Abstract
Ganoderma lucidum (GL) is a kind of edible fungus with various functions and a precious medicinal material with a long history. Ganoderma lucidum polysaccharide (GLP) is one of the main bioactive substances in GL, with anti-tumor, anti-oxidation, anti-cancer, and other biological activities. GLP is closely related to human health, and the research on GLP is getting deeper. This paper reviewed the extraction and purification methods of GLP, the relationship between structure and activity, and the qualitative and quantitative methods. This review provides solutions for the analysis and application of GLP. At the same time, some new methods for extraction, purification and analysis of GLP, the relationship between advanced structures and activity, and future applications of and research into GLP were emphasized. As a kind of bioactive macromolecule, GLP has unique functional properties. Through the comprehensive summary of the extraction, purification, and analysis of GLP and its future prospects, we hope that this review can provide valuable reference for the further study of GLP.
1. Introduction of Ganoderma lucidum Polysaccharide
Ganoderma lucidum (GL) is a group of fungi belongs to basidiomycete. It is widely distributed in tropical, subtropical, and temperate regions of Europe, America, Africa, and Asia. GL is mainly composed of ash (0.72%~1.77%), carbohydrates (21.83%~27.78%), fats (1.1%~8.3%), fibers (59%~65%), proteins (7%~8%), etc. [1]. It has been reported that there are several active constituents in GL, such as triterpenes, polysaccharides, steroids, fatty acids, amino acids, nucleosides, proteins, alkaloids, inorganic elements, etc. [2]. Most of the above constituents have been proven to have numerous health benefits due to their immunomodulatory effect [3,4], antioxidant [5], anti-tumor [6,7], anti-cancer [8,9,10,11,12], treatment of diabetes [13], prevention of metabolic syndrome cardiovascular disease [14], anti-obesity [15], and regulation of intestinal microorganisms properties [16]. These varied effects enhance the overall medicinal value of GL.
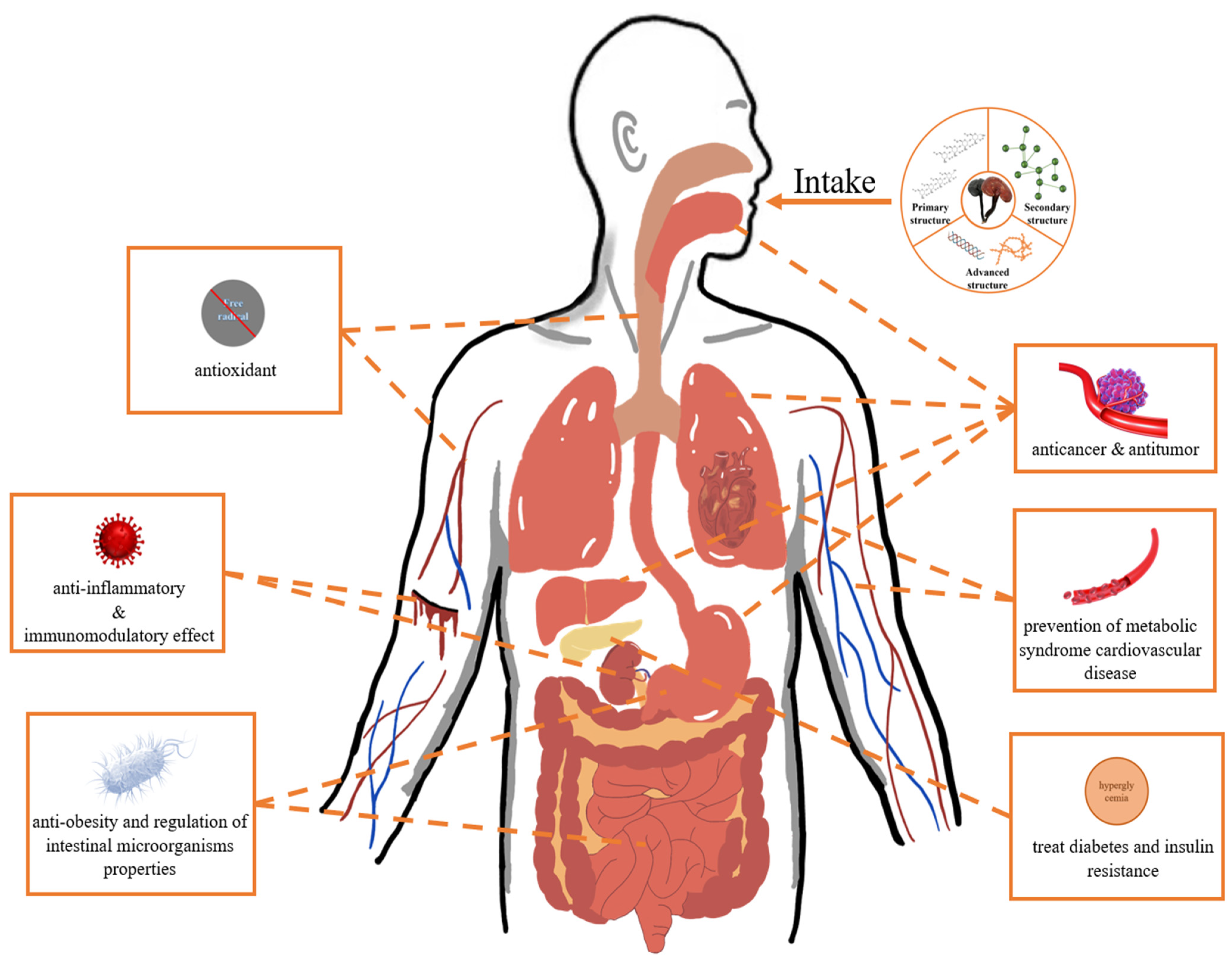
Ganoderma lucidum polysaccharide (GLP), one of the representatives of active ingredients in GL, is a substance extracted from GL spore powder or fruiting bodies [17]. As a natural biomacromolecule, GLP has been widely used in food, medicine, and health products due to its beneficial effects on human health (Figure 1). This promotes more intensive research on the structures, activities, and applications of GLPs. Most recently, OGLP-CMC/SA hydrogel was synthesized by using oxidized GLP, sodium alginate, and carboxymethyl chitosan as a matrix. This hydrogel has good mechanical properties, antioxidant properties, and biocompatibility, and it can effectively reduce inflammation and promote epidermal growth so as to heal diabetic wounds [18]. In another study, a dissolvable microneedle patch was synthesized using WSG, a polysaccharide extracted from GL, which inhibits the viability and mobility of melanoma cells, with two biocompatible compounds, PVA and PVP. This patch can significantly inhibit the growth of melanoma, breaking through the conventional treatment [19].
Figure 1.
Relationship between activity of GLP and human health. GLP can protect human health through a variety of mechanisms, such as inhibiting the growth and proliferation of tumor cells, reducing activity of various enzymes, improving human blood pressure and cholesterol levels, and eliminating free radicals.
In the past few years, the importance of GLP has received many detailed reviews. For example, GLPs can be used as novel neuroprotective agents [20], as well as in biosynthesis [21] and clinical research [1]. All these indicate the importance of GLP research, but the latest research results of GLPs, such as the new methods of preparation and detection and the relationship between advanced structure and biological activity, have not been summarized. These new research contents can not only improve the shortcomings of traditional methods but also provide scientific direction for further research on GLP in the future. Therefore, this review will not only summarize the past valuable research but also summarize the latest cutting-edge research results and put forward the prospect of future research directions for GLP.
And as we carry out this work, we find there are still several unsolved problems related to the structures and functions of GLPs. Firstly, the structure of GLPs is diverse and complex, and most of the current research focuses on their primary structure. The conformations and three-dimensional (3D) structures of GLPs are still seldom investigated. Secondly, GLPs are mostly applied in drugs and health care products, but only several species are legal in the food industry. The National Health Commission and the State Administration for Market Regulation said, in a document released in 2023, that G. lucidum karst and Ganoderma sinense can be eaten as food and Chinese medicine. Further investigation on toxicology and food safety analysis of various species of GL may promote more applications of GLPs in food products. Thirdly, the contents of GLPs are varied depending on the species, original sources, and growth stages of GL. There is a trend towards developing real-time monitoring procedures and image systems to detect the content of GLPs. The most advanced machine-learning technology is potentially critical for the construction of databases related to bioactive ingredients in GL. In this paper, we will briefly summarize preparations, structure–activity relationships, and analysis technology of GLPs. At the same time, we will try to propose ideas that may solve the above-presented problems. We will investigate spectroscopic techniques applied to the characterization of the advanced structures of GLPs, such as near-infrared, hyperspectral, and mass spectrometry. Several new advanced and integrated methods are described, such as mass spectrometry with imaging systems and spectroscopic techniques with machine learning for rapid and nondestructive detection. It is hoped that we can provide novel ideas for future research on GLPs.
2. Preparation of Ganoderma lucidum Polysaccharide
2.1. Extraction of GLP
GLP mainly comes from the fruiting body and mycelium, and most of it exists in the cell wall. Therefore, in the process of extracting GLPs, it is necessary to consider how to effectively break the cell wall and prevent the degradation of GLPs to ensure their extraction rate. At present, there are four methods used to extract polysaccharides from GL, including physical methods, chemical methods, biological enzymatic methods [22], and mechanical crushing. The physical methods mainly include the water extraction method [23,24], ultrasonic assisted extraction method [25,26], and microwave method [27]. The chemical method is mainly the alkali extraction method [28]. The mechanical crushing method is the use of a twin-screw extruder for extrusion blasting [29]. Those methods have long been investigated and applied in the preparation of GLPs. There are many reviews that detail the specific steps and principles of these traditional methods [30,31,32]. Therefore, we will not explore each method in detail in this review. In order to improve the extraction efficiency of a single method, many researchers will combine two methods, such as ultrasound-assisted enzyme method [33], ultrasonic microwave-assisted method [34], and vacuum-microwave extraction [35]. This combination can indeed improve the extraction rate of GLP, but it greatly increases the extraction time.
Most recently, several new strategies have been created to extract GLPs. The first method is the fermentation method [36]. It uses the transformation of microorganisms (Bacillus and Saccharomyces cerevisiae) to extract bioactive substances more gently, which can help to retain the natural active ingredients in the extract and reduce the toxic side effects. At the same time, microorganisms will produce a variety of active enzymes (cellulase and protease) in the metabolic process to achieve synergistic effects. Compared with the above traditional methods, it has a higher utilization rate of raw materials and a milder action condition. The second method is ternary deep eutectic solvent extraction [37]. It is the synthesis of DESs by the molar ratio of choline chloride, guaiacol and lactic acid at 1:1:1. The key parameters were optimized by response surface method, and the maximum extraction rate was 94.72 g/mg. In this process, there is a triple hydrogen bond interaction and high binding energy between DESs and glucose, which is the main reason for improving the extraction rate. DESs has good cycle stability and high recovery rate, reducing the consumption of raw materials and environmental pollution [38]. The third method is continuous phase transition extraction [39]. The researchers first applied the technique to GLPs extraction. In the extraction process, continuous fresh solvent enhances the concentration gradient and increase the mass transfer rate, thus extracting more polysaccharides. Compared with the hot water method and ultrasonic method, the extraction rate of polysaccharide after CPTE was 3.34 times and 2.68 times. This method has the advantages of time-saving and high efficiency, so it is a promising extraction method.
