06 | 4.28 | Lys | 29.46 | 1.43 |
| Gly | 156.45 | 7.57 | Arg | 64.58 | 3.09 |
| Ala | 78.87 | 3.86 | Cys | 1.17 | 0.05 |
| Val | 17.43 | 0.81 | Met | 8.41 | 0.31 |
| Wavenumber [cm−1] | 3278 | 1628 | 1537 | 1243 |
|---|---|---|---|---|
| Molecular Action | Stretching and Oscillation of N-H | Oscillation and Stretching of C=O and C-N | N-H Bending | N-H Bending |
| Uncross-linked gelatin | ||||
| bovine | 10.91 | 4.12 | 3.82 | 4.97 |
| porcine | 13.71 | 5.19 | 4.98 | 6.35 |
| chicken | 9.21 | 3.53 | 4.89 | 6.16 |
| Cross-linked gelatin | ||||
| bovine | 11.31 | 4.32 | 4.17 | 5.24 |
| porcine | 18.58 | 6.69 | 6.17 | 8.16 |
| chicken | 13.72 | 5.07 | 3.33 | 4.43 |
| Uncross-linked Gelatin Fibers | ||||||
|---|---|---|---|---|---|---|
| Time [h] | Swelling Index | Solubility [%] | ||||
| Bovine | Porcine | Chicken | Bovine | Porcine | Chicken | |
| 0.17 | 14.3 ± 0.4 | 18.5 ± 0.2 | 13.0 ± 0.3 | 44.8 ± 0.3 a,c,e | 34.6 ± 0.4 a,c,e | 35.7 ± 0.5 a,c,e |
| 0.50 | 15.7 ± 0.2 | 0.0 ± 0.0 | 13.5 ± 0.1 | 54.8 ± 0.2 a,c,e | 100.0 ± 0.0 a,c,e | 47.5 ± 0.4 a,c,e |
| 1.00 | 0.0 ± 0.0 | – | 0.0 ± 0.0 | 100.0 ± 0.0 a,c,e | – | 100.0 ± 0.0 a,c,e |
| Crossed-linked gelatin fibers | ||||||
| 0.17 | 5.8 ± 0.3 | 4.3 ± 0.1 | 4.2 ± 0.3 | 13.4 ± 0.4 b,d,f | 15.0 ± 0.3 b,d,f | 28.3 ± 0.5 b,d,f |
| 0.50 | 7.8 ± 0.1 | 8.1 ± 0.2 | 4.7 ± 0.2 | 12.4 ± 0.2 b,d,f | 18.2 ± 0.7 b,d,f | 12.8 ± 0.7 b,d,f |
| 1.00 | 6.3 ± 0.4 | 9.2 ± 0.4 | 5.8 ± 0.1 | 14.9 ± 0.4 b,d,f | 11.2 ± 0.6 b,d,f | 8.1 ± 0.4 b,d,f |
| 3 | 7.1 ± 0.1 | 6.2 ± 0.2 | 5.0 ± 0.3 | 32.8 ± 0.3 b,d,f | 18.5 ± 0.5 b,d,f | 23.3 ± 0.2 b,d,f |
| 6 | 6.2 ± 0.3 | 6.3 ± 0.2 | 5.2 ± 0.2 | 24.5 ± 0.8 b,d,f | 21.5 ± 0.3 b,d,f | 22.4 ± 0.5 b,d,f |
| 12 | 6.9 ± 0.2 | 9.5 ± 0.4 | 6.0 ± 0.2 | 18.0 ± 0.4 b,d,f | 11.9 ± 0.2 b,d,f | 27.9 ± 0.6 b,d,f |
| 24 | 7.7 ± 0.2 | 12.4 ± 0.3 | 6.4 ± 0.4 | 21.4 ± 0.3 b,d,f | 17.8 ± 0.5 b,d,f | 21.2 ± 0.5 b,d,f |
| 48 | 10.0 ± 0.6 | 11.7 ± 0.1 | 5.0 ± 0.3 | 23.9 ± 0.7 b,d,f | 27.7 ± 0.4 b,d,f | 21.3 ± 0.4 b,d,f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinek, J.; Mokrejš, P.; Pavlačková, J.; Hřibová, M.; Pokorný, P.; Janáčová, D.; Gál, R. Characterization of Fibers Prepared by Centrifugal Spinning from Biotechnologically Derived Chicken Gelatin. Foods 2024, 13, 2630. https://doi.org/10.3390/foods13162630
Martinek J, Mokrejš P, Pavlačková J, Hřibová M, Pokorný P, Janáčová D, Gál R. Characterization of Fibers Prepared by Centrifugal Spinning from Biotechnologically Derived Chicken Gelatin. Foods. 2024; 13(16):2630. https://doi.org/10.3390/foods13162630
Chicago/Turabian StyleMartinek, Jakub, Pavel Mokrejš, Jana Pavlačková, Martina Hřibová, Pavel Pokorný, Dagmar Janáčová, and Robert Gál. 2024. "Characterization of Fibers Prepared by Centrifugal Spinning from Biotechnologically Derived Chicken Gelatin" Foods 13, no. 16: 2630. https://doi.org/10.3390/foods13162630






