Extraction, Identification, and Antioxidant Activity of Flavonoids from Hylotelephium spectabile (Boreau) H. Ohba
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Standard Curve Plotting
2.3. Single-Factor Test for Total Flavonoid Extraction
2.4. Optimization of Extraction Conditions for Total Flavonoid Compounds via Response Surface Analysis
2.5. Isolation and Purification of Flavonoids and Structure Identification for H. spectabile
2.5.1. Selection of Resin Types
2.5.2. Optimum Macroporous Resin Kinetic Curve Experiment
2.5.3. Purification of Total Flavonoids from H. spectabile Macroporous Resin—Single-Factor Experiment
2.6. HPLC-MS Analysis of AB-8 Macroporous Resin Purification
2.6.1. Test Material
2.6.2. Chromatographic Conditions
2.6.3. Mass Spectrum Condition
2.7. Analysis of Antioxidant Activity
2.7.1. Preparation of the Sample Solution after Purification and Extraction
2.7.2. Total Reducing Capacity Assay
2.7.3. DPPH Radical-Scavenging Activity Assay
2.7.4. Hydroxyl Radical-Scavenging Ability Assay
2.7.5. ABTS+ Radical-Scavenging Ability Assay
2.8. Method of Analysis
3. Results and Discussion
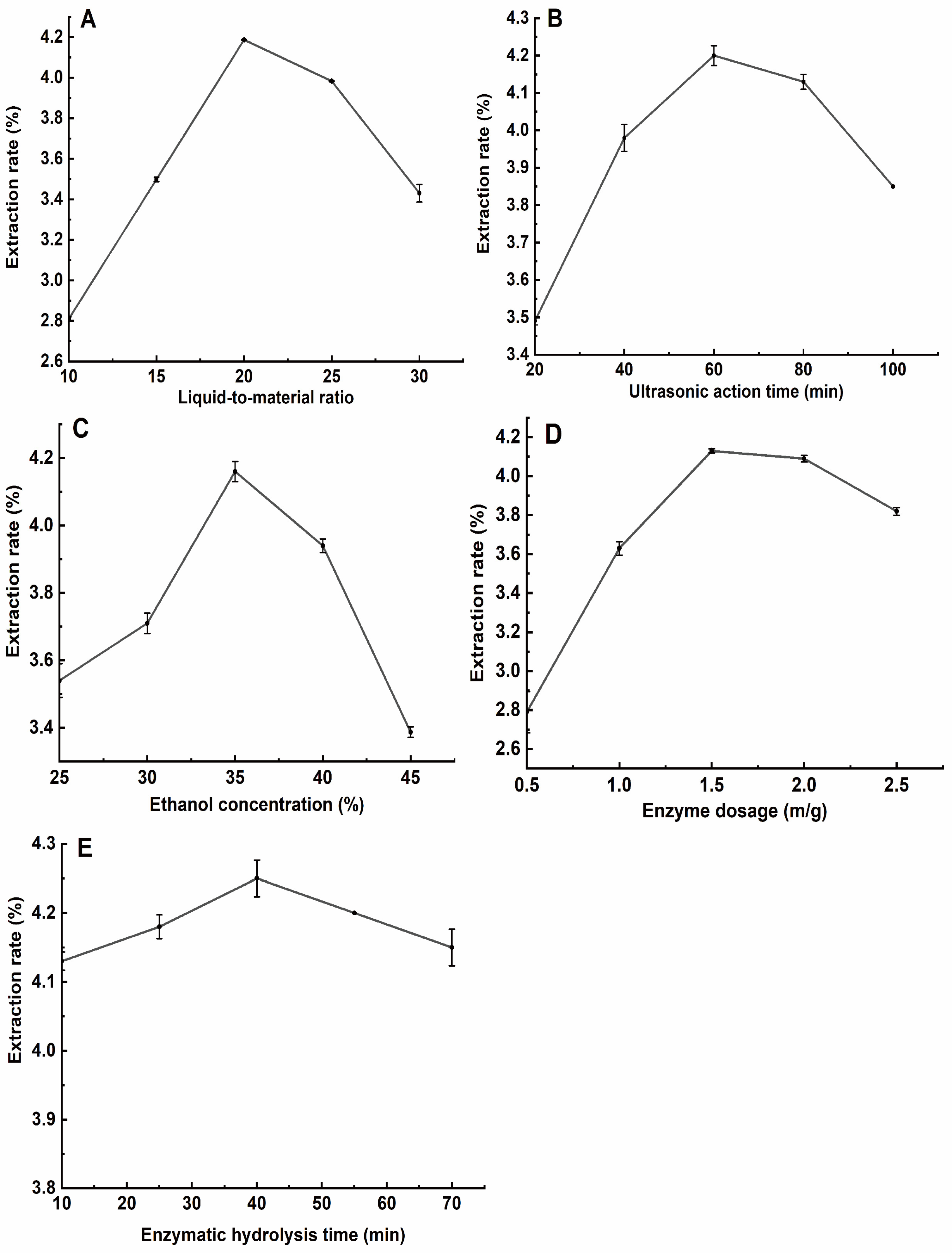
3.1. Single-Factor Test Results for the Extraction of Total Flavonoids from H. spectabile
3.1.1. Effect of Liquid–Material Ratio on the Extraction Rate of Total Flavonoids from H. spectabile
3.1.2. Effect of Ultrasonic Extraction Time on the Rate of Total Flavonoid Extraction from H. spectabile
3.1.3. Effect of Ethanol Volume Fraction on the Rate of Total Flavonoid Extraction from H. spectabile
3.1.4. Effects of the Amount of Complex Enzyme on the Rate of Total Flavonoid Extraction from H. spectabile
3.1.5. Effect of Enzymolysis Time on the Rate of Total Flavonoid Extraction from H. spectabile
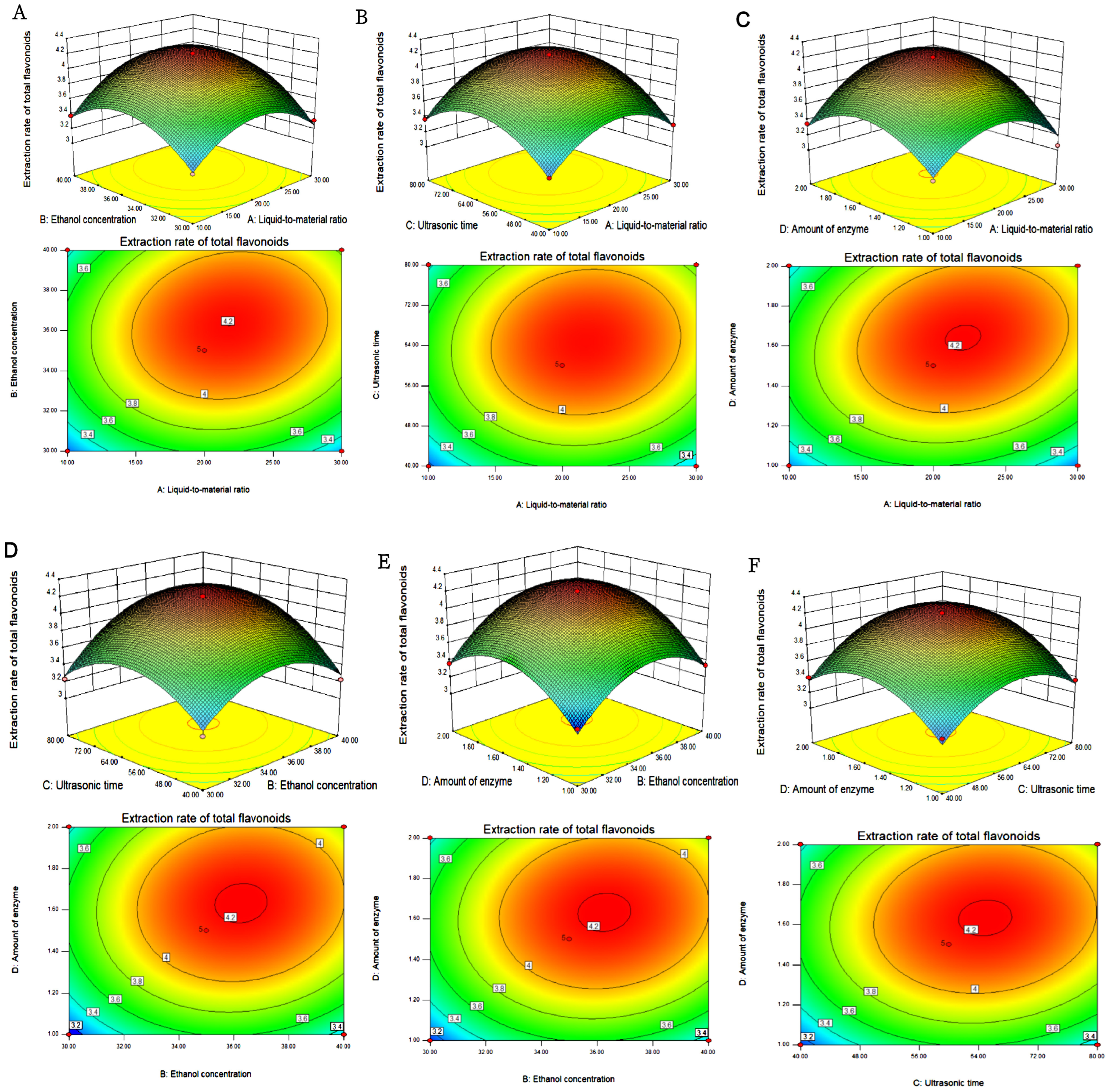
3.2. Optimization Results for a Response Surface Experiment for the Extraction of Total Flavonoids from H. spectabile
3.2.1. Establishment and Significance Test of the Response Mode
3.2.2. Analyzing Response Surface Maps and Contour Maps
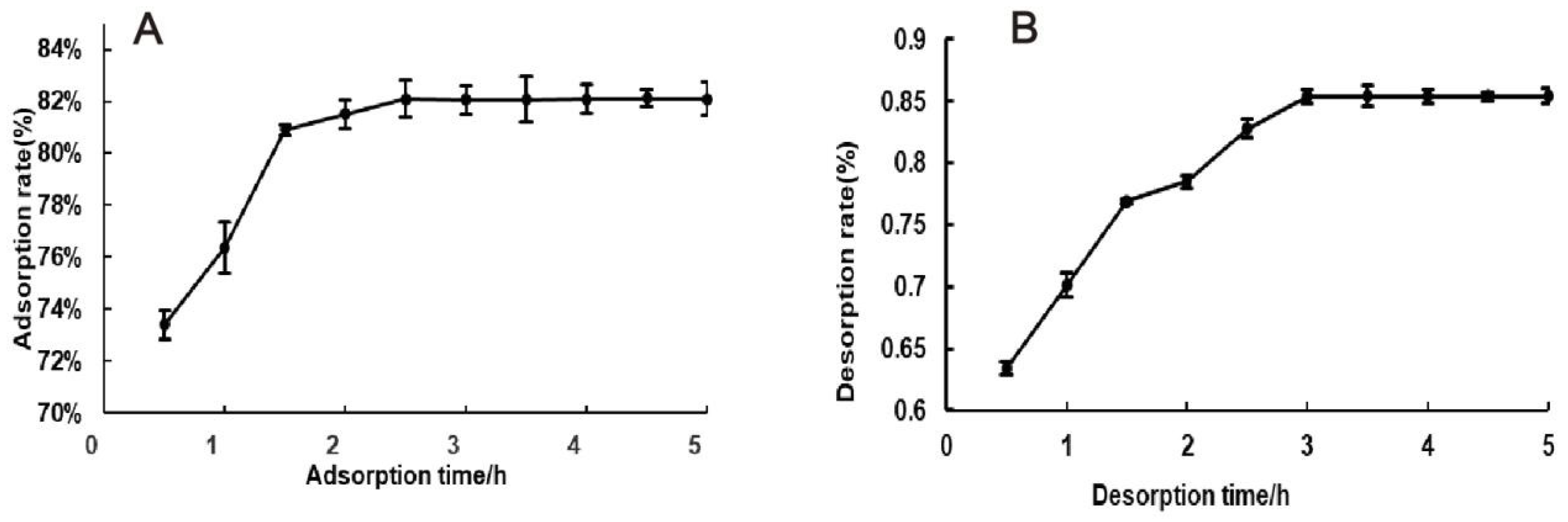
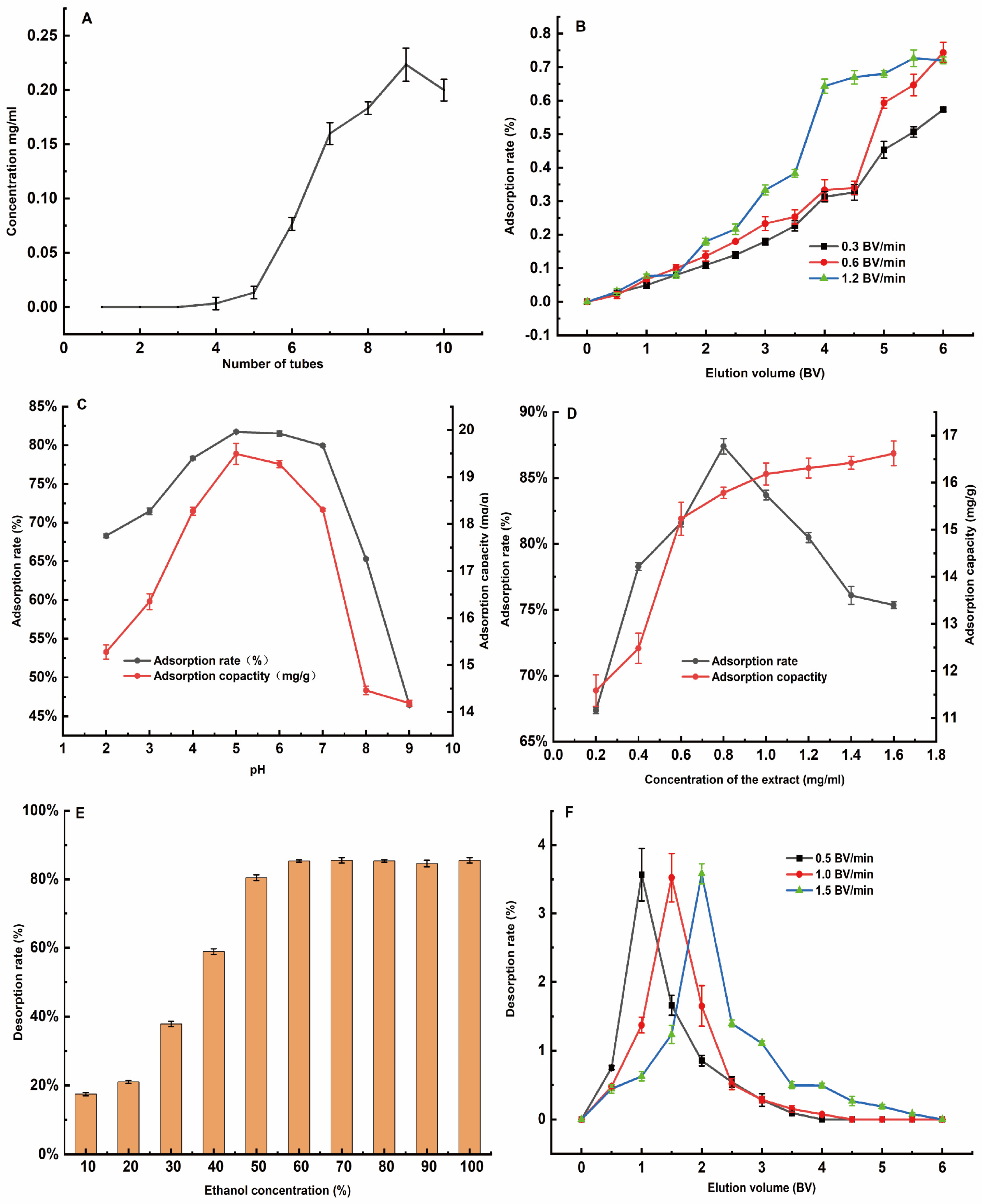
3.3. Separation and Purification of Total Flavonoids from H. spectabile
3.3.1. Screening of Macroporous Resins
3.3.2. Static Adsorption and Desorption Kinetic Curves of AB-8
3.3.3. Single-Factor Adsorption Test Results
3.3.4. Results of the Elution Single-Factor Test
3.4. Identifying the Composition of the Total Flavonoids of H. spectabile
3.4.1. HPLC-MS Identification and Analysis of Flavonoids
3.4.2. Flavonoid Content Analysis
3.5. Study of the Antioxidant Activity of the Flavonoids in H. spectabile
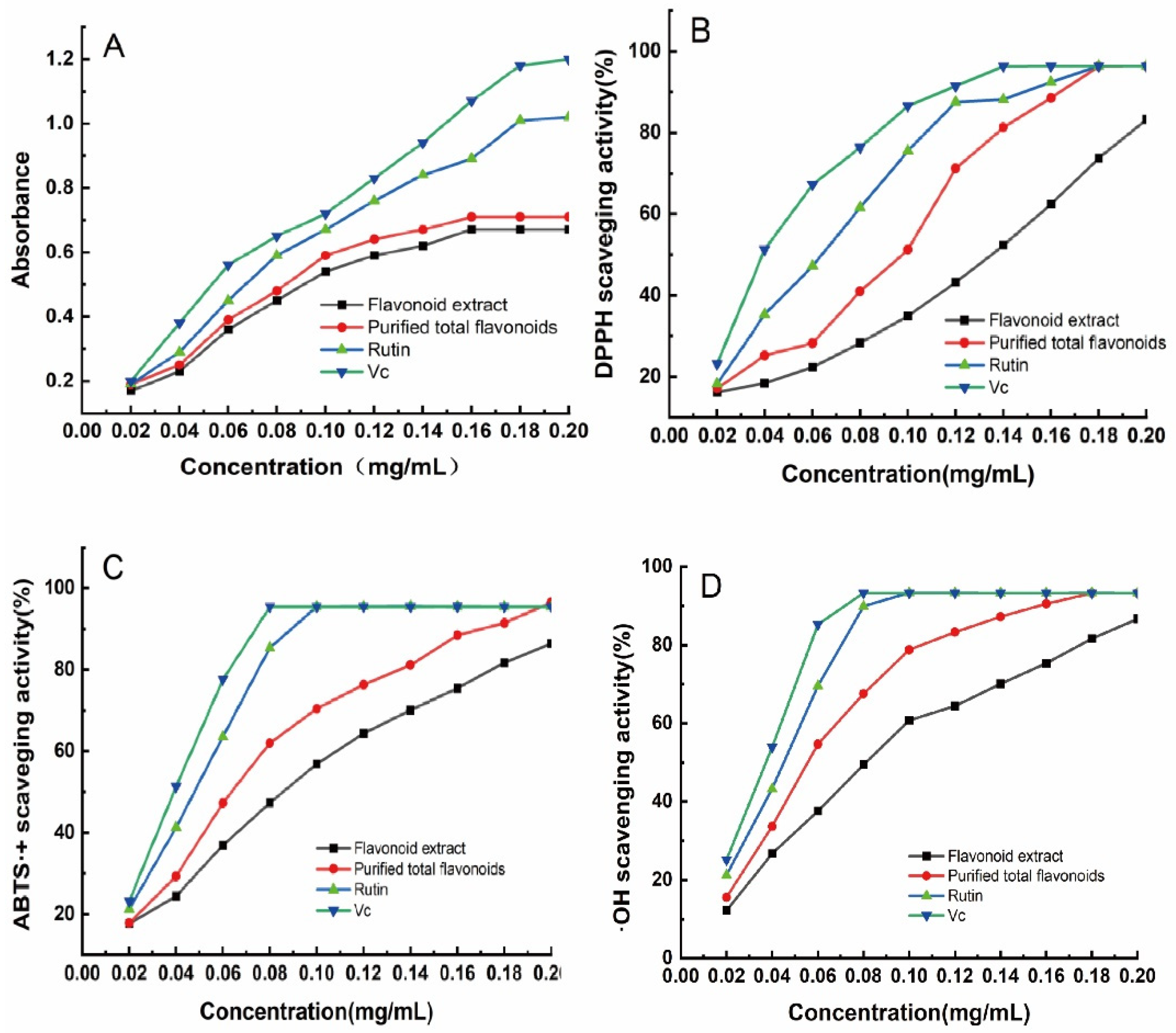
3.5.1. Total Reducing Capacity of H. spectabile Flavonoid Extract
3.5.2. DPPH Free Radical-Scavenging Ability of H. spectabile Flavonoid Extract
3.5.3. ABTS·+ Free Radical-Scavenging Ability of H. spectabile Flavonoid Extract
3.5.4. ·OH Free Radical-Scavenging Ability of H. spectabile Flavonoid Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baraldi, R.; Neri, L.; Costa, F.; Facini, O.; Rapparini, F.; Carriero, G. Ecophysiological and Micromorphological Characterization of Green Roof Vegetation for Urban Mitigation. Urban For. Urban Green. 2019, 37, 24–32. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Medicinal, Non-Medicinal, Biopesticides, Color- and Dye-Yielding Plants; Secondary Metabolites and Drug Principles; Significance of Medicinal Plants; Use of Medicinal Plants in the Systems of Traditional and Complementary and Alternative Medicines (CAMs). In Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1: Pharmacognosy; Alamgir, A.N.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 61–104. ISBN 978-3-319-63862-1. [Google Scholar]
