Bioaccessibility of Mineral Nutrients in Plain Green Spanish-Style Manzanilla Table Olives Packaged in Nutrient Salt Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Physicochemical Analysis of Brines and Fruits
2.3. In Vitro Digestion of Olives
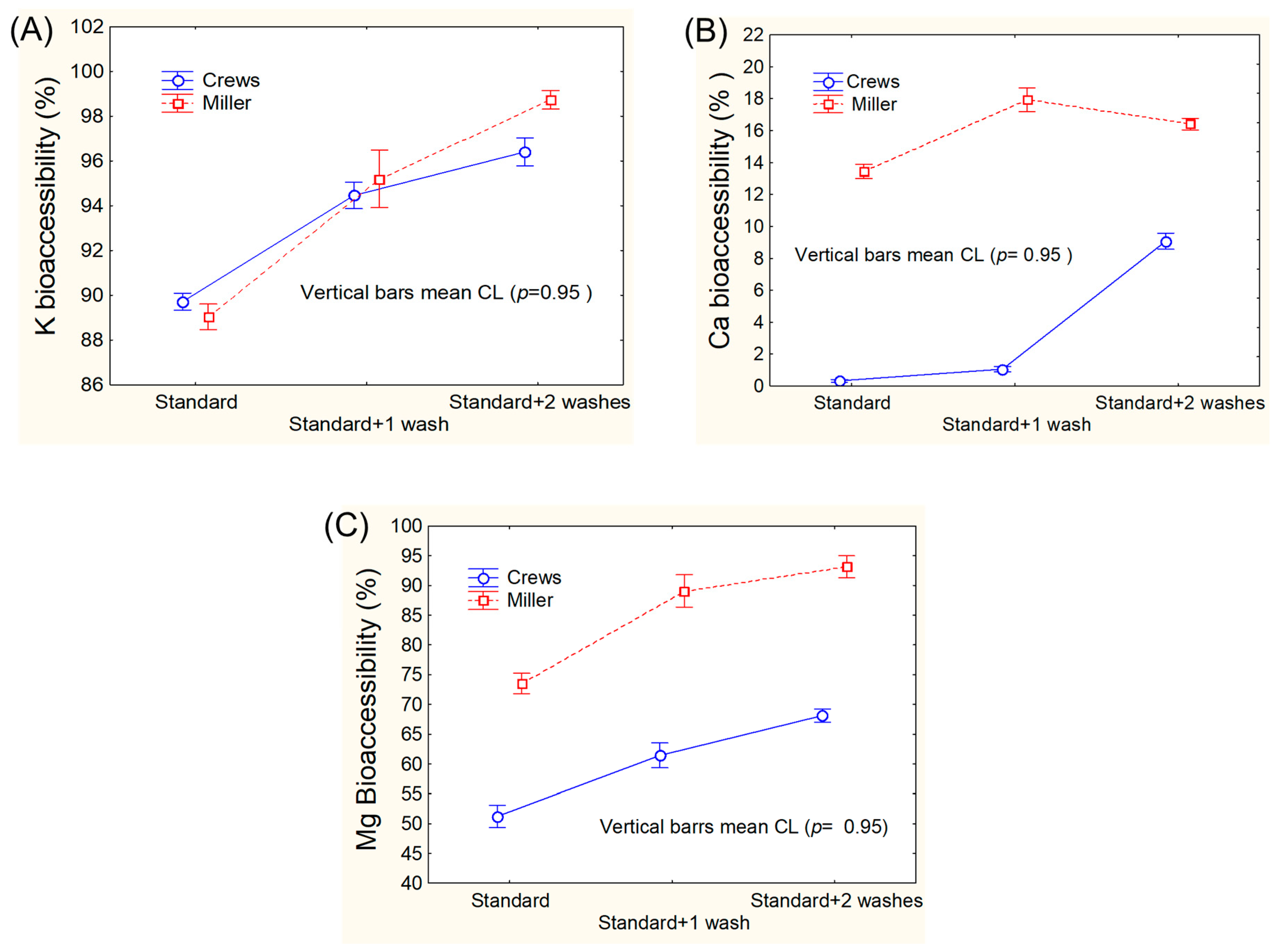
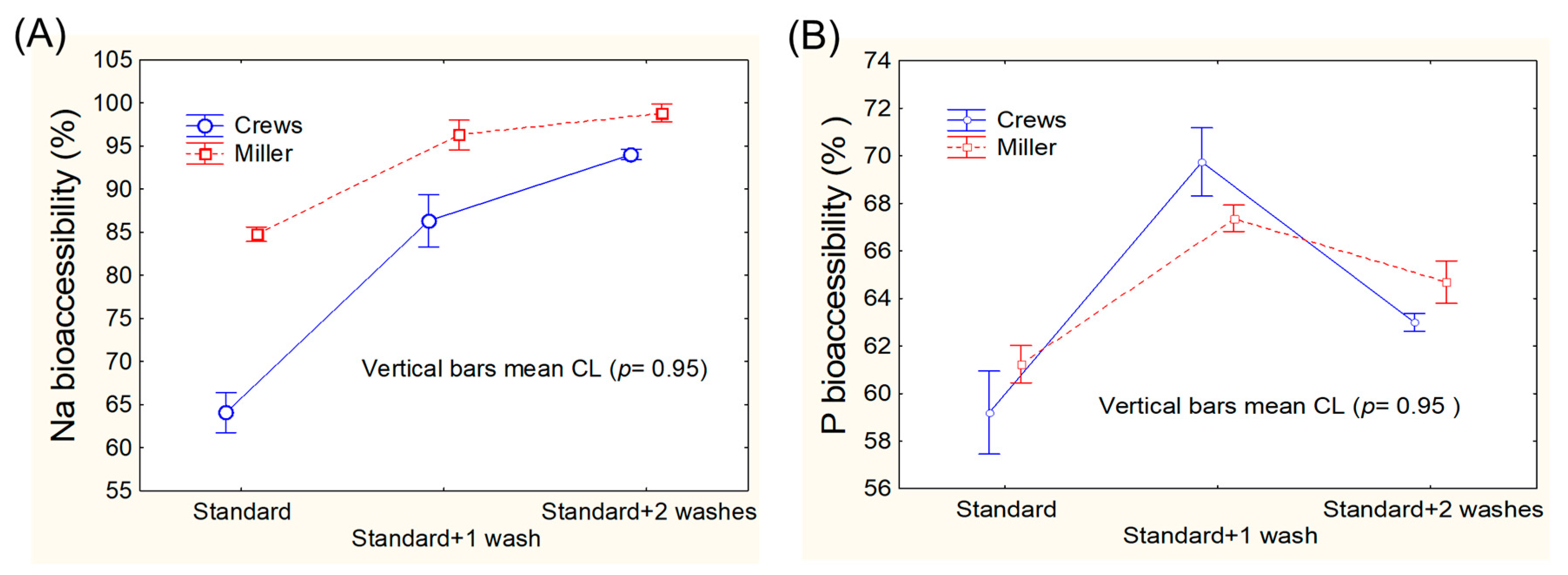
2.3.1. Miller’s Protocol
2.3.2. Crews’ Protocol
2.3.3. Post-Digestion Re-Extractions
2.4. Wet Mineralisation
2.5. Mineral Analysis
2.6. Mineral Recovery and Bioaccessibility Estimation
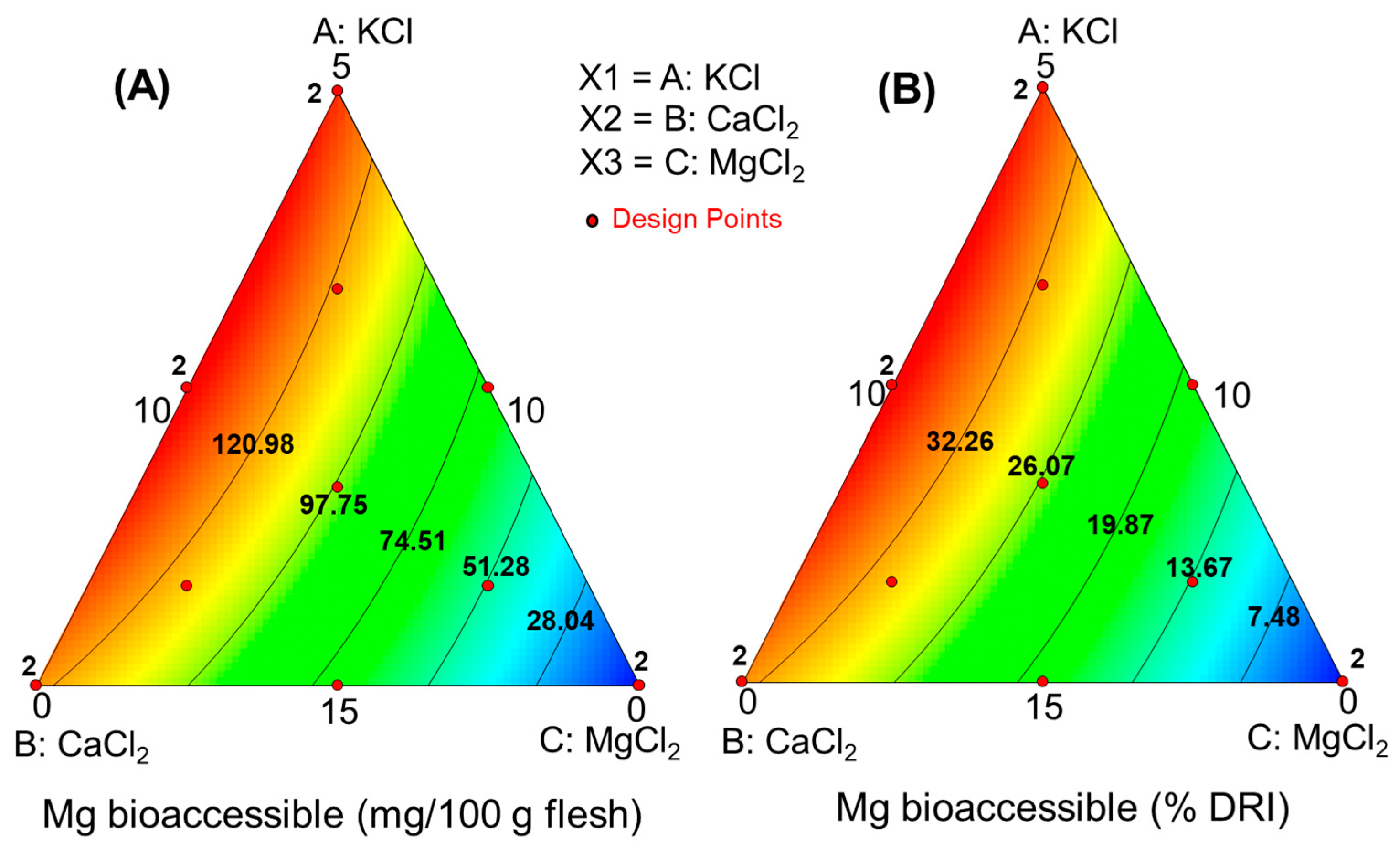
2.7. Mineral Nutrient Bioaccessibility, Recovery, and RDI Contribution According to Chloride Salt Mixtures
2.8. Statistical Analysis
3. Results
3.1. Assays for Adapting Mineral Bioaccessibility Protocols to Green Table Olives
