Psidium guajava Seed Oil Reduces the Severity of Colitis Induced by Dextran Sulfate Sodium by Modulating the Intestinal Microbiota and Restoring the Intestinal Barrier
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Methods
2.2.1. Recording of Body Weight, Disease Activity Index, and Organ Indexes
2.2.2. Morphologic Observation of Colon Tissue
2.2.3. Measurement of Serum Biochemical Indicator
2.2.4. Determination of mRNA Expression Levels of Related Factors in Colonic Tissues
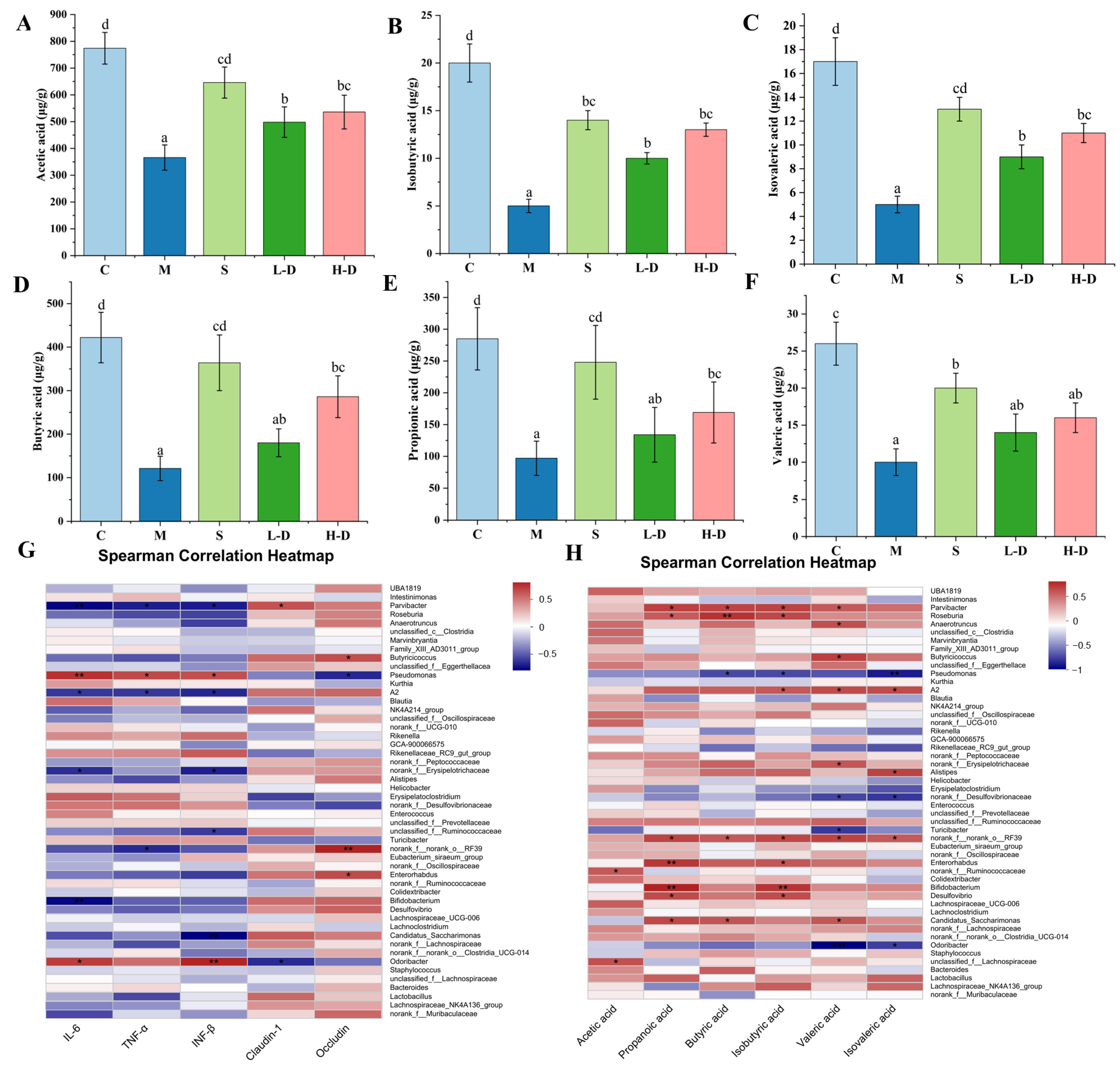
2.2.5. Determination of Short-Chain Fatty Acids in the Cecum
2.2.6. 16S rRNA Sequencing of the Mouse Gut Microbiome
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Psidium guajava Seed Oil on Body Weight and DAI Scores of Colitis Mice
3.2. Effect of Psidium guajava Seed Oil on Colonic Organ Index in Colitis Mice
3.3. Effect of Psidium guajava Seed Oil on the Morphology of Colon Tissue in Mice with Colitis
3.4. Effects of Psidium guajava Seed Oil on the Colonic Tissue Structure of Mice with Colitis
3.5. Effects of Psidium guajava Seed Oil on the Ultrastructure of Colon in Colitis Mice
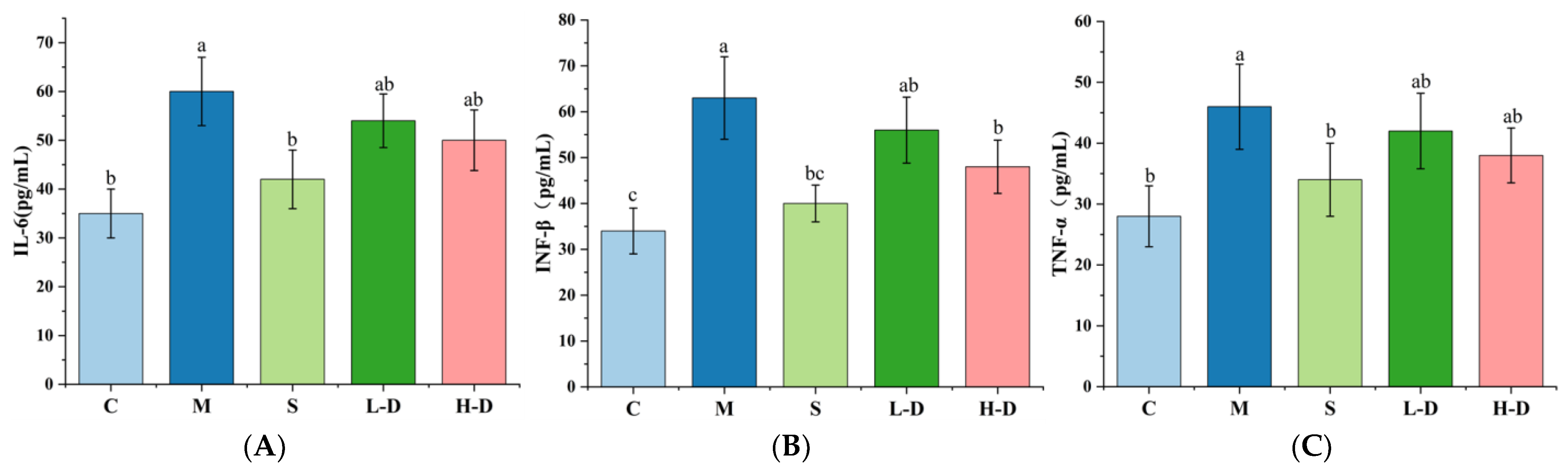
3.6. Effect of Psidium guajava Seed Oil on Inflammatory Factors in Serum and Colonic Tissues of Mice
3.7. Effect of Psidium guajava Seed Oil on the Expression Level of Colon Related mRNA Genes
3.8. Effect of Guarana Acid Oil on the Composition of Intestinal Microbiota of Mice with Colitis
3.8.1. Visualization of TKSO Distribution in Fecal Samples of Colitis Mice
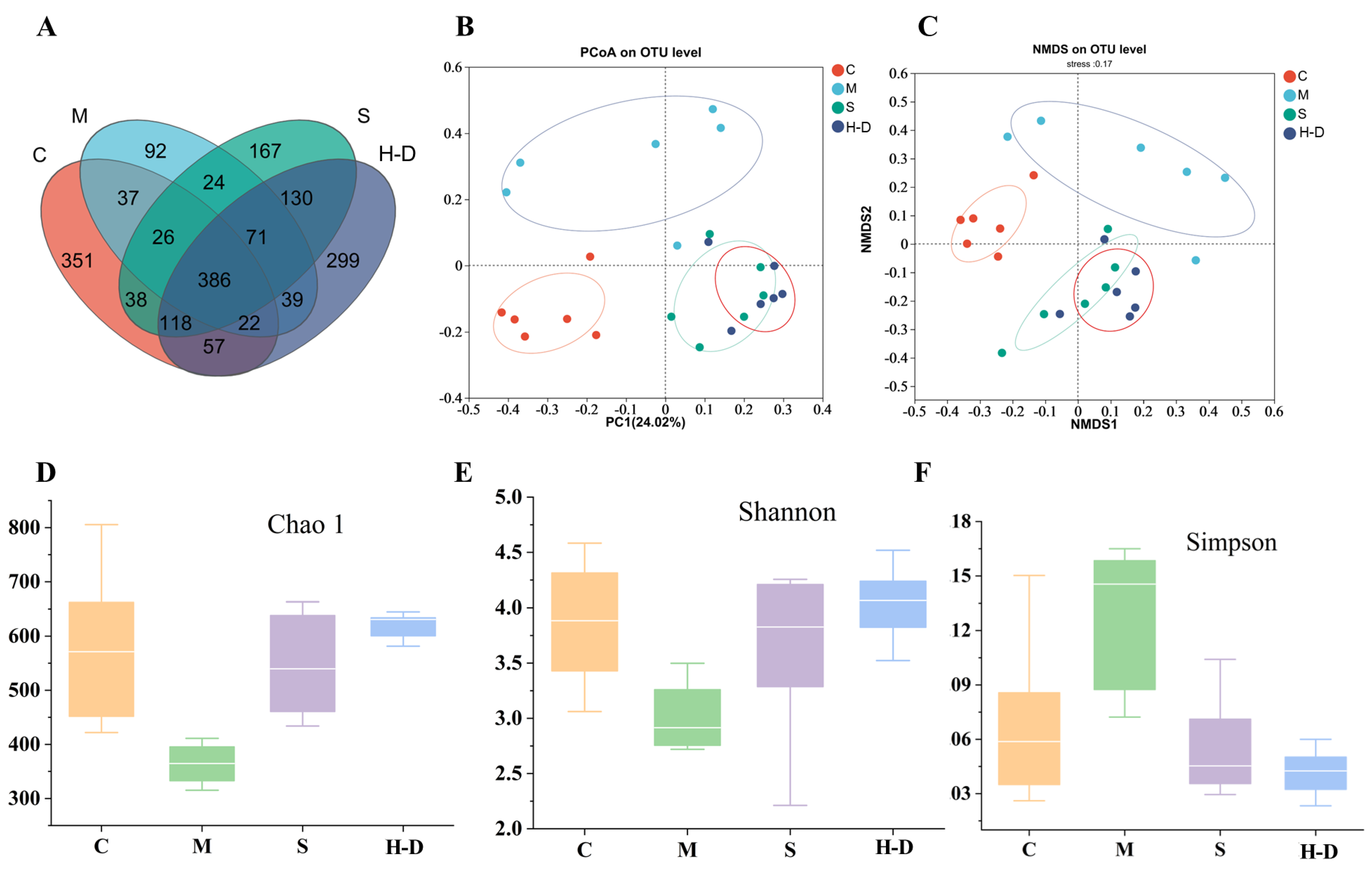
3.8.2. Effect of TKSO on Diversity in Colony Mice
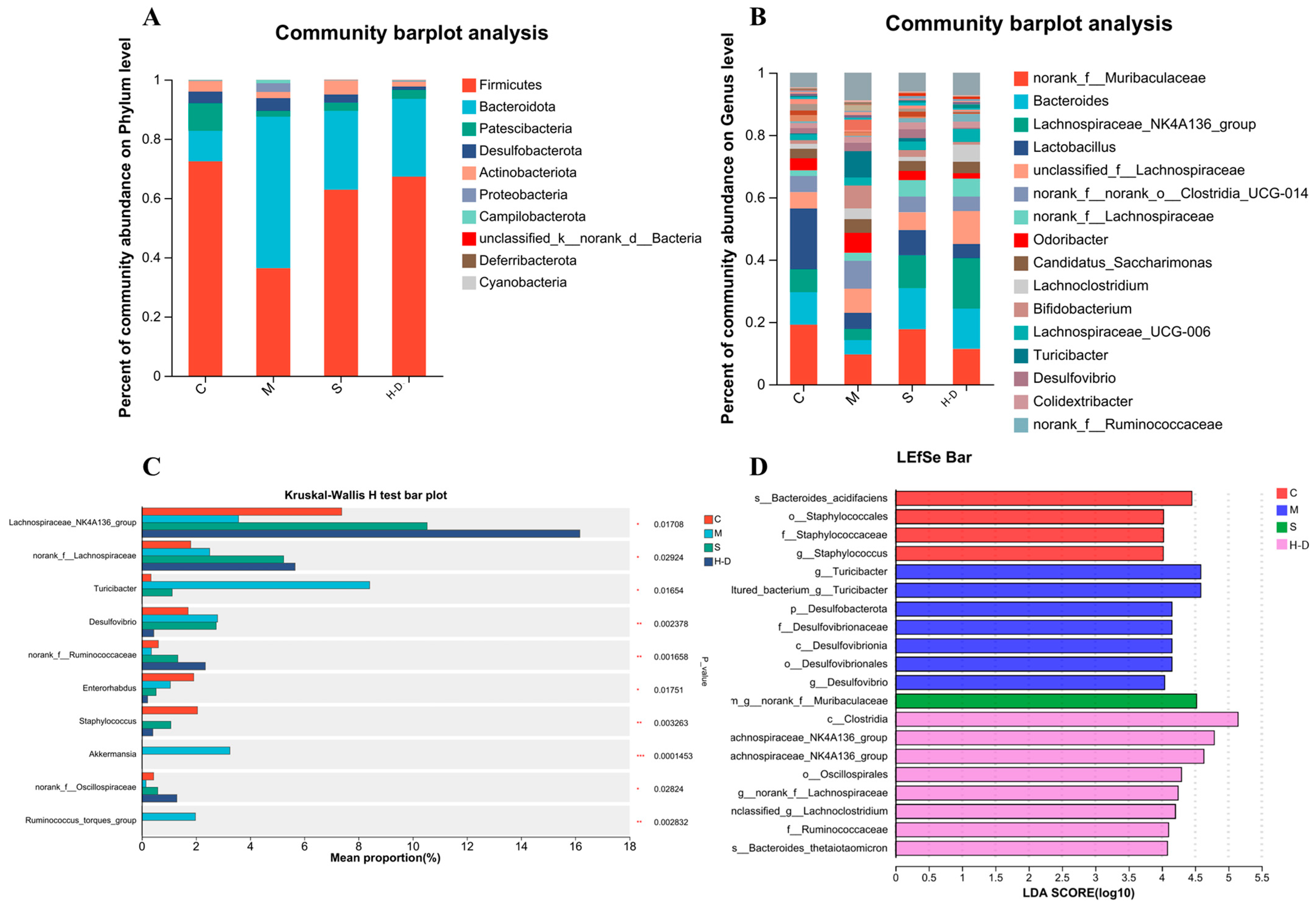
3.8.3. Composition and Abundance Analysis at the Gate Level
3.8.4. Composition and Abundance Analysis at the Genus Level
