Comparison of Volatile Compounds in Jingshan Green Tea Scented with Different Flowers Using GC-IMS and GC-MS Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Collection
2.2. Chemicals and Reagents
2.3. Volatile Compounds Detected via HS–GC–IMS
2.4. Volatile Compounds Detected via SBSE–GC–MS
2.5. Calculation of ROAV
2.6. Statistical Analysis
3. Results and Discussion
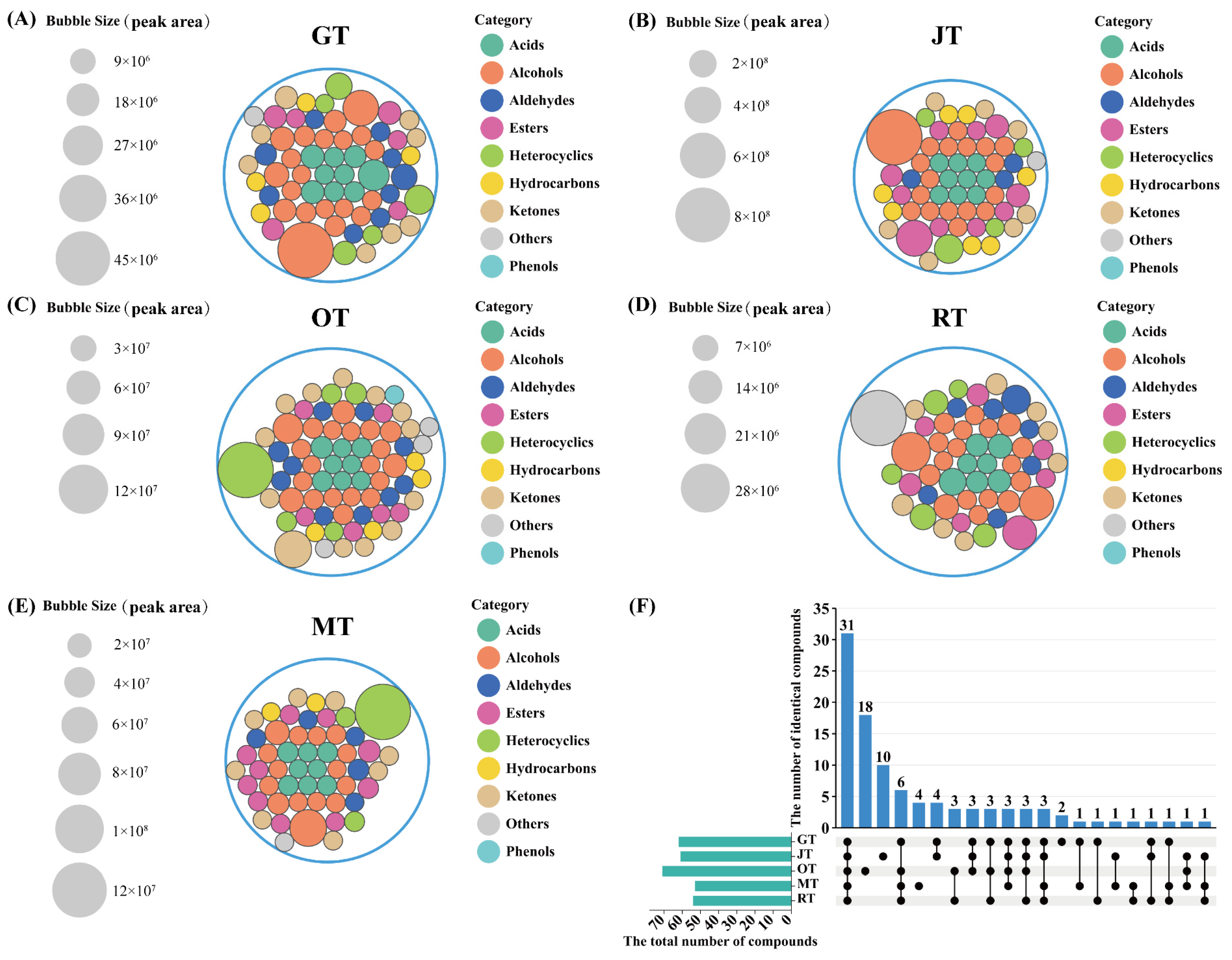
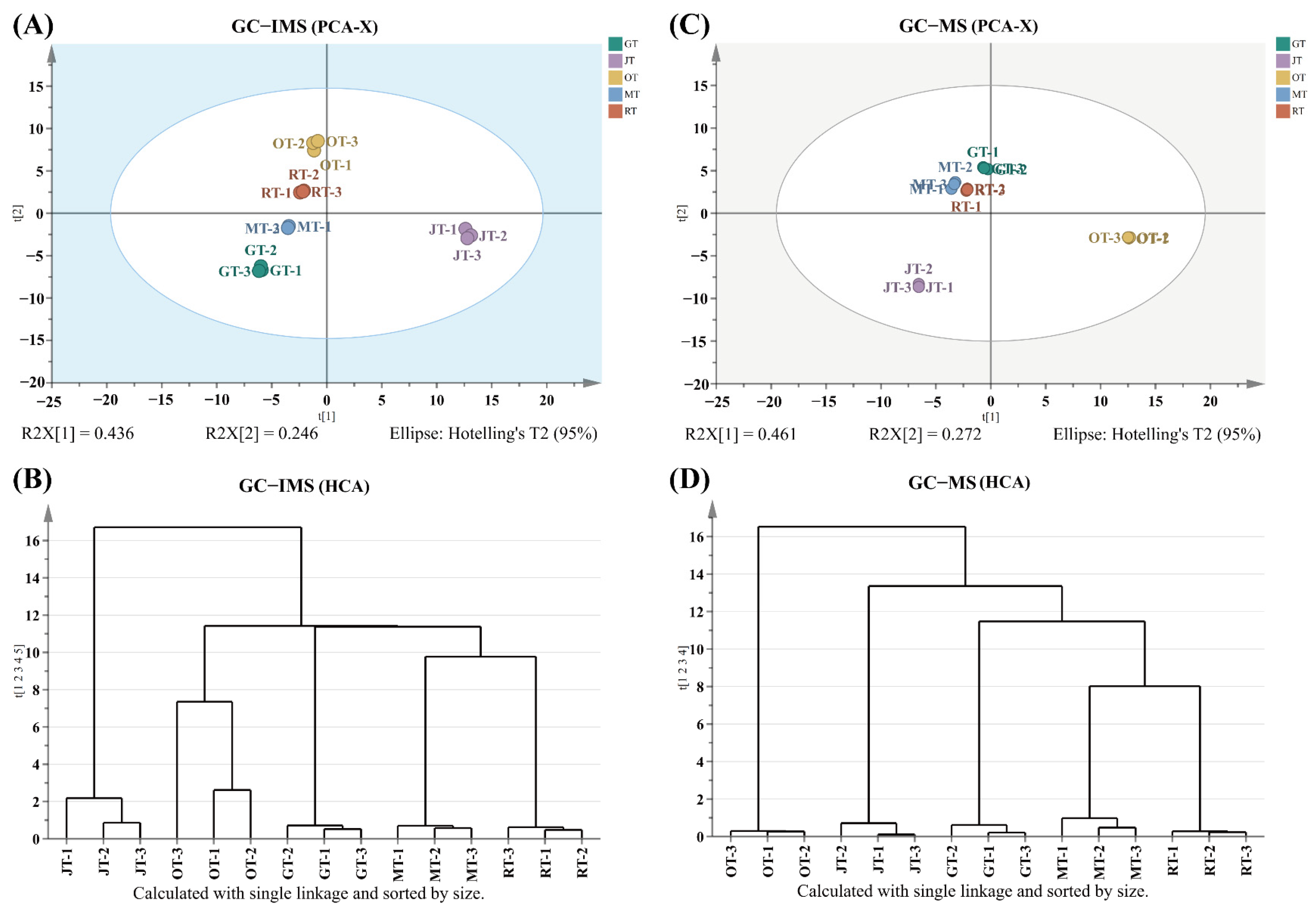
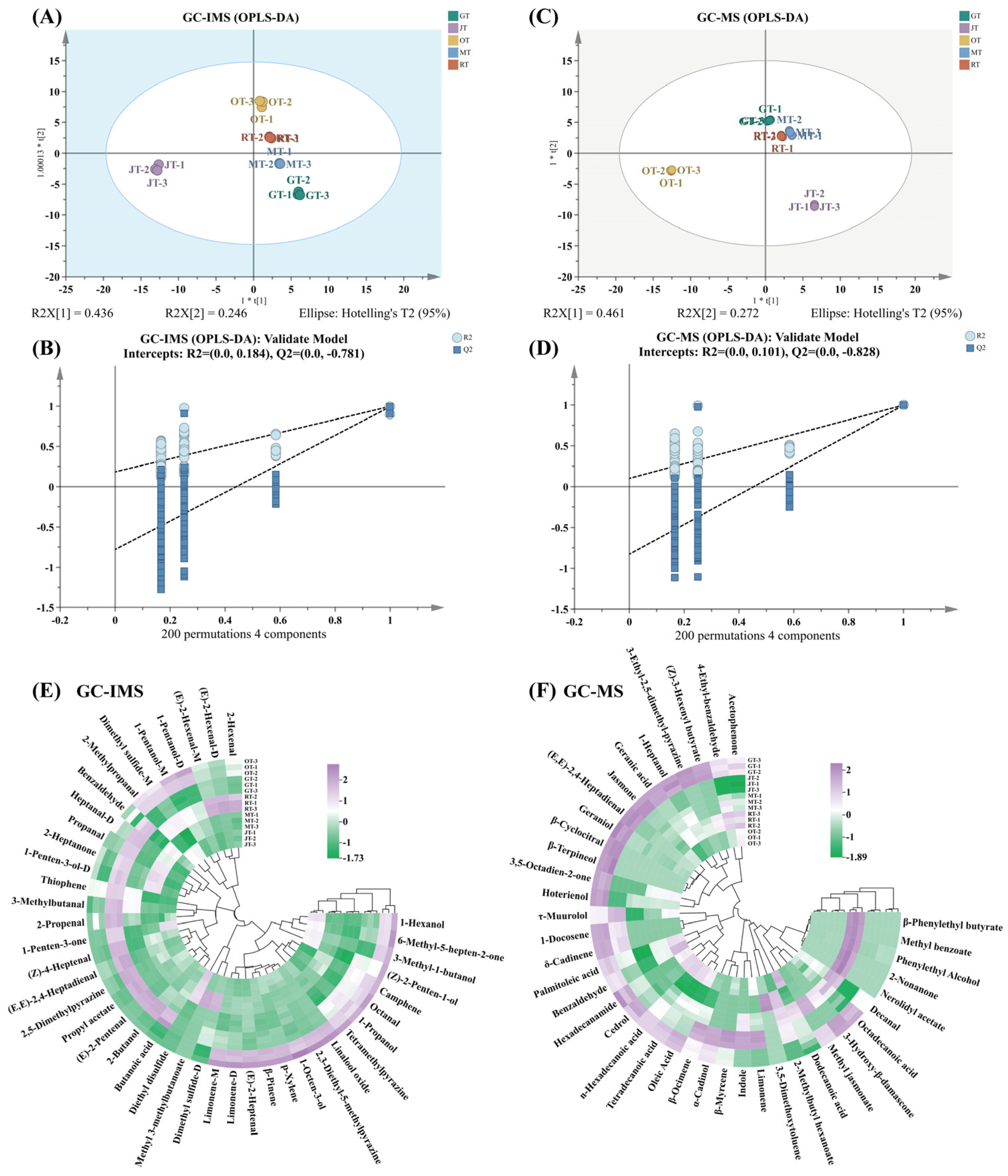
3.1. Profiles of Volatile Compounds of Different Tea Samples
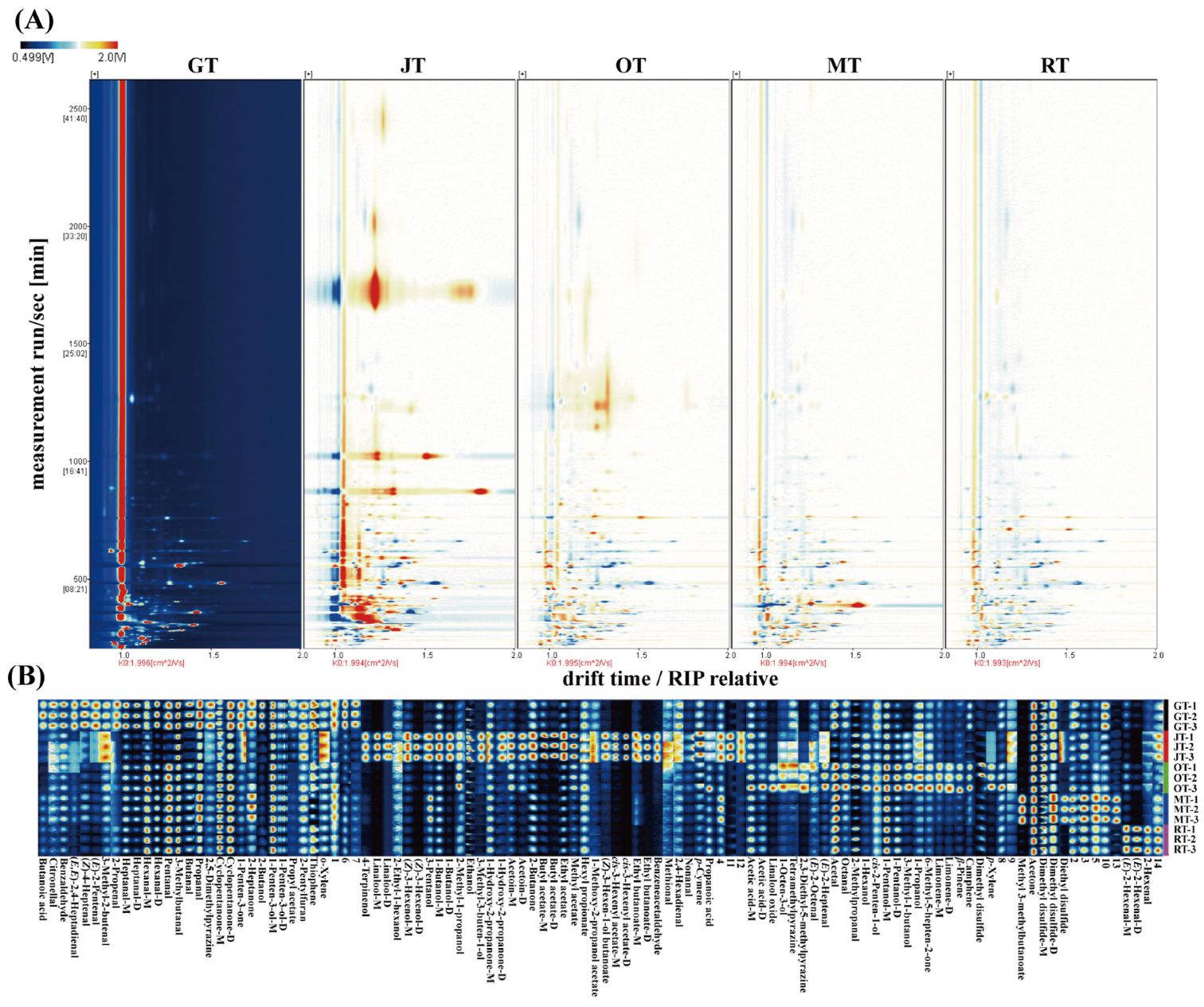
3.1.1. Identification of Volatile Compounds via HS–GC–IMS
3.1.2. Identification of Volatile Compounds via SBSE–GC–MS
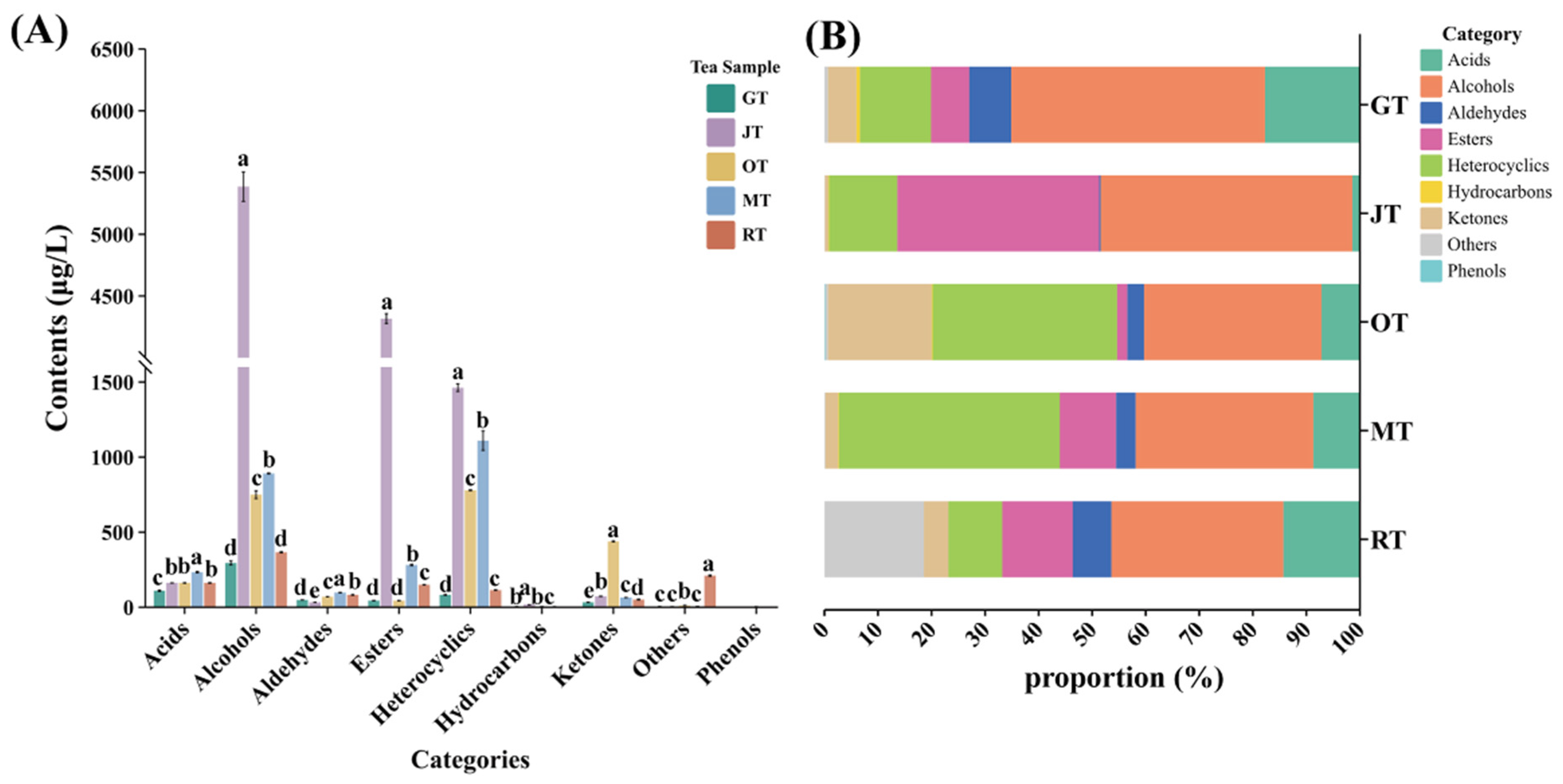
3.2. Comparison of Volatile Compounds among Different Tea Samples
3.3. Screening for Characteristic Volatile Compounds
Key Aroma Compound Analysis Based on ROAV
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, X.Y.; Ho, C.-T.; Schwab, W.; Wan, X.C. Aroma profiles of green tea made with fresh tea leaves plucked in summer. Food Chem. 2021, 363, 130328. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Hao, M.; Zhang, X.C.; He, Y.F.; Chen, X.S.; Taylor, E.W.; Zhang, J.S. Potential of green tea EGCG in neutralizing SARS-CoV-2 Omicron variant with greater tropism toward the upper respiratory tract. Trends Food Sci. Technol. 2022, 132, 40–53. [Google Scholar] [CrossRef]
- Hu, X.L.; He, J.; Qiao, L.; Wang, C.; Wang, Y.; Yu, R.X.; Xu, W.; Wang, F.; Yang, S.H.; Zhang, X.C.; et al. Multifunctional dual network hydrogel loaded with novel tea polyphenol magnesium nanoparticles accelerates wound repair of mrsa infected diabetes. Adv. Funct. Mater. 2024, 34, 2312140. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, N.F.; Yu, T.Z.; Gao, J.; Fan, Y.L.; Wang, W.Y.; Wang, J.H.; Wu, Y.D.; Zhang, J.X.; Ning, J.M. The enhancement of flowery-like aroma in green tea under optimized processing conditions by sensory-directed flavor analysis. Food Chem. X 2024, 22, 101427. [Google Scholar] [CrossRef]
- Wang, Y.G.; Huang, Z.F.; Zhou, T.Y.; Li, C.; Sun, Y.L.; Pang, J. Progress of research on aroma absorption mechanism and aroma fixation pathway of jasmine green tea. J. Sci. Food Agric. 2024, 6, 13656. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, C.Z.; Tian, C.Y.; Xu, K.; Lai, Z.X.; Lin, Y.L.; Guo, Y.Q. Volatilomics Analysis of Jasmine Tea during Multiple Rounds of Scenting Processes. Foods 2023, 12, 812. [Google Scholar] [CrossRef]