- Gunathilaka, T.L.; Samarakoon, K.W.; Ranasinghe, P.; Peiris, L.D.C. In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva. Molecules 2019, 24, 3708. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Jin, C.; Yin, X.; Wang, S. Chemical Constituents of Hylotelephium Erythrostictum. Chem. Nat. Compd. 2021, 57, 1141–1143. [Google Scholar] [CrossRef]
- Boriollo, M.F.G.; Marques, M.B.; da Silva, T.A.; da Silva, J.J.; Dias, R.A.; Silva Filho, T.H.N.; Melo, I.L.R.; dos Santos Dias, C.T.; Bernardo, W.L.d.C.; de Mello Silva Oliveira, N.; et al. Antimicrobial Potential, Phytochemical Profile, Cytotoxic and Genotoxic Screening of Sedum Praealtum A. DC. (Balsam). BMC Complement. Med. Ther. 2020, 20, 133. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Nguyen Tram Anh, M.; Van Hung, P.; Thi Lan Phi, N. Optimized Conditions for Flavonoid Extraction from Pomelo Peel Byproducts under Enzyme- and Ultrasound-Assisted Extraction Using Response Surface Methodology. J. Food Qual. 2021, 2021, 6666381. [Google Scholar] [CrossRef]
- Yun, S.-B.; Lee, Y.; Lee, N.K.; Jeong, E.-J.; Jeong, Y.-S. Optimization of Microwave Extraction Conditions for Antioxidant Phenolic Compounds from Ligustrum lucidum Aiton Using Response Surface Methodology. J. Korean Soc. Food Sci. Nutr. 2014, 43, 570–576. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, M.; Gao, L.; Bao, Y.; Li, P.; Wang, M.; Shao, T.; Wang, G.; Liu, C. Optimal extraction of polysaccharides from Stevia rebaudiana roots for protection against hydrogen peroxide-induced oxidative damage in RAW264.7 cells. Nat. Prod. Res. 2023, 4, 1–5. [Google Scholar] [CrossRef]
- Wang, X.-H.; Wang, J.-P. Effective Extraction with Deep Eutectic Solvents and Enrichment by Macroporous Adsorption Resin of Flavonoids from Carthamus tinctorius L. J. Pharmaceut. Biomed. 2019, 176, 112804. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.-M.; Guo, C.-Y.; Zhang, S.-M.; Liu, C.-L.; Zhang, D.-S.; Bai, X.-M. Ultrasound-Assisted Extraction of Total Flavonoids from Inula Helenium. Pharmacogn. Mag. 2012, 8, 166–170. [Google Scholar] [CrossRef]
- Cui, L.; Ma, Z.; Wang, D.; Niu, Y. Ultrasound-Assisted Extraction, Optimization, Isolation, and Antioxidant Activity Analysis of Flavonoids from Astragalus membranaceus Stems and Leaves. Ultrason. Sonochem. 2022, 90, 106190. [Google Scholar] [CrossRef]
- Hao, C.; Chen, L.; Dong, H.; Xing, W.; Xue, F.; Cheng, Y. Extraction of Flavonoids from Scutellariae Radix Using Ultrasound-Assisted Deep Eutectic Solvents and Evaluation of Their Anti-Inflammatory Activities. ACS Omega 2020, 5, 23140–23147. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chu, X.; Su, J.; Fu, X.; Kan, Q.; Wang, X.; Zhang, X. Enzyme-Assisted Ultrasonic Extraction of Total Flavonoids from Acanthopanax senticosus and Their Enrichment and Antioxidant Properties. Processes 2021, 9, 1708. [Google Scholar] [CrossRef]