3.8.5. Correlation Analysis of Short-Chain Fatty Acids, Colon Biochemical Indicators and Intestinal Microbiota
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care Clin. Off. Pract. 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Kumar, L.; O’Morain, N.; Sheridan, J.; Coe, C.; Egan, C.; Jones, F.; Cullen, G.; Doran, P.; Leyden, J.; Galligan, M.; et al. P483 Golimumab response in Ulcerative Colitis as early as week 2: What can we take from this? J. Crohn’s Colitis 2023, 17, i614–i615. [Google Scholar] [CrossRef]
- Mei, Z.; Huang, X.; Zhang, H.; Cheng, D.; Xu, X.; Fang, M.; Hu, J.; Liu, Y.; Liang, Y.; Mei, Y. Chitin derivatives ameliorate DSS-induced ulcerative colitis by changing gut microbiota and restoring intestinal barrier function. Int. J. Biol. Macromol. 2022, 202, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Basso, P.J.; Fonseca, M.T.; Bonfá, G.; Alves, V.B.; Sales-Campos, H.; Nardini, V.; Cardoso, C.R. Association among genetic predisposition, gut microbiota, and host immune response in the etiopathogenesis of inflammatory bowel disease. Braz. J. Med. Biol. Res. = Rev. Bras. De Pesqui. Medicas E Biol. 2014, 47, 727–737. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, L.; Zhao, W.; Zhao, W.; Han, X.; Niu, J.; Li, R.; Zhao, C. Flaxseed oil alleviates dextran sulphate sodium-induced ulcerative colitis in rats. J. Funct. Foods 2020, 64, 103602. [Google Scholar] [CrossRef]
- Jiang, Q.; Jiang, C.; Lu, H.; Zhou, T.; Hu, W.; Ping Tan, C.; Feng, Y.; Shen, G.; Xiang, X.; Chen, L. Camellia oil alleviates DSS-induced colitis in mice by regulating the abundance of intestinal flora and suppressing the NF-κB signaling pathway. J. Funct. Foods 2023, 108, 105777. [Google Scholar] [CrossRef]