- Meng, X.; Wang, F.; Fu, C.H.; Zeng, L.; Chen, Z.H.; Du, Q.Z.; Feng, Z.H.; Yin, J.F.; Xu, Y.Q. Effect of osmanthus hydrolat on the aroma quality and volatile components of osmanthus black tea. Food Chem. X 2024, 23, 101564. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Wang, Q.W.; Xie, J.L.; Deng, Y.L.; Zhu, J.Y.; Xie, Z.W.; Yuan, H.B.; Jiang, Y.W. Uncovering the dynamic alterations of volatile components in sweet and floral aroma black tea during processing. Foods 2024, 13, 728. [Google Scholar] [CrossRef]
- Rong, Y.T.; Xie, J.L.; Yuan, H.B.; Wang, L.L.; Liu, F.Q.; Deng, Y.L.; Jiang, Y.W.; Yang, Y.Q. Characterization of volatile metabolites in Pu-erh teas with different storage years by combining GC–E–Nose, GC–MS, and GC–IMS. Food Chem. X 2023, 18, 100693. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zhang, Y.; Hu, C.B.; Yu, B.; Wan, C.X.; Chen, B.; Lu, L.R.; Yuan, L.R.; Wu, Z.H.; Chen, H.B. The flavor substances changes in Fuliang green tea during storage monitoring by GC–MS and GC–IMS. Food Chem. X 2024, 21, 101047. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Schwab, W.; Ho, C.-T.; Song, C.K.; Wan, X.C. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC–MS and GC–IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Huang, W.J.; Sheng, C.Y.; Wu, Y.D.; Lu, M.X.; Li, T.H.; Zhang, J.X.; Wei, Y.M.; Wang, Y.J.; Ning, J.M. Contribution of tea stems to large-leaf yellow tea aroma. Food Chem. 2024, 460, 140472. [Google Scholar] [CrossRef]
- Yan, H.; Li, W.X.; Zhu, Y.L.; Lin, Z.Y.; Chen, D.; Zhang, Y.; Lv, H.P.; Dai, W.D.; Ni, D.J.; Lin, Z.; et al. Comprehensive comparison of aroma profiles and chiral free and glycosidically bound volatiles in Fujian and Yunnan white teas. Food Chem. 2024, 448, 139067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Zhang, J.X.; Liu, S.T.; Li, T.H.; Wei, Y.M.; Gu, Z.; Su, Z.C.; Ning, J.M.; Wang, Y.J.; Hou, Z.W. Characterization of the key volatile compounds in longjing tea (Camellia sinensis) with different aroma types at different steeping temperatures by GC–MS and GC–IMS. LWT-Food Sci. Technol. 