Currently, GLPs are extracted by using traditional methods regularly, but it may lead to less polysaccharide and more impurities. For example, the water extraction method does not completely destroy the cell structure. Alkali extraction will use organic reagents that may cause pollution to the environment, and the type and concentration of alkali should be considered when using this method to avoid polysaccharide degradation. Although the effects of the ultrasonic-assisted method and microwave-assisted method are better than the first two, they have no selectivity in the extraction process and make it easy to produce more impurities. As a mild method, enzyme extraction has high extraction efficiency, but its price is high, and the conditions of enzyme action need to be strictly controlled to avoid its inactivation. The mechanical method is seldom used at present because its parameters are difficult to control. For these cutting-edge methods, although the extraction efficiency is better than that of traditional methods, they are in the embryonic stage and need more time to verify their feasibility. Different extraction methods have different effects, which may affect the variety, structure, and biological function of polysaccharides, so it is necessary to select the appropriate extraction method according to many factors. With the development of efficient green concepts, researchers can not only optimize on the basis of traditional methods but must also develop more new extraction techniques. These are the research focuses in polysaccharide extraction in the future. As for the advantages and disadvantages of the above-mentioned GLP extraction methods, we summarize them briefly in Table 1.

Table 1.
The extraction methods of GLP.
2.2. Separation and Purification of GLP
Extraction procedures are essential to obtain GLPs, which are then still filled with various impurities like proteins, pigments, and other organic small molecules [48]. Therefore, further purification procedures are critical to obtain highly purified and structurally homogeneous GLPs. In this section, we will investigate in detail the several steps of the separation and purification of GLPs, as well as the commonly used methods.
The presence of proteins affects the quality of the polysaccharide and its physiological activity and may also influence the subsequent structural analysis of the polysaccharide, etc. Therefore, the extracted polysaccharides need to be deproteinized to obtain purified polysaccharides. One most commonly used method, called the “Sevag” method, is based on the interaction of organic solvents and salts to form a precipitate of proteins in solution for separation and purification [49]. Another method is that of using trichloroacetic acid as a denaturant to remove proteins from polysaccharides [50]. This method is based on a basic principle, in which proteins are desaturated under acidic conditions. While conformation of proteins is modified and insoluble salts are generated, more hydrophobic groups exposed those favorable for forming precipitates. However, the above two methods require the use of organic reagents, which may cause degradation of the polysaccharides [51], affecting the subsequent determination of their characterization of their structure. The third method is the protease method. In this, the polysaccharides are not mixed with organic reagents but rather enzymes to break the connecting bonds between polysaccharides and proteins, thus causing less contamination [52]. Moreover, after deproteinization with protease, the total carbohydrate recovery and the efficiency was high, and the antioxidant activity of GLPs could still be maintained at a high level [51]. The high cost of enzymes makes it difficult to realize industrial applications.
The pigment mainly comes from the polysaccharide itself and extraction process residues, and it will affect the detection results of the subsequent experiments, especially the color reaction of the experiments [48]. Meanwhile, formation of pigments is a major factor in the structural characterization and bioactive complexity of polysaccharides [53]. The most-used method of removing colorants is the activated carbon adsorption method. Activated carbon is an adsorbent material with a highly microporous structure, and organic and inorganic substances and other impurities in the object can be adsorbed on its surface to achieve purification. It captures pigments in polysaccharides mainly by van der Waals and electrostatic forces, but the selectivity coefficients for pigments and polysaccharides are poor [54]. Another method is to use hydrogen peroxide decolorization, which has a strong oxidation capacity and can break the double bonds in colored substances [55]. The third method is to use macroporous adsorption resins, which can adsorb pigments through their large surface area. In addition, macroporous resins have the advantages of good stability, low cost, and high adsorption efficiency. The resin is also regenerable, making it more suitable for polysaccharide decolorization than the previous two methods [56].
Deproteinization and decolorization are only for the removal of proteins and pigments. There are still many small molecules in polysaccharides, especially some inorganic salts, monosaccharides, and oligosaccharides. Ultrafiltration is a pressurized membrane separation technology in which small molecule impurities pass through a film with certain-sized pores under a pressure difference and polysaccharides are retained, thus realizing the purpose of separation and removal of impurities [57,58]. Dialysis is also a membrane separation method that uses diffusion pressure to expel small molecule impurities out of a semi-permeable membrane while large molecule polysaccharides remain in the membrane [59,60].
Graded precipitation [61] is a method that uses ethanol, methanol, and propanol as precipitants and precipitates the precipitate according to the different solubilities of polysaccharides in organic solvents of different concentrations. The second method is column chromatography, including anion exchange method, gel chromatography, and macroporous resin column chromatography. To obtain the homogeneous polysaccharide, GLPs can be purified by anion exchange combined with gel column [62]. The third method, ultrafiltration, is a pressure-driven membrane separation technique that removes small molecule solutes and solvents.
The above methods are commonly used for GLPs’ separation and purification, but their shortcomings could not be ignored. In deproteinization, the “Sevag” method needs to be repeated several times to achieve the desired results. Like trichloroacetic acid, the “Sevag” method leaves toxic chemical residues and may causes polysaccharide degradation. In the decolorization process, the selectivity coefficient of activated carbon for polysaccharide and pigment is poor [63], which makes it easy to cause the loss of polysaccharides. The strong oxidation power of hydrogen peroxide will cause the degradation of polysaccharide and reduce its molecular weight. Furthermore, the molecular weights of polysaccharides are different, and the resolution of ultrafiltration is low, so it is necessary to consider the molecular weights of polysaccharides and select different conditions. With the updating of technology, some new separation and purification techniques have appeared. For example, repeated freeze–thaw treatment causes insoluble aggregation and precipitation of proteins by causing complex changes in the buffering environment [64,65]. Dialdehyde cellulose is a modified polysaccharide that can be used to form a Schiff base by binding to proteins, thereby removing proteins from some crude polysaccharides [66]. Compared with traditional methods, these two methods have higher deproteinization efficiency and polysaccharide extraction rate, and the reagents and treatment processes used are greener and safer, avoiding environmental pollution. Besides these new methods of deproteinization, asymmetrical flow field-flow fractionation (AF4), ultrafiltration and the “Sevag” method are combined to improve the purification process of GLP [67]. AF4 provides sample component separation based only on its hydrodynamic size. The upper wall of the AF4 channel is an impermeable polycarbonate glass plate, and the bottom channel plate is permeable. The bottom channel plate and ultrafiltration membrane form an accumulation wall, which allows free passage of the carrier liquid and small molecules with sizes smaller than the MWCO. This method combines a variety of methods and uses deionized water throughout the research process, avoiding the process of dialysis desalination and greatly improving the efficiency while retaining the original structure of GLPs.
There is no specific standard for the extraction, separation, and purification of GLPs. Different treatment methods have different advantages and disadvantages, and the polysaccharides obtained are also different. GLP is an edible active substance, so the whole process needs to be green and safe as the first principle. Therefore, avoiding using harmful organic solvents or using less-toxic ones throughout the whole process is advisable to avoid subsequent products containing toxicity and limit their application. Secondly, efficiency should be considered. GLP has a broad application prospect in medicine and health food and will inevitably achieve large-scale industrial production with further development. When other conditions permit, efficient methods can be beneficial to economic returns. Therefore, exploring more green and efficient methods to obtain safe, large-quantity and high-purity GLPs is still the focus of future research.
3. Structure of Ganoderma lucidum Polysaccharide
3.1. Structure from Primary to Quaternary
It has been shown that structure of natural polysaccharides may be categorized into primary, secondary, tertiary, and quaternary structures [20]. The primary structure mainly includes the composition of the monosaccharides, the way in which neighboring sugar groups are connected, the allosteric configuration, and the presence or absence of branches [68]. The composition of monosaccharides of GLP from different sources may vary, with most of them mainly containing glucose and galactose and some of them also containing mannose, arabinose, and fucose [21]. These polysaccharide compounds can be categorized as homopolysaccharides and heteropolysaccharides according to the composition of the monosaccharides [3]. Polysaccharides can also be categorized into α-type and β-type based on the isotropic structure of monosaccharides. As the main active structure, β-type polysaccharides have received much attention from researchers [69]. However, there are few studies on the activity of α-type polysaccharides. Monosaccharides are linked to each other by different glycosidic bonds such as (1→3), (1→4), and (1→6). This is the main reason that most GLPs present high biological activities.
The composition of monosaccharides, types of glycosidic bonds, and anisotropic structures described above are only based on primary structural analyses of GLPs. The term “advanced structures” primarily includes secondary, tertiary, and quaternary structures of GLPs. Secondary structures refer to various types of polymers with hydrogen bonding between chains of polysaccharides. At present, the secondary structures found in GLPs are single helical structures [70,71], rigid chain conformation [72], and linear and short-rod conformation [73]. Tertiary structures are further twisted and folded on the basis of the secondary structure format to form the spatial conformation with a certain shape and size. Researchers used HPSEC-MALLS-RI to identify a polysaccharide in GL with a compact sphere chain conformation, formed by stacked multiple chains [74]. Another study found that GLP is circular aggregates formed by intertwined chains and has a triple helix structure in aqueous solution [75]. The quaternary structures are the polymer formed by the combination of non-covalent bonding between macromolecular chains. Due to technical limitations, there is no research to clarify the quaternary structure type of GLPs, which is also one of the problems to be overcome in the future.
3.2. Structure with or without Activity
The structure of GLP is closely related to its biological activity. The main factors affecting the structure and bioactivity of GLP include monosaccharide composition, glycosidic bond, side chain composition, and molecular weight [76]. According to the composition of monosaccharides, polysaccharides can be divided into homopolysaccharides and heteropolysaccharides. Homopolysaccharides can significantly inhibit tumor growth and down-regulate proliferating cell nuclear antigen markers [77]. Heteropolysaccharides also show good anti-tumor, immune stimulation, and other activities because heteropolysaccharides are different monosaccharides combined in different proportions or ways. One of the key ingredients is mannose, which helps human organisms recognize toxic carbohydrates, thus stimulating the immune system to produce cytokines [78]. It has been reported that heteropolysaccharides exhibit higher immunomodulatory effects than homopolysaccharides [73]. Furthermore, the active polysaccharides isolated from GL are mainly β-(1→3), β-(1→4), and β-(1→6) types of glucans [79]. β-(1→3)-D-glucan is not only resistant to solid tumors [80] but also acts as an antimicrobial agent [79]. The low-molecular-weight β-(1→3)-glucan also reduces the formation of reactive oxygen and inhibits acidic and neutral sphingomyelinase activity [81]. Purified β-(1→3)-glucan also has an immunizing effect [72,82]. β-(1→6)-D-glucan has also been reported to have antitumor activity and activation of NKs [83]. Besides glucan, other monosaccharides can be linked by different glycosidic bonds, such as β-(1→3)-D-glucose [84], β-(1→3)-D-glucopyranosyl [7], β-(1→6)-D-glucopyranosyl [84], β-(1→3)-glucohexaose [85], etc., and exhibit different activities. Of course, there are α-types of polysaccharides that have been isolated, but due to their water-insoluble nature, their activity has been less studied. The isolation of α-(1→3)-D-glucan from GL substrates, a water-insoluble polysaccharide, was chemically modified by researchers to improve its water solubility. The anti-tumor [86], antioxidant [87], and immunomodulatory [88] activities of α-(1→3)-D-glucan were significantly enhanced after chemical modification. In addition to the differences in monosaccharide composition and glycoside bond, the molecular weight also affects the activity of polysaccharides. In one study, a homopolysaccharide with a molecular weight of 44.4 kDa was isolated from the fruiting body of GL and showed good antitumor activity in mice [77]. Another study isolated a homopolysaccharide with a molecular weight of 1013 kDa, which also has anti-tumor effects [89]. From these two studies, it can be seen that no matter the size of the molecular weight of polysaccharide, it has a certain biological activity. However, one report suggested that the greater the molecular weight of GLP, the higher its biological activity [90]. Therefore, there is still disagreement about which molecular weight has the best biological activity, and further research is needed. We summarize information about the primary structure of some GLPs with respect to their biological activities and other aspects in Table 2.
