Mild Approach for the Formulation of Chestnut Flour-Enriched Snacks: Influence of Processing Parameters on the Preservation of Bioactive Compounds of Raw Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients, Chemicals, and Reagents
2.2. Thermogravimetric Analysis (TGA) of Flours
2.3. Preparation of Expanded Snacks-Experimental Design
2.4. Characterization of Expanded Snacks
2.4.1. Snack Density and Expansion Degree
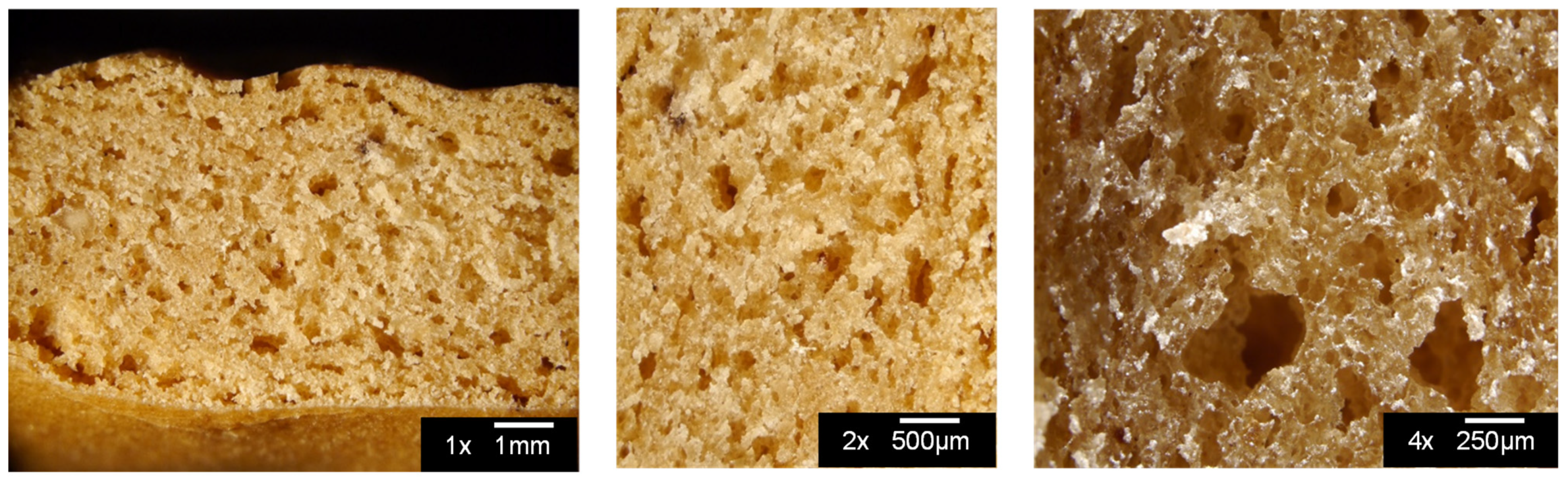
2.4.2. Microstructure
2.4.3. Extraction of Phenolic Compounds
2.4.4. Determination of Total Phenol Content (TPC)
2.4.5. Determination of ABTS Radical Scavenging Capacity
2.4.6. Volatile Molecules by Gas Chromatography/Mass Spectrometry
2.4.7. Storage Studies
Moisture Content
Texture Measurement
2.5. Statistical Analysis
3. Results and Discussion
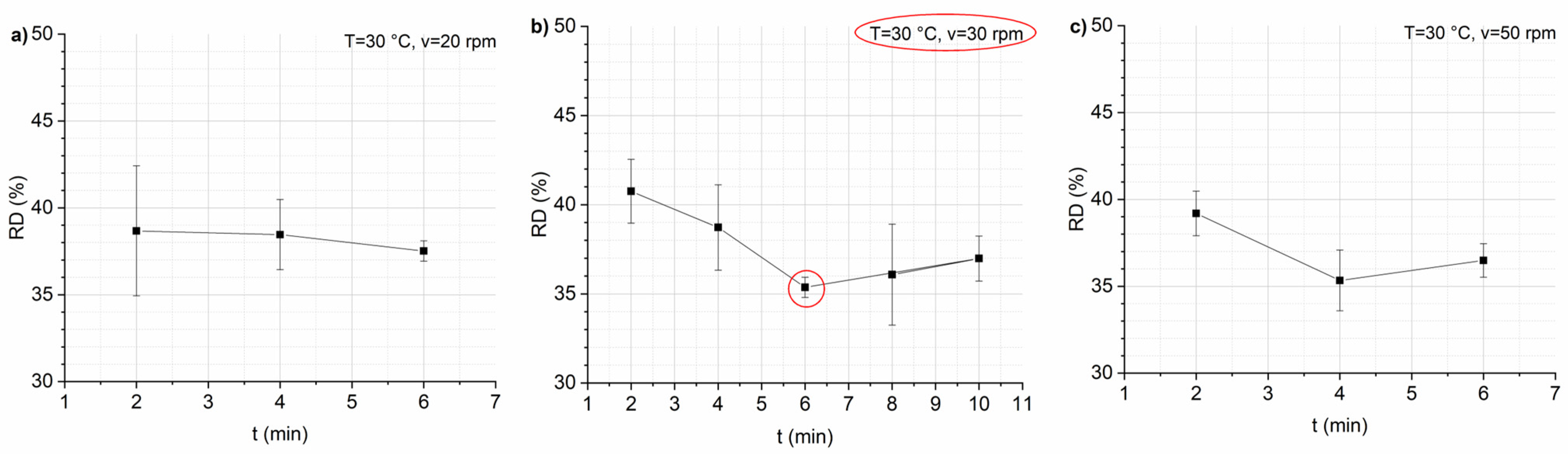
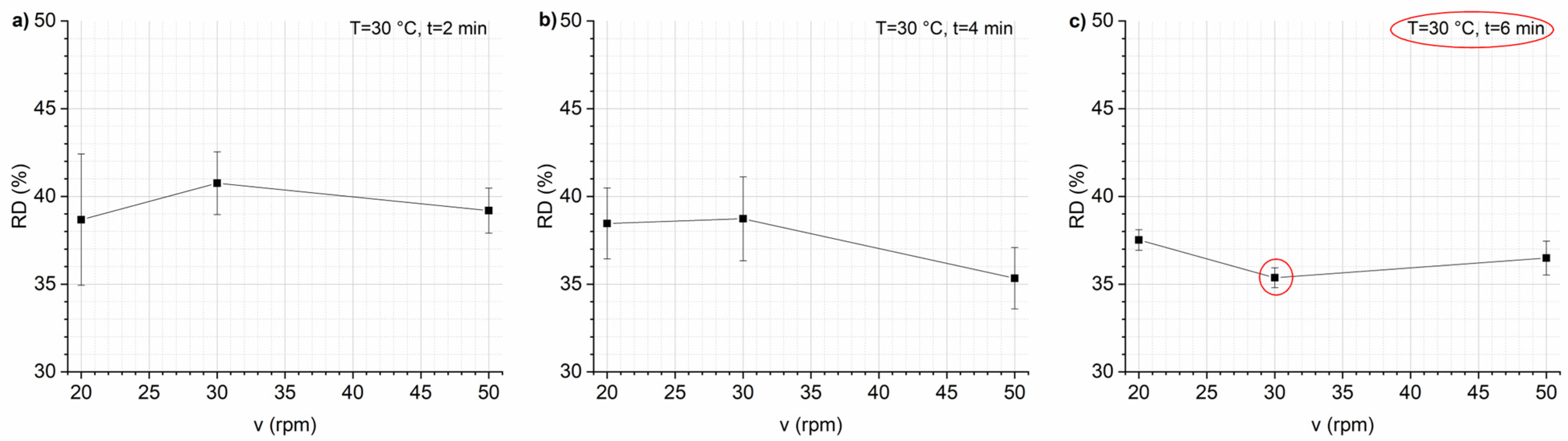
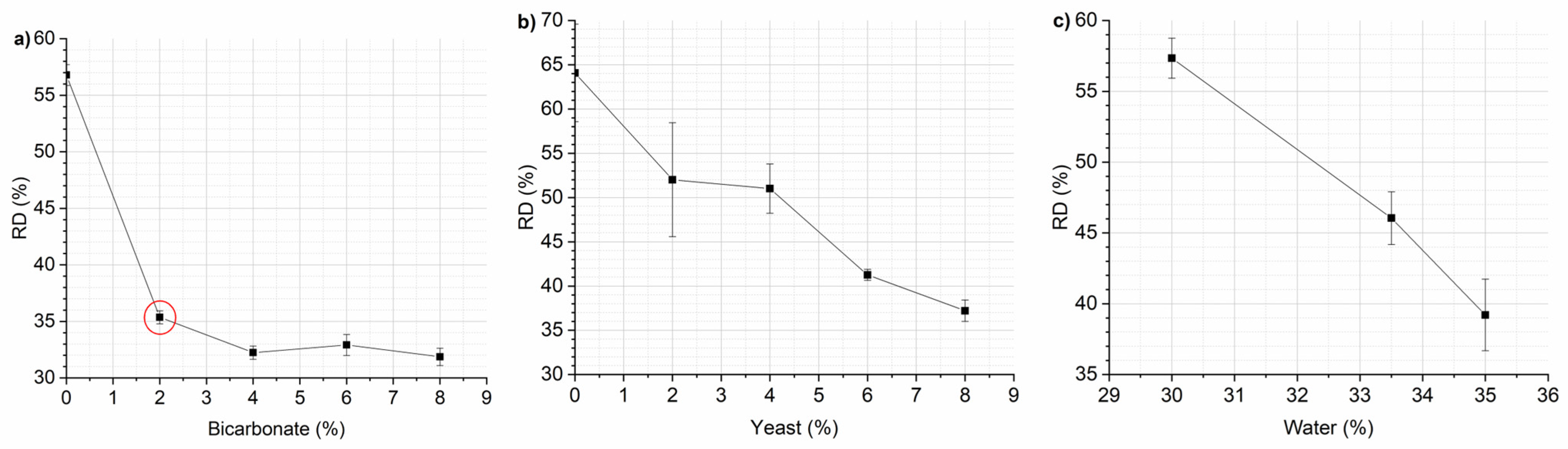
3.1. Optimization of Mixing Parameters and Recipe
3.2. Processing Influence on TPC and Antioxidant Activity
3.3. Volatile Compounds Determination
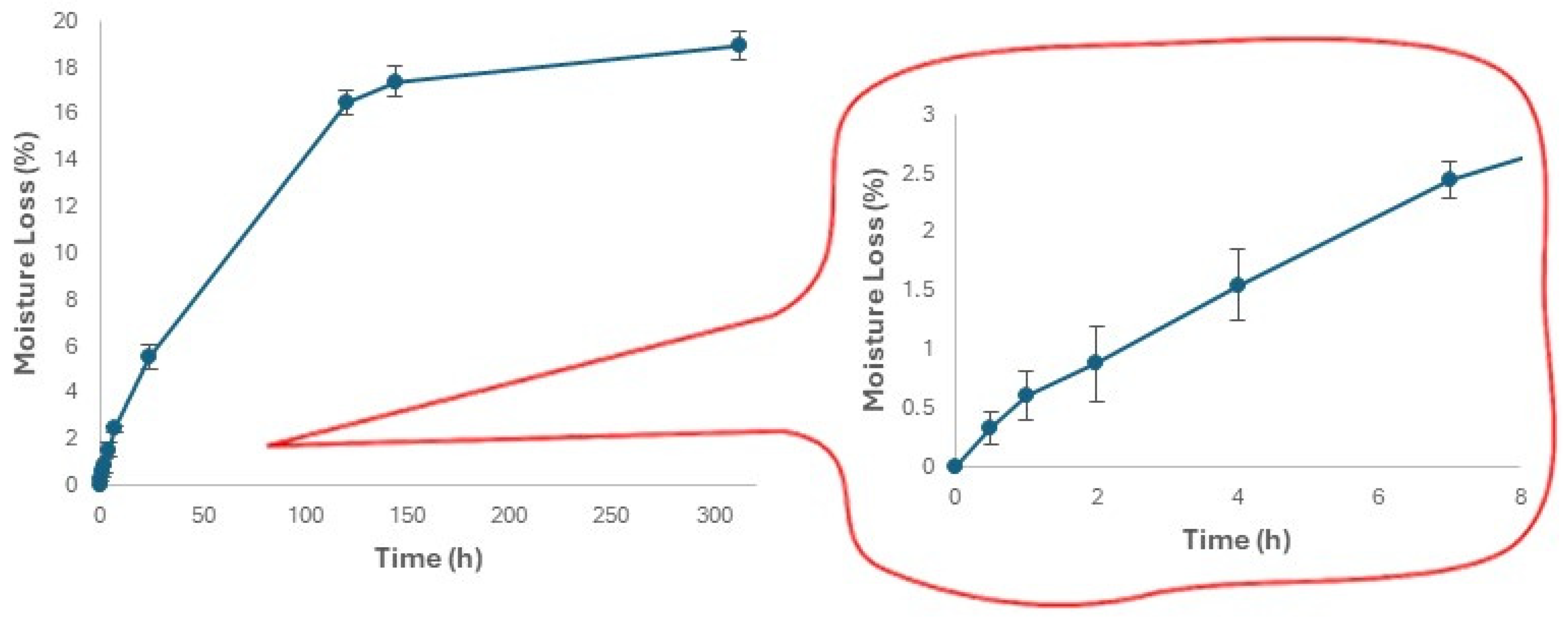
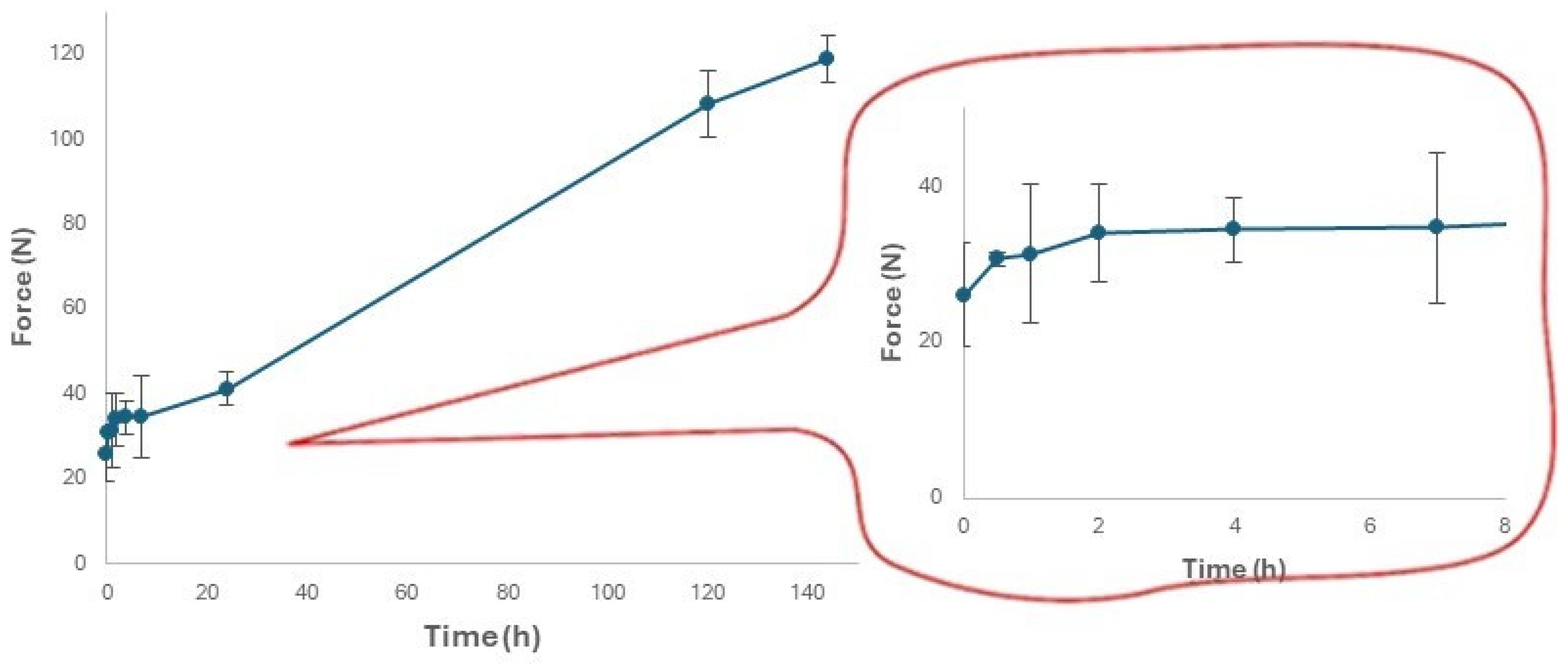
3.4. Changes in the Moisture Content and Hardness of Expanded Snacks during Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ainsworth, P.; Plunkett, A. Reducing salt in snack products. In Reducing Salt in Foods: Practical Strategies; Kilcast, D., Angus, F., Eds.; Woodhead Publishing: New York, NY, USA, 2007; pp. 296–315. [Google Scholar]
- Riaz, M.N. Snack Foods: Processing. In Encyclopedia of Food Grains, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 414–422. [Google Scholar]
- Petrontino, A.; Frem, M.; Fucilli, V.; Labbate, A.; Tria, E.; Bozzo, F. Ready-to-Eat Innovative Legumes Snack: The Influence of Nutritional Ingredients and Labelling Claims in Italian Consumers’ Choice and Willingness-to-Pay. Nutrients 2023, 15, 1799. [Google Scholar] [CrossRef]
- Carcea, M.; Narducci, V.; Turfani, V.; Giannini, V. Polyphenols in Raw and Cooked Cereals/Pseudocereals/Legume Pasta and Couscous. Foods 2017, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Grains and grain fractions. In Snack Foods: Processing and Technology; Bhattacharya, S., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 17–74. [Google Scholar]