- Hou, M.; Hu, W.; Wang, A.; Xiu, Z.; Shi, Y.; Hao, K.; Sun, X.; Cao, D.; Lu, R.; Sun, J. Ultrasound-Assisted Extraction of Total Flavonoids from Pteris cretica L.: Process Optimization, HPLC Analysis, and Evaluation of Antioxidant Activity. Antioxidants 2019, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Hapsari, S.; Yohed, I.; Kristianita, R.A.; Jadid, N.; Aparamarta, H.W.; Gunawan, S. Phenolic and Flavonoid Compounds Extraction from Calophyllum inophyllum Leaves. Arab. J. Chem. 2022, 15, 103666. [Google Scholar] [CrossRef]
- Kobus, Z.; Pecyna, A.; Buczaj, A.; Krzywicka, M.; Przywara, A.; Nadulski, R. Optimization of the Ultrasound-Assisted Extraction of Bioactive Compounds from Cannabis sativa L. Leaves and Inflorescences Using Response Surface Methodology. Appl. Sci. 2022, 12, 6747. [Google Scholar] [CrossRef]
- Cui, H.; Lu, T.; Wang, M.; Zou, X.; Zhang, Y.; Yang, X.; Dong, Y.; Zhou, H. Flavonoids from Morus alba L. Leaves: Optimization of Extraction by Response Surface Methodology and Comprehensive Evaluation of Their Antioxidant, Antimicrobial, and Inhibition of α-Amylase Activities through Analytical Hierarchy Process. Molecules 2019, 24, 2398. [Google Scholar] [CrossRef]
- Li, Y.; Dai, M.; Wang, L.; Wang, G. Polysaccharides and glycosides from Aralia echinocaulis protect rats from arthritis by modulating the gut microbiota composition. J. Ethnopharmacol. 2021, 269, 113749. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Gong, G.; Li, F.; Ren, H.; Liu, Y. Adsorption and Desorption Properties of Macroporous Resins for Flavonoids from the Extract of Chinese Wolfberry (Lycium barbarum L.). Food Bioprod. Process. 2015, 93, 148–155. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, H.; Shao, Q.; Bilia, A.R.; Wang, Y.; Zhao, X. Enrichment and Purification of the Bioactive Flavonoids from Flower of Abelmoschus manihot (L.) Medic Using Macroporous Resins. Molecules 2018, 23, 2649. [Google Scholar] [CrossRef]
- Li, R.; Xia, Z.; Tian, Y.; Guan, M.; Zheng, Y.; Bin, L.; Dong, J.; Jiang, Q.; Du, L.; Li, M. Purification of Total Flavonoids from Ginkgo Biloba Flowers with Resin Column Chromatography and Evaluation of Antioxidant Activities in Vitro. Prep. Biochem. Biotechnol. 2023, 53, 308–316. [Google Scholar] [CrossRef]
- Hou, M.; Hu, W.; Xiu, Z.; Jiang, A.; Men, L.; Hao, K.; Sun, X.; Cao, D. Preparative Purification of Total Flavonoids from Sophora tonkinensis Gagnep. by Macroporous Resin Column Chromatography and Comparative Analysis of Flavonoid Profiles by HPLC-PAD. Molecules 2019, 24, 3200. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, M.; Sun-Waterhouse, D.; Zhuang, M.; Chen, H.; Feng, M.; Lin, L. Absorption and Desorption Behaviour of the Flavonoids from Glycyrrhiza glabra L. Leaf on Macroporous Adsorption Resins. Food Chem. 2015, 168, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Mottaghipisheh, J.; Iriti, M. Sephadex® LH-20, Isolation, and Purification of Flavonoids from Plant Species: A Comprehensive Review. Molecules 2020, 25, 4146. [Google Scholar] [CrossRef] [PubMed]
- Erpel, F.; Camilo, C.; Mateos, R.; Ricardo Pérez-Correa, J. A Macroporous Resin Purification Process to Obtain Food-Grade Phlorotannin-Rich Extracts with α-Glucosidase Inhibitory Activity from Chilean Brown Seaweeds: An UHPLC-MSn Profiling. Food Chem. 2023, 402, 134472. [Google Scholar] [CrossRef]