3.2. Mineral Content in the Pulp of Green Spanish-Style Table Olives Packaged in Chloride Salt Mixtures
3.3. Bioaccessibility of Mineral Nutrients in Green Spanish-Style Table Olives Packaged with Chloride Salt Mixtures
3.3.1. Mineral Concentrations in Different Fractions after In Vitro Digestion
3.3.2. Mineral Nutrient Bioaccessibility after In Vitro Digestion
3.3.3. Bioaccessibility and Nutritional Labelling of Minerals
- Model parameters: significant, p < 0.0001; lack of fit, p = 0.04508; precision, 53
- Amount bioac. (mg/100 g pulp)
- Contribution to RDI (%) =
- Model parameters: significant, p < 0.0001; lack of fit, p = 0.4508; precision, 53
- Amount bioac. (mg/100 g pulp)
- Contribution to RDI (%) =
- Model parameters: significant, p < 0.0001; lack of fit, p = 0.2158; precision, 95.16
- Amount bioac. (mg/100 g pulp) =
- Contribution to RDI (%) =
3.3.4. Comparison between Usual Mineral Nutrition Labelling and that Based on Bioaccessibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IOC, International Olive Council. Online Reference Included in World Table Olives Figures: Production. 2022. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 17 May 2024).
- Garrido Fernández, A.; Fernández Díez, M.J.; Adams, R.M. Table Olive Production and Processing; Chapman & Hall: London, UK, 1997. [Google Scholar]
- IOC, International Olive Council. Trade Standards Applying to Table Olives. COI/OT/NC No. 1. December 2004. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-OT-NC1-2004-Eng.pdf (accessed on 26 June 2024).