- Morales, P.; Berrios, J.D.J.; Varela, A.; Burbano, C.; Cuadrado, C.; Muzquiz, M.; Pedrosa, M.M. Novel fiber-rich lentil flours as snack-type functional foods: An extrusion cooking effect on bioactive compounds. Food Funct. 2015, 6, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Baik, B.-K. Antioxidant activity and phenolic content of lentils (Lens culinaris), chickpeas (Cicer arietinum L.), peas (Pisum sativum L.) and soybeans (Glycine max), and their quantitative changes during processing. Int. J. Food Sci. Technol. 2008, 43, 1971–1978. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef] [PubMed]
- Mitrović, J.; Nikolić, N.; Karabegović, I.; Lazić, M.; Nikolić, L.; Savić, S.; Pešić, M.; Šimurina, O.; Stojanović-Krasić, M. The effect of thermal processing on the content and antioxidant capacity of free and bound phenolics of cookies enriched by nettle (Urtica dioica L.) seed flour and extract. Food Sci. Technol. 2021, 42, e62420. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Ruiz-Armenta, X.A.; Zazueta-Morales, J.d.J.; Aguilar-Palazuelos, E.; Delgado-Nieblas, C.I.; López-Diaz, A.; Camacho-Hernández, I.L.; Gutiérrez-Dorado, R.; Martínez-Bustos, F. Effect of extrusion on the carotenoid content, physical and sensory properties of snacks added with bagasse of naranjita fruit: Optimization process. CYTA J. Food 2018, 16, 172–180. [Google Scholar] [CrossRef]
- Moraru, C.I.; Kokini, J. Nucleation and expansion during extrusion and microwave heating of cereal foods. Compr. Rev. Food Sci. Food Saf. 2003, 2, 147–165. [Google Scholar] [CrossRef]
- Tovar-Jiménez, X.; Caro-Corrales, J.; Gómez-Aldapa, C.A.; Zazueta-Morales, J.; Limón-Valenzuela, V.; Castro-Rosas, J.; Hernández-Ávila, J.; Aguilar-Palazuelos, E. Third generation snacks manufactured from orange by-products: Physicochemical and nutritional characterization. J. Food Sci. Technol. 2015, 52, 6607–6614. [Google Scholar] [CrossRef]
- Pathak, N.; Kochhar, A. Extrusion technology: Solution to Develop Quality Snacks for Malnourished Generation. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 1293–1307. [Google Scholar] [CrossRef]
- Abilmazhinov, Y.; Bekeshova, G.; Nesterenko, A.; Dibrova, Z.; Ermolaev, V.; Ponomarev, E.; Vlasova, V. A review on the improvement of extruded food processing equipment: Extrusion cooking in food processing. Food Sci. Technol. 2023, 43, e80621. [Google Scholar] [CrossRef]
