Advancements in Non-Thermal Processing Technologies for Enhancing Safety and Quality of Infant and Baby Food Products: A Review
Abstract
1. Introduction
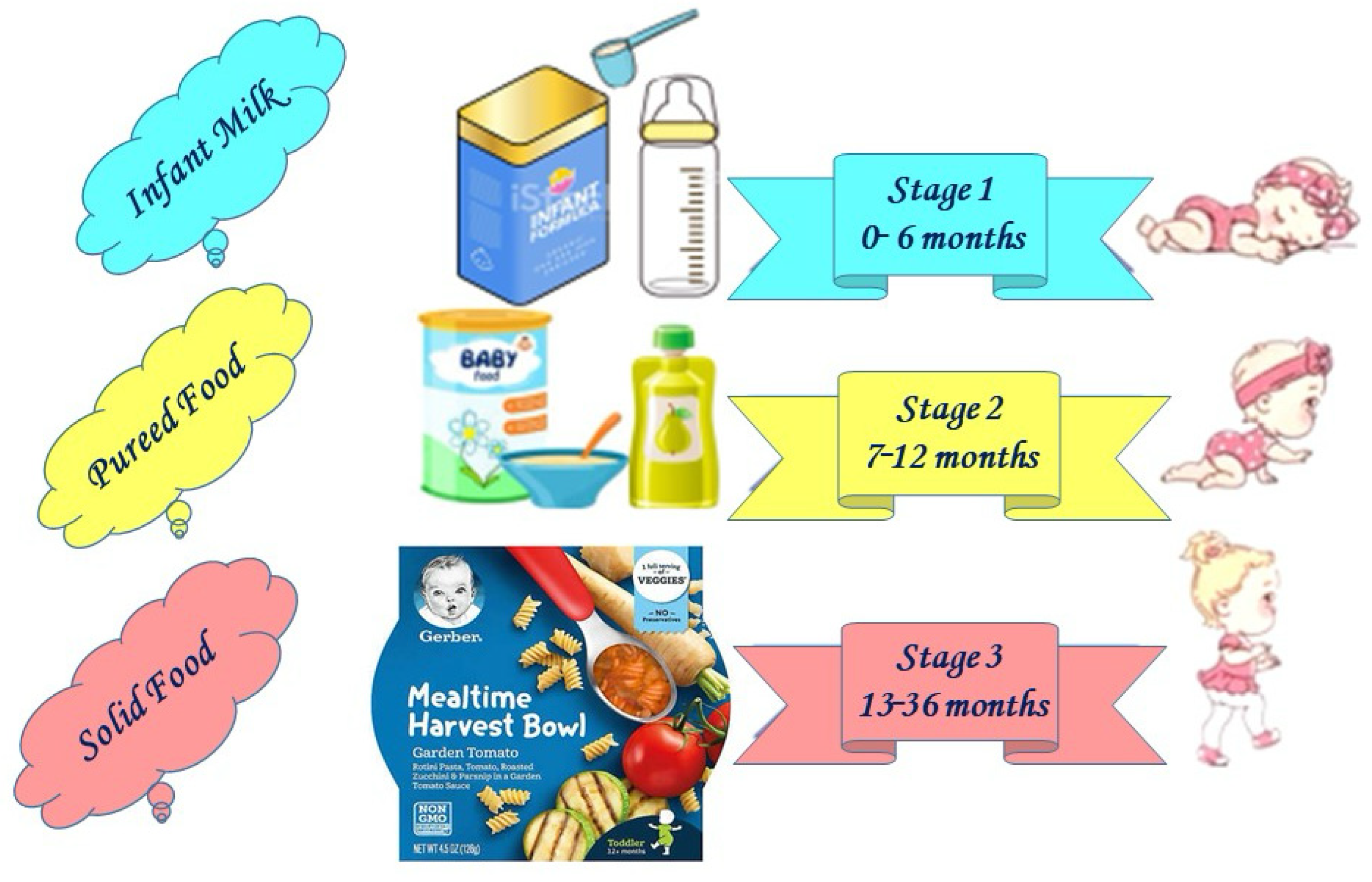
2. Infant and Young Children’s Food Categories
2.1. Infant Formula
2.2. Complementary Foods
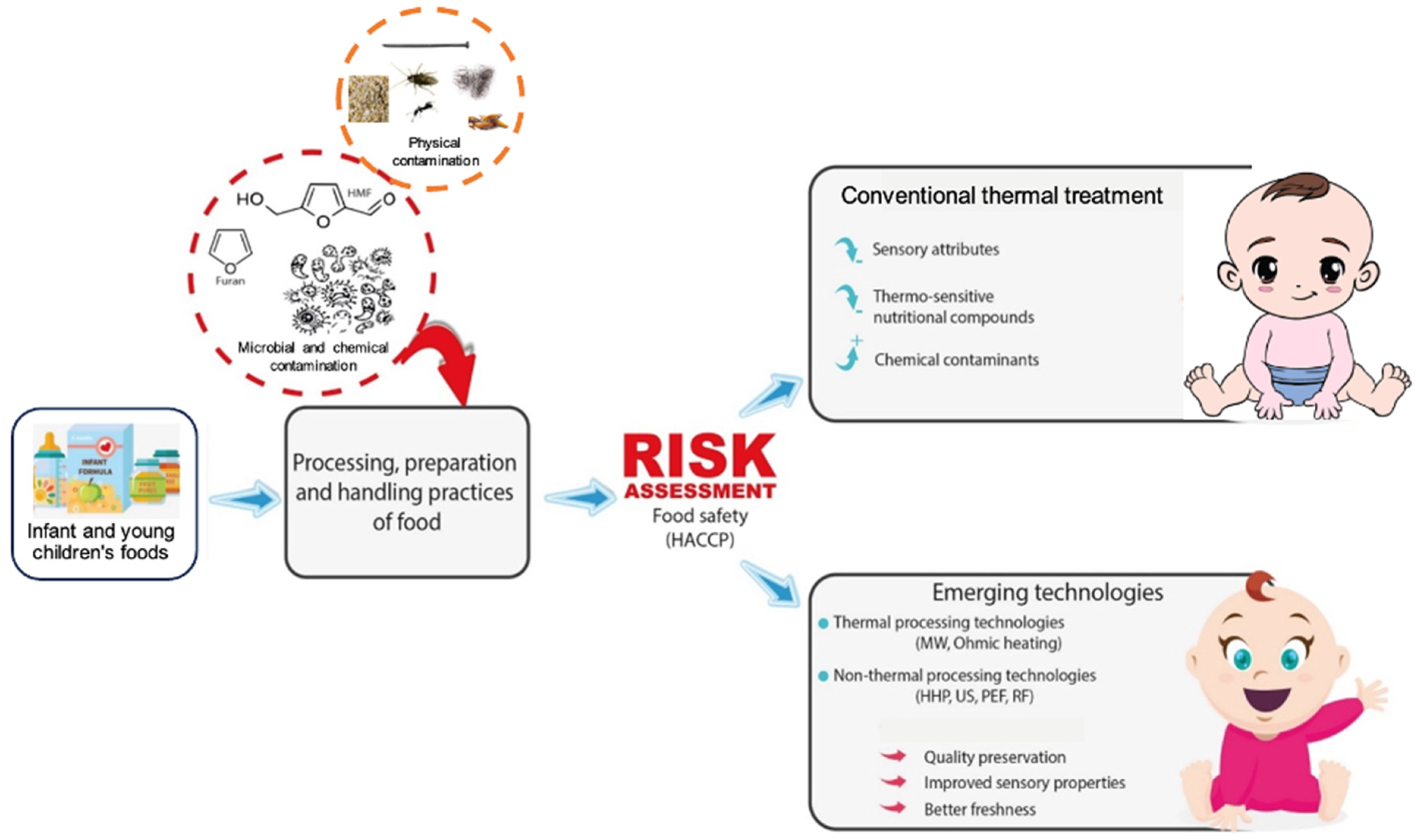
3. Assessment of Hazards and Safety Measures in Infant and Baby Foods
3.1. Microbial Hazards
3.2. Physical Hazards
3.3. Chemical Hazards
3.3.1. Furan
3.3.2. 5-Hydroxymethylfurfural (HMF)
3.3.3. Acrylamide, 3-MCPD Esters, and Glycidyl Esters
4. Conventional Thermal Processing
5. Non-Thermal Processing Technologies
5.1. High-Pressure Processing (HPP) Technology
5.2. Radio Frequency (RF) Technology
5.3. Ultrasound (US) Technology
5.4. Pulsed Electric Field (PEF) Technology
6. Regulatory Landscape of Non-Thermal Processing Technologies
7. Conclusions and Future Trends
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BFs | Baby foods |
| CAGR | Compound annual growth rate |
| FAO | Food and agriculture organization |
| FPCs | Food processing contaminants |
| FOF | Follow-on formula |
| FPC | Food-processing contaminants |
| FUF | Follow-up formula |
| HACCP | Hazard analysis critical control point |
| HHP | High hydrostatic pressure |
| HMF | 5-hydroxymethyl-2-furfural |
| HPP | High-pressure processing |
| HPTS | High-pressure thermal sterilization |
| IB | Infant botulism |
| IFs | Infant formulas |
| MWH | Microwave heating |
| OH | Ohmic heating |
| PATS | Pressure assisted thermal sterilization |
| PEF | Pulsed electric field |
| PFUF | Powdered follow-up formulas |
| PIF | Powdered infant formula |
| PIFM | Powdered infant formula milk |
| RDA | Recommended dietary Allowance |
| RF | Radio frequency |
| RPIFM | Reconstituted powdered infant formula milk |
| UHT | Direct ultrahigh temperature |
| US | Ultrasound |
References