2024, 200, 116183. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, Y.X.; Chen, Y.L.; Xiao, L.K.; Zhang, X.L.; Yang, C.H.W.; Li, Z.J.; Zhu, M.Z.; Liu, Z.H.; Wang, Y.L. Discrimination and characterization of the volatile profiles of five Fu brick teas from different manufacturing regions by using HS–SPME–GC–MS and HS–GC–IMS. Curr. Res. Food Sci. 2022, 5, 1788–1807. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zhang, Y.; Yan, F.; Tang, Y.; Yu, B.; Chen, B.; Lu, L.R.; Yuan, L.R.; Wu, Z.H.; Chen, H.B. Monitoring changes in the volatile compounds of tea made from summer tea leaves by GC–IMS and HS–SPME–GC–MS. Foods 2023, 12, 146. [Google Scholar] [CrossRef]
- Gil, C.S.; Lim, S.T.; Lim, Y.J.; Jung, K.-H.; Na, J.-K.; Eom, S.H. Volatile content variation in the petals of cut roses during vase life. Sci. Hortic. 2020, 261, 108960. [Google Scholar] [CrossRef]
- Scalliet, G.; Piola, F.; Douady, C.J.; Réty, S.; Raymond, O.; Baudino, S.; Bordji, K.; Bendahmane, M.; Dumas, C.; Cock, J.M.; et al. Scent evolution in Chinese roses. Proc. Natl. Acad. Sci. USA 2008, 105, 5927–5932. [Google Scholar] [CrossRef]
- Liu, N.F.; Shen, S.S.; Huang, L.F.; Deng, G.J.; Wei, Y.M.; Ning, J.M.; Wang, Y.J. Revelation of volatile contributions in green teas with different aroma types by GC–MS and GC–IMS. Food Res. Int. 2023, 169, 112845. [Google Scholar] [CrossRef] [PubMed]
- Bera, P.; Kotamreddy, J.N.R.; Samanta, T.; Maiti, S.; Mitra, A. Inter-specific variation in headspace scent volatiles composition of four commercially cultivated jasmine flowers. Nat. Prod. Res. 2015, 29, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- An, H.M.; Ou, X.C.; Zhang, Y.B.; Li, S.; Xiong, Y.F.; Li, Q.; Huang, J.N.; Liu, Z.H. Study on the key volatile compounds and aroma quality of jasmine tea with different scenting technology. Food Chem. 2022, 385, 132718. [Google Scholar] [CrossRef]
- Wang, Y.J.; Deng, G.J.; Huang, L.F.; Ning, J.M. Sensory-directed flavor analysis reveals the improvement in aroma quality of summer green tea by osmanthus scenting. Food Chem. X 2024, 23, 101571. [Google Scholar] [CrossRef]
- Zhou, H.C.; Hou, Z.W.; Wang, D.X.; Ning, J.M.; Wei, S. Large scale preparation, stress analysis, and storage of headspace volatile condensates from Jasminum sambac flowers. Food Chem. 2019, 286, 170–178. [Google Scholar] [CrossRef]