- Wang, Y.-J.; Yeh, T.-L.; Shih, M.-C.; Tu, Y.-K.; Chien, K.-L. Dietary sodium intake and risk of cardiovascular disease: A systematic review and dose-response meta-analysis. Nutrients 2020, 12, 2934. [Google Scholar] [CrossRef]
- CDC, U.S. Centers for Disease Control and Prevention. About Sodium and Health. Health Risks. 2024. Available online: https://www.cdc.gov/salt/about/index.html (accessed on 24 June 2024).
- US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf (accessed on 26 June 2024).
- European Parliament and the Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Off. J. Eur. Union 2011, L304/18–L304/63. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:304:0018:0063:en:PDF (accessed on 26 June 2024).
- European Commission, Directorate-General Health and Consumers. Implementation of the EU Salt Reduction Framework: Results of Members States Survey; Publications Office of the European Union: Luxembourg, 2012; ISBN 978-92-79-23821-5. Available online: https://health.ec.europa.eu/publications/implementation-eu-salt-reduction-framework-results-member-states-survey_en (accessed on 26 June 2024).
- Rocha, J.; Borges, N.; Pinho, O. Table olives and health: A review. J. Nutr. Sci. 2020, 9, e57. [Google Scholar] [CrossRef] [PubMed]
- López, A.; García, P.; Garrido, A. Multivariate characterisation of table olives according to their mineral nutrient composition. Food Chem. 2008, 106, 369–378. [Google Scholar] [CrossRef]
- Moreno-Baquero, J.M.; Bautista-Gallego, J.; Garrido-Fernández, A.; López-López, A. Mineral content and sensory characteristics of Gordal green table olives fermented in chloride salt mixtures. J. Food Sci. 2012, 77, S107–S114. [Google Scholar] [CrossRef]
- Bautista Gallego, J.; Arroyo López, F.N.; Romero Gil, V.; Rodríguez Gómez, F.; García García, P.; Garrido Fernández, A. Chloride salt mixtures affect Gordal cv. green Spanish-style table olive fermentation. Food Microbiol. 2011, 28, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Moreno-Baquero, J.M.; Garrido-Fernández, A. Impact of salts mixtures on the physicochemical and sensory characteristics of Spanish-style Manzanilla green table olives during packaging. Foods 2023, 12, 3561. [Google Scholar] [CrossRef]
- López-López, A.; Moreno-Baquero, J.M.; Garrido-Fernández, A. Relationships between Na, K, and Ca mineral nutrients in brine and table olive flesh. Nutritional labelling implications. LWT-Food Sci. Technol. 2023, 189, 115546. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Hondrodimou, O.; Mallouchos, A.; Nychas, G.-J.E. A study on the implications of NaCl reduction in the fermentation profile of Conservolea natural black olives. Food Microbiol. 2011, 28, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Ambra, R.; Lucchetti, S.; Moneta, E.; Peparaio, M.; Nardo, N.; Baiamonte, I.; Di Costanzo, M.G.; Civitelli, E.S.; Pastore, G. Effect of partial substitution of sodium with potassium chloride in the fermenting brine on organoleptic characteristics and bioactive molecules occurrence in table olives debittered using Spanish and Castelvetrano methods. Int. J. Food Sci. Technol. 2017, 52, 662–670. [Google Scholar] [CrossRef]
- López-López, A.; Moreno-Baquero, J.M.; Garrido Fernandez, A. In vitro bioaccessibility of ripe table olive mineral nutrients. Foods 2020, 9, 275. [Google Scholar] [CrossRef]
- Crews, H.M.; Burrell, J.A.; McWeeny, D.J. Preliminary enzymolysis studies on trace element extractability from food. J. Sci. Food Agric. 1983, 34, 997–1004. [Google Scholar] [CrossRef]