- Fragkou, P.C.; Karaviti, D.; Zemlin, M.; Skevaki, C. Impact of Early Life Nutrition on Children’s Immune System and Noncommunicable Diseases Through Its Effects on the Bacterial Microbiome, Virome and Mycobiome. Front. Immunol. 2021, 12, 644269. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.S.; Santos, M.E. A Thousand Days-A programme for vulnerable early childhood in Argentina: Targeting, dropout risk factors and correlates of time to graduation. Child Care Health Dev. 2023, 49, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Barker, D. The Developmental Origins of Chronic Adult Disease. Acta Paediatr. Suppl. 2004, 93, 26–33. [Google Scholar] [CrossRef]
- Floris, R.; Lambers, T.; Alting, A.; Kiers, J. Trends in Infant Formulas: A Dairy Perspective. In Improving the Safety and Quality of Milk: Improving Quality in Milk Products; Griffiths, M.W., Ed.; Woodhead Publishing: Cambridge, UK; Limited, University of Guelph: Guelph, ON, Canada, 2010; pp. 454–474. [Google Scholar] [CrossRef]
- Patel, J.K.; Rouster, A.S. Infant Nutrition Requirements and Options; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Maldonado-Pereira, L.; Barnaba, C.; Medina-Meza, I.G. Dietary Exposure Assessment of Infant Formula and Baby Foods’ Oxidized Lipids in the US Population. Food Chem. Toxicol. 2023, 172, 113552. [Google Scholar] [CrossRef] [PubMed]
- Infant and Toddler Nutrition|Nutrition|CDC. Available online: https://www.cdc.gov/nutrition/infantandtoddlernutrition/index.html (accessed on 19 April 2023).
- Boué, G.; Cummins, E.; Guillou, S.; Antignac, J.P.; Le Bizec, B.; Membré, J.M. Public Health Risks and Benefits Associated with Breast Milk and Infant Formula Consumption. Crit. Rev. Food Sci. Nutr. 2018, 58, 126–145. [Google Scholar] [CrossRef]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Kent, R.M.; Fitzgerald, G.F.; Hill, C.; Stanton, C.; Ross, R.P. Novel Approaches to Improve the Intrinsic Microbiological Safety of Powdered Infant Milk Formula. Nutrients 2015, 7, 1217–1244. [Google Scholar] [CrossRef]
- Intelligence, M.; Mordor Intelligence. Obtenido de Mercado De Productos Cosméticos Y de Cuidado Personal Orgánicos: Crecimiento, Tendencias Y Pronósticos (2023–2028). Available online: https://www.mordorintelligence.com/es/industry-reports/organic-personal-care-and-cosmetic-product-market (accessed on 23 April 2024).
- Baby Food Market Size, Analysis|Global Report To 2032. Available online: https://www.gminsights.com/industry-analysis/baby-food-market (accessed on 18 April 2023).