- Altan, A.; McCarthy, K.L.; Maskan, M. Effect of extrusion process on antioxidant activity, total phenolics and β-glucan content of extrudates developed from barley-fruit and vegetable by-products. Int. J. Food Sci. Technol. 2009, 44, 1263–1271. [Google Scholar] [CrossRef]
- Delgado-Licon, E.; Ayala, A.L.M.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Atienzo-Lazos, M.; Drzewiecki, J.; Martínez-Sánchez, C.E.; Gorinstein, S. Influence of extrusion on the bioactive compounds and the antioxidant capacity of the bean/corn mixtures. Int. J. Food Sci. Nutr. 2009, 60, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Blandino, M.; Bresciani, A.; Locatelli, M.; Loscalzo, M.; Travaglia, F.; Vanara, F.; Marti, A. Pulse type and extrusion conditions affect phenolic profile and physical properties of extruded products. Food Chem. 2023, 403, 134369. [Google Scholar] [CrossRef]
- Angelino, D.; Cossu, M.; Marti, A.; Zanoletti, M.; Chiavaroli, L.; Brighenti, F.; Del Rio, D.; Martini, D. Bioaccessibility and Bioavailability of Phenolic Compounds in Bread: A Review. Food Funct. J. 2017, 8, 2368–2393. [Google Scholar] [CrossRef]
- Pasqualone, A.; Costantini, M.; Coldea, T.E.; Summo, C. Use of Legumes in Extrusion Cooking: A Review. Foods 2020, 9, 958. [Google Scholar] [CrossRef]
- Martin, A.; Schmidt, V.; Osen, R.; Bez, J.; Ortner, E.; Mittermaier, S. Texture, sensory properties and functionality of extruded snacks from pulses and pseudocereal proteins. J. Sci. Food Agric. 2020, 102, 5011–5021. [Google Scholar] [CrossRef]
- Paciulli, M.; Rinaldi, M.; Cavazza, A.; Ganino, T.; Rodolfi, M.; Chiancone, B.; Chiavaro, E. Effect of chestnut flour supplementation on physico-chemical properties and oxidative stability of gluten-free biscuits during storage. LWT Food Sci. Technol. 2018, 98, 451–457. [Google Scholar] [CrossRef]
- Rachwa-Rosiak, D.; Nebesny, E.; Budryn, G. Chickpeas-composition, nutritional value, health benefits, application to bread and snacks: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1137–1145. [Google Scholar] [CrossRef]
- Herrera, A.C.; Gonzalez de Mejia, E. Feasibility of commercial breadmaking using chickpea as an ingredient: Functional properties and potential health benefits. J. Food Sci. 2021, 86, 2208–2224. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Cirlini, M.; Morini, E.; Rinaldi, M.; Ganino, T.; Chiavaro, E. Effect of chestnut flour supplementation on physico-chemical properties and volatiles in bread making. LWT-Food Sci. Technol. 2013, 53, 233–239. [Google Scholar] [CrossRef]