In addition to the primary structure being related to the activity of GLPs, there is a definite link between its advanced structure and activity [91]. The triple helix structure is the most common type of advanced structure of polysaccharides and the most studied [92]. Researchers demonstrated that GLP with triple-helical structure can modulate immune activity through RAW264.7 macrophage in a cell model study in vitro [75]. There are also some GLPs that show linear or short rod conformation, while the short rod conformation has more immunomodulatory activity than the linear one [73]. This may be due to the different compositional ratio of glucose in the formation of polymers. GLP can also form a rigid chain conformation in aqueous solution [72]. The polysaccharide changes conformation from an ordered structure to a single-chain structure when the NaOH concentration is greater than 0.15 M in an alkaline solution or in an aqueous solution at 135 °C or above. This transition is consistent with experimental results with other edible mushroom polysaccharides [93], and it is hypothesized that the biological activity of this polysaccharide is related to the rigid chain conformation. However, this conformational shift may result in a decrease or loss of its activity [94]. Because of the limited technology and the hindrance of the complex structure of polysaccharides, the relationship between advanced structure and biological activity has been less studied, and this is one of the challenges to be overcome in the future.

Table 2.
The structures and bioactivities of GLPs.
Table 2.
The structures and bioactivities of GLPs.
| Type | Backbone | Name | Mw | Monosaccharide Composition Ratio | Bioactivity | Raw Source | Reference |
|---|---|---|---|---|---|---|---|
| α | α-(1→6)-D-galactopyranosyl α-(1,2,6)-D-galactopyranosyl | LZ-D-1 | 2.8 × 104 Da | L-Fuc:D-Glc:D-Gal = 1:1:5 | Immunity: Stimulate proliferation of mouse spleen lymphocytes in vitro | Chongming/ Shanghai | [95] |
| α-(1→4)-D-glucan | LB-B1 | 9.3 × 103 Da | Only D-glucose | — | — | [96] | |
| α-(1,6)-Galp | PSG-2 | 6.9 × 104 Da | Galactose:Fucose:Glucose = 8:1:1 | — | Ganzhou/ Jiangxi | [97] | |
| β | β-(1→3)-D-glucan | GL-IV-I | 1.33 × 105 Da | — | — | Longyan/Fujian | [71] |
| GLP20 | 3.75 × 106 Da | — | Immunity: Increase NO production of RAW264.7 macrophages | Shanghai | [72] | ||
| PSGL-I-1A | 7.18 × 105 Da | Only D-glucose | Immunity: Affect T lymphocyte-stimulating activity | Shanxi | [98] | ||
| β- (1→3)-D-glucosyl | LB-NB | 4.7 × 104 Da | Only D-glucose | Immunity: Remarkable stimulation of proliferation of T-cells in vitro | Shanghai | [70] | |
| β-(1→3)-glucose | PSG-1 | — | Glu:Mannose:Galactose = 9:1:1 | — | — | [99] | |
| β-(1→3)-D-glucopyranosyl | SP | 1.0 × 104 Da | — | Immunity: Enhancement of lymphocyte proliferation and antibody production | — | [100] | |
| β-(1→6)-D-glucan | PGL | 1.26 × 105 Da | Only D-glucose | Immunity: Had an immunosuppressive effect on antibody production and lymphocyte proliferation | — | [101] | |
| β-(1→3)-(1→6)-D-glucan | GTM5 | 1.76 × 106 Da | Glc | Antitumor | Wuhan | [80] | |
| GTM6 | 1.61 × 106 Da | Glc:Man = 3.83:1 | Antitumor | Wuhan | [80] | ||
| β-(1→3)-(1→4)-(1→6)-D-glucopyranosyl β-(1→6)-D-mannopyranosyl | PL-4 | 2.0 × 105 Da | Mannose:Glc = 1:13 | Immunity: Enhanced the proliferation of T- and B-lymphocytes in vitro | Shanxi | [102] | |
| β-(1→3)-(1→6)-D-glucopyranosyl | Ganoderans B | 7.4 × 103 Da | — | Reduced the blood glucose concentration | Kyoto/Japan | [103] | |
| β-(1→3)-(1→4)-(1→6)-D-glucan | GLSWA-I | 1.57 × 105 Da | — | Significantly promoted dinitrochlorobenzene-induced delayed-type ear swelling in mice | Shanghai | [104] | |
| β-(1,6)-D-Glcp | GLSA50-1B | 1.03 × 105 Da | Only Glucose | — | Shanghai | [105] | |
| α, β | α-(1→4)-D-glucan β-(1→3)-D-glucan | GTM3 | 4.65 × 106 Da | Glc | Antitumor: Exhibited significant inhibition ratio beyond 50% | Wuhan | [80] |
| GTM4 | 4.68 × 106 Da | Glc | Antitumor | Wuhan | [80] | ||
| α-(1→4)-D-glucopyranosyl β-(1→6)-D-galactopyranosyl | PL-1 | 8.3 × 103 Da | Rha:Gal:Glc = 1:4:13 | Immunity: Enhanced the proliferation of T- and B-lymphocytes in vitro | Shanxi | [102] | |
| α-(1→6)-D-galactopyranosyl β-(1→3)-(1→6)-D-glucopyranosyl | Ganoderans C | 5.8 × 103 Da | D-glucose:D-galactose = 24:1 | Reduced the blood glucose concentration | Kyoto/Japan | [103] | |
| α-(1,6)-galactopyranosyl α-(1,2,6)-galactopyranosyl β-(1,3)-glucopyranosyl β-(1,4,6)-glucopyranosyl | LZ-C-1 | 7.0 × 103 Da | L-Fuc, D-Glc, D-Gal | — | Shanghai | [106] | |
| α-(1→6)-D-glucopyranosyl β-(1→3)-D-glucopyranosyl β-(1→3,6)-D-glucopyranosyl | GSG | 1.43 × 105 Da | — | Immunity: Stimulating effects on murine lymphocyte proliferation | Jilin | [107] | |
| Not well-known | 1,2,6-galactose 1,3-glucose 1,6-galactose | GLPCW-II | 1.2 × 104 Da | D-Glc:L-Fuc:D-Gal = 1.00:1.09:4.09 | Stimulated the proliferation of mouse spleen lymphocytes | Shanghai | [108] |
| — | PSG-1 | 1.013 × 106 Da | Glucose:Mannose:Galactose = 4.91:1:1.28 | — | Ganzhou/ Jiangxi | [97] | |
| 1,3-glucosyl | GSG | 8.0 × 103 Da | Only D-glucose | Immunity: Potentiated the Con A-induced proliferative response of splenocytes | — | [109] | |
| — | GLIS | — | D-glucose:D-galactose:D-mannose = 3.0:1:1 | Immunity | — | [110] | |
| — | SeGLP-2B-1 | 1.06 × 106 Da | Glucose:Rhamnose:Xylose:Galactose = 1.000:0.652:0.443:0.227 | Anticancer | — | [111] | |
| Glucan | PL-3 | 6.3 × 104 Da | Glucan | Immunity: Enhanced the proliferation of T- and B-lymphocytes in vitro | Shanxi | [102] | |
| — | GTM1 | 6.28 × 105 Da | Galactose:Mannose = 1.85:1 | Antitumor | Wuhan | [80] | |
| — | GTM2 | 8.18 × 105 Da | Galactose:Glc = 1:1.36 | Antitumor | Wuhan | [80] |
4. Analysis of Ganoderma lucidum Polysaccharide
Polysaccharide is an important active substance in GL, which has many biological activities and can promote human health. The content of polysaccharides in GL has always been one of the key concerns of consumers, and its content will change with the growth region and growth stage of GL. How to achieve non-destructive, rapid, and real-time detection of polysaccharide content is a future development trend. The structure of polysaccharides is complex and diverse. Different monosaccharides and different glycosidic bonds can form a variety of primary structures, and different primary structures can establish different advanced structures and conformations. So, figuring out its structure is a challenge. In this section, we will review the qualitative and quantitative methods of GLPs, with emphasis on some novel methods.
4.1. Quantitative Analysis of GLP
We summarize all appliable analysis methods for quantifying GLP in Figure 2. At present, the traditional methods used for the detection of GLPs content mainly include the phenol sulfuric acid method [112,113] and anthrone sulfuric acid method [114], both of which utilize the principle of chromogenic reaction for detection. Their results are susceptible to various factors, and they require complex pre-treatment and the use of organic reagents that can easily cause environmental pollution [115]. HPLC [116] is beginning to be applied for detecting the content of GLPs because of its high sensitivity and accuracy. High-performance size exclusion chromatography (HPSEC) is a type of HPLC and is a good method for quantitative analysis. The polysaccharide content in GL can be accurately analyzed by combining it with multi-angle laser scattering [117]. However, it requires a large amount of expensive organic substances and laborious pre-treatment. The determination of GLPs content using the above method is quite time-consuming and inefficient when the sample amount is too large. In order to realize non-destructive and rapid detection, it is essential to find some novel and rapid detection methods.
Machine learning is an increasingly attractive technology that is widely applied in various areas, such as medicine [118,119], materials chemistry [120,121], and biology [122]. Most recently, several reviews [123,124] investigated the potential application of machine learning in food science and industry. It is obvious that this advanced technology will help to solve many scientific and technological problems during research on food topics. Detection of food constituents is a sophisticated procedure with a series unit process. As we discussed above, the traditional method requires the sample to be crushed and then extracted, separated, and purified before quantitative analysis, which is very complicated and cumbersome. Therefore, machine learning has been combined with many technologies, such as hyperspectral imaging [125] and infrared spectroscopy (mid-infrared spectroscopy [126] and near-infrared spectroscopy [127]), to analyze GLPs with high efficiency, fast data analysis, and little or no sample preparation, followed by machine learning to extract data features to build models. This cutting-edge approach enables real-time monitoring of polysaccharide content in GL regardless of species, region, and growth stage and provides a viable method for improving the quality and economic value of GL, with promising applications in large-scale cultivation and high-throughput detection.