- Gan, J.; Zhang, X.; Ma, C.; Sun, L.; Feng, Y.; He, Z.; Zhang, H. Purification of Polyphenols from Phyllanthus emblica L. Pomace Using Macroporous Resins: Antioxidant Activity and Potential Anti-Alzheimer’s Effects. J. Food Sci. 2022, 87, 1244–1256. [Google Scholar] [CrossRef]
- Li, C.; Wang, E.; Elshikh, M.S.; Alwahibi, M.S.; Wang, W.; Wu, G.; Shen, Y.; Abbasi, A.M.; Shan, S. Extraction and Purification of Total Flavonoids from Gnaphalium affine D. Don and Their Evaluation for Free Radicals’ Scavenging and Oxidative Damage Inhabitation Potential in Mice Liver. Arab. J. Chem. 2021, 14, 103006. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, P.; Dai, Y.; Luo, X.; Manickam, S.; Li, D.; Han, Y.; Loke Show, P. Bridge between Mass Transfer Behavior and Properties of Bubbles under Two-Stage Ultrasound-Assisted Physisorption of Polyphenols Using Macroporous Resin. Chem. Eng. J. 2022, 436, 135158. [Google Scholar] [CrossRef]
- Shen, D.; Labreche, F.; Wu, C.; Fan, G.; Li, T.; Dou, J.; Zhu, J. Ultrasound-Assisted Adsorption/Desorption of Jujube Peel Flavonoids Using Macroporous Resins. Food Chem. 2022, 368, 130800. [Google Scholar] [CrossRef]
- Liu, X.; Yan, S.; Zhou, H.; Wu, H.; Wang, S.; Yong, X.; Zhou, J. Separation and Purification of Glabridin from a Deep Eutectic Solvent Extract of Glycyrrhiza glabra Residue by Macroporous Resin and Its Mechanism. Sep. Purif. Technol. 2023, 315, 123731. [Google Scholar] [CrossRef]
- Li, G.; Yan, H.; Liu, X. Simultaneous Purification and Separation of Syringoside and Oleuropein from Syringa oblata Lindl. Extract Using Macroporous Resin. J. Chem. 2019, 2019, 2924548. [Google Scholar] [CrossRef]
- Wang, X.-H.; Wang, J.-P. Ultrasonic-Assisted Extraction and Enrichment of the Flavonoids from Salicornia Europaea Leaves Using Macroporous Resins and Response Surface Methodology. Chem. Pap. 2023, 77, 2769–2781. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in Antioxidant Flavonoids of Stamen Extracts from Nymphaea lotus L. Using Ultrasonic-Assisted Extraction and Macroporous Resin Adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Cheng, J.; Zhao, J.; Shi, R.; He, L.; Li, Q.; Chen, Y. Efficient Purification of Flavonoids from Bamboo Shoot Residues of Phyllostachys edulis by Macroporous Resin and Their Hypoglycemic Activity. Food Chem. X 2022, 16, 100505. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-H.; Tong, Q.; Han, J.-J.; Bao, B.-Y.-M.-Q.-E.; Wu, J.-S.; Han, N.-R.-C.-K.-T.; Dai, N.-Y.-T.; Wu, R.-J. Orthogonal Test Design for Optimization of the Isolation and Purification of Total Favonoids from Artemisia Frigida Willd Using Macroporous Resin Chromatography. Afr. J. Pharm. Pharmacol. 2016, 10, 192–199. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, W.; Wang, C.; Yang, X.; Lou, Y.; Xia, X.; Xu, H. Optimization of Enzyme-Assisted Extraction and Purification of Flavonoids from Pinus Koraiensis Nut-Coated Film and Antioxidant Activity Evaluation. Molecules 2021, 26, 1950. [Google Scholar] [CrossRef]
- Purwaningsih, I.; Fathiah, F.; Amaliyah, N.; Kuswiyanto, K. The Phenolic, Flavonoid, and Anthocyanin Content From Methanol Extract of Senggani Fruit and Its Antioxidant Activity. Indones. J. Chem. Res. 2023, 10, 195–202. [Google Scholar] [CrossRef]