- Hu, K.; Liu, J.; Li, B.; Liu, L.; Gharibzahedi, S.M.T.; Su, Y.; Jiang, Y.; Tan, J.; Wang, Y.; Guo, Y. Global Research Trends in Food Safety in Agriculture and Industry from 1991 to 2018: A Data-Driven Analysis. Trends Food Sci. Technol. 2019, 85, 262–276. [Google Scholar] [CrossRef]
- Murphy, E.G.; Fenelon, M.A.; Roos, Y.H.; Hogan, S.A. Decoupling Macronutrient Interactions During Heating of Model Infant Milk Formulas. J. Agric. Food Chem. 2014, 62, 10585–10593. [Google Scholar] [CrossRef]
- Xiong, K.; Li, M.M.; Chen, Y.Q.; Hu, Y.M.; Jin, W. Formation and Reduction of Toxic Compounds Derived from the Maillard Reaction During the Thermal Processing of Different Food Matrices. J. Food Prot. 2024, 87, 100338. [Google Scholar] [CrossRef]
- Boz, H. Furosine in Cereal Products—A Review. J. Stored Prod. Res. 2023, 102, 102114. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, H.; Chen, Y.F.; Pan, J.J.; Liu, A.D.; Pan, F.; Zhang, J.B.; Zhong, H.N. Risk Assessment of MOAH and MOSH in Infants and Young Children. Biomed. Environ. Sci. 2019, 32, 130–133. [Google Scholar] [CrossRef] [PubMed]
- ESPGHAN Committee on Nutrition. Preparation and Handling of Powdered Infant Formula: A Commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 320–322. [Google Scholar] [CrossRef]
- Maryniak, N.Z.; Sancho, A.I.; Hansen, E.B.; Bøgh, K.L. Alternatives to Cow’s Milk-Based Infant Formulas in the Prevention and Management of Cow’s Milk Allergy. Foods 2022, 11, 926. [Google Scholar] [CrossRef]
- Dipasquale, V.; Serra, G.; Corsello, G.; Romano, C. Standard and Specialized Infant Formulas in Europe: Making, Marketing, and Health Outcomes. Nutr. Clin. Pract. 2020, 35, 273–281. [Google Scholar] [CrossRef]
- Masum, A.K.M.; Chandrapala, J.; Huppertz, T.; Adhikari, B.; Zisu, B. Production and Characterization of Infant Milk Formula Powders: A Review. Dry. Technol. 2021, 39, 1492–1512. [Google Scholar] [CrossRef]
- Saeeduddin, M.; Abid, M.; Jabbar, S. Quality Assessment of Pear Juice Under Ultrasound and Commercial Pasteurization Processing Conditions. LWT—Food Sci. Technol. 2015, 64, 452–458. [Google Scholar] [CrossRef]
- Vella, C.; Attard, E. Consumption of Minerals, Toxic Metals and Hydroxymethylfurfural: Analysis of Infant Foods and Formulae. Toxics 2019, 7, 33. [Google Scholar] [CrossRef]
- Préstamo, G.; Sanz, P.D.; Fonberg-Broczek, M.; Arroyo, G. High Pressure Response of Fruit Jams Contaminated with Listeria monocytogenes. Lett. Appl. Microbiol. 1999, 28, 313–316. [Google Scholar] [CrossRef]
- Batool, Z.; Chen, J.H.; Liu, B.; Chen, F.; Wang, M. Review on Furan as a Food Processing Contaminant: Identifying Research Progress and Technical Challenges for Future Research. J. Agric. Food Chem. 2023, 71, 5093–5106. [Google Scholar] [CrossRef]
- Hamed, N.F.; Alamri, S.A.; Hamdi, N.H. Overview of the Updates in Nutrient Profiles, Types, Indications and Side Effects of Infant Formula. Arch. Pharm. Pract. 2022, 13, 54–61. [Google Scholar] [CrossRef]
- Heyman, M.B. Committee on Nutrition. Lactose Intolerance in Infants, Children, and Adolescents. Pediatrics 2006, 118, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Bourdeau, T.; Affolter, M.; Dupuis, L.; Panchaud, A.; Lahrichi, S.; Merminod, L.; Martin-Paschoud, C.; Adams, R.; Nutten, S.; Blanchard, C. Peptide Characterization and Functional Stability of a Partially Hydrolyzed Whey-Based Formula Over Time. Nutrients 2021, 13, 3011. [Google Scholar] [CrossRef]
- Aggett, P.J.; Agostoni, C.; Goulet, O.; Hernell, O.; Koletzko, B.; Lafeber, H.L.; Michaelsen, K.F.; Milla, P.; Rigo, J.; Weaver, L.T. Antireflux or Antiregurgitation Milk Products for Infants and Young Children: A Commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Żołnowska, M.; Berni Canani, R. Effects of an Extensively Hydrolyzed Formula Supplemented with Two Human Milk Oligosaccharides on Growth, Tolerability, Safety and Infection Risk in Infants with Cow’s Milk Protein Allergy: A Randomized, Multi-Center Trial. Nutrients 2022, 14, 530. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, J.; Greer, F. Use of Soy Protein-Based Formulas in Infant Feeding. Pediatrics 2008, 121, 1062–1068. [Google Scholar] [CrossRef]
- Łoś-Rycharska, E.; Kieraszewicz, Z.; Czerwionka-Szaflarska, M. Medium Chain Triglycerides (MCT) Formulas in Paediatric and Allergological Practice. Gastroenterol. Rev. 2016, 11, 226–231. [Google Scholar] [CrossRef]
- Ojha, S.; Dorling, J. Feed Thickeners in Gastro-Oesophageal Reflux in Infants. BMJ Paediatr Open 2018, 2, e000262. [Google Scholar] [CrossRef]
- Thomas, D.W.; Greer, F.R. Committee on Nutrition; Section on Gastroenterology, Hepatology, and Nutrition. Probiotics and Prebiotics in Pediatrics. Pediatrics 2010, 126, 1217–1231. [Google Scholar] [CrossRef]
- Donnet-Hughes, A.; Schiffrin, E.; Haschke, F.; Fichot, M.C.; Huber-Haag, K.J. Infant Formula with Probiotics. US Patent No. 8,377,430B2, 2013. [Google Scholar]
- Broderick, J.; Bussa, J.; O’Rourke, J.S. Mead Johnson Nutrition Company: A Controversy Over Enfamil (A); The Eugene D. Fanning Center for Business Communication, Mendoza College of Business, University of Notre Dame: Notre Dame, IN, USA, 2012. [Google Scholar]
- Kulwa, K.B.M.; Mamiro, P.S.; Kolsteren, P.W. Nutrition Education Package Focusing on Infant and Young Child Feeding in Tanzania. J. Nutr. Educ. Behav. 2023, 55, 493–508. [Google Scholar] [CrossRef]
- Crawley, H.; Westland, S.; Weston, S. Specialised Infant Milks in the UK: Infants 0–6 Months–Information for Health Professionals. 2017. Available online: https://gpifn.org.uk/wp-content/uploads/2016/09/2ef94-specialised_milk_sep19_final.pdf (accessed on 18 December 2023).
- Pina-Perez, M.C.; Martínez, A.; Rodrigo, D. New Advances in Infant Feeding: New Products and Novel Technologies. Recent. Pat. Food Nutr. Agric. 2016, 8, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Fortune Business Insights. Vegetable Puree Market Size, Growth, Trends & Forecast 2028. Available online: https://www.fortunebusinessinsights.com/vegetable-puree-market-104383 (accessed on 8 June 2023).