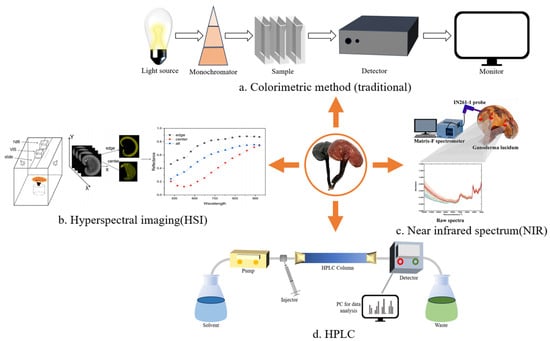
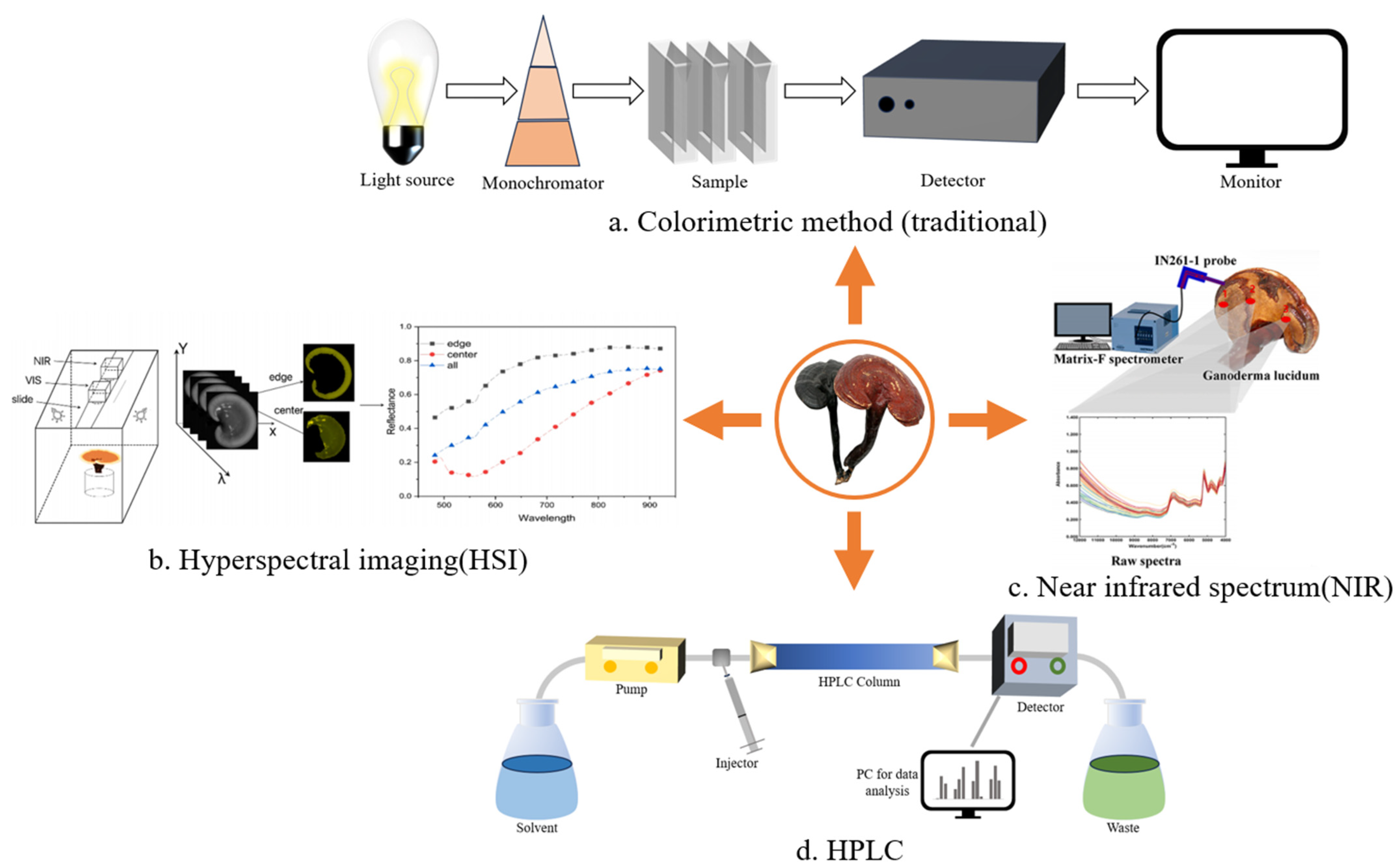
Figure 2.
Methods for quantitative analysis of GLP. (a) The content of polysaccharide was calculated by measuring the absorbance of the sample using colorimetric reaction. (b) In a dark environment, the samples are photographed by halogen lamp and hyperspectral camera, and then the relationship between reflectance and wavelength was obtained by software processing and the polysaccharide content was finally calculated. (c) The infrared spectrum of GL was collected by probe, the performing band was selected by synergy interval partial least squares, and the model was optimized by ant lion optimization algorithm to predict the total polysaccharide content. (d) The chromatogram was obtained by HPLC, and the content of GLPs was deduced by calculating the peak area. (b,c) are reproduced from [125,127,128,129].
Figure 2.
Methods for quantitative analysis of GLP. (a) The content of polysaccharide was calculated by measuring the absorbance of the sample using colorimetric reaction. (b) In a dark environment, the samples are photographed by halogen lamp and hyperspectral camera, and then the relationship between reflectance and wavelength was obtained by software processing and the polysaccharide content was finally calculated. (c) The infrared spectrum of GL was collected by probe, the performing band was selected by synergy interval partial least squares, and the model was optimized by ant lion optimization algorithm to predict the total polysaccharide content. (d) The chromatogram was obtained by HPLC, and the content of GLPs was deduced by calculating the peak area. (b,c) are reproduced from [125,127,128,129].
4.2. Qualitative Analysis of GLP
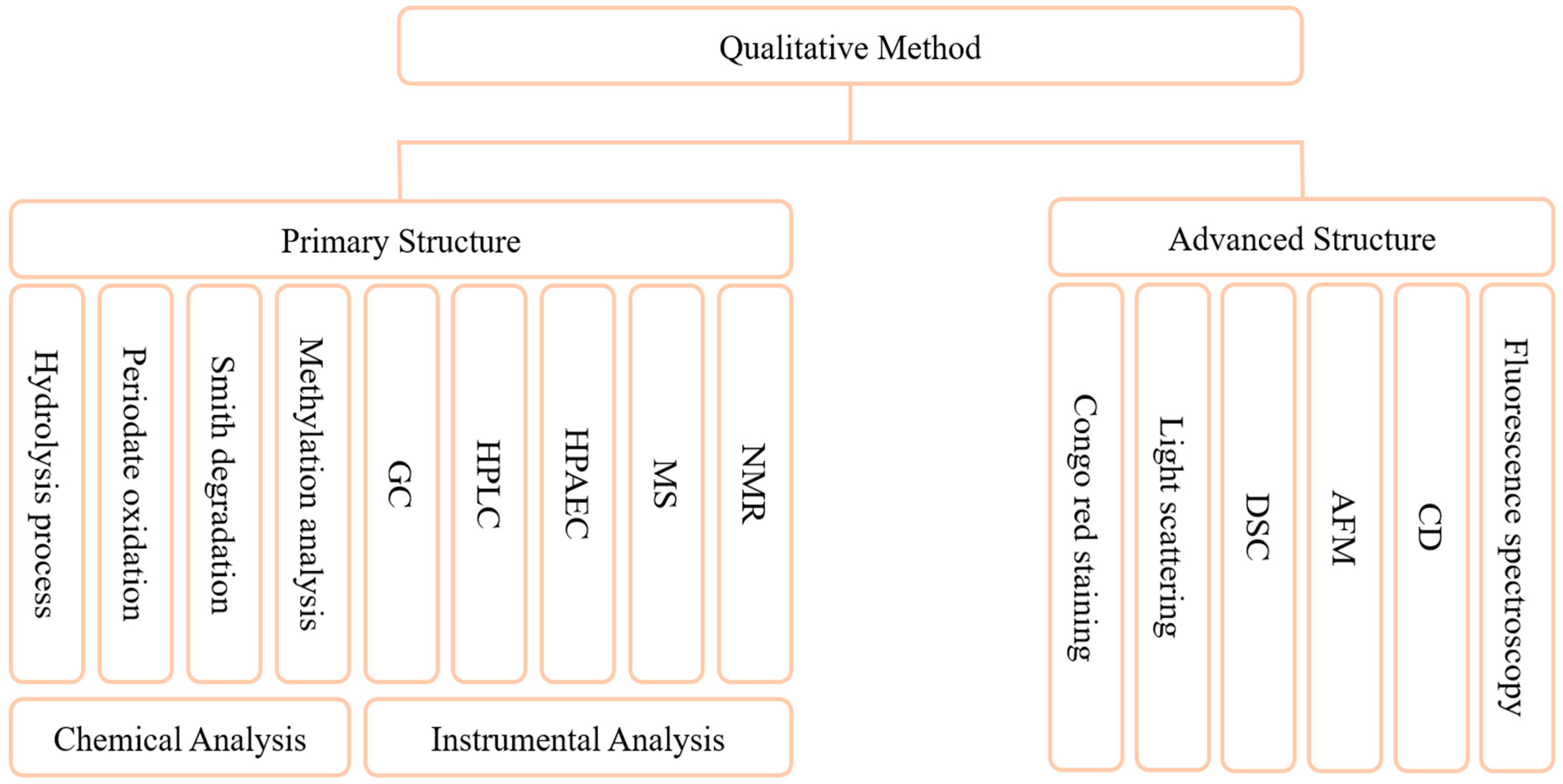
The methods for characterization of the primary structure of GLPs have been well established (Figure 3). Characterization of the structure of GLPs is mainly based on two strategies, chemical and instrumental. Commonly, chemical analysis methods include hydrolysis process, periodate oxidation, “Smith” degradation, and methylation analysis. The hydrolysis method includes complete acid hydrolysis, partial acid hydrolysis, etc., which are mainly used to analyze the composition of polysaccharide chains. Acid hydrolysis may lead to excessive degradation of monosaccharides under harsh conditions or prolonged treatment, so it is essential to explore the optimal hydrolysis conditions for polysaccharides [130]. Periodate oxidation is a common method for analyzing the structure of polysaccharides, and the position and type of glycosidic bond can be determined according to the consumption of periodic acid [128]. The methylation analysis mainly characterizes the linking mode of sugar residues but can degrade polysaccharides. Some studies have achieved complete methylation by improving the scheme, without causing obvious degradation [131]. Instrumental analytical methods mainly include GC, HPLC, HPAEC, MS, and NMR. Compared with chemical methods, this kind of method is more accurate and convenient [129,132]. Researchers have characterized GLPs through a variety of instrumental methods and elucidated the fine structural characteristics of its main chain and branch chain [99]. Another study analyzed the composition of Panax species polysaccharides by GC-MS as Rha, Ara, GalA, Man, Glc, and Gal [133]. The composition of monosaccharides, the composition of main and branched chains, and the determination of the type of glycosidic bond can be obtained by the above methods. Fewer methods are used to characterize advanced structures, mainly Congo red staining [134], light scattering, differential scanning calorimetry (DSC) [72], atomic force microscopy (AFM), circular dichroism (CD), and fluorescence spectroscopy. Congo red staining is the most convenient method to study the change of polysaccharide morphological chain, and it does not require significant equipment. CD is an effective method to study the three-dimensional structure of biological macromolecules, which can provide information about the absolute configuration and conformation of molecules [135]. The light scattering method can accurately reflect the change of chain conformation, but it requires high purity of the sample and solvent used. AFM only needs a small number of samples to analyze the morphology of polysaccharide chains, but it has high requirements for instrument equipment. At present, the methods used to determine the conformation of polysaccharides still have the problems of being time-consuming, high-cost and low-accuracy. It is a popular strategy to combine two different methods and equipment to study the conformation of polysaccharides [136]. The development of combining computer-aided and analytical methods for molecular modeling has begun [137]. This will provide a new more accurate and simpler strategy for the resolution of advanced structures of polysaccharides.