- Miller, D.D.; Schricker, B.R.; Rasmussen, R.R.; Van Campen, D. An in vitro method for estimation of iron availability from meals. Am. J. Clin. Nutr. 1981, 34, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- Crews, H.M.; Burrell, J.A.; McWeeny, D.J. Trace element solubility from food following enzymolysis. Z. Lebensm. Unters Forsch. 1985, 180, 221–226. [Google Scholar] [CrossRef]
- Crews, H.M.; Burrell, J.A.; McWeeny, D.J. Comparison of trace element solubility from food items treated separately and in combination. Z. Lebensm. Unters Forsch. 1985, 180, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulos, N. GBC 932/933 Atomic Absorption Spectrophotometers; Operation Manual: Braeside, VIC, Australia, 1994. [Google Scholar]
- AOAC. Phosphorous in fruits and fruits products no. 970.39. In Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC International: Gaithersburg, MD, USA, 1993. [Google Scholar]
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- StatSoft. Statistica for Windows (Computer Program Manual); StatSoft: Tulsa, OK, USA, 2015. [Google Scholar]
- Farquhar, W.B.; Edwards, D.G.; Jurkovitz, C.T.; Weintraub, W.S. Dietary sodium and health: More than just blood pressure. J. Am. Coll. Cardiol. 2015, 65, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.W.; Bagar, S.; Jerums, G.; Ekinci, E.I. Sodium and its role in cardiovascular disease-The debate continues. Front. Endocrinol. 2016, 7, 264. [Google Scholar] [CrossRef]
- Collins, M.; Mason, H.; O’Flaherty, M.; Guzman-Castillo, M.; Critchley, J.; Capewell, S. An economic evaluation of salt reduction policies to reduce coronary heart disease in England: A policy modeling study. Value Health 2014, 17, 517–524. [Google Scholar] [CrossRef]
- Brenes, M.; García, P.; Garrido, A. Influence of salts and pH on the firmness of olives in acid conditions. J. Food Qual. 1994, 17, 335–346. [Google Scholar] [CrossRef]
- Jiménez, A.; Heredia, A.; Guillén, R.; Fernández-Bolaños, J. Correlation between soaking conditions, cation content of cell wall, and olive firmness during “Spanish green olive” processing. J. Agric. Food Chem. 1997, 45, 1653–1658. [Google Scholar] [CrossRef]
- García-Serrano, P.; Romero, C.; Medina, E.; García-García, P.; De Castro, A.; Brenes, M. Effect of calcium on the preservation of green olives intended for black ripe olive processing under free-sodium chloride conditions. LWT-Food Sci. Technol. 2020, 118, 108870. [Google Scholar] [CrossRef]
- García Serrano, P.; Romero, C.; García García, P.; Brenes, M. Influence of the type of calcium salt on the cation absorption and firmness of black ripe olives. Int. J. Food Sci. Technol. 2021, 56, 919–926. [Google Scholar] [CrossRef]
- Del Pino, A.M.; Regni, L.; Reale, L.; Micheli, M.; Datti, A.; Proietti, P.; Palmerini, C.A. Selenium preserves cytosolic-Ca2+ homeostasis in olive callus cells during oxidative stress. Plant Cell Tiss. Organ Cult. 2023, 154, 519–525. [Google Scholar] [CrossRef]
- Cardoso, S.; Coimbra, M.A.; Lopes da Silva, J.A. Calcium-mediated gelation of an olive pomace pectic extract. Carbohyd. Polym. 2003, 52, 125–133. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Mafra, I.; Soares, M.R.; Evtuguin, D.V.; Coimbra, M.A. Dimeric calcium complexes of arabinan-rich pectic polysaccharides from Olea europaea L. cell walls. Carbohyd. Polym. 2006, 65, 535–543. [Google Scholar] [CrossRef]
- Uusi-Rasi, K.; Kärkkäinen, M.U.M.; Lamberg-Allardt, C.J.E. Calcium intake in health maintenance-a systematic review. Food Nutr. Res. 2013, 57, 21082. [Google Scholar] [CrossRef]
- Pu, F.; Chen, N.; Xue, S. Calcium intake, calcium homeostasis and health. Food Sci. Hum. Wellness 2016, 5, 8–16. [Google Scholar] [CrossRef]