- Torra, M.; Belorio, M.; Ayuso, M.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L.; Gómez, M. Chickpea and Chestnut Flours as Non-Gluten Alternatives in Cookies. Foods 2021, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Frakolaki, G.; Giannou, V.; Topakas, E.; Tzia, C. Chemical characterization and breadmaking potential of spelt versus wheat flour. J. Cereal Sci. 2018, 79, 50–56. [Google Scholar] [CrossRef]
- Martín-Diana, A.B.; García-Casas, M.J.; Martínez-Villaluenga, C.; Frías, J.; Peñas, E.; Rico, D. Wheat and Oat Brans as Sources of Polyphenol Compounds for Development of Antioxidant Nutraceutical Ingredients. Foods 2021, 10, 115. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin-Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Crescente, G.; Cascone, G.; Petruzziello, A.; Bouymajane, A.; Volpe, M.G.; Russo, G.L.; Moccia, S. A Comparative Study between Microwave Hydrodiffusion and Gravity (MHG) and Ultrasound-Assisted Extraction (UAE): Chemical and Biological Characterization of Polyphenol-Enriched Extracts from Aglianico Grape Pomace. Foods 2023, 12, 2678. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Di Renzo, T.; Boscaino, F.; Nazzaro, F.; Fratianni, F.; Aponte, M. Lactic Acid Bacteria Biota and Aroma Profile of Italian Traditional Sourdoughs from the Irpinian Area in Italy. Front. Microbiol. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Aksenova, O.; Kulikova, M. Development of an extruded potato snack using recycled materials. KnE Life Sci. 2020, 59, 359–370. [Google Scholar] [CrossRef]
- Gómez, A.; Ferrero, C.; Calvelo, A.; Añón, M.; Puppo, M. Effect of Mixing Time on Structural and Rheological Properties of Wheat Flour Dough for Breadmaking. Int. J. Food Prop. 2011, 14, 583–598. [Google Scholar] [CrossRef]
- Launay, B.; Lisch, J.M. Twin-screw extrusion cooking of starches: Flow behaviour of starch pastes, expansion and mechanical properties of extrudates. J. Food Eng. 1983, 2, 259–280. [Google Scholar] [CrossRef]
- Pansawat, N.; Jangchud, K.; Jangchud, A.; Wuttijumnong, P.; Saalia, F.K.; Eitenmiller, R.R.; Phillips, R.D. Effects of extrusion conditions on secondary extrusion variables and physical properties of fish, rice-based snacks. LWT Food Sci. Technol. 2008, 41, 632–641. [Google Scholar] [CrossRef]