- Yao, S.; Lu, H.; Zhou, T.; Jiang, Q.; Jiang, C.; Hu, W.; Li, M.; Tan, C.P.; Feng, Y.; Du, Q.; et al. Sciadonic acid attenuates high-fat diet-induced bone metabolism disorders in mice. Food Funct. 2024, 15, 4490–4502. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Rodríguez-Varela, L.I.; Parada-Alfonso, F. Guava (Psidium guajava L.) seed oil obtained with a homemade supercritical fluid extraction system using supercritical CO2 and co-solvent. J. Supercrit. Fluids 2011, 56, 238–242. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, S.; Zhou, Z.; Akoh, C.C. Physicochemical Properties and Volatile Profiles of Cold-Pressed Trichosanthes kirilowii Maxim Seed Oils. Int. J. Food Prop. 2016, 19, 1765–1775. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Wang, Z.-X.; Wei, S.-S.; Liu, X.-Q.; He, J.-B.; Zhang, W.-N.; Du, J. Trichosanthes kirilowii Maxim seed kernel oil: The optimization of ultrasound-assisted extraction and evaluation of its potential as a novel biodiesel feedstock. Sustain. Chem. Pharm. 2023, 31, 100903. [Google Scholar] [CrossRef]
- de Souza, T.d.S.; Ferreira, M.F.d.S.; Menini, L.; Souza, J.R.C.d.L.; Bernardes, C.d.O.; Ferreira, A. Chemotype diversity of Psidium guajava L. Phytochemistry 2018, 153, 129–137. [Google Scholar] [CrossRef]
- Sun, S.; Guo, J.; Duan, X. Biodiesel preparation from Phoenix tree seed oil using ethanol as acyl acceptor. Ind. Crops Prod. 2019, 137, 270–275. [Google Scholar] [CrossRef]
- Pariza, M.W. Perspective on the safety and effectiveness of conjugated linoleic acid1234. Am. J. Clin. Nutr. 2004, 79, 1132S–1136S. [Google Scholar] [CrossRef] [PubMed]
- Fuke, G.; Nornberg, J.L. Systematic evaluation on the effectiveness of conjugated linoleic acid in human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1–7. [Google Scholar] [CrossRef]