- Appel, L.J.; Baker, D.H.; Bar-Or, O.; Minaker, K.L.; Curtis Morris, R., Jr.; Resnick, L.M.; Sawka, M.N.; Volpe, S.L.; Weinberger, M.H.; Whelton, P.K. Panel on Dietary Reference Intakes for Electrolytes and Water Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. In Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Agostoni, C.; Przyrembel, H. The Timing of Introduction of Complementary Foods and Later Health. World Rev. Nutr. Diet. 2013, 108, 63–70. [Google Scholar] [CrossRef]
- Ayeni, K.I.; Sulyok, M.; Krska, R.; Warth, B.; Ezekiel, C.N. Mycotoxins in Complementary Foods Consumed by Infants and Young Children Within the First 18 Months of Life. Food Control 2023, 144, 109328. [Google Scholar] [CrossRef]
- Abeshu, M.A.; Lelisa, A.; Geleta, B. Complementary Feeding: Review of Recommendations, Feeding Practices, and Adequacy of Homemade Complementary Food Preparations in Developing Countries—Lessons from Ethiopia. Front. Nutr. 2016, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Microbiological Specifications for Foods (ICMSF). Pathogens and Indicator Organisms in Powdered Infant Formula. In Microorganisms in Foods 7: Microbiological Testing in Food Safety Management; Springer: Berlin/Heidelberg, Germany, 2018; pp. 345–355. [Google Scholar]
- Kim, S.A.; Oh, S.W.; Lee, Y.M.; Imm, J.Y.; Hwang, I.G.; Kang, D.H.; Rhee, M.S. Microbial Contamination of Food Products Consumed by Infants and Babies in Korea. Lett. Appl. Microbiol. 2011, 53, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, H.M.W.; Wells-Bennik, M.H.J.; Zwietering, M.H. Natural Diversity in Heat Resistance of Bacteria and Bacterial Spores: Impact on Food Safety and Quality. Ann. Rev. Food Sci. Technol. 2018, 9, 383–410. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ Panel); Koutsoumanis, K.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. The Efficacy and Safety of High-Pressure Processing of Food. EFSA J. 2022, 20, e07128. [Google Scholar] [CrossRef]
- Torrents-Masoliver, B.; Sandjong, D.; Jofré, A.; Ribas-Agustí, A.; Muñoz, I.; Felipe, X.; Castellari, M.; Meurillon, M.; den Besten, H.M.W.; Engel, E.; et al. Hazard Control Through Processing and Preservation Technologies for Enhancing the Food Safety Management of Infant Food Chains. Glob. Pediatr. 2022, 2, 100014. [Google Scholar] [CrossRef]
- Girma, K.; Tilahun, Z.; Haimanot, D. Review on Milk Safety with Emphasis on Its Public Health. World J. Dairy. Food Sci. 2014, 9, 166–183. [Google Scholar] [CrossRef]
- Malik, S.; Krishnaswamy, K.; Mustapha, A. Hazard Analysis and Risk-Based Preventive Controls (HARPC): Current Food Safety and Quality Standards for Complementary Foods. Foods 2021, 10, 2199. [Google Scholar] [CrossRef]
- Fraqueza, M.J.; Barreto, A.S. HACCP: Hazard Analysis and Critical Control Points. In Handbook of Fermented Meat and Poultry; Toldrá, F., Hui, Y.H., Astiasarán, I., Sebranek, J.G., Talon, R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 469–485. [Google Scholar]
- Gharibzahedi, S.M.T.; Hernández-Ortega, C.; Welti-Chanes, J.; Putnik, P.; Barba, F.J.; Mallikarjunan, K.; Escobedo-Avellaneda, Z.; Roohinejad, S. High Pressure Processing of Food-Grade Emulsion Systems: Antimicrobial Activity, and Effect on the Physicochemical Properties. Food Hydrocoll. 2019, 87, 307–320. [Google Scholar] [CrossRef]
- Guo, W.; Sanjeevi, P.; Gharibzahedi, S.M.T.; Guo, Y.; Wang, Y. Effects of Temperature and Fluid Velocity on Beer Pasteurization in Open and Closed Loop Heating Systems: Numerical Modeling and Simulation. Int. J. Food Eng. 2020, 16, 20190313. [Google Scholar] [CrossRef]
- Martín-Carrasco, I.; Carbonero-Aguilar, P.; Dahiri, B.; Moreno, I.M.; Hinojosa, M. Comparison Between Pollutants Found in Breast Milk and Infant Formula in the Last Decade: A Review. Sci. Total Environ. 2023, 875, 162461. [Google Scholar] [CrossRef] [PubMed]
- Nik Azmi, N.N.A.; Tan, T.C.; Ang, M.Y.; Leong, Y.H. Occurrence and Risk Assessment of 3-Monochloropropanediols Esters (3-MCPDE), 2-Monochloropropanediol Esters (2-MCPDE), and Glycidyl Esters (GE) in Commercial Infant Formula Samples from Malaysia. Food Addit. Contam. Part A 2023, 40, 212–221. [Google Scholar] [CrossRef]
- Hradecky, J.; Kludska, E.; Belkova, B.; Wagner, M.; Hajslova, J. Ohmic Heating: A Promising Technology to Reduce Furan Formation in Sterilized Vegetable and Vegetable/Meat Baby Foods. Innov. Food Sci. Emerg. Technol. 2017, 43, 1–6. [Google Scholar] [CrossRef]
- Di Pietro, G.; Forcucci, F.; Chiarelli, F. Endocrine Disruptor Chemicals and Children’s Health. Int. J. Mol. Sci. 2023, 24, 2671. [Google Scholar] [CrossRef] [PubMed]
- Mogol, B.A.; Gökmen, V. Thermal Process Contaminants: Acrylamide, Chloropropanols and Furan. Curr. Opin. Food Sci. 2016, 7, 86–92. [Google Scholar] [CrossRef]
- Mesías, M.; Wagner, M.; George, S.; Morales, F.J. Impact of Conventional Sterilization and Ohmic Heating on the Amino Acid Profile in Vegetable Baby Foods. Innov. Food Sci. Emerg. Technol. 2016, 34, 24–28. [Google Scholar] [CrossRef]
- IARC Monographs on the Identification of Carcinogenic Hazards to Humans—International Agency For Research On Cancer. Available online: https://monographs.iarc.who.int/ (accessed on 8 May 2023).
- Gül Akıllıoğlu, H.; Savaş Bahçeci, K.; Gökmen, V. Investigation and Kinetic Evaluation of Furan Formation in Tomato Paste and Pulp During Heating. Food Res. Int. 2015, 78, 224–230. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risks for Public Health Related to the Presence of Furan and Methylfurans in Food. EFSA J. 2017, 15, e05005. [Google Scholar] [CrossRef]
- van der Fels-Klerx, H.J.; Edwards, S.G.; Kennedy, M.C.; O‘Hagan, S.; O’Mahony, C.; Scholz, G.; Steinberg, P.; Chiodini, A. A Framework to Determine the Effectiveness of Dietary Exposure Mitigation to Chemical Contaminants. Food Chem. Toxicol. 2014, 74, 360–371. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Update on Furan Levels in Food from Monitoring Years 2004–2010 and Exposure Assessment. EFSA J. 2011, 9, 2347. [Google Scholar] [CrossRef]
- Başaran, B. The Evaluation of Childhood Foods and Infant Formula Exposure to Furan, Chloropropanols and Acrylamide Contamination by Food Processing. In Infant Feeding—Breast Versus Formula; Jaber, I., Al-Zwaini, Al-Ani, Z.R., Hurley, W., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Jestoi, M.; Järvinen, T.; Järvenpää, E.; Tapanainen, H.; Virtanen, S.; Peltonen, K. Furan in the Baby-Food Samples Purchased from the Finnish Markets—Determination with SPME-GC-MS. Food Chem. 2009, 117, 522–528. [Google Scholar] [CrossRef]