- Lin, Y.P.; Wang, Y.; Huang, Y.B.; Song, H.L.; Yang, P. Aroma Identification and Classification in 18 Kinds of Teas (Camellia sinensis) by Sensory Evaluation, HS–SPME–GC–IMS–GC × GC–MS, and Chemometrics. Foods 2023, 12, 2433. [Google Scholar] [CrossRef]
- Ma, J.Q.; Wang, Y.J.; Li, J.Y.; Zhang, J.X.; Wei, Y.M.; Yan, Y.X.; Wang, H.P.; Pan, Y.; Xiong, Z.C.; Wang, R.J.; et al. Aroma formation mechanism by the drying step during Congou black tea processing: Analyses by HP–SPME and SAFE with GC–MS. LWT-Food Sci. Technol. 2024, 198, 116019. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, Q.F.; Jiang, R.G.; Liu, Y.; Xie, H.; Ou, X.C.; Li, Q.; Liu, Z.H.; Huang, J.A. Characteristic volatiles of Fu brick tea formed primarily by extracellular enzymes during Aspergillus cristatus fermentation. Food Res. Int. 2024, 177, 113854. [Google Scholar] [CrossRef]
- Gao, Y.X.; Lei, Z.Y.; Huang, J.G.; Sun, Y.M.; Liu, S.; Yao, L.P.; Liu, J.X.; Liu, W.X.; Liu, Y.N.; Chen, Y. Characterization of Key Odorants in Lushan Yunwu Tea in Response to Intercropping with Flowering Cherry. Foods 2024, 13, 1252. [Google Scholar] [CrossRef]
- Xie, J.L.; Wang, L.L.; Deng, Y.L.; Yuan, H.B.; Zhu, J.Y.; Jiang, Y.W.; Yang, Y.Q. Characterization of the key odorants in floral aroma green tea based on GC–E–Nose, GC–IMS, GC–MS and aroma recombination and investigation of the dynamic changes and aroma formation during processing. Food Chem. 2023, 427, 136641. [Google Scholar] [CrossRef]
- Shang, C.Q.; Hu, Y.M.; Deng, C.H.; Hu, K.J. Rapid determination of volatile constituents of Michelia alba flowers by gas chromatography–mass spectrometry with solid-phase microextraction. J. Chromatogr. A 2002, 942, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Koksal, N.; Saribas, R.; Kafkas, E.; Aslancan, H.; Sadighazadi, S. Determination of volatile compounds of the first rose oil and the first rose water by HS–SPME–GC–MS techniques. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 145–150. [Google Scholar] [CrossRef]
- Zhou, L.J.; Yu, C.; Cheng, B.X.; Han, Y.; Luo, L.; Pan, H.T.; Zhang, Q.X. Studies on the volatile compounds in flower extracts of Rosa odorata and R. chinensis. Ind. Crops Prod. 2020, 146, 112143. [Google Scholar] [CrossRef]