- Hayati, E.K.; Ningsih, R.; Latifah, L. Antioxidant Activity of Flavonoid from Rhizome Kaemferia galanga L. Extract. ALCHEMY J. Chem. 2015, 4, 127–137. [Google Scholar] [CrossRef][Green Version]
- Akbar, A.; Soekamto, N.H.; Firdaus; Bahrun. Total Phenolics and Flavonoids Level of N-Hexane, Ethyl Acetate and Methanol Extracts of Sargassum sp. along with Their Antioxidant Activity by DPPH Method. AIP Conf. Proc. 2022, 2638, 060009. [Google Scholar] [CrossRef]
- Zhai, K.; Wang, W.; Zheng, M.; Khan, G.J.; Wang, Q.; Chang, J.; Dong, Z.; Zhang, X.; Duan, H.; Gong, Z.; et al. Protective effects of Isodon Suzhouensis extract and glaucocalyxin A on chronic obstructive pulmonary disease through SOCS3–JAKs/STATs pathway. Food Front. 2023, 4, 511–523. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the Antioxidant Activity of Total Flavonoids Extract from Persimmon (Diospyros kaki L.) Leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Muhammad, S.; Chen, A.; Chen, P.; Wang, J.; Yang, C.; Yuan, H.; Wang, Z. An Experimental Study Exploring the Influencing Factors for Ultrasonic-Assisted Extraction of Flavonoid Compounds from Leaves of Amorpha fruticosa L. J. For. Res. 2019, 30, 1735–1741. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, P.; Baquero, L.P.; Larrota, H.R. Chapter 14—Flavonoids: Potential Therapeutic Agents by Their Antioxidant Capacity. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 265–288. ISBN 978-0-12-814774-0. [Google Scholar]
- Prasetyaningrum, A.; Widayat, W.; Jos, B.; Ratnawati, R.; Riyanto, T.; Prinanda, G.R.; Monde, B.U.L.; Susanto, E.E. Optimization of Sequential Microwave-Ultrasonic-Assisted Extraction of Flavonoid Compounds from Moringa Oleifera. Trends Sci. 2023, 20, 6401. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Li, S.; Yang, J. Effects of chrysin (5,7-dihydroxyflavone) on vascular remodeling in hypoxia-induced pulmonary hypertension in rats. Chin. Med. 2015, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zeng, H.; Chen, W.; Zheng, S.; Luo, J.; Jiang, H.; Yang, B.; Farag, M.A.; Lou, H.; Song, L.; et al. Effects of tree age on flavonoids and antioxidant activity in Torreya grandis nuts via integrated metabolome and transcriptome analyses. Food Front. 2023, 4, 358–367. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; He, X.; Wang, S.; Cao, S.; Xia, Z.; Xian, H.; Qin, L.; Mao, W. Structure and Anticoagulant Property of a Sulfated Polysaccharide Isolated from the Green Seaweed Monostroma angicava. Carbohydr. Polym. 2017, 159, 195–206. [Google Scholar] [CrossRef]
- Xiang, X.; Qing, Y.; Li, S.; Kwame, A.W.; Wang, M.; Ren, J. The study of single-cell dynamics contributes to the evaluation of food-derived antioxidant capacity. eFood 2023, 4, e66. [Google Scholar] [CrossRef]
- Liu, D.; Sun, Q.; Xu, J.; Li, N.; Lin, J.; Chen, S.; Li, F. Purification, Characterization, and Bioactivities of a Polysaccharide from Mycelial Fermentation of Bjerkandera fumosa. Carbohydr. Polym. 2017, 167, 115–122. [Google Scholar] [CrossRef]
- Fathy, H.M.; Abd El-Maksoud, A.A.; Cheng, W.; Elshaghabee, F.M.F. Value-Added Utilization of Citrus Peels in Improving Functional Properties and Probiotic Viability of Acidophilus-Bifidus-Thermophilus (ABT)-Type Synbiotic Yoghurt during Cold Storage. Foods 2022, 11, 2677. [Google Scholar] [CrossRef]