- Santonicola, S.; Mercogliano, R. Occurrence and Production of Furan in Commercial Foods. Italian J. Food Sci. 2016, 28, 155–177. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) Levels in Honey and Other Food Products: Effects on Bees and Human Health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Prata, R.; Petrarca, M.H.; Filho, J.T.; Godoy, H.T. Simultaneous Determination of Furfural, 5-Hydroxymethylfurfural and 4-Hydroxy-2,5-Dimethyl-3(2H)-Furanone in Baby Foods Available in the Brazilian Market. J. Food Compos. Anal. 2021, 99, 103874. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bin-Jumah, M.; Othman, S.I.; Khafaga, A.F.; Shaheen, H.M.; Samak, D.; Shehata, A.M.; Allam, A.A.; Abd El-Hack, M.E. The Toxicological Aspects of the Heat-Borne Toxicant 5-Hydroxymethylfurfural in Animals: A Review. Molecules 2020, 25, 1941. [Google Scholar] [CrossRef]
- Sabater, C.; Montilla, A.; Ovejero, A.; Prodanov, M.; Olano, A.; Corzo, N. Furosine and HMF Determination in Prebiotic-Supplemented Infant Formula from Spanish Market. J. Food Compos. Anal. 2018, 66, 65–73. [Google Scholar] [CrossRef]
- Er Demirhan, B.; Demirhan, B.; Sönmez, C.; Torul, H.; Tamer, U.; Yentür, G. Short Communication: Determination of Potential 5-Hydroxymethyl-2-Furaldehyde and 2-Furaldehyde Compounds in Follow-On Milks and Infant Formulas Using the High-Performance Liquid Chromatography Method. J. Dairy Sci. 2015, 98, 818–822. [Google Scholar] [CrossRef]
- Boyaci-Gunduz, C.P. Acrylamide Exposure of Infants and Toddlers Through Baby Foods and Current Progress on Regulations. Curr. Opin. Food Sci. 2022, 46, 100849. [Google Scholar] [CrossRef]
- Abt, E.; Robin, L.P.; McGrath, S.; Srinivasan, J.; DiNovi, M.; Adachi, Y.; Chirtel, S. Acrylamide Levels and Dietary Exposure from Foods in the United States, an Update Based on 2011–2015 Data. Food Addit Contam. Part A 2019, 36, 1475–1490. [Google Scholar] [CrossRef]
- EFSA: European Food Safety Authority; EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on Acrylamide in Food; EPSA: Parma, Italy, 2015; p. 321. [Google Scholar]
- Spungen, J.H.; MacMahon, S.; Leigh, J.; Flannery, B.; Kim, G.; Chirtel, S.; Smegal, D. Estimated US Infant Exposures to 3-MCPD Esters and Glycidyl Esters from Consumption of Infant Formula. Food Addit. Contam. Part A 2018, 35, 1085–1092. [Google Scholar] [CrossRef]
- Beekman, J.K.; Popol, S.; Granvogl, M.; MacMahon, S. Occurrence of 3-Monochloropropane-1, 2-Diol (3-MCPD) Esters and Glycidyl Esters in Infant Formulas from Germany. Food Addit. Contam. Part A 2021, 38, 1656–1671. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Fromberg, A. Monochloropropanediol and Glycidyl Esters in Infant Formula and Baby Food Products on the Danish Market: Occurrence and Preliminary Risk Assessment. Food Control 2020, 110, 106980. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Hernández-Hernández, H.M.; Villanueva-Rodríguez, S.J. Current Status of Emerging Food Processing Technologies in Latin America: Novel Thermal Processing. Innov. Food Sci. Emerg. Technol. 2018, 50, 196–206. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Miskeen, S.; Lee, B.H.; Oh, D.H. Hurdle Technology: A Novel Approach for Enhanced Food Quality and Safety—A Review. Food Control 2017, 73, 1426–1444. [Google Scholar] [CrossRef]
- Roux, S.; Courel, M.; Birlouez-Aragon, I.; Municino, F.; Massa, M.; Pain, J.P. Comparative Thermal Impact of Two UHT Technologies, Continuous Ohmic Heating and Direct Steam Injection, on the Nutritional Properties of Liquid Infant Formula. J. Food Eng. 2016, 179, 36–43. [Google Scholar] [CrossRef]
- Kelleher, C.M.; Tobin, J.T.; O’Mahony, J.A.; Kelly, A.L.; O’Callaghan, D.J.; McCarthy, N.A. A Comparison of Pilot-Scale Supersonic Direct Steam Injection to Conventional Steam Infusion and Tubular Heating Systems for the Heat Treatment of Protein-Enriched Skim Milk-Based Beverages. Innov. Food Sci. Emerg. Technol. 2019, 52, 282–290. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Guo, M. Processing Technology for Infant Formula. In Human Milk Biochemistry and Infant Formula Manufacturing Technology; Guo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 211–229. [Google Scholar] [CrossRef]
- Montagne, D.H.; Van Dael, P.; Skanderby, M.; Hugelshofer, W. Infant Formulae—Powders and Liquids. In Dairy Powders and Concentrated Products; Tamime, A.Y., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 294–331. [Google Scholar] [CrossRef]
- Henry, M.; Fouladkhah, A. Outbreak History, Biofilm Formation, and Preventive Measures for Control of Cronobacter sakazakii in Infant Formula and Infant Care Settings. Microorganisms 2019, 7, 77. [Google Scholar] [CrossRef]
- Aaliya, B.; Valiyapeediyekkal Sunooj, K.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; Sabu, S.; Sasidharan, A.; Theingi Hlaing, M.; George, J. Recent Trends in Bacterial Decontamination of Food Products by Hurdle Technology: A Synergistic Approach Using Thermal and Non-Thermal Processing Techniques. Food Res. Int. 2021, 147, 110514. [Google Scholar] [CrossRef]
- Rinaldi, M.; Langialonga, P.; Dhenge, R.; Aldini, A.; Chiavaro, E. Quality Traits of Apple Puree Treated with Conventional, Ohmic Heating and High-Pressure Processing. Eur. Food Res. Technol. 2021, 247, 1679–1688. [Google Scholar] [CrossRef]
- Sun, X.; Tan, Y.; Hao, J.; Jiang, Y.; Yang, Y.; Wang, X.; Wang, S.; Wang, Q.; Wang, L. Effects of Different Thermal Treatments on Structural and Physicochemical Properties of Model Infant Formula. SSRN Electron. J. 2023. [Google Scholar] [CrossRef]
- Kultur, G.; Misra, N.N.; Barba, F.J.; Koubaa, M.; Gökmen, V.; Alpas, H. Effect of High Hydrostatic Pressure on Background Microflora and Furan Formation in Fruit Purée Based Baby Foods. J. Food Sci. Technol. 2018, 55, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Kultur, G.; Misra, N.N.; Barba, F.J.; Koubaa, M.; Gökmen, V.; Alpas, H. Microbial Inactivation and Evaluation of Furan Formation in High Hydrostatic Pressure (HHP) Treated Vegetable-Based Infant Food. Food Res. Int. 2017, 101, 17–23. [Google Scholar] [CrossRef]