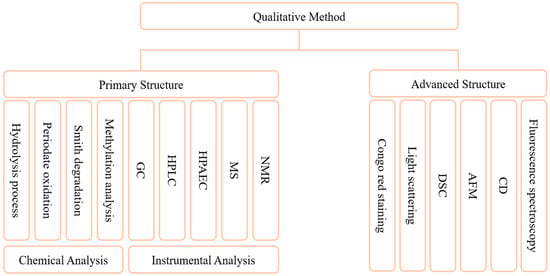
Figure 3.
Qualitative methods of GLPs. These methods are used to analyze the composition of monosaccharides, the structure of main chain and branch chain, the judgment of the type of glycosidic bond, and the advanced structures.
5. Conclusions and Future Perspectives
GLPs have many biological activities and have positive effects on human health, so they have become a hot research topic at home and abroad. This article summarized the extraction and purification methods of GLPs, the relationship between structure and activity, and the qualitative and quantitative analysis methods of GLPs, but the research on GLPs needs to be further in-depth. According to the problems we found in the process of review, such as the complex structure of GLPs, the content being changeable, and the applications being too few, the following suggestions are put forward, hoping to provide useful value for future research on GLPs.
- Some GLPs are insoluble in water, so it is difficult to satisfy all the chemical properties as well as exhibit satisfactory biological activity [17]. In order to solve this problem, their physical properties and chemical structure can be changed by chemical modification [138,139]. Nanoparticles prepared from natural polysaccharides have a special structure that enables the active ingredient to be encapsulated in a polymer matrix and precisely transported to a specific site for releasing [17]. GLPs can also be combined with other materials to form composite materials to improve their biological activity [140,141]. The study of chemical modification and nano delivery systems can make some GLPs show more obvious biological activity and increase their applications.
- The structure and activity of polysaccharides are closely related [142,143], but most of the current studies are on the relationship between primary structures and biological activity, and there are few studies on advanced structures and conformations. In future studies, we need to characterize these advanced structures and conformations with more novel methods and advanced instruments and elucidate their relationship with activity. This will provide us with more information about the biology of GLPs and scientific basis for their potential applications.
- As the main active substance of GL, polysaccharide has attracted more and more attention due to its remarkable biological activity. However, the scarcity of natural resources, the restriction of growth conditions, and the difficulty of controlling the stability of yield hinder its development and application. It would be a good solution to obtain GL strains with high polysaccharide content by artificial breeding with gene technology [144,145,146]. Of course, it is also possible to cultivate disease-resistant and pest-resistant GL through these technologies to improve the overall quality of GL.
- GL has different species, and their growth environments are not the same, which causes great differences in the content, efficacy, and structure of their bioactive ingredients. In order to facilitate researchers to understand information more quickly and clearly, it is essential to establish a shared database. This database can collect experimental data on GLPs from the whole world, and visitors can browse these data to explore the principles and mechanisms behind it and further promote the vigorous development of GL research.
Author Contributions
Conceptualization, Y.Z.; investigation, Y.Z., P.T. and H.L.; writing—original draft preparation, Y.Z.; writing—review and editing, D.Z., X.C., R.M. and J.P.; visualization, Y.Z., R.M. and J.P.; supervision, R.M. and J.P.; project administration, R.M. and J.P.; funding acquisition, R.M. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China (2023YFF1000800).
Data Availability Statement
No new date was created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, S.; Zhang, S.; Peng, B.; Tan, D.; Wu, M.; Wei, J.; Wang, Y.; Luo, H. Ganoderma lucidum: A comprehensive review of phytochemistry, efficacy, safety and clinical study. Food Sci. Hum. Wellness 2024, 13, 568–596. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, Q.; Wang, H.; Zhao, M.; Liu, S.; Nie, S.; Xie, M. Immunomodulatory effect of Ganoderma atrum polysaccharides on Th17/Treg balance. J. Funct. Foods 2018, 45, 215–222. [Google Scholar] [CrossRef]
- Tan, W.-C.; Kuppusamy, U.R.; Phan, C.-W.; Tan, Y.-S.; Raman, J.; Anuar, A.M.; Sabaratnam, V. Ganoderma neo-japonicum Imazeki revisited: Domestication study and antioxidant properties of its basidiocarps and mycelia. Sci. Rep. 2015, 5, 12515. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Q.; He, Y.-M. The effect of Ganoderma lucidum extract on immunological function and identify its anti-tumor immunostimulatory activity based on the biological network. Sci. Rep. 2018, 8, 12680. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Shi, L.; Ding, K. Structure elucidation and anti-tumor activity in vivo of a polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Int. J. Biol. Macromol. 2019, 141, 693–699. [Google Scholar] [CrossRef]
- de Camargo, M.R.; Inacio, K.K.; Frazon, T.F.; Amôr, N.G.; Corrêa, L.E.; Costa, F.C.; Quagliato, E.N.; Rodini, C.O.; Lara, V.S. Ganoderma lucidum polysaccharides associated with 5-Fluorouracil impair OSCC tumorigenesis in vitro. Pharmacol. Res.-Mod. Chin. Med. 2023, 9, 100310. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, R.; Yao, J.; Xi, C.; Du, S. Ganoderma lucidum Polysaccharide Enhanced the Antitumor Effects of 5-Fluorouracil against Gastric Cancer through Its Upregulation of NKG2D/MICA. Int. J. Polym. Sci. 2019, 2019, 4564213. [Google Scholar] [CrossRef]
- Han, W.; Chen, H.; Zhou, L.; Zou, H.; Luo, X.; Sun, B.; Zhuang, X. Polysaccharides from Ganoderma sinense-rice bran fermentation products and their anti-tumor activities on non-small-cell lung cancer. BMC Complement. Med. Ther. 2021, 21, 169. [Google Scholar] [CrossRef]
- Li, G.-L.; Tang, J.-F.; Tan, W.-L.; Zhang, T.; Zeng, D.; Zhao, S.; Ran, J.-H.; Li, J.; Wang, Y.-P.; Chen, D.-L. The anti-hepatocellular carcinoma effects of polysaccharides from Ganoderma lucidum by regulating macrophage polarization via the MAPK/NF-κB signaling pathway. Food Funct. 2023, 14, 3155–3168. [Google Scholar] [CrossRef]
- de Camargo, M.R.; Frazon, T.F.; Inacio, K.K.; Smiderle, F.R.; Amôr, N.G.; Dionísio, T.J.; Santos, C.F.; Rodini, C.O.; Lara, V.S. Ganoderma lucidum polysaccharides inhibit in vitro tumorigenesis, cancer stem cell properties and epithelial-mesenchymal transition in oral squamous cell carcinoma. J. Ethnopharmacol. 2022, 286, 114891. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Gabryelska, K.; Grabarczyk, M. Mushrooms of the Genus Ganoderma Used to Treat Diabetes and Insulin Resistance. Molecules 2019, 24, 4075. [Google Scholar] [CrossRef] [PubMed]
- Klupp, N.L.; Kiat, H.; Bensoussan, A.; Steiner, G.Z.; Chang, D.H. A double-blind, randomised, placebo-controlled trial of Ganoderma lucidum for the treatment of cardiovascular risk factors of metabolic syndrome. Sci. Rep. 2016, 6, 29540. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, L.; Zhai, Q.; Chu, C.; Wang, S.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Combined Ganoderma lucidum polysaccharide and ciprofloxacin therapy alleviates Salmonella enterica infection, protects the intestinal barrier, and regulates gut microbiota. Food Funct. 2023, 14, 6896–6913. [Google Scholar] [CrossRef]
- Kou, F.; Ge, Y.; Wang, W.; Mei, Y.; Cao, L.; Wei, X.; Xiao, H.; Wu, X. A review of Ganoderma lucidum polysaccharides: Health benefit, structure-activity relationship, modification, and nanoparticle encapsulation. Int. J. Biol. Macromol. 2023, 243, 125199. [Google Scholar] [CrossRef]
- Li, F.; Liu, T.; Liu, X.; Han, C.; Li, L.; Zhang, Q.; Sui, X. Ganoderma lucidum polysaccharide hydrogel accelerates diabetic wound healing by regulating macrophage polarization. Int. J. Biol. Macromol. 2024, 260, 129682. [Google Scholar] [CrossRef]
- Lo, H.-C.; Lin, T.-E.; Lin, C.-Y.; Wang, W.-H.; Chen, Y.-C.; Tsai, P.-H.; Su, J.-C.; Lu, M.-K.; Hsu, W.-H.; Lin, T.-Y. Targeting TGFβ receptor-mediated snail and twist: WSG, a polysaccharide from Ganoderma lucidum, and it-based dissolvable microneedle patch suppress melanoma cells. Carbohydr. Polym. 2024, 341, 122298. [Google Scholar] [CrossRef]
- Liu, X.; Yang, L.; Li, G.; Jiang, Y.; Zhang, G.; Ling, J. A novel promising neuroprotective agent: Ganoderma lucidum polysaccharide. Int. J. Biol. Macromol. 2023, 229, 168–180. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Cui, F.-J.; Sun, L.; Zan, X.-Y.; Sun, W.-J. Recent advances in Ganoderma lucidum polysaccharides: Structures/bioactivities, biosynthesis and regulation. Food Biosci. 2023, 56, 103281. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, J.; Wang, C.; Liu, L.; Li, B.; Zhou, J. Optimization of enzymatic extraction of polysaccharides from Ganoderma lucidum using response surface methodology. Sci. Technol. Food Ind. 2016, 37, 238–244. [Google Scholar] [CrossRef]
- Yin, Y.; Yao, Q. Extraction Process of Ganoderma lucidum Polysaccharides. Adv. Microbiol. 2018, 7, 26–37. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, J.; Zheng, F.; Yao, J.; Wang, T.; Jiang, S.; Pang, M. Study on extraction of polysaccharides from Ganoderma lucidum by hot compressed water and its antioxidant activities. J. Food Sci. Technol. 2018, 36, 58–62. [Google Scholar] [CrossRef]