- Campbell, C.; Nanjundaswamy, A.K.; Njiti, V.; Xia, Q.; Chukwuma, F. Value-Added Probiotic Development by High-Solid Fermentation of Sweet Potato with Saccharomyces Boulardii. Food Sci. Nutr. 2017, 5, 633–638. [Google Scholar] [CrossRef]
- Bisson, G.; Marino, M.; Poletti, D.; Innocente, N.; Maifreni, M. Turbidimetric Definition of Growth Limits in Probiotic Lactobacillus Strains from the Perspective of an Adaptation Strategy. J. Dairy Sci. 2021, 104, 12236–12248. [Google Scholar] [CrossRef] [PubMed]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing Total Phenolics, Total Flavonoids Contents and Antioxidant Activity of Moringa oleifera Leaf Extract by the Appropriate Extraction Method. Ind. Crop. Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Pei, C.; Junxiu, B.a.O.; Fei, W.; Shouwu, T.; Haifeng, L.I.U.; Hongbin, L.I. Extraction of Total Flavonoids from Green Cotton Fiber by Ultrasonic-Based Response Surface Optimization. Xinjiang Agric. Sci. 2024, 61, 576. [Google Scholar] [CrossRef]
- Huiduan, L.; Jianzhong, Y. Study on Extract Methodology of Total Flavonoids from Ginger and Hydroxyl Radicals Scavenging Effect. Am. J. Chem. Biochem. Eng. 2017, 1, 7–16. [Google Scholar] [CrossRef]
- Chu, Q.; Yu, L.; Zheng, Z.; Chen, M.; Hua, Z.; Hang, M.; Li, Y.; Li, X.; Liu, Y.; Yang, Y.; et al. Apios americana Medik Flowers Extract Protects PC12 cells against H2O2 Induced Neurotoxicity via Regulating Autophagy. Food Chem. Toxicol. 2019, 124, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, K.; Wang, L.-J.; Yin, G.; Wang, J.; Wang, Y.; Jin, Y.-B.; Li, Q.; Wang, T.-J. HPLC–MS/MS Determination of Flavonoids in Gleditsiae Spina for Its Quality Assessment. J. Sep. Sci. 2018, 41, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, C.; Deng, J.; Xia, T.; Zhang, X.; Xue, S.; Song, M.K.; Olatunji, O.J. Schisandrin B ameliorates adjuvant-induced arthritis in rats via modulation of inflammatory mediators, oxidative stress, and HIF-1α/VEGF pathway. J. Pharm. Pharmacol. 2024, 76, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]

| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A Liquid-to-material ratio (mL/g) | 15:1 | 20:1 | 25:1 |
| B Ethanol concentration (%) | 30 | 35 | 40 |
| C Ultrasonic extraction time (min) | 40 | 60 | 80 |
| D Amount of enzyme (%) | 1.0 | 1.5 | 2 |
| Macroporous Resin | Polarity | Surface Area (m2/g) | Pore Size (nm) |
|---|---|---|---|
| AB-8 | Weak polarity | 450–530 | 13.0–14.0 |
| D101 | Non-polar | 600–700 | 10.0–12.0 |
| S-8 | Polarity | 90–150 | 25.0–30.0 |
| HPD-600 | Polarity | 480–520 | 8.0 |
| NKA-9 | Polarity | 170–250 | 15.5–16.5 |
| HPD-826 | Polarity | 500–600 | 9.0–10.0 |
| X-5 | Non-polar | 500–550 | 29.0–30.0 |
| ADS-17 | Non-polar | 90–150 | 25.0–30.0 |
| Time | Flow Rate | %B |
|---|---|---|
| Initial | 0.300 | 10.0 |
| 1.00 | 0.300 | 10.0 |
| 14.00 | 0.300 | 90.0 |
| 15.00 | 0.300 | 90.0 |
| 15.10 | 0.300 | 10.0 |
| 18.00 | 0.300 | 10.0 |
| Experiment Number | A | B | C | D | Total Flavonoid Extraction Rate Y (%) |
|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 0 | 3.166 |
| 2 | −1 | 0 | 1 | 0 | 3.369 |
| 3 | 0 | 0 | 0 | 0 | 4.109 |
| 4 | 0 | 1 | −1 | 0 | 3.226 |
| 5 | −1 | 0 | −1 | 0 | 3.169 |
| 6 | 1 | 1 | 0 | 0 | 3.88 |
| 7 | 0 | −1 | −1 | 0 | 3.136 |
| 8 | 1 | 0 | 1 | 0 | 3.76 |
| 9 | −1 | 0 | 0 | −1 | 3.18 |
| 10 | 0 | −1 | 1 | 0 | 3.236 |
| 11 | 0 | 1 | 0 | −1 | 3.345 |