- Li, H.; Zhu, K.; Zhou, H.; Peng, W. Effects of High Hydrostatic Pressure Treatment on Allergenicity and Structural Properties of Soybean Protein Isolate for Infant Formula. Food Chem. 2012, 132, 808–814. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Zu, S.; Wu, X.; Shi, A.; Zhang, J.; Wang, Q.; He, N. Effects of High Hydrostatic Pressure on the Conformational Structure and Gel Properties of Myofibrillar Protein and Meat Quality: A Review. Foods 2021, 10, 1872. [Google Scholar] [CrossRef] [PubMed]
- Cetin-Karaca, H.; Morgan, M.C. Antimicrobial Efficacy of Cinnamaldehyde, Chitosan and High Pressure Processing against Cronobacter sakazakii in Infant Formula. J. Food Saf. 2020, 40, e12845. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Stockmann, R.; Terefe, N.S. Effect of Citric Acid and High Pressure Thermal Processing on Enzyme Activity and Related Quality Attributes of Pear Puree. Innov. Food Sci. Emerg. Technol. 2018, 45, 196–207. [Google Scholar] [CrossRef]
- Sevenich, R.; Kleinstueck, E.; Crews, C.; Anderson, W.; Pye, C.; Riddellova, K.; Hradecky, J.; Moravcova, E.; Reineke, K.; Knorr, D. High-Pressure Thermal Sterilization: Food Safety and Food Quality of Baby Food Puree. J. Food Sci. 2014, 79, M230–M237. [Google Scholar] [CrossRef]
- Sevenich, R.; Bark, F.; Kleinstueck, E.; Crews, C.; Pye, C.; Hradecky, J.; Reineke, K.; Lavilla, M.; Martinez-de-Maranon, I.; Briand, J.C.; et al. The Impact of High Pressure Thermal Sterilization on the Microbiological Stability and Formation of Food Processing Contaminants in Selected Fish Systems and Baby Food Puree at Pilot Scale. Food Control 2015, 50, 539–547. [Google Scholar] [CrossRef]
- Gratz, M.; Sevenich, R.; Hoppe, T. Gentle Sterilization of Carrot-Based Purees by High-Pressure Thermal Sterilization and Ohmic Heating and Influence on Food Processing Contaminants and Quality Attributes. Front. Nutr. 2021, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ismail, M.; Farid, M. Processing of Baby Food Using Pressure-Assisted Thermal Sterilization (PATS) and Comparison with Thermal Treatment. High. Press. Res. 2017, 37, 579–593. [Google Scholar] [CrossRef]
- Pina-pérez, M.C.; Aliaga, D.R.; Bernat, C.F.; Enguidanos, M.R.; López, A.M. Inactivation of Enterobacter sakazakii by Pulsed Electric Field in Buffered Peptone Water and Infant Formula Milk. Int. Dairy J. 2007, 17, 1441–1449. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Rodrigo, D.; López, A.M. Sub-lethal Damage in Cronobacter sakazakii Subsp. sakazakii Cells after Different Pulsed Electric Field Treatments in Infant Formula Milk. Food Control 2009, 20, 1145–1150. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Martínez-López, A.; Rodrigo, D. Cocoa Powder as a Natural Ingredient Revealing an Enhancing Effect to Inactivate Cronobacter Sakazakii Cells Treated by Pulsed Electric Fields in Infant Milk Formula. Food Control 2013, 32, 87–92. [Google Scholar] [CrossRef]
- Lin, Y.; Subbiah, J.; Chen, L.; Verma, T.; Liu, Y. Validation of Radio Frequency Assisted Traditional Thermal Processing for Pasteurization of Powdered Infant Formula Milk. Food Control 2020, 109, 106897. [Google Scholar] [CrossRef]
- Michael, M.; Phebus, R.K.; Thippareddi, H.; Thippareddi, H.; Subbiah, J.; Birla, S.L.; Schmidt, K.A. Validation of Radio-Frequency Dielectric Heating System for Destruction of Cronobacter sakazakii and Salmonella Species in Nonfat Dry Milk. J. Dairy Sci. 2014, 97, 7316–7324. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, G.; Xie, Y.; Liu, Y. Effects of Radio Frequency on Physicochemical Properties of Powdered Infant Formula Milk as Compared with Conventional Thermal Treatment. LWT 2020, 134, 110194. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Tang, J.; Wang, S.; Wang, L.; Zhu, G.; Li, X.; Liu, Y. Thermal Inactivation of Cronobacter sakazakii ATCC 29544 in Powdered Infant Formula Milk Using Thermostatic Radio Frequency. Food Control 2020, 114, 107270. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Lin, Y.; Xie, Y.; Wang, S.; Gao, Z.; Liu, Y. Comparison of Cronobacter sakazakii Thermal Death Kinetics in Powdered Infant Formula Milk Using Hot Water and Combined Radio Frequency and Hot Air Treatment. In Proceedings of the 2016 American Society of Agricultural and Biological Engineers Annual International Meeting, ASABE 2016, Orlando, FL, USA, 17–20 July 2016. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, Z.; Wang, S.; Wang, L.; Xie, Y.; Liu, Y. Dielectric Properties of Powdered Infant Formula Milk as Influenced by Frequency, Temperature and Main Components. In Proceedings of the 2016 American Society of Agricultural and Biological Engineers Annual International Meeting, ASABE 2016, Orlando, FL, USA, 17–20 July 2016. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, Y.; Zheng, Y.; Zhu, H.; Liu, Z.; Jiao, S. Assessment of Radio Frequency Heating on Composition, Microstructure, Flowability and Rehydration Characteristics of Milk Powder. Food Sci. Technol. 2017, 37, 544–551. [Google Scholar] [CrossRef]
- Lin, Y.; Ai, Z.; Liu, Y.; Tang, J.; Wang, S.; Gao, Z. Dielectric Loss Mechanism of Powdered Infant Formula Milk. Innov. Food Sci. Emerg. Technol. 2022, 76, 102950. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Chen, Y.; Pandiselvam, R.; Liu, Y. Surface Free Fat Bridging Contributes to the Stickiness of Powdered Infant Formula Milk Pasteurized by Radio Frequency Dry Heat Treatment. J. Food Eng. 2022, 323, 111001. [Google Scholar] [CrossRef]
- Adekunte, A.; Valdramidis, V.P.; Tiwari, B.K.; Slone, N.; Cullen, P.J.; Donnell, C.P.O.; Scannell, A. Resistance of Cronobacter sakazakii in Reconstituted Powdered Infant Formula During Ultrasound at Controlled Temperatures: A Quantitative Approach on Microbial Responses. Int. J. Food Microbiol. 2010, 142, 53–59. [Google Scholar] [CrossRef]
- Luo, M.; Shan, K.; Zhang, M. Application of Ultrasound Treatment for Improving the Quality of Infant Meat Puree. Ultrason. Sonochem. 2021, 80, 105831. [Google Scholar] [CrossRef]
- Djekic, I.; Sanjuán, N.; Clemente, G.; Jambrak, A.R.; Djukić-Vuković, A.; Brodnjak, U.V.; Pop, E.; Thomopoulos, R.; Tonda, A. Review on Environmental Models in the Food Chain—Current Status and Future Perspectives. J. Clean. Prod. 2018, 176, 1012–1025. [Google Scholar] [CrossRef]