- Jamieson, S.; Wallace, C.E.; Das, N.; Bhattacharyya, P.; Bishayee, A. Guava (Psidium guajava L.): A glorious plant with cancer preventive and therapeutic potential. Crit. Rev. Food Sci. Nutr. 2022, 63, 192–223. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lee, H.J.; Cho, H.Y.; Kim, Y.J. Role of the Conjugated Linoleic Acid in the Prevention of Cancer. Crit. Rev. Food Sci. Nutr. 2005, 45, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.S.; Cha, Y.-S.; Kim, K.-A. Protective Effect of Perilla Oil Against Dextran Sodium Sulfate-Induced Colitis in Mice Challenged with a High-Fat Diet. J. Med. Food 2022, 25, 1021–1028. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Q.; Jiang, C.; Lu, H.; Hu, W.; Yu, S.; Li, M.; Tan, C.P.; Feng, Y.; Xiang, X.; et al. Sciadonic acid attenuates high-fat diet-induced obesity in mice with alterations in the gut microbiota. Food Funct. 2023, 14, 2870–2880. [Google Scholar] [CrossRef]
- Tanideh, N.; Sadeghi, F.; Amanat, S.; Firoozi, D.; Noorafshan, A.; Iraji, A.; Koohi-Hosseinabadi, O. Protection by pure and genistein fortified extra virgin olive oil, canola oil, and rice bran oil against acetic acid-induced ulcerative colitis in rats. Food Funct. 2020, 11, 860–870. [Google Scholar] [CrossRef]
- Balaha, M.; Kandeel, S.; Elwan, W. Garlic oil inhibits dextran sodium sulfate-induced ulcerative colitis in rats. Life Sci. 2016, 146, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Shan, C.; Ma, T.; Geng, S.; Ning, D. Walnut oil alleviates DSS–induced colitis in mice by inhibiting NLRP3 inflammasome activation and regulating gut microbiota. Microb. Pathog. 2021, 154, 104866. [Google Scholar] [CrossRef]