- Suksomboon, A.; Limroongreungrat, K.; Sangnark, A.; Thititumjariya, K.; Noomhorm, A. Effect of extrusion conditions on the physicochemical properties of a snack made from purple rice (Hom Nil) and soybean flour blend. Int. J. Food Sci. Technol. 2011, 46, 201–208. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, N.; Kaur, A.; Singh, B. Effect of extrusion on physicochemical properties, digestibility, and phenolic profiles of grit fractions obtained from dry milling of normal and waxy corn. J. Food Sci. 2017, 82, 1101–1109. [Google Scholar] [CrossRef]
- Charunuch, C.; Limsangouan, N.; Prasert, W.; Wongkrajang, K. Optimization of extrusion conditions for ready-to-eat breakfast cereal enhanced with defatted rice bran. Int. Food Res. J. 2014, 21, 713–722. [Google Scholar]
- Kaur, A.; Kaur, S.; Singh, M.; Singh, N.; Shevkani, K.; Singh, B. Effect of banana flour, screw speed and temperature on extrusion behaviour of corn extrudates. J. Food Sci. Technol. 2015, 52, 276–4285. [Google Scholar] [CrossRef]
- Alam, M.S.; Pathania, S.; Sharma, A. Optimization of the extrusion process for development of high fibre soybean-rice ready-to-eat snacks using carrot pomace and cauliflower trimmings. LWT Food Sci. Technol. 2016, 74, 135–144. [Google Scholar] [CrossRef]
- Altan, A.; Maskan, M. Development of Extruded Food by Utilizing Food Industry By-Products. In Advances in Food Extrusion Technology; Maskan, M., Altan, A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 121–159. [Google Scholar]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds Biological Activity; InTech: Rijeka, Croatia, 2017; pp. 1–24. [Google Scholar]
- Abdel-Aal, E.-S.M.; Rabalski, I. Effect of baking on free and bound phenolic acids in wholegrain bakery products. J. Cereal Sci. 2013, 57, 312–318. [Google Scholar] [CrossRef]
- Yu, L.; Beta, T. Identification and Antioxidant Properties of Phenolic Compounds during Production of Bread from Purple Wheat Grains. Molecules 2015, 20, 15525–15549. [Google Scholar] [CrossRef]
- Konopka, I.; Tańska, M.; Faron, A.; Czaplicki, S. Release of free ferulic acid and changes in antioxidant properties during the wheat and rye bread making process. Food Sci. Biotechnol. 2014, 23, 831–840. [Google Scholar] [CrossRef]
- Gélinas, P.; McKinnon, C.M. Effect of wheat variety, farming site, and bread-baking on total phenolics. Int. J. Food Sci. Technol. 2006, 41, 329–332. [Google Scholar] [CrossRef]