| 12 | 0 | 1 | 0 | 1 | 3.796 |
| 13 | 0 | −1 | 0 | 1 | 3.367 |
| 14 | 0 | 0 | 0 | 0 | 4.207 |
| 15 | 0 | 0 | −1 | −1 | 3.188 |
| 16 | 0 | 0 | −1 | 1 | 3.399 |
| 17 | 0 | 0 | 0 | 0 | 4.206 |
| 18 | −1 | 0 | 0 | 1 | 3.35 |
| 19 | 0 | 0 | 0 | 0 | 4.206 |
| 20 | 0 | 0 | 1 | −1 | 3.365 |
| 21 | 0 | 1 | 1 | 0 | 3.838 |
| 22 | 1 | 0 | 0 | 1 | 3.775 |
| 23 | 1 | −1 | 0 | 0 | 3.316 |
| 24 | 0 | 0 | 0 | 0 | 4.108 |
| 25 | −1 | 1 | 0 | 0 | 3.378 |
| 26 | 0 | −1 | 0 | −1 | 3.167 |
| 27 | 0 | 0 | 1 | 1 | 3.846 |
| 28 | 1 | 0 | 0 | −1 | 3.358 |
| 29 | 1 | 0 | −1 | 0 | 3.298 |
| Source of Variance | Sum of Squares | Degrees of Freedom | Mean Squared | F-Number | p | Significance |
|---|---|---|---|---|---|---|
| Model | 3.94 | 14 | 0.28 | 52.94 | <0.0001 | *** |
| A | 0.18 | 1 | 0.18 | 34.06 | <0.0001 | *** |
| B | 0.36 | 1 | 0.36 | 67.41 | <0.0001 | *** |
| C | 0.33 | 1 | 0.33 | 62.50 | <0.0001 | *** |
| D | 0.41 | 1 | 0.41 | 77.86 | <0.0001 | *** |
| AB | 0.03 | 1 | 0.03 | 5.82 | 0.0301 | * |
| AC | 0.02 | 1 | 0.02 | 3.22 | 0.0942 | * |
| AD | 0.07 | 1 | 0.07 | 14.05 | 0.0022 | * |
| BC | 0.07 | 1 | 0.07 | 12.31 | 0.0035 | * |
| BD | 0.02 | 1 | 0.02 | 2.96 | 0.1074 | * |
| CD | 0.02 | 1 | 0.02 | 3.42 | 0.0855 | * |
| A2 | 1.02 | 1 | 1.02 | 191.65 | <0.0001 | *** |
| B2 | 0.92 | 1 | 0.92 | 173.72 | <0.0001 | *** |
| C2 | 0.94 | 1 | 0.94 | 176.15 | <0.0001 | *** |
| D2 | 0.93 | 1 | 0.93 | 175.45 | <0.0001 | *** |
| Residual error | 0.075 | 14 | 5.323 × 10−3 | |||
| Missing fit | 0.063 | 10 | 6.303 × 10−3 | 2.19 | 0.2333 | |
| Pure error | 0.011 | 4 | 2.872 × 10−3 | |||
| Total deviation | 4.02 | 28 | ||||
| R2 | 0.9815 | |||||
| R2Adj | 0.9629 |
| Resin Type | Adsorption Rate (%) | Desorption Rate (%) |
|---|---|---|
| ADS-17 | 58.36% | 89.72% |
| D101 | 60.63% | 97.67% |
| X-5 | 68.52% | 89.55% |
| S-8 | 75.46% | 84.97% |
| HPD-600 | 77.14% | 83.02% |
| HPD-826 | 77.55% | 83.98% |
| NKA-9 | 78.22% | 80.23% |
| AB-8 | 81.70% | 85.35% |
| (A) | ||||
| Number | Substance Name | Peak Time (min) | Peak Area (m/z) | Content (ng/mL) |
| 1 | Fisetin | 6.51 | 8,260,000 | 33,317.94651 |
| 2 | Myricitrin | 8.74 | 70,100,000 | 17,386.46613 |
| 3 | Phloretin | 9.77 | 23,200,000 | 11,933.10595 |
| 4 | Luteolin | 8.92 | 1,670,000 | 6065.768006 |
| 5 | Quercitrin | 10.2 | 45,900,000 | 2907.228018 |
| 6 | Astragalin | 7.88 | 51,400,000 | 2774.128377 |
| 7 | L-Epicatechin | 8.48 | 24,000,000 | 2547.56823 |
| 8 | Kaempferol | 8.28 | 1,370,000 | 2026.947908 |
| 9 | Neohesperidin | 7.99 | 71,400,000 | 1604.706504 |
| 10 | Rutin | 8.01 | 12,200,000 | 1555.740433 |
| (B) | ||||
| Number | Substance Name | Peak Time (min) | Peak Area (mAU/min) | Content (ng/mL) |
| 1 | Fisetin | 6.51 | 4,160,000 | 12,042.10526 |
| 2 | Myricitrin | 8.74 | 38,600,000 | 6870.550162 |
| 3 | Luteolin | 8.92 | 1,960,000 | 5109.004739 |
| 4 | Phloretin | 9.77 | 12,200,000 | 4503.355705 |
| 5 | Quercitrin | 10.2 | 56,200,000 | 2554.545455 |
| 6 | Kaempferol | 8.28 | 2,350,000 | 2495.173745 |
| 7 | Rutin | 8.01 | 22,500,000 | 2059.06822 |
| 8 | Astragalin | 7.88 | 43,600,000 | 1688.732394 |
| 9 | L-Epicatechin | 8.48 | 13,700,000 | 1043.628809 |
| 10 | Vitexin | 7.50 | 793,000 | 843.6170213 |
| (C) | ||||
| Number | Substance Name | Peak Time (min) | Peak Area (mAU/min) | Content (ng/mL) |
| 1 | Rutin | 8.01 | 18,600,000 | 2208.913897 |