- Misra, N.N.; Koubaa, M.; Roohinejad, S.; Juliano, P.; Alpas, H.; Inácio, R.S.; Saraiva, J.A.; Barba, F.J. Landmarks in the Historical Development of Twenty First Century Food Processing Technologies. Food Res. Int. 2017, 97, 318–339. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Pataro, G.; Tiwari, B. Guidelines on Reporting Treatment Conditions for Emerging Technologies in Food Processing. Crit. Rev. Food Sci. Nutr. 2022, 62, 5925–5949. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Saxena, V.K.; Dutta, S. Novel Thermal and Non-Thermal Processing of Watermelon Juice. Trends Food Sci. Technol. 2019, 93, 234–243. [Google Scholar] [CrossRef]
- Akdemie Evrendilek, G. Principles of High Pressure Processing and Its Equipment. In Non-Thermal Food Processing Operations; Jafari, S.M., Therdthai, N., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 301–318. [Google Scholar] [CrossRef]
- Park, S.H.; Balasubramaniam, V.M.; Sastry, S.K.; Lee, J. Pressure-Ohmic-Thermal Sterilization: A Feasible Approach for the Inactivation of Bacillus amyloliquefaciens and Geobacillus stearothermophilus Spores. Innov. Food Sci. Emerg. Technol. 2013, 19, 115–123. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B. Effects of High Hydrostatic Pressure on the Quality and Functionality of Protein Isolates, Concentrates, and Hydrolysates Derived from Pulse Legumes: A Review. Trends Food Sci. Technol. 2021, 107, 466–479. [Google Scholar] [CrossRef]
- Barba, F.J.; Terefe, N.S.; Buckow, R.; Knorr, D.; Orlien, V. New Opportunities and Perspectives of High Pressure Treatment to Improve Health and Safety Attributes of Foods. A Review. Food Res. Int. 2015, 77, 725–742. [Google Scholar] [CrossRef]
- Silva, F.V.M. Evelyn Pasteurization of Food and Beverages by High Pressure Processing (HPP) at Room Temperature: Inactivation of Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Salmonella, and Other Microbial Pathogens. Appl. Sci. 2023, 13, 1193. [Google Scholar] [CrossRef]
- Palmers, S.; Grauwet, T.; Celus, M.; Kebede, B.T.; Hendrickx, M.E.; Van Loey, A. Furan Formation as a Function of Pressure, Temperature and Time Conditions in Spinach Purée. LWT—Food Sci. Technol. 2015, 64, 565–570. [Google Scholar] [CrossRef]
- Patel, Y.K.; Patel, K.K. High Pressure Processing. In Novel Technologies in Food Science; Chhikara, N., Panghal, A., Chaudhary, G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 479–509. [Google Scholar] [CrossRef]
- Barba, F.J.; Koubaa, M.; do Prado-Silva, L.; Orlien, V.; de Souza Sant’Ana, A. Mild Processing Applied to the Inactivation of the Main Foodborne Bacterial Pathogens: A Review. Trends Food Sci. Technol. 2017, 66, 20–35. [Google Scholar] [CrossRef]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Režek Jambrak, A.; Granato, D.; Montesano, D.; Bursać Kovačević, D. Novel Food Processing and Extraction Technologies of High-Added Value Compounds from Plant Materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Sevenich, R.; Mathys, A. Continuous Versus Discontinuous Ultra-High-Pressure Systems for Food Sterilization with Focus on Ultra-High-Pressure Homogenization and High-Pressure Thermal Sterilization: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 646–662. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Sonar, C.R.; Patel, J.; Albahr, Z.; Sablani, S.S. High Pressure-Assisted Thermal Sterilization of Low-Acid Fruit and Vegetable Purees: Microbial Safety, Nutrient, Quality, and Packaging Evaluation. Food Control 2020, 114, 107233. [Google Scholar] [CrossRef]
- IFSH Receives FDA Acceptance of Pressure Enhanced Sterilization Process for Commercial Production of Multi-Component Shelf-Stable Foods. Available online: https://www.food-safety.com/articles/2681-ifsh-receives-fda-acceptance-of-pressure-enhanced-sterilization-process-for-commercial-production-of-multicomponent-shelf-stable-foods (accessed on 13 July 2024).
- Bu, G.; Li, T. High Hydrostatic Pressure Treatment Reduces the Potential Antigenicity of Β-Conglycinin by Changing the Protein Structure During In Vitro Digestion. J. Sci. Food Agric. 2022, 102, 4025–4034. [Google Scholar] [CrossRef]
- Cetin-Karaca, H.; Morgan, M.C. Inactivation of Bacillus cereus Spores in Infant Formula by Combination of High Pressure and Trans-Cinnamaldehyde. LWT 2018, 97, 254–260. [Google Scholar] [CrossRef]
- Mariadon Shanlang Pathaw, P.; Mukhim, C.; Rani, S.; Kamble, D.B.; Swer, T.L. Principles of Radiofrequency Processing in the Food Industry. In Emerging Thermal Processes in the Food Industry; Jafari, S.M., Ed.; Woodhead Publishing: Sawston, UK, 2023; pp. 289–312. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Leone, F.; Bressan, F.; Battaglia, G.; Veccia, T.; Chiofalo, V. Radio Frequency Heating of Milk–Effects on Quality, Safety, and Shelf Life Assessed Using Artificial Senses and Chemometric Tools. Electronics 2018, 7, 402. [Google Scholar] [CrossRef]
- Pugliese, A.; Cabassi, G.; Chiavaro, E.; Paciulli, M.; Carini, E.; Mucchetti, G. Physical Characterization of Whole and Skim Dried Milk Powders. J. Food Sci. Technol. 2017, 54, 3433–3442. [Google Scholar] [CrossRef]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, Applications, and Comparison of Thermal (Pasteurization, Sterilization, and Aseptic Packaging) against Non-Thermal (Ultrasounds, UV Radiation, Ozonation, High Hydrostatic Pressure) Technologies in Food Processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Faisal Manzoor, M.; Ali, M.; Muhammad Aadil, R.; Ali, A.; Goksen, G.; Li, J.; Zeng, X.A.; Proestos, C. Sustainable Emerging Sonication Processing: Impact on Fungicide Reduction and the Overall Quality Characteristics of Tomato Juice. Ultrason. Sonochem. 2023, 94, 106313. [Google Scholar] [CrossRef]
- Carrillo-Lopez, L.M.; Luna-Rodriguez, L.; Alarcon-Rojo, A.D.; Huerta-Jimenez, M. High Intensity Ultrasound Homogenizes and Improves Quality of Beef Longissimus dorsi. Food Sci. Technol. 2018, 39, 332–340. [Google Scholar] [CrossRef]
- Chavan, P.; Sharma, P.; Sharma, S.R.; Mittal, T.C.; Jaiswal, A.K. Application of High-Intensity Ultrasound to Improve Food Processing Efficiency: A Review. Foods 2022, 11, 122. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, H.; Mei, J.; Xie, J.; Shao, C. Advances in Application of Ultrasound in Meat Tenderization: A Review. Front. Sustain. Food Syst. 2022, 6, 1–17. [Google Scholar] [CrossRef]
- Roohinejad, S.; Oey, I.; Everett, D.W.; Niven, B.E. Evaluating the Effectiveness of β-Carotene Extraction from Pulsed Electric Field-Treated Carrot Pomace Using Oil-in-Water Microemulsion. Food Bioprocess Technol. 2014, 7, 3336–3348. [Google Scholar] [CrossRef]