- Leenhardt, F.; Lyan, B.; Rock, E.; Boussard, A.; Potus, J.; Chanliaud, E.; Remesy, C. Wheat Lipoxygenase Activity Induces Greater Loss of Carotenoids than Vitamin E during Breadmaking. J. Agric. Food Chem. 2006, 54, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Holtekjølen, A.K.; Bævre, A.B.; Rødbotten, M.; Berg, H.; Knutsen, S.H. Antioxidant properties and sensory profiles of breads containing barley flour. Food Chem. 2008, 110, 414–421. [Google Scholar] [CrossRef]
- Ross, C.F.; Hoye, C.; Fernandez-Plotka, V.C. Influence of Heating on the Polyphenolic Content and Antioxidant Activity of Grape Seed Flour. J. Food Sci. 2011, 76, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Alfeo, V.; Bravi, E.; Ceccaroni, D.; Perretti, G.; Marconi, O.; Sileoni, V. Effect of Baking Time and Temperature on Nutrients and Phenolic Compounds Content of Fresh Sprouts Breadlike Product. Foods 2020, 9, 1447. [Google Scholar] [CrossRef] [PubMed]
- Barbarisi, C.; De Vito, V.; Pellicano, M.P.; Boscaino, F.; Balsamo, S.; Laurino, C.; Sorrentino, G.; Volpe, M.G. Bread chemical and nutritional characteristics as influenced by food grade sea water. Int. J. Food Prop. 2019, 22, 280–289. [Google Scholar] [CrossRef]
- Yadav, U.; Singh, R.R.B.; Arora, S. Evaluation of quality changes in nutritionally enriched extruded snacks during storage. J. Food Sci. Technol. 2018, 55, 3939–3948. [Google Scholar] [CrossRef]
- Yu, X.; Martin, S.E.; Schmidt, S.J. Exploring the problem of mold growth and the efficacy of various mold inhibitor methods during moisture sorption isotherm measurements. J. Food Sci. 2008, 73, 69–81. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Moisture sorption isotherms and evaluation of quality changes in extruded snacks during storage. LWT 2016, 74, 448–455. [Google Scholar] [CrossRef]
- Reddy, M.K.; Kuna, A.; Devi, N.L.; Krishnaiah, N.; Kaur, C.; Nagamalleswari, Y. Development of extruded Ready-To-Eat (RTE) snacks using corn, black gram, roots, and tuber flour blends. J. Food Sci. Technol. 2014, 51, 1929–1937. [Google Scholar] [CrossRef]
- Alam, S.; Pathania, S.; Kumar, S.; Sharma, A. Studies on Storage Stability of Carrot Pomace-Chickpea Incorporated Rice Based Snacks. Agric. Res. J. 2015, 52, 73–79. [Google Scholar] [CrossRef]