- Mashtoub, S.; Howarth, G.S. Emu Oil and zinc monoglycerolate independently reduce disease severity in a rat model of ulcerative colitis. BioMetals 2023, 36, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ahn, M.; Choi, Y.; Kang, T.; Kim, J.; Lee, N.H.; Kim, G.O.; Shin, T. Alpha-Linolenic Acid Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Inflammation 2020, 43, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Aldamarany, W.A.S.; Taocui, H.; Liling, D.; Mei, H.; Yi, Z.; Zhong, G. Perilla, sunflower, and tea seed oils as potential dietary supplements with anti-obesity effects by modulating the gut microbiota composition in mice fed a high-fat diet. Eur. J. Nutr. 2023, 62, 2509–2525. [Google Scholar] [CrossRef]
- Pan, X.; Liu, P.; Zhang, Y.-J.; Zhang, H.-K.; Wei, H.; Jiang, J.-Y.; Hui, Y.; Shang, E.-X.; Li, W.-W.; Wang, Y.; et al. Carboxymethyl chitosan-TK resistant starch complex ameliorates type 2 diabetes by regulating the gut microbiota. Int. J. Biol. Macromol. 2023, 253, 126930. [Google Scholar] [CrossRef]
- Shao, X.; Liu, L.; Zhou, Y.; Zhong, K.; Gu, J.; Hu, T.; Yao, Y.; Zhou, C.; Chen, W. High-fat diet promotes colitis-associated tumorigenesis by altering gut microbial butyrate metabolism. Int. J. Biol. Sci. 2023, 19, 5004–5019. [Google Scholar] [CrossRef]
- Petelin, A.; Šik Novak, K.; Hladnik, M.; Bandelj, D.; Baruca Arbeiter, A.; Kramberger, K.; Kenig, S.; Jenko Pražnikar, Z. Helichrysum italicum (Roth) G. Don and Helichrysum arenarium (L.) Moench Infusion Consumption Affects the Inflammatory Status and the Composition of Human Gut Microbiota in Patients with Traits of Metabolic Syndrome: A Randomized Comparative Study. Foods 2022, 11, 3277. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, W.; Shen, Z.; Li, M.; Yin, J.; Tang, Y.; Zhou, X.; Zhu, X.; Sun, Z. The association between alpha diversity of gut microbiota, neuroimaging markers and cognitive function in cerebral small vessel disease. Brain Res. 2024, 1827, 148757. [Google Scholar] [CrossRef]