- Zeng, L.T.; Zhou, Y.; Gui, J.D.; Fu, X.M.; Mei, X.; Zhen, Y.P.; Ye, T.X.; Du, B.; Dong, F.; Watanabe, N.; et al. Formation of volatile tea constituent indole during the oolong tea manufacturing process. J. Agric. Food Chem. 2016, 64, 5011–5019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.C.; Niu, Y.W.; Xiao, Z.B. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC–MS–O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC×GC–qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef]
- Hattori, S.; Takagaki, H.; Fujimori, T. Evaluation of Japanese green tea extract using GC–O with original aroma simultaneously input to the sniffing port method (OASIS). Food Sci. Technol. Res. 2003, 9, 350–352. [Google Scholar] [CrossRef]
- Shi, Y.L.; Zhu, Y.; Ma, W.J.; Lin, Z.; Lv, H.P. Characterisation of the volatile compounds profile of Chinese pan-fried green tea in comparison with baked green tea, steamed green tea, and sun-dried green tea using approaches of molecular sensory science. Curr. Res. Food Sci. 2022, 5, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Huang, L.F.; Deng, G.J.; Ning, J.M. The shaking and standing processing improve the aroma quality of summer black tea. Food Chem. 2024, 454, 139772. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Zhao, H.Y.; Jiang, G.L.; Chen, J.; Yi, J.W.; Zhou, C.X.; Luo, D.H. The flavour of wheat gluten hydrolysate after Corynebacterium Glutamicum fermentation: Effect of degrees of hydrolysis and fermentation time. Food Chem. 2024, 458, 140238. [Google Scholar] [CrossRef]
- Xu, H.R.; Pan, S.; Wang, J.B.; Ye, T.C.; Yan, M.; Liang, X.M.; Qian, G.L.; Yan, T.C.; Xin, G. Comparative characterization of volatile organic compounds and aroma profiles in 10 Actinidia arguta cultivars by gas chromatography–mass spectrometry (GC–MS), sensory analysis, and odor activity value (OAV) combined with chemometrics. J. Food Compos. Anal. 2024, 133, 106450. [Google Scholar] [CrossRef]
- Hao, Z.L.; Feng, J.; Chen, Q.L.; Lin, H.Z.; Zhou, X.H.; Zhuang, J.Y.; Wang, J.Y.; Tan, Y.P.; Sun, Z.L.; Wang, Y.F.; et al. Comparative volatiles profiling in milk-flavored white tea and traditional white tea Shoumei via HS–SPME–GC–TOFMS and OAV analyses. Food Chem. X 2023, 18, 100710. [Google Scholar] [CrossRef]