- Kang, Q.; Chen, S.; Li, S.; Wang, B.; Liu, X.; Hao, L.; Lu, J. Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction. Int. J. Biol. Macromol. 2019, 124, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhang, W.; Liu, S. Optimization of ultrasonic-assisted extraction of polysaccharides and triterpenoids from the medicinal mushroom Ganoderma lucidum and evaluation of their in vitro antioxidant capacities. PLoS ONE 2020, 15, e0244749. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Smiderle, F.R.; Morales, D.; Iacomini, M.; Soler-Rivas, C. Strengths and weaknesses of the aniline-blue method used to test mushroom (1→3)-β-d-glucans obtained by microwave-assisted extractions. Carbohydr. Polym. 2019, 217, 135–143. [Google Scholar] [CrossRef]
- Zhang, J.J.; Chen, F.F.; Yan, Z.P.; Li, C.; Sun, E.; Luo, Y.; Tan, X.B. Alkali extraction of Ganoderma lucidum β-glucan and its anti-tumor immune regulation. Acta Pharm. Sin. 2020, 55, 512–521. [Google Scholar] [CrossRef]
- Zheng, Y.; Shuangshi, L.; Guowei, Y.; Yuepeng, W.; Zhongzhi, L.; Limin, H. Effect of twin-screw extrusion on polysaccharide extraction from Ganoderma lucidum. Sci. Technol. Food Ind. 2017, 8, 280–283. [Google Scholar] [CrossRef]
- Sun, Y.; He, H.; Wang, Q.; Yang, X.; Jiang, S.; Wang, D. A Review of Development and Utilization for Edible Fungal Polysaccharides: Extraction, Chemical Characteristics, and Bioactivities. Polymers 2022, 14, 4454. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.-C.; Chang, J.-S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Do, D.T.; Lam, D.H.; Nguyen, T.; Phuong Mai, T.T.; Phan, L.T.M.; Vuong, H.T.; Nguyen, D.V.; Linh, N.T.T.; Hoang, M.N.; Mai, T.P.; et al. Utilization of Response Surface Methodology in Optimization of Polysaccharides Extraction from Vietnamese Red Ganoderma lucidum by Ultrasound-Assisted Enzymatic Method and Examination of Bioactivities of the Extract. Sci. World J. 2021, 2021, 7594092. [Google Scholar] [CrossRef]
- Xu, N.; Sun, Y.H.; Guo, X.L.; Liu, C.; Mao, Q.; Hou, J.M. Optimization of ultrasonic-microwave synergistic extraction of polysaccharides from Morchella conica. J. Food Process. Preserv. 2017, 42, 13423. [Google Scholar] [CrossRef]
- Song, C.F.; Wang, S.G.; Yang, J.; Cui, Z.W.; Gu, Y.H. Optimization of Vacuum-Microwave Radiation Pretreatment on Extraction of Ganoderma Polysaccharides. Math. Probl. Eng. 2015, 2015, 792832. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Q.; Chai, N. 灵芝多糖的发酵提取方法、灵芝多糖组合物及其应用. CN114672527A, 28 June 2022. [Google Scholar]
- Li, R.; Shi, G.; Chen, L.; Liu, Y. Polysaccharides extraction from Ganoderma lucidum using a ternary deep eutectic solvents of choline chloride/guaiacol/lactic acid. Int. J. Biol. Macromol. 2024, 263, 130263. [Google Scholar] [CrossRef]
- Cai, C.; Wang, Y.; Yu, W.; Wang, C.; Li, F.; Tan, Z. Temperature-responsive deep eutectic solvents as green and recyclable media for the efficient extraction of polysaccharides from Ganoderma lucidum. J. Clean. Prod. 2020, 274, 123047. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Hou, T.; An, S.; Guo, B.; Liu, C.; Hu, L.; Huang, Y.; Zhang, S.; Song, M.; et al. Extraction kinetics, physicochemical properties and immunomodulatory activity of the novel continuous phase transition extraction of polysaccharides from Ganoderma lucidum. Food Funct. 2021, 12, 9708–9718. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, W. Optimization of Extraction Process of Polysaccharide from Lucidum Spores Using Response Surface Analysis. Food Res. Dev. 2017, 38, 51–55. [Google Scholar] [CrossRef]
- Huang, S.-Q.; Li, J.-W.; Wang, Z.; Pan, H.-X.; Chen, J.-X.; Ning, Z.-X. Optimization of Alkaline Extraction of Polysaccharides from Ganoderma lucidum and Their Effect on Immune Function in Mice. Molecules 2010, 15, 3694–3708. [Google Scholar] [CrossRef]
- Shao, P.; Feng, J.; Sun, P.; Xiang, N.; Lu, B.; Qiu, D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Res. Int. 2020, 137, 109376. [Google Scholar] [CrossRef]
- Du, B.; Jeepipalli, S.P.K.; Xu, B. Critical review on alterations in physiochemical properties and molecular structure of natural polysaccharides upon ultrasonication. Ultrason. Sonochem. 2022, 90, 106170. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, J.; Shen, M.; Chen, Y.; Yu, Q.; Xie, J. Structure, function and advance application of microwave-treated polysaccharide: A review. Trends Food Sci. Technol. 2022, 123, 198–209. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Miao, S.; Lu, X. Advances in Enzymatic Extraction of Polysaccharides. Sci. Technol. Food Ind. 2021, 42, 351–358. [Google Scholar] [CrossRef]
- Lazou, A.E. Food extrusion: An advanced process for innovation and novel product development. Crit. Rev. Food Sci. Nutr. 2022, 64, 4532–4560. [Google Scholar] [CrossRef]
- Zhou, J.; Qin, L.; Zhang, C.; Luo, S.; Cui, S. Study on Extraction of Lentinan by Orthogonal Optimization of Twin-Screw Extrusion Pretreatment and Microwave-Assisted Extraction. Process Technol. 2020, 5, 89–94+99. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, J.; Lan, W.; Yu, L.; Bi, Y.; Song, S.; Xiong, B.; Wang, H. Polysaccharide decolorization: Methods, principles of action, structural and functional characterization, and limitations of current research. Trends Food Sci. Technol. 2023, 138, 284–296. [Google Scholar] [CrossRef]
- Ke, L.; Duan, X.; Cui, J.; Song, X.; Ma, W.; Zhang, W.; Liu, Y.; Fan, Y. Research progress on the extraction technology and activity study of Epimedium polysaccharides. Carbohydr. Polym. 2023, 306, 120602. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Yang, X.; Zhou, H. The Deproteinization, Antioxidant Acticities and Inhibitory Effect on α-Amylase of Polysaccharides from Corn Silk. Am. J. Biochem. Biotechnol. 2019, 15, 83–90. [Google Scholar] [CrossRef]
- Zeng, X.; Li, P.; Chen, X.; Kang, Y.; Xie, Y.; Li, X.; Xie, T.; Zhang, Y. Effects of deproteinization methods on primary structure and antioxidant activity of Ganoderma lucidum polysaccharides. Int. J. Biol. Macromol. 2019, 126, 867–876. [Google Scholar] [CrossRef]
- Mengting, L.; Metsawur, M.; Tong, L.; Ping, X.; Shulan, S.; Jinao, D. Study on the Enzymatic Deproteinization Technology, Composition Analysis and Immunomodulatory Activity of Isatidis Radix Polysaccharides. J. Nanjing Univ. Tradit. Chin. Med. 2024, 40, 379–390. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, R.; Sun, J.; Duan, Y.; Zhou, H.; Zhou, W.; Li, G. Static decolorization of polysaccharides from the leaves of Rhododendron dauricum: Process optimization, characterization and antioxidant activities. Process Biochem. 2022, 121, 113–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Campbell, R.; Drake, M.; Zhong, Q. Decolorization of Cheddar cheese whey by activated carbon. J. Dairy Sci. 2015, 98, 2982–2991. [Google Scholar] [CrossRef]
- Shao, L.; Sun, Y.; Liang, J.; Li, M.; Li, X. Decolorization affects the structural characteristics and antioxidant activity of polysaccharides from Thesium chinense Turcz: Comparison of activated carbon and hydrogen peroxide decolorization. Int. J. Biol. Macromol. 2020, 155, 1084–1091. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, H.; Li, Y.; Wu, M.; Yu, M.; Sun, X. Optimized purification process of polysaccharides from Carex meyeriana Kunth by macroporous resin, its characterization and immunomodulatory activity. Int. J. Biol. Macromol. 2019, 132, 76–86. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, B.X. Ganoderma and Health; Springer: Singapore, 2019. [Google Scholar]
- Kang, J.; Hua, X.; Yang, R.; Chen, Y.; Yang, H. Characterization of natural low-methoxyl pectin from sunflower head extracted by sodium citrate and purified by ultrafiltration. Food Chem. 2015, 180, 98–105. [Google Scholar] [CrossRef]
- Du, J.; Li, J.; Zhu, J.; Huang, C.; Bi, S.; Song, L.; Hu, X.; Yu, R. Structural characterization and immunomodulatory activity of a novel polysaccharide from Ficus carica. Food Funct. 2018, 9, 3930–3943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Z.; Mei, H.; Xu, J.; Zhou, T.; Cheng, F.; Wang, K. Angelica sinensis polysaccharide nanoparticles as a targeted drug delivery system for enhanced therapy of liver cancer. Carbohydr. Polym. 2019, 219, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cao, D.; Ji, H.; Xu, H.; Feng, Y.; Liu, A. Physicochemical characterization, rheological properties, and hypolipidemic and antioxidant activities of compound polysaccharides in Chinese herbal medicines by fractional precipitation. Int. J. Biol. Macromol. 2023, 242, 124838. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Sudharsan, S.; Sivasankar, P.; Sivakumar, L.; Vairamani, S.; Shanmugam, A. Isolation and chemical characteristics of rhamnose enriched polysaccharide from Grateloupia lithophila. Carbohydr. Polym. 2018, 195, 486–494. [Google Scholar] [CrossRef]
- Tang, W.; Liu, D.; Yin, J.-Y.; Nie, S.-P. Consecutive and progressive purification of food-derived natural polysaccharide: Based on material, extraction process and crude polysaccharide. Trends Food Sci. Technol. 2020, 99, 76–87. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Cui, S.-H.; Zha, X.-Q.; Bansal, V.; Xue, L.; Li, X.-L.; Hao, R.; Pan, L.-H.; Luo, J.-P. Jellyfish skin polysaccharides: Extraction and inhibitory activity on macrophage-derived foam cell formation. Carbohydr. Polym. 2014, 106, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Huang, S.; Chen, J.; Wang, B.; He, L.; Zhang, L.; Li, S.; Wang, J.; Wu, J.; Lai, X.; et al. A novel green method for deproteinization of polysaccharide from Cipangopaludina chinensis by freeze-thaw treatment. J. Clean. Prod. 2017, 142, 3409–3418. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Zheng, Y.; He, Z.; Guan, P.; He, X.; Hui, L.; Dai, Y. Study of Schiff base formation between dialdehyde cellulose and proteins, and its application for the deproteinization of crude polysaccharide extracts. Ind. Crops Prod. 2018, 112, 532–540. [Google Scholar] [CrossRef]