- Huang, F.; Zhao, R.; Xia, M.; Shen, G.X. Impact of Cyanidin-3-Glucoside on Gut Microbiota and Relationship with Metabolism and Inflammation in High Fat-High Sucrose Diet-Induced Insulin Resistant Mice. Microorganisms 2020, 8, 1238. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Yi, H.; Han, X.; Chi, C. Identification and characterization of plantaricin Q7, a novel plantaricin produced by Lactobacillus plantarum Q7. LWT—Food Sci. Technol. 2016, 71, 386–390. [Google Scholar] [CrossRef]
- Dong, J.; Ping, L.; Zhang, K.; Tang, H.; Liu, J.; Liu, D.; Zhao, L.; Evivie, S.E.; Li, B.; Huo, G. Immunomodulatory effects of mixed Lactobacillus plantarum on lipopolysaccharide-induced intestinal injury in mice. Food Funct. 2022, 13, 4914–4929. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.; Yuan, Q.; Hao, W.; Wang, Y.; Wu, D.; Chen, X.; Wang, S. Agaricus bisporus polysaccharides ameliorate ulcerative colitis in mice by modulating gut microbiota and its metabolism. Food Funct. 2024, 15, 1191–1207. [Google Scholar] [CrossRef]
- Zhao, H.; Mo, Q.; Kulyar, M.F.; Guan, J.; Zhang, X.; Luo, X.; Li, J. Metagenomic Analysis Reveals A Gut Microbiota Structure and Function Alteration between Healthy and Diarrheic Juvenile Yaks. Animals 2024, 14, 1181. [Google Scholar] [CrossRef]
- Lin, T.-C.; Soorneedi, A.; Guan, Y.; Tang, Y.; Shi, E.; Moore, M.D.; Liu, Z. Turicibacter fermentation enhances the inhibitory effects of Antrodia camphorata supplementation on tumorigenic serotonin and Wnt pathways and promotes ROS-mediated apoptosis of Caco-2 cells. Front. Pharmacol. 2023, 14, 1203087. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Goodson, M.L.; Vang, W.; Rutkowsky, J.; Kalanetra, K.; Bhattacharya, M.; Barile, D.; Raybould, H.E. Human milk oligosaccharide 2’-fucosyllactose supplementation improves gut barrier function and signaling in the vagal afferent pathway in mice. Food Funct. 2021, 12, 8507–8521. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wang, X.; Zhou, Y.; Zhao, X.; Chen, M.; Zhao, H.; Cao, W. Schisandra chinensis Bee Pollen Ameliorates Colitis in Mice by Modulating Gut Microbiota and Regulating Treg/Th17 Balance. Foods 2024, 13, 585. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, L.; Wang, R.; Chen, Y.; Deng, S.; Shen, G.; Liu, S.; Xiang, X. Hypoglycemic mechanism of Tegillarca granosa polysaccharides on type 2 diabetic mice by altering gut microbiota and regulating the PI3K-akt signaling pathway. Food Sci. Hum. Wellness 2024, 13, 842–855. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Q.; Lu, H.; Jiang, C.; Hu, W.; Yu, S.; Xiang, X.; Tan, C.P.; Feng, Y.; Zhang, J.; et al. Antidiabetic effect of sciadonic acid on type 2 diabetic mice through activating the PI3K-AKT signaling pathway and altering intestinal flora. Front. Nutr. 2022, 9, 1053348. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Fu, H.; Yang, H.K.; Xu, W.; Wang, J.; Yang, S.-T. Butyric acid: Applications and recent advances in its bioproduction. Biotechnol. Adv. 2018, 36, 2101–2117. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
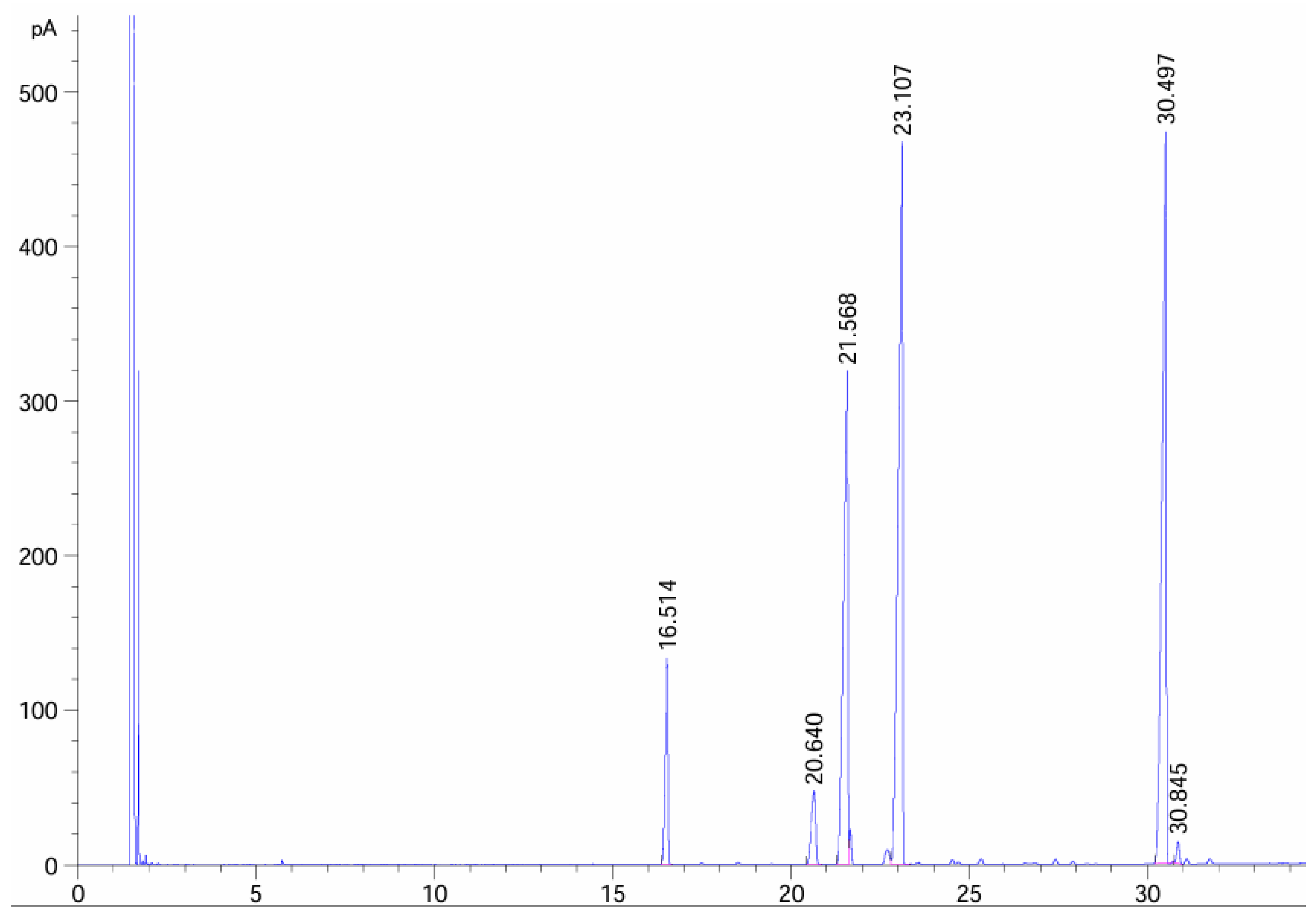



| Composition | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C22:2 |
|---|---|---|---|---|---|---|
| Relative content (%) | 6.016 | 3.291 | 22.448 | 33.177 | 31.761 | 0.559 |
| Retain time (min) | 16.514 | 20.64 | 21.568 | 23.107 | 30.497 | 30.846 |
| Gene | Primer Sequence | |
|---|---|---|
| Forward Primer | Reverse Primer | |