| 2 | Myricitrin | 8.74 | 7,410,000 | 1711.591195 |
| 3 | Quercitrin | 10.2 | 7,400,000 | 436.5024289 |
| 4 | Neohesperidin | 7.99 | 18,800,000 | 393.4991381 |
| 5 | Protocatechuic acid | 7.37 | 19,600,000 | 338.7242852 |
| 6 | Hesperidin | 8.00 | 2,030,000 | 271.3283015 |
| 7 | Astragalin | 7.88 | 5,120,000 | 257.3486722 |
| 8 | Glycitin | 11.5 | 2,880,000 | 84.59146169 |
| 9 | Protocatechualdehyde | 7.20 | 1,260,000 | 82.50577772 |
| 10 | Tangeretin | 12.1 | 4,490,000 | 80.31816182 |
| (D) | ||||
| Number | Substance Name | Peak Time (min) | Peak Area (mAU/min) | Content (ng/mL) |
| 1 | Fisetin | 6.51 | 8,030,000 | 61,360.33017 |
| 2 | Myricitrin | 8.74 | 76,200,000 | 35,803.23223 |
| 3 | Phloretin | 9.77 | 24,800,000 | 24,165.2424 |
| 4 | Luteolin | 8.92 | 2,930,000 | 20,160.9461 |
| 5 | Kaempferol | 8.28 | 6,140,000 | 17,209.34555 |
| 6 | Vitexin | 7.50 | 2,390,000 | 6711.708735 |
| 7 | Quercitrin | 10.2 | 55,700,000 | 6683.370977 |
| 8 | Astragalin | 7.88 | 56,200,000 | 5746.107077 |
| 9 | L-Epicatechin | 8.48 | 25,000,000 | 5027.22768 |
| 10 | Rutin | 8.01 | 20,500,000 | 4952.279327 |
| (E) | ||||
| Number | Substance Name | Peak Time (min) | Peak Area (mAU/min) | Content (ng/mL) |
| 1 | Fisetin | 6.51 | 8,260,000 | 33,317.94651 |
| 2 | Myricitrin | 8.74 | 70,100,000 | 17,386.46613 |
| 3 | Phloretin | 9.77 | 23,200,000 | 11,933.10595 |
| 4 | Luteolin | 8.92 | 1,670,000 | 6065.768006 |
| 5 | Quercitrin | 10.2 | 45,900,000 | 2907.228018 |
| 6 | Astragalin | 7.88 | 51,400,000 | 2774.128377 |
| 7 | L-Epicatechin | 8.48 | 24,000,000 | 2547.56823 |
| 8 | Kaempferol | 8.28 | 1,370,000 | 2026.947908 |
| 9 | Neohesperidin | 7.99 | 71,400,000 | 1604.706504 |
| 10 | Rutin | 8.01 | 12,200,000 | 1555.740433 |
| Sample Name | Fisetin | Myricetin | Luteolin | Rutin | Kaempferide | Phloretin |
|---|---|---|---|---|---|---|
| A | 13,965.77 | 202.60 | 5046.74 | 2376.83 | 455.00 | 1984.23 |
| B | 12,042.11 | 183.71 | 5109.00 | 2059.07 | 405.91 | 4503.3 |
| C | 67.99 | 6.52 | 45.33 | 2208.91 | 8.16 | 14.32 |
| D | 61,360.33 | 1323.14 | 20,160.95 | 4952.28 | 1642.04 | 24,165.24 |
| E | 33,317.95 | 619.44 | 6065.77 | 1555.74 | 651.31 | 11,933.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Wu, X.; Yin, Q.; Dong, Z.; Zheng, L.; Qian, Y.; Sun, Y.; Chen, Z.; Zhai, K. Extraction, Identification, and Antioxidant Activity of Flavonoids from Hylotelephium spectabile (Boreau) H. Ohba. Foods 2024, 13, 2652. https://doi.org/10.3390/foods13172652
Li N, Wu X, Yin Q, Dong Z, Zheng L, Qian Y, Sun Y, Chen Z, Zhai K. Extraction, Identification, and Antioxidant Activity of Flavonoids from Hylotelephium spectabile (Boreau) H. Ohba. Foods. 2024; 13(17):2652. https://doi.org/10.3390/foods13172652
Chicago/Turabian StyleLi, Na, Xiao Wu, Qin Yin, Zeng Dong, Lele Zheng, Yihui Qian, Yulu Sun, Ziping Chen, and Kefeng Zhai. 2024. "Extraction, Identification, and Antioxidant Activity of Flavonoids from Hylotelephium spectabile (Boreau) H. Ohba" Foods 13, no. 17: 2652. https://doi.org/10.3390/foods13172652
APA StyleLi, N., Wu, X., Yin, Q., Dong, Z., Zheng, L., Qian, Y., Sun, Y., Chen, Z., & Zhai, K. (2024). Extraction, Identification, and Antioxidant Activity of Flavonoids from Hylotelephium spectabile (Boreau) H. Ohba. Foods, 13(17), 2652. https://doi.org/10.3390/foods13172652