- Guo, Y.; Ye, H.; Wang, H.; Wang, Q.; Fan, S.; Dou, H. Asymmetrical flow field-flow fractionation combined with ultrafiltration: A novel and high-efficiency approach for separation, purification, and characterization of Ganoderma lucidum polysaccharides. Talanta 2023, 253, 124053. [Google Scholar] [CrossRef]
- Diener, M.; Adamcik, J.; Sánchez-Ferrer, A.; Jaedig, F.; Schefer, L.; Mezzenga, R. Primary, Secondary, Tertiary and Quaternary Structure Levels in Linear Polysaccharides: From Random Coil, to Single Helix to Supramolecular Assembly. Biomacromolecules 2019, 20, 1731–1739. [Google Scholar] [CrossRef]
- Jin, Y.; Li, P.; Wang, F. β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef]
- Bao, X.; Dong, Q.; Fang, J. Structure and Conformation Behavior of a Glucan from Spores of Ganoderma lucidum (Fr.) Karst. Acta Biochim. Biophys. Sin. 2000, 32, 557–561. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L. Structure and chain conformation of five water-soluble derivatives of a β-d-glucan isolated from Ganoderma lucidum. Carbohydr. Res. 2009, 344, 105–112. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Tang, Q.; Yang, Y.; Guo, Q.; Wang, Q.; Wu, D.; Cui, S.W. Physicochemical characterization of a high molecular weight bioactive β-d-glucan from the fruiting bodies of Ganoderma lucidum. Carbohydr. Polym. 2014, 101, 968–974. [Google Scholar] [CrossRef]
- Li, J.; Gu, F.; Cai, C.; Hu, M.; Fan, L.; Hao, J.; Yu, G. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2020, 143, 806–813. [Google Scholar] [CrossRef]
- Luo, H.-j.; Zhang, Y.-k.; Wang, S.-z.; Lin, S.-q.; Wang, L.-f.; Lin, Z.-x.; Lu, G.-d.; Lin, D.-m. Structural characterization and anti-oxidative activity for a glycopeptide from Ganoderma lucidum fruiting body. Int. J. Biol. Macromol. 2024, 261, 129793. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Kan, Q.; Song, M.; Hou, T.; An, S.; Lin, H.; Chen, H.; Hu, L.; Xiao, J.; et al. Extraction, Structural Characterization, and Immunomodulatory Activity of a High Molecular Weight Polysaccharide from Ganoderma lucidum. Front. Nutr. 2022, 9, 846080. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ren, Q.; Wang, S.; Gao, J.; Shen, C.; Zhang, S.; Wang, Y.; Guan, F. Chemical Modification of Polysaccharides: A Review of Synthetic Approaches, Biological Activity and the Structure–Activity Relationship. Molecules 2023, 28, 6073. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Wu, X. Ganoderma lucidum polysaccharide (GLP) enhances antitumor immune response by regulating differentiation and inhibition of MDSCs via a CARD9-NF-κB-IDO pathway. Biosci. Rep. 2020, 40, BSR20201170. [Google Scholar] [CrossRef] [PubMed]
- Ruthes, A.C.; Smiderle, F.R.; Iacomini, M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydr. Polym. 2016, 136, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.A.; Wani, A.H.; War, J.M.; Bhat, M.Y. Major Bioactive Properties of Ganoderma Polysaccharides: A Review. Asian J. Pharm. Clin. Res. 2021, 14, 11–24. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, L.; Zeng, F.; Kennedy, J.F. Structure and antitumor activities of the water-soluble polysaccharides from Ganoderma tsugae mycelium. Carbohydr. Polym. 2005, 59, 385–392. [Google Scholar] [CrossRef]
- Kao, P.-F.; Wang, S.-H.; Hung, W.-T.; Liao, Y.-H.; Lin, C.-M.; Yang, W.-B. Structural Characterization and Antioxidative Activity of Low-Molecular-Weights Beta-1,3-Glucan from the Residue of Extracted Ganoderma lucidum Fruiting Bodies. J. Biomed. Biotechnol. 2012, 2012, 673764. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Liang, Y.-C.; Lee, S.-S.; Chiang, B.-L. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-κB and p38 mitogen-activated protein kinase pathways. J. Leukoc. Biol. 2005, 78, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Firenzuoli, F.; Gori, L.; Lombardo, G. The Medicinal Mushroom Agaricus blazei Murrill: Review of Literature and Pharmaco-Toxicological Problems. Evid.-Based Complement. Altern. Med. 2007, 5, 3–15. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, W.; Yi, Y.; Yang, G.; Wu, Z.; Yi, J.; Kong, F. Synthesis of β-(1→6)-branched β-(1→3) glucohexaose and its analogues containing an α-(1→3) linked bond with antitumor activity. Bioorgan. Med. Chem. 2003, 11, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Wiater, A.; Paduch, R.; Choma, A.; Pleszczyńska, M.; Siwulski, M.; Dominik, J.; Janusz, G.; Tomczyk, M.; Szczodrak, J. Biological study on carboxymethylated (1→3)-α-d-glucans from fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2012, 51, 1014–1023. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.-Q.; Nie, S.-P.; Wang, Y.-X.; Cui, S.W.; Xie, M.-Y. Sulfated modification, characterization and property of a water-insoluble polysaccharide from Ganoderma atrum. Int. J. Biol. Macromol. 2015, 79, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Duan, J.; Fang, X.; Fang, J. Chemical modifications of the (1→3)-α-D-glucan from spores of Ganoderma lucidum and investigation of their physicochemical properties and immunological activity. Carbohydr. Res. 2001, 336, 127–140. [Google Scholar] [CrossRef]
- Zhang, S.; Nie, S.; Huang, D.; Huang, J.; Feng, Y.; Xie, M. A Polysaccharide from Ganoderma atrum Inhibits Tumor Growth by Induction of Apoptosis and Activation of Immune Response in CT26-Bearing Mice. J. Agric. Food Chem. 2014, 62, 9296–9304. [Google Scholar] [CrossRef]
- Lu, J.; He, R.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Tan, Y.; Wang, S.; Chen, H.; Zhou, A. Progress in understanding the structure-activity relationship and hypoglycemic mechanism of polysaccharides. Food Sci. 2021, 42, 355–363. [Google Scholar] [CrossRef]
- Meng, Y.; Lyu, F.; Xu, X.; Zhang, L. Recent Advances in Chain Conformation and Bioactivities of Triple-Helix Polysaccharides. Biomacromolecules 2020, 21, 1653–1677. [Google Scholar] [CrossRef]
- Liang, Z.; Yin, Z.; Liu, X.; Ma, C.; Wang, J.; Zhang, Y.; Kang, W. A glucomannogalactan from Pleurotus geesteranus: Structural characterization, chain conformation and immunological effect. Carbohydr. Polym. 2022, 287, 119346. [Google Scholar] [CrossRef]
- Guo, X.; Kang, J.; Xu, Z.; Guo, Q.; Zhang, L.; Ning, H.; Cui, S.W. Triple-helix polysaccharides: Formation mechanisms and analytical methods. Carbohydr. Polym. 2021, 262, 117962. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, J.; Zhou, K.; Yang, Y.; Zhou, S.; Jia, W.; Hao, R.; Pan, Y. Purification, NMR Study and Immunostimulating Property of a Fucogalactan from the Fruiting Bodies of Ganoderma lucidum. Planta Medica 2008, 74, 1730–1734. [Google Scholar] [CrossRef]
- Bao, X.; Fang, J. Islation and Structural Determination of a Glucan from the Spores of Ganoderma lucidum. Acta Bot. Sin. 2001, 43, 312–315. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, S.-P.; Yin, J.-Y.; Wang, Y.-X.; Xie, M.-Y. Structural characterization of a heterogalactan purified from fruiting bodies of Ganoderma atrum. Food Hydrocoll. 2014, 36, 339–347. [Google Scholar] [CrossRef]
- Bao, X.-F.; Zhen, Y.; Ruan, L.; Fang, J.-N. Purification, Characterization, and Modification of T Lymphocyte Stimulating Polysaccharide from Spores of Ganoderma lucidum. Chem. Pharm. Bull. 2002, 50, 623–629. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, S.; Cui, S.W.; Xu, M.; Ding, H.; Xie, M. Characterization of a bioactive polysaccharide from Ganoderma atrum: Re-elucidation of the fine structure. Carbohydr. Polym. 2017, 158, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Liu, C.; Fang, J.; Li, X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydr. Res. 2001, 332, 67–74. [Google Scholar] [CrossRef]
- Bao, X.; Fang, J.; Li, X. Structural Characterization and Immunomodulating Activity of a Complex Glucan from Spores of Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2014, 65, 2384–2391. [Google Scholar] [CrossRef]
- Bao, X.-F.; Wang, X.-S.; Dong, Q.; Fang, J.-N.; Li, X.-Y. Structural features of immunologically active polysaccharides from Ganoderma lucidum. Phytochemistry 2002, 59, 175–181. [Google Scholar] [CrossRef]
- Tomoda, M.; Gonda, R.; Kasahara, Y.; Hikino, H. Glycan Structures Of Ganoderans B And C, Hypoglycemic Glycans Of Ganoderma lucidum Fruit Bodies. Phytochemistry 1986, 25, 2817–2820. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yu, H.; Zhou, S.; Zhang, Z.; Wu, D.; Yan, M.; Tang, Q.; Zhang, J. Structural characterization and immuno-enhancing activity of a highly branched water-soluble β-glucan from the spores of Ganoderma lucidum. Carbohydr. Polym. 2017, 167, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wang, Y.; Shi, L.; Yao, J.; Li, J.; Ma, F.; Ding, K. A novel water-soluble β-d-glucan isolated from the spores of Ganoderma lucidum. Carbohydr. Res. 2012, 353, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, J.; Yang, Y.; Zhou, S.; Liu, Y.; Tang, Q.; Du, X.; Chen, H.; Pan, Y. Structural characterisation of a heteropolysaccharide by NMR spectra. Food Chem. 2009, 112, 962–966. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Zhang, L. Structure and immunological activity of a novel polysaccharide from the spores of Ganoderma lucidum. Afr. J. Biotechnol. 2011, 10, 10923–10929. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, J.; Ye, X.; Tang, Q.; Liu, Y.; Gong, C.; Du, X.; Pan, Y. Structural elucidation of the polysaccharide moiety of a glycopeptide (GLPCW-II) from Ganoderma lucidum fruiting bodies. Carbohydr. Res. 2008, 343, 746–752. [Google Scholar] [CrossRef]