| IL-4 | CATGGCGTCCCTTCTCCTGTG | GTTGTCATCCTGCTCTTCTTTCTCG |
| IL-6 | GTGGTATCCTCTGTGAAGTCTCCTC | TTCTTGGGACTGATGCTGGTGAC |
| TNF-α | GTGGTTTGTGAGTGTGAGGGTCTG | CGCTCTTCTGTCTACTGAACTTCG |
| Occludin | CCTCTGACCTTGAGTGTGGATGAC | CCTCTGACCTTGAGTGTGGATGAC |
| Claudin-1 | GTGTCCTACTTTCCTGCTCCTGTC | AGAAGGTGTTGGCTTGGGATAAGG |
| β-actin | TTGTAGAAGGTGTGGTGCCAGATC | GATGGTGGGAATGGGTCAGAAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Shen, G.; Lu, H.; Jiang, C.; Hu, W.; Jiang, Q.; Xiang, X.; Wang, Z.; Chen, L. Psidium guajava Seed Oil Reduces the Severity of Colitis Induced by Dextran Sulfate Sodium by Modulating the Intestinal Microbiota and Restoring the Intestinal Barrier. Foods 2024, 13, 2668. https://doi.org/10.3390/foods13172668
Zhang H, Shen G, Lu H, Jiang C, Hu W, Jiang Q, Xiang X, Wang Z, Chen L. Psidium guajava Seed Oil Reduces the Severity of Colitis Induced by Dextran Sulfate Sodium by Modulating the Intestinal Microbiota and Restoring the Intestinal Barrier. Foods. 2024; 13(17):2668. https://doi.org/10.3390/foods13172668
Chicago/Turabian StyleZhang, Hanwen, Guoxin Shen, Hongling Lu, Chenkai Jiang, Wenjun Hu, Qihong Jiang, Xingwei Xiang, Zongxing Wang, and Lin Chen. 2024. "Psidium guajava Seed Oil Reduces the Severity of Colitis Induced by Dextran Sulfate Sodium by Modulating the Intestinal Microbiota and Restoring the Intestinal Barrier" Foods 13, no. 17: 2668. https://doi.org/10.3390/foods13172668
APA StyleZhang, H., Shen, G., Lu, H., Jiang, C., Hu, W., Jiang, Q., Xiang, X., Wang, Z., & Chen, L. (2024). Psidium guajava Seed Oil Reduces the Severity of Colitis Induced by Dextran Sulfate Sodium by Modulating the Intestinal Microbiota and Restoring the Intestinal Barrier. Foods, 13(17), 2668. https://doi.org/10.3390/foods13172668