- Dar, B.N.; Sharma, S.; Nayik, G.A. Effect of storage period on physiochemical, total phenolic content and antioxidant properties of bran enriched snacks. J. Food Meas. Charact. 2016, 10, 755–761. [Google Scholar] [CrossRef]
- Nazir, F.; Salim, R.; Naik, H.R.; Hussain, S.Z. Studies on storage stability of apricot and date incorporated rice based extruded snack. Int. J. Chem. Stud. 2017, 5, 2016–2018. [Google Scholar]
- Ding, Q.B.; Ainsworth, P.; Plunkett, A.; Tucker, G.; Marson, H. The effect of extrusion conditions on the functional and physical properties of wheat-based expanded snacks. J. Food Eng. 2006, 73, 142–148. [Google Scholar] [CrossRef]
- Li, H.Y.; Gilbert, R.G. Starch molecular structure: The basis for an improved understanding of cooked rice texture. Carbohydr. Polym. 2018, 195, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Federici, E.; Gentilucci, V.; Bernini, V.; Vittadini, E.; Pellegrini, N. Ready to eat shelf-stable brown rice in pouches: Effect of moisture content on product’s quality and stability. Eur. Food Res. Technol. 2021, 247, 2677–2685. [Google Scholar] [CrossRef] [PubMed]

| Test Number | T (°C) | v (rpm) | t (min) |
|---|---|---|---|
| 1 | 30 | 20 | 6 |
| 2 | 30 | 20 | 4 |
| 3 | 30 | 20 | 2 |
| 4 | 30 | 30 | 6 |
| 5 | 30 | 30 | 4 |
| 6 | 30 | 30 | 2 |
| 7 | 30 | 50 | 6 |
| 8 | 30 | 50 | 4 |
| 9 | 30 | 50 | 2 |
| 10 | 50 | 20 | 6 |
| 11 | 50 | 20 | 4 |
| 12 | 50 | 20 | 2 |
| 13 | 50 | 30 | 6 |
| 14 | 50 | 30 | 4 |
| 15 | 50 | 30 | 2 |
| 16 | 50 | 50 | 6 |
| 17 | 50 | 50 | 4 |
| 18 | 50 | 50 | 2 |
| 19 | 30 | 30 | 8 |
| 20 | 30 | 30 | 10 |
| Dough | Snack | |
|---|---|---|
| Aldehydes | ||
| 2-methylbutanal | 8.2 ± 0.4 | nd |
| 3-methylbutanal | 19.9 ± 1.8 | nd |
| Hexanal | 1543.7 ± 124.1 | 1607.2 ± 18.6 |
| Nonanal | 3.0 ± 0.2 | nd |
| 2-octenal | 6.2 ± 0.1 | nd |
| 2-Nonenal, (Z)- | 4.0 ± 0.4 | nd |
| Benzaldehyde | 10.1 ± 0.6 | 1.2 ± 0.0 |
| Octanal | 4.9 ± 0.5 | nd |
| Total | 1600.0 ± 126.6 | 1608.4 ± 18.6 |
| Alcohols | ||
| Ethyl alcohol | 1560.7 ± 81.9 | 1258.7 ± 11.4 |
| 1-Penten-3-ol | 217.8 ± 0.1 | nd |
| isoamyl alcohol | 83.9 ± 4.4 | nd |
| 1-Pentanol | 188.8 ± 9.0 | nd |
| 2-Penten-1-ol, (Z) | 139.4 ± 1.3 | 6.5 ± 0.4 |
| 1-Hexanol | 498.9 ± 2.9 | 27.9 ± 0.3 |
| 3-Hexen-1-ol, (z) | 3.5 ± 0.1 | nd |
| 1-octen-3-ol | 31.2 ± 0.8 | 1.8 ± 0.1 |
| Benzyl Alcohol | 4.4 ± 0.1 | 2.6 ± 0.2 |
| Phenylethyl Alcohol | 7.4 ± 0.4 | 3.6 ± 0.3 |
| Total | 2736.1 ± 63.8 | 1301.0 ± 11.6 |
| Ketones | ||