- Guo, L.; Xie, J.; Ruan, Y.; Zhou, L.; Zhu, H.; Yun, X.; Jiang, Y.; Lü, L.; Chen, K.; Min, Z.; et al. Characterization and immunostimulatory activity of a polysaccharide from the spores of Ganoderma lucidum. Int. Immunopharmacol. 2009, 9, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, Q.; Zimmerman-Kordmann, M.; Reutter, W.; Fan, H. Activation of B lymphocytes by GLIS, a bioactive proteoglycan from Ganoderma lucidum. Life Sci. 2002, 71, 623–638. [Google Scholar] [CrossRef]
- Shang, D.; Zhang, J.; Wen, L.; Li, Y.; Cui, Q. Preparation, Characterization, and Antiproliferative Activities of the Se-Containing Polysaccharide SeGLP-2B-1 from Se-Enriched Ganoderma lucidum. J. Agric. Food Chem. 2009, 57, 7737–7742. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Wu, J.; Weng, L.; Zhang, H.; Zhang, J.; Wu, A. An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydr. Polym. 2020, 227, 115332. [Google Scholar] [CrossRef]
- Zhao, H.; Lai, C.-J.-S.; Yu, Y.; Wang, Y.-n.; Zhao, Y.-J.; Ma, F.; Hu, M.; Guo, J.; Wang, X.; Guo, L. Acidic hydrolysate fingerprints based on HILIC-ELSD/MS combined with multivariate analysis for investigating the quality of Ganoderma lucidum polysaccharides. Int. J. Biol. Macromol. 2020, 163, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.; O’Connor, N.; Jose, D.; Barrett, A.; Regan, F. Selection and optimization of protein and carbohydrate assays for the characterization of marine biofouling. Anal. Methods 2020, 12, 2228–2236. [Google Scholar] [CrossRef]
- Li, L.-F.; Zhang, Q.-W.; Han, Q.-B. Recent advances in qualitative and quantitative analysis of polysaccharides in natural medicines: A critical review. J. Pharm. Biomed. Anal. 2022, 220, 115016. [Google Scholar] [CrossRef]
- Han, Z.; Zhao, Y.; Liu, H.; Li, R. Determination of Ganoderma lucidum Polysaccharide by Reversed-phase High Performance Liquid Chromatography. J. Agric. Sci. Technol. 2009, 11, 65–67. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, S.; Feng, N.; Liu, Y.; Wang, J.; Tang, Q.; Han, W.; Zhang, J. Directional Harvest of Ganoderma lucidum ‘Hunong No. 1’ for High Triterpenoids and Water Soluble Polysaccharides. Acta Edulis Fungi 2023, 30, 113–122. [Google Scholar] [CrossRef]
- Goodwin, N.L.; Choong, J.J.; Hwang, S.; Pitts, K.; Bloom, L.; Islam, A.; Zhang, Y.Y.; Szelenyi, E.R.; Tong, X.; Newman, E.L.; et al. Simple Behavioral Analysis (SimBA) as a platform for explainable machine learning in behavioral neuroscience. Nat. Neurosci. 2024, 27, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Widman, A.J.; Shah, M.; Frydendahl, A.; Halmos, D.; Khamnei, C.C.; Øgaard, N.; Rajagopalan, S.; Arora, A.; Deshpande, A.; Hooper, W.F.; et al. Ultrasensitive plasma-based monitoring of tumor burden using machine-learning-guided signal enrichment. Nat. Med. 2024, 30, 1655–1666. [Google Scholar] [CrossRef]
- Puszkarska, A.M.; Taddese, B.; Revell, J.; Davies, G.; Field, J.; Hornigold, D.C.; Buchanan, A.; Vaughan, T.J.; Colwell, L.J. Machine learning designs new GCGR/GLP-1R dual agonists with enhanced biological potency. Nat. Chem. 2024. [Google Scholar] [CrossRef]
- Li, B.; Raji, I.O.; Gordon, A.G.R.; Sun, L.; Raimondo, T.M.; Oladimeji, F.A.; Jiang, A.Y.; Varley, A.; Langer, R.S.; Anderson, D.G. Accelerating ionizable lipid discovery for mRNA delivery using machine learning and combinatorial chemistry. Nat. Mater. 2024, 23, 1002–1008. [Google Scholar] [CrossRef]
- Mathis, N.; Allam, A.; Tálas, A.; Kissling, L.; Benvenuto, E.; Schmidheini, L.; Schep, R.; Damodharan, T.; Balázs, Z.; Janjuha, S.; et al. Machine learning prediction of prime editing efficiency across diverse chromatin contexts. Nat. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Zeng, X.; Cao, R.; Xi, Y.; Li, X.; Yu, M.; Zhao, J.; Cheng, J.; Li, J. Food flavor analysis 4.0: A cross-domain application of machine learning. Trends Food Sci. Technol. 2023, 138, 116–125. [Google Scholar] [CrossRef]
- Ji, H.; Pu, D.; Yan, W.; Zhang, Q.; Zuo, M.; Zhang, Y. Recent advances and application of machine learning in food flavor prediction and regulation. Trends Food Sci. Technol. 2023, 138, 738–751. [Google Scholar] [CrossRef]
- Liu, Y.; Long, Y.; Liu, H.; Lan, Y.; Long, T.; Kuang, R.; Wang, Y.; Zhao, J. Polysaccharide prediction in Ganoderma lucidum fruiting body by hyperspectral imaging. Food Chem. X 2022, 13, 100199. [Google Scholar] [CrossRef]
- Ma, Y.; He, H.; Wu, J.; Wang, C.; Chao, K.; Huang, Q. Assessment of Polysaccharides from Mycelia of genus Ganoderma by Mid-Infrared and Near-Infrared Spectroscopy. Sci. Rep. 2018, 8, 10. [Google Scholar] [CrossRef]
- Ni, H.; Fu, W.; Wei, J.; Zhang, Y.; Chen, D.; Tong, J.; Chen, Y.; Liu, X.; Luo, Y.; Xu, T. Non-destructive detection of polysaccharides and moisture in Ganoderma lucidum using near-infrared spectroscopy and machine learning algorithm. LWT 2023, 184, 115001. [Google Scholar] [CrossRef]
- Pandeirada, C.O.; Achterweust, M.; Janssen, H.G.; Westphal, Y.; Schols, H.A. Periodate oxidation of plant polysaccharides provides polysaccharide-specific oligosaccharides. Carbohydr. Polym. 2022, 291, 119540. [Google Scholar] [CrossRef]
- Yao, H.Y.; Wang, J.Q.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, W.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Monosaccharide composition analysis of polysaccharides from natural sources: Hydrolysis condition and detection method development. Food Hydrocoll. 2021, 116, 106641. [Google Scholar] [CrossRef]
- Nagar, S.; Lakhera, A.K.; Kumar, V. Upgrading Methylation Method for Structural Studies of Polysaccharides: Case Analysis of a Bioactive Polysaccharide from Acacia tortilis. J. Biol. Act. Prod. Nat. 2020, 10, 70–85. [Google Scholar] [CrossRef]
- Zheng, Y.; Yan, J.; Cao, C.; Liu, Y.; Yu, D.; Liang, X. Application of chromatography in purification and structural analysis of natural polysaccharides: A review. J. Sep. Sci. 2023, 46, e2300368. [Google Scholar] [CrossRef]
- Cheong, K.-L.; Wu, D.-T.; Deng, Y.; Leong, F.; Zhao, J.; Zhang, W.-J.; Li, S.-P. Qualitation and quantification of specific polysaccharides from Panax species using GC–MS, saccharide mapping and HPSEC-RID-MALLS. Carbohydr. Polym. 2016, 153, 47–54. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Ren, H.; Pang, M.; Sui, X.; Du, X. Extraction, Purification, Structural Characterization and Antioxidant Activity of Polysaccharides from the Fruiting Body of Guanxian Ganoderma lucidum. Sci. Technol. Food Ind. 2023, 44, 81–89. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Zhao, D. Structural analysis of biomacromolecules using circular dichroism spectroscopy. In Advanced Spectroscopic Methods to Study Biomolecular Structure and Dynamics; Academic Press: San Diego, CA, USA, 2023; pp. 77–103. [Google Scholar]
- Fu, Y.-L.; Shi, L. Methods of study on conformation of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2024, 263, 130275. [Google Scholar] [CrossRef] [PubMed]
- Kuttel, M.M.; Ståhle, J.; Widmalm, G. CarbBuilder: Software for building molecular models of complex oligo- and polysaccharide structures. J. Comput. Chem. 2016, 37, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Liu, Y.; Yang, Y.; Wang, R.; Li, T. Advances in sulfonated modification and bioactivity of polysaccharides. Int. J. Biol. Macromol. 2023, 253, 126400. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Zhao, X.; Tang, Q.; Dernedde, J.; Zhang, J.; Fan, H. Anti-inflammatory properties of GLPss58, a sulfated polysaccharide from Ganoderma lucidum. Int. J. Biol. Macromol. 2018, 107, 486–493. [Google Scholar] [CrossRef]
- Zheng, D.; Zhao, J.; Li, Y.; Zhu, L.; Jin, M.; Wang, L.; Liu, J.; Lei, J.; Li, Z. Self-Assembled pH-Sensitive Nanoparticles Based on Ganoderma lucidum Polysaccharide–Methotrexate Conjugates for the Co-delivery of Anti-tumor Drugs. ACS Biomater. Sci. Eng. 2021, 7, 3764–3773. [Google Scholar] [CrossRef]
- Zhang, S.; Pang, G.; Chen, C.; Qin, J.; Yu, H.; Liu, Y.; Zhang, X.; Song, Z.; Zhao, J.; Wang, F.; et al. Effective cancer immunotherapy by Ganoderma lucidum polysaccharide-gold nanocomposites through dendritic cell activation and memory T cell response. Carbohydr. Polym. 2019, 205, 192–202. [Google Scholar] [CrossRef]
- Chen, S.K.; Wang, X.; Guo, Y.Q.; Song, X.X.; Yin, J.Y.; Nie, S.P. Exploring the partial degradation of polysaccharides: Structure, mechanism, bioactivities, and perspectives. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4831–4870. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Lai, Z.; Hu, X.; Wang, L.; Wang, X.; Li, Z.; Gao, M.; Yang, Y.; Wang, Q.; et al. Effect of monosaccharide composition and proportion on the bioactivity of polysaccharides: A review. Int. J. Biol. Macromol. 2024, 254, 127955. [Google Scholar] [CrossRef]
- Liu, S.-R.; Ke, B.-R.; Zhang, W.-R.; Liu, X.-R.; Wu, X.-P. Breeding of new Ganoderma lucidum strains simultaneously rich in polysaccharides and triterpenes by mating basidiospore-derived monokaryons of two commercial cultivars. Sci. Hortic. 2017, 216, 58–65. [Google Scholar] [CrossRef]
- Tang, C.; Tan, Y.; Zhang, J.; Zhou, S.; Honda, Y.; Zhang, H. A Novel Strain Breeding of Ganoderma lucidum UV119 (Agaricomycetes) with High Spores Yield and Strong Resistant Ability to Other Microbes’ Invasions. Foods 2023, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, M.; Zhao, L.; Chen, L.; Ding, Z. Novel Insights into the Mechanism Underlying High Polysaccharide Yield in Submerged Culture of Ganoderma lucidum Revealed by Transcriptome and Proteome Analyses. Microorganisms 2023, 11, 772. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).