- Wang, M.Q.; Ma, W.J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC–MS), gas chromatography-olfactometry (GC–O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef]
- Qin, M.X.; Zhou, J.T.; Luo, Q.Q.; Zhu, J.Y.; Yu, Z.; Zhang, D.; Ni, D.J.; Chen, Y.Q. The key aroma components of steamed green tea decoded by sensomics and their changes under different withering degree. Food Chem. 2024, 439, 138176. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Li, S.Y.; Du, X.; Xu, W.; Bian, J.L.; Chen, S.X.; He, C.L.; Xu, J.Y.; Ye, S.R.; Feng, D.J.; et al. Insights into momentous aroma dominating the characteristic flavor of jasmine tea. Food Sci. Nutr. 2023, 11, 7841–7854. [Google Scholar] [CrossRef]
- Wei, Y.M.; Zhang, J.X.; Li, T.H.; Zhao, M.J.; Song, Z.S.; Wang, Y.J.; Ning, J.M. GC–MS, GC–O, and sensomics analysis reveals the key odorants underlying the improvement of yellow tea aroma after optimized yellowing. Food Chem. 2024, 431, 137139. [Google Scholar] [CrossRef]
- Lin, Y.P.; Huang, Y.B.; Liu, X.; Pan, Y.N.; Feng, X.Y.; Guo, H.W.; Li, X.L.; Tao, Y.K.; Chen, P.; Chu, Q. Uncovering the Shuixian tea grades hierarchy in Chinese national standard: From sensory evaluation to microstructure and volatile compounds analysis. Food Chem. 2024, 459, 140342. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.X.; Han, Q.D.; Dong, G.Z.; Wang, B.; Zhang, J.F.; Lei, S.M.; Liu, Y.G. Quality assessment of rose tea with different drying methods based on physicochemical properties, HS–SPME–GC–MS, and GC–IMS. J. Food Sci. 2023, 88, 1378–1391. [Google Scholar] [CrossRef]
- Meng, X.; Wang, J.Q.; Wang, F.; Gao, Y.; Fu, C.H.; Du, Q.; Feng, Z.H.; Chen, J.X.; Yin, J.F.; Xu, Y.Q. Moisture content of tea dhool for the scenting process affects the aroma quality and volatile compounds of osmanthus black tea. Food Chem. 2024, 438, 138051. [Google Scholar] [CrossRef] [PubMed]
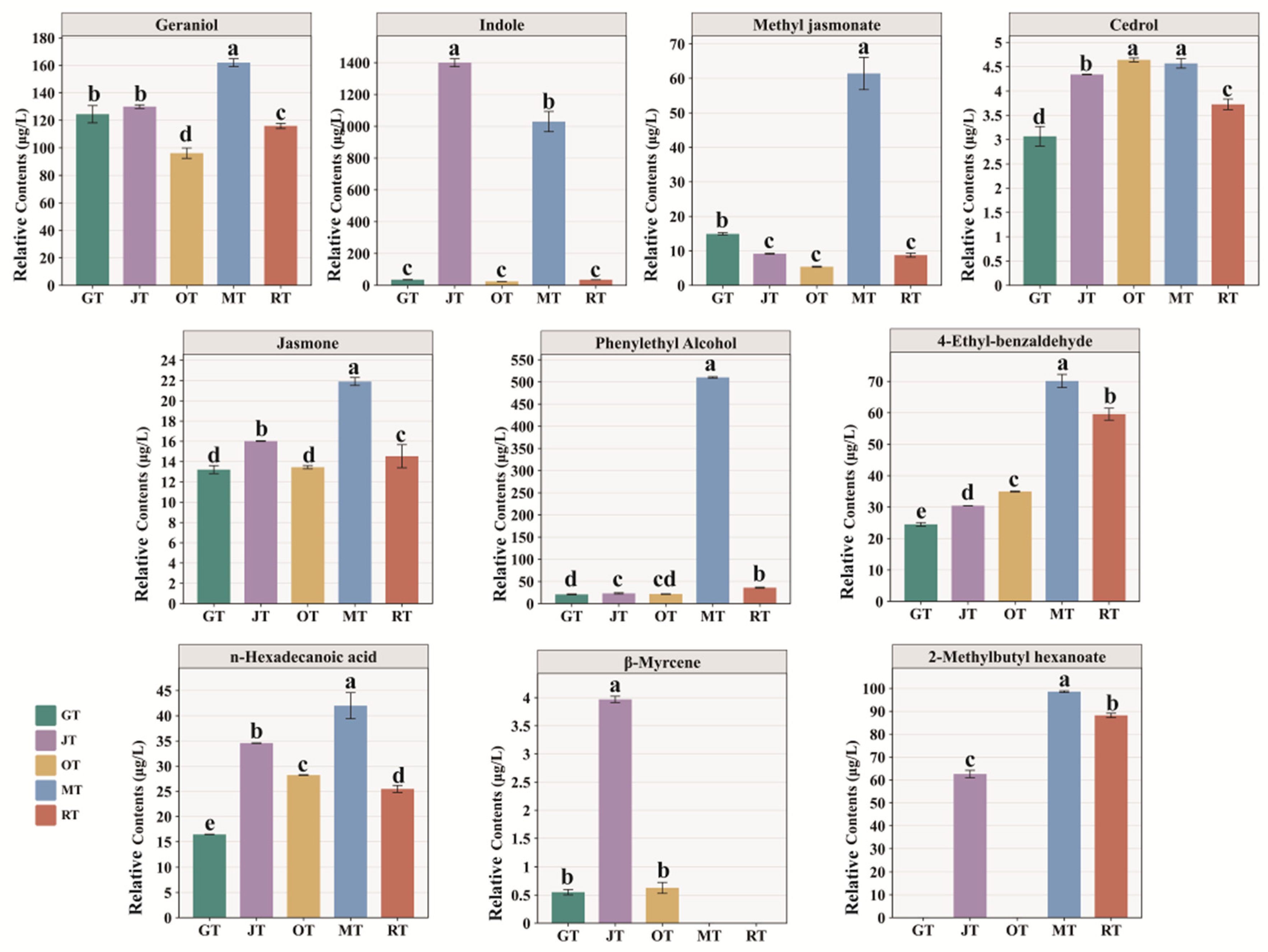
| No. | CAS | Compound | RI a | Threshold (μg·L−1) b | Aroma Description c | Identification Basis d | ROAV e | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GT | JT | OT | MT | RT | |||||||
| 1 | 100-52-7 | Benzaldehyde | 972 | 150 | Fruity, almond-like | MS/RI | 0.01 | 0.01 | 0.03 | n.d. | 0.02 |
| 2 | 111-70-6 | 1-Heptanol | 985 | 425 | Green, sweet | MS/RI | 0.00 | n.d. | n.d. | 0.00 | 0.00 |