| 2-Propanone | 249.0 ± 19.3 | 287.1 ± 17.0 |
| 2,3-pentanedione | 9.0 ± 0.3 | nd |
| 2-heptanone | nd | 22.6 ± 1.4 |
| 3-hydroxy-2-butanone | 21.7 ± 1.6 | 32.8 ± 1.6 |
| 6-methyl-5-hepten-2-one | 6.5 ± 0.6 | nd |
| Total | 286.2 ± 18.1 | 342.6 ± 20.1 |
| Esters | ||
| Ethyl Acetate | 96.7 ± 9.9 | 758.5 ± 27.0 |
| Isoamyl acetate | nd | 20.3 ± 0.5 |
| Total | 96.7 ± 9.9 | 778.8 ± 26.5 |
| Acids | ||
| Acetic acid | 12.4 ± 0.7 | 4.5 ± 0.4 |
| Hexanoic acid | nd | 3.7 ± 0.2 |
| Heptanoic acid | nd | 6.4 ± 0.4 |
| Nonanoic acid | nd | 8.5 ± 0.7 |
| Total | 12.4 ± 0.7 | 23.2 ± 0.9 |
| Terpenoids | ||
| Limonene | 243.9 ± 18.4 | 274.8 ± 6.4 |
| Eucalyptol | 23.9 ± 0.5 | nd |
| g-terpinene | 14.6 ± 0.7 | 18.5 ± 2.1 |
| o-cymene | 5.6 ± 0.1 | 7.8 ± 0.6 |
| Camphor | 2.3 ± 0.2 | nd |
| Linalool | 15.3 ± 0.3 | 5.5 ± 0.3 |
| 4-terpineol | 1.2 ± 0.1 | nd |
| verbenone | 1.7 ± 0.1 | nd |
| Total | 308.4 ± 20.1 | 306.5 ± 4.0 |
| Pyrazines | ||
| 2,5-dimethyl-pyrazine | nd | 42.1 ± 2.8 |
| 2,6-dimethyl-pyrazine | nd | 16.8 ± 1.1 |
| 2,3-dimethyl-pyrazine | nd | 1.6 ± 0.1 |
| 2-ethyl-5-methyl-pyrazine | nd | 9.3 ± 0.9 |
| Total | nd | 69.8 ± 5.0 |
| Furans | ||
| 2-pentyl-furan | 19.2 ± 1.5 | 38.9 ± 3.3 |
| 2-Furanmethanol | 1.9 ± 0.1 | 3.0 ± 0.0 |
| Total | 21.2 ± 1.6 | 41.9 ± 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascone, G.; Oliviero, M.; Sorrentino, L.; Crescente, G.; Boscaino, F.; Sorrentino, A.; Volpe, M.G.; Moccia, S. Mild Approach for the Formulation of Chestnut Flour-Enriched Snacks: Influence of Processing Parameters on the Preservation of Bioactive Compounds of Raw Materials. Foods 2024, 13, 2651. https://doi.org/10.3390/foods13172651
Cascone G, Oliviero M, Sorrentino L, Crescente G, Boscaino F, Sorrentino A, Volpe MG, Moccia S. Mild Approach for the Formulation of Chestnut Flour-Enriched Snacks: Influence of Processing Parameters on the Preservation of Bioactive Compounds of Raw Materials. Foods. 2024; 13(17):2651. https://doi.org/10.3390/foods13172651
Chicago/Turabian StyleCascone, Giovanni, Maria Oliviero, Luigi Sorrentino, Giuseppina Crescente, Floriana Boscaino, Andrea Sorrentino, Maria Grazia Volpe, and Stefania Moccia. 2024. "Mild Approach for the Formulation of Chestnut Flour-Enriched Snacks: Influence of Processing Parameters on the Preservation of Bioactive Compounds of Raw Materials" Foods 13, no. 17: 2651. https://doi.org/10.3390/foods13172651