- Roohinejad, S.; Everett, D.W.; Oey, I. Effect of Pulsed Electric Field Processing on Carotenoid Extractability of Carrot Purée. Int. J. Food Sci. Technol. 2014, 49, 2120–2127. [Google Scholar] [CrossRef]
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Bursać Kovačević, D. Pulsed Electric Fields as an Alternative to Thermal Processing for Preservation of Nutritive and Physicochemical Properties of Beverages: A Review. J. Food Process Eng. 2018, 41, e12638. [Google Scholar] [CrossRef]
- Oey, I.; Giteru, S.; Leong, S.Y. Methods and Protocols for Pulsed Electric Fields Treatment of Foods. In Emerging Food Processing Technologies; Gavahian, M., Ed.; Springer: Humana, NY, USA, 2022; pp. 1–29. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Sonne, A.M.; Grunert, K.G.; Banati, D.; Pollák-Tóth, A.; Lakner, Z.; Olsen, N.V.; Žontar, T.P.; Peterman, M. Consumer Perception of the Use of High-Pressure Processing and Pulsed Electric Field Technologies in Food Production. Appetite 2009, 52, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Dharini, M.; Mahendran, R. Status of International Regulations for Non-Thermal Processing of Foods. In Non-Thermal Technologies for the Food Industry; CRC Press: Boca Raton, FL, USA, 2024; pp. 345–354. [Google Scholar]
- Rodrigo, D.; Sampedro, F.; Silva, A.; Palop, A.; Martínez, A. New Food Processing Technologies as a Paradigm of Safety and Quality. Br. Food J. 2010, 112, 467–475. [Google Scholar] [CrossRef]
- Leong, S.Y.; Duque, S.M.M.; Conde, L.A.; Khrisanapant, P.; Oey, I. Addressing the Opportunities of Non-Thermal Food Processing Technologies in the ASEAN Region Context. Int. J. Food Sci. Technol. 2024; early view. [Google Scholar] [CrossRef]
| Type of Infant/Baby Foods | Infants’ Foods Available | Features | Stage | Ref. |
|---|---|---|---|---|
| First infant formula milk (IFM) powder/liquid |  |
| Stage 1 | [22] |
| Follow-on formula (stage 2) |  |
| Stage 2 | [23,24] |
| Growing up milk GUM (toddler milk)/follow-up formula (FUF) |  |
| Stage 3 | [25] |
| Hungry baby formulas |  |
| Stage 1, 2 | [26] |
| Preterm formula |  |
| Stage 1 | [22] |
| Lactose-free formula |  |
| Stage 1, 2, 3 | [27] |
| Comfort formula |  |
| Stage 1, 2, 3 | [28,29] |
| Extensively hydrolyzed formula |  |
| Stage 1, 2, 3 | [30] |
| Hypoallergenic or elemental formula |  |
| Stage 1, 2, 3 | [30] |
| SOYA alternative milk formula |  |
| Stage 3 | [31] |
| MCT formula |  |
| Stage 1, 2 | [32] |
| Anti-reflux or pre-thickened formulas |  |
| Stage 1, 2 | [33] |
| Probiotic infant formula |  |
| Stage 1, 2, 3 | [34,35] |
| PKU formula |  |
| Stage 1, 2, 3 | [36] |
| Puree meal (animal foods) |  |
| Stage 2, 3 | [37] |
| Organic infant formula |  |
| Stage 1, 2, 3 | [38] |
| Complementary foods: cereal/ porridge |  |
| Stage 2, 3 | [39] |
| Complementary foods: fruit/vegetable puree |  |
| Stage 2,3 | [40] |
| Baby water |  |
| Stage 1, 2, 3 | [41] |
| Technology | Treatment Conditions | Food System or Product Substrate | Novel Processing Effects/Applications | Ref. |
|---|---|---|---|---|
| HHP | 200, 300, and 400 MPa; 25, 35, and 45 °C; 5, 10, and 15 min | BFs Fruit puree |
| [90] |
| HHP | 200, 300, and 400 MPa; 25, 35, and 45 °C; 5, 10, and 15 min | BFs Vegetable puree |
| [91] |
| HHP | 300 MPa; 20 °C; 5, 10, 15, and 20 min | IFs Based on soy protein isolate (SPI) |
| [92] |
| HHP | 400 MPa, 20 °C, 20 min | BFs Fruit jam |
| [93] |
| HPP-TC | 600 MPa, 5 min + TC (0.1%) Storage: 23 and 7 °C | RPIFM |
| [94] |
| HPP CH + TC | 600 MPa, 5 min + TC (0.1%) Storage at 7, 23, and 45 °C | IFs |
| [94] |
| HPTP | 600 MPa, 90 °C, 5 min | BFs Pear puree |
| [95] |
| HPTS | 600 MPa, 90–121 °C, 0.45–28 min | BFs Vegetable puree |
| [96] |
| HPTS | 600 MPa, 100–115 °C, 0.45–28 min | BFs Vegetable puree |
| [97] |
| PES-OH | PES: 600 MPa; 105, 110, and 121 °C for 5–10 min OH: 12 kHz; 115, 121, 125, and 130 °C for 3, 7, 14, and 21 min | BFs Carrot based puree |
| [98] |
| PATS | 135 MPa, 140 °C | BFs Apple puree |
| [99] |
| PEF | 10 to 40 kV/cm/60–3895 µs/25 °C | PIFM |
| [100] |
| PEF | 15 and 35 kV/cm Storage: 8 °C, 12 h | PIFM |
| [101] |
| PEF + CocoanOX 12%, CCX | 15, 25, and 35 kV/cm | Cacao milk |
| [102] |
| RF-TTP | 6 kW, 27.12 MHz, 65 °C, after 21 h | PIFM |
| [103] |
| RFDH | 3 kW; 27.12 MHz; at 75, 80, 85, or 90 °C; for 0 to 80 min | PIFM Nonfat dry milk (NDM) |
| [104] |
| RF-HA | 6 kW; 27.12 MHz; 65 and 70 °C | PIFM |
| [105] |
| RF-HW | 6 kW; 27.12 MHz; 65 and 70 °C | PIFM |
| [106] |
| RF-HW | 60, 65, 70 and 75 °C | PIFM |
| [107] |
| RF-MWH | 10 MHz–3 GHz; 0.36–0.54 g/cm3; 20–80 °C | PIFM |
| [108] |
| RF | 27.1 MHz; 6 kW; 90 °C; 5 and 10 min | PIFM |
| [109] |
| RF | 10 to 3000 MHz, 20–80 °C | PIFM |
| [110] |
| RF | 70 °C for 0, 23.3, 46.6, 69.9, 93.2, and 116.5 min | PIFM |
| [111] |
| US | 20 kHz; 24.4, 30.5, 42.7, 54.9, and 61 μm; 25, 35, and 50 °C; 0, 4, 8, 12, 16, and 20 min | IFs |
| [112] |
| US | 750 W; 20 kHz; 25, 45, and 65 °C; 10 min | Pear puree |
| [22] |
| US | 200, 400, or 600 W; 20 kHz; 4 °C; 15, 30, and 45 min | Meat puree |
| [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasdar, N.; Mostashari, P.; Greiner, R.; Khelfa, A.; Rashidinejad, A.; Eshpari, H.; Vale, J.M.; Gharibzahedi, S.M.T.; Roohinejad, S. Advancements in Non-Thermal Processing Technologies for Enhancing Safety and Quality of Infant and Baby Food Products: A Review. Foods 2024, 13, 2659. https://doi.org/10.3390/foods13172659
Pasdar N, Mostashari P, Greiner R, Khelfa A, Rashidinejad A, Eshpari H, Vale JM, Gharibzahedi SMT, Roohinejad S. Advancements in Non-Thermal Processing Technologies for Enhancing Safety and Quality of Infant and Baby Food Products: A Review. Foods. 2024; 13(17):2659. https://doi.org/10.3390/foods13172659
Chicago/Turabian StylePasdar, Nasim, Parisa Mostashari, Ralf Greiner, Anissa Khelfa, Ali Rashidinejad, Hadi Eshpari, Jim M. Vale, Seyed Mohammad Taghi Gharibzahedi, and Shahin Roohinejad. 2024. "Advancements in Non-Thermal Processing Technologies for Enhancing Safety and Quality of Infant and Baby Food Products: A Review" Foods 13, no. 17: 2659. https://doi.org/10.3390/foods13172659
APA StylePasdar, N., Mostashari, P., Greiner, R., Khelfa, A., Rashidinejad, A., Eshpari, H., Vale, J. M., Gharibzahedi, S. M. T., & Roohinejad, S. (2024). Advancements in Non-Thermal Processing Technologies for Enhancing Safety and Quality of Infant and Baby Food Products: A Review. Foods, 13(17), 2659. https://doi.org/10.3390/foods13172659