| 3 | 123-35-3 | β-Myrcene | 1002 | 1.2 | Geranium-like | MS/RI/Std | 0.41 | 2.60 | 0.60 | n.d. | n.d. |
| 4 | 4313-03-5 | (E,E)-2,4-Heptadienal | 1023 | 10 | Fatty, nutty, hay, green | MS/RI | 0.98 | n.d. | 0.33 | 0.20 | 0.22 |
| 5 | 138-86-3 | Limonene | 1038 | 200 | Citrus, lemon, orange-like | MS/RI | n.d. | 0.01 | 0.01 | 0.00 | n.d. |
| 6 | 13877-91-3 | β-Ocimene | 1059 | 34 | Citrus | MS/RI | 0.02 | 0.07 | 0.02 | 0.04 | n.d. |
| 7 | 98-86-2 | Acetophenone | 1077 | 65 | Sweet | MS/RI | 0.09 | 0.06 | 0.18 | 0.15 | 0.23 |
| 8 | 13360-65-1 | 3-Ethyl-2,5-dimethyl-pyrazine | 1090 | 25 | Earthy | MS/RI | 0.02 | n.d. | n.d. | n.d. | 0.01 |
| 9 | 821-55-6 | 2-Nonanone | 1102 | 41 | Earthy, herbal | MS/RI | n.d. | n.d. | n.d. | 0.03 | n.d. |
| 10 | 38284-27-4 | 3,5-Octadien-2-one | 1104 | 150 | n.f. | MS/RI | 0.02 | n.d. | 0.03 | n.d. | 0.02 |
| 11 | 93-58-3 | Methyl benzoate | 1105 | 73 | Violet-like, floral | MS/RI | n.d. | n.d. | n.d. | 0.21 | n.d. |
| 12 | 7299-41-4 | β-Terpineol | 1107 | 330 | n.f. | MS/RI | 0.01 | n.d. | n.d. | n.d. | n.d. |
| 13 | 29957-43-5 | Hoterienol | 1115 | 110 | Fresh, floral, fruity | MS/RI | 0.12 | n.d. | 0.09 | 0.09 | 0.11 |
| 14 | 1960/12/8 | Phenylethyl alcohol | 1125 | 140 | Floral, honey-like | MS/RI | 0.13 | 0.13 | 0.18 | 2.48 | 0.24 |
| 15 | 16491-36-4 | (Z)-3-Hexenyl butyrate | 1195 | 500 | Fruity | MS/RI | 0 | n.d. | n.d. | n.d. | 0 |
| 16 | 112-31-2 | Decanal | 1213 | 9.3 | Citrus-like | MS/RI | 0.07 | n.d. | 0.17 | 0.27 | 0.16 |
| 17 | 4748-78-1 | 4-Ethyl-benzaldehyde | 1222 | 40 | Almond-like | MS/RI | 0.54 | 0.6 | 1 | 1.19 | 1.41 |
| 18 | 432-25-7 | β-Cyclocitral | 1227 | 3 | Fruity | MS/RI | 0.78 | n.d. | n.d. | n.d. | n.d. |
| 19 | 2601-13-0 | 2-Methylbutyl hexanoate | 1229 | 30 | n.f. | MS/RI | n.d. | 1.64 | n.d. | 2.23 | 2.79 |
| 20 | 106-24-1 | Geraniol | 1263 | 1.1 | Rose-like, citrus-like | MS/RI/Std | 100 | 92.86 | 100 | 100 | 100 |
| 21 | 4179-19-5 | 3,5-Dimethoxytoluene | 1275 | n.f. | Sweet | MS/RI | - | - | - | - | - |
| 22 | 120-72-9 | Indole | 1306 | 11 | Fecal, mothball-like | MS/RI/Std | 2.87 | 100 | 2.55 | 63.58 | 3.05 |
| 23 | 4698/8/2 | Geranic acid | 1372 | 10000 | Sour | MS/RI | 0 | 0 | n.d. | 0 | 0 |
| 24 | 488-10-8 | Jasmone | 1406 | 7 | Celery-like | MS/RI | 1.67 | 1.8 | 2.2 | 2.12 | 1.97 |
| 25 | 103-52-6 | β-Phenylethyl butyrate | 1457 | 376 | Fruity, sweet | MS/RI | n.d. | n.d. | n.d. | 0.05 | n.d. |
| 26 | 483-76-1 | δ-Cadinene | 1533 | n.f. | Fresh, woody | MS/RI | - | - | - | - | - |
| 27 | 143-07-7 | Dodecanoic acid | 1581 | 100 | n.f. | MS/RI | 0.1 | 0.28 | 0.44 | 0.42 | 0.21 |
| 28 | 77-53-2 | Cedrol | 1612 | 0.5 | Woody, sweet | MS/RI | 5.42 | 6.82 | 10.62 | 6.2 | 7.07 |
| 29 | 102488-09-5 | 3-Hydroxy-β-damascone | 1626 | n.f. | n.f. | MS/RI | - | - | - | - | - |
| 30 | 19912-62-0 | τ-Muurolol | 1654 | n.f. | Herbal | MS/RI | - | - | - | - | - |
| 31 | 1211-29-6 | Methyl jasmonate | 1658 | 3 | Floral, jasmine, green | MS/RI | 4.4 | 2.4 | 2.06 | 13.9 | 2.78 |
| 32 | 481-34-5 | α-Cadinol | 1667 | n.f. | Herbal, woody | MS/RI | - | - | - | - | - |
| 33 | 2306-78-7 | Nerolidyl acetate | 1683 | n.f. | Floral | MS/RI | - | - | - | - | - |
| 34 | 544-63-8 | Tetradecanoic acid | 1769 | 10 | Coconut-like | MS/RI | 0.43 | 0.51 | 0.84 | 0.63 | n.d. |
| 35 | 373-49-9 | Palmitoleic acid | 1944 | 11 | n.f. | MS/RI | 0.4 | 0.55 | 0.8 | n.d. | 0.66 |
| 36 | 1957/10/3 | n-Hexadecanoic acid | 1963 | 10 | Creamy | MS/RI | 1.45 | 2.72 | 3.23 | 2.85 | 2.42 |
| 37 | 112-80-1 | Oleic acid | 2136 | 200 | Fatty | MS/RI | 0.02 | 0.02 | 0.03 | 0.03 | n.d. |
| 38 | 1957/11/4 | Octadecanoic acid | 2155 | 100 | n.f. | MS/RI | 0.03 | 0.05 | 0.05 | 0.07 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Z.; Chen, Z.; Li, L.; Chen, H.; Zhang, H.; Liu, S.; Zhang, R.; Song, Q.; Chen, Y.; Su, Z.; et al. Comparison of Volatile Compounds in Jingshan Green Tea Scented with Different Flowers Using GC-IMS and GC-MS Analyses. Foods 2024, 13, 2653. https://doi.org/10.3390/foods13172653
Hou Z, Chen Z, Li L, Chen H, Zhang H, Liu S, Zhang R, Song Q, Chen Y, Su Z, et al. Comparison of Volatile Compounds in Jingshan Green Tea Scented with Different Flowers Using GC-IMS and GC-MS Analyses. Foods. 2024; 13(17):2653. https://doi.org/10.3390/foods13172653
Chicago/Turabian StyleHou, Zhiwei, Ziyue Chen, Le Li, Hongping Chen, Huiyuan Zhang, Sitong Liu, Ran Zhang, Qiyue Song, Yuxuan Chen, Zhucheng Su, and et al. 2024. "Comparison of Volatile Compounds in Jingshan Green Tea Scented with Different Flowers Using GC-IMS and GC-MS Analyses" Foods 13, no. 17: 2653. https://doi.org/10.3390/foods13172653