- Soares, J.H. 12 Phosphorus bioavailability. In Bioavailability of Nutrients for Animals. Amino Acids, Minerals, and Vitamins; Ammerman, C.B., Baker, D.H., Lewis, A.J., Eds.; Academic Press: Cambridge, MA, USA, 1995; pp. 257–294. [Google Scholar] [CrossRef]
- McCarty, M.F. Lower bioavailability of plant-derived phosphorus. Am. J. Clin. Nutr. 2014, 99, 966–967. [Google Scholar] [CrossRef]
- Naismith, D.J.; Braschi, A. An investigation into the bioaccessibility of potassium in unprocessed fruits and vegetables. Int. J. Food Sci. Nutr. 2008, 59, 438–450. [Google Scholar] [CrossRef]
- Houston, M. The role of magnesium in hypertension and cardiovascular disease. J. Clin. Hypertens. 2011, 13, 843–847. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition; Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; et al. Scientific advice related to nutrient profiling for the development of harmonised mandatory front-of-pack nutrition labelling and the setting of nutrient profiles for restricting nutrition and health claims on foods. EFSA J. 2022, 20, 7259. [Google Scholar] [CrossRef]
- Veronese, N.; Demurtas, J.; Pesolillo, G.; Celotto, S.; Barnini, T.; Calusi, G.; Caruso, M.G.; Notarnicola, M.; Reddavide, R.; Stubbs, B.; et al. Magnesium and health outcomes: An umbrella review of systematic reviews and meta-analysis of observational and intervention studies. Eur. J. Nutr. 2020, 59, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Melse-Boonstra, A. Bioavailability of micronutrients from nutrient-dense whole foods: Zooming in on dairy, vegetables, and fruits. Front. Nutr. 2020, 7, 101. [Google Scholar] [CrossRef]
- Pongrac, P.; Kelemen, M.; Vogel-Mikus, K.; Vavpetic, P.; Pelicon, P.; Zurga, P.; Vidovic, N.; Paskovic, M.P.; Smiljana, G.B.; Lukic, I.; et al. Tissue specific calcium and magnesium allocation to explain differences in bulk concentration in leaves of one-year-old seedling of two olive (Olea europaea L.) cultivars. Plant Physiol. Biochem. 2023, 194, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Zhang, B.; Bozdar, B.; Chachar, S.; Rai, M.; Li, J.; Li, Y.; Haya, F.; Chachar, Z.; Tu, P. The power of magnesium: Unlocking the potential for increase yield, quality, and stress tolerance of horticultural crops. Front. Plant Sci. 2023, 14, 1285512. [Google Scholar] [CrossRef]



| Run | [NaCl] (g/L) | [KCl] (g/L) | [CaCl2] (g/L) | [MgCl2] (g/L) |
|---|---|---|---|---|
| 1 | 25 | 5.0 | 10.0 | 10.0 |
| 2 | 25 | 15.0 | 10.0 | 0.0 |
| 3 | 25 | 15.0 | 0.0 | 10.0 |
| 4 | 25 | 5.0 | 10.0 | 10.0 |
| 5 | 25 | 10.0 | 5.0 | 10.0 |
| 6 | 25 | 15.0 | 5.0 | 5.0 |
| 7 | 25 | 8.3 | 8.3 | 8.3 |
| 8 | 25 | 15.0 | 0.0 | 10.0 |
| 9 | 25 | 15.0 | 10.0 | 0.0 |
| 10 | 25 | 13.3 | 3.3 | 8.3 |
| 11 | 25 | 13.3 | 8.3 | 3.3 |
| 12 | 25 | 11.7 | 6.7 | 6.7 |
| 13 | 25 | 10.0 | 5.0 | 10.0 |
| 14 | 25 | 10.0 | 10.0 | 5.0 |
| Control | 50 | 0.0 | 0.0 | 0.0 |
| Run | Fraction | [Na] mg/kg | [K] mg/kg | [Ca] mg/kg | [Mg] mg/kg | [P] mg/kg | Average Weight (g) |
|---|---|---|---|---|---|---|---|
| 1 | Raw material | 6301.01 (21.64) | 1571.64 (10.02) | 4611.70 (3.80) | 1570.25 (1.12) | 59.25 (0.62) | 2.0022 (0.0012) |
| Supernatant | 1176.90 (5.43) | 108.02 (0.37) | 62.86 (0.11) | 86.18 (0.15) | 16.64 (1.40) | 33.2526 (0.3170) | |
| Solid residue | 245.58 (9.32) | 23.69 (0.95) | 2876.60 (192.76) | 151.89 (10.59) | 26.88 (0.06) | 2.6554 (0.3630) | |
| Blank | 1041.36 (5.21) | 21.57 (0.02) | 1.94 (0.02) | 4.62 (0.02) | 23.47 (0.11) | 26.3000 (N/A) | |
| 2 | Raw material | 6398.18 (32.66) | 5003.36 (4.47) | 4583.18 (4.49) | 39.12 (1.15) | 65.12 (0.52) | 2.0212 (0.0004) |
| Supernatant | 1374.04 (3.58) | 311.67 (1.17) | 63.50 (0.17) | 5.27 (0.01) | 27.44 (0.52) | 33.9886 (0.0093) | |
| Solid residue | 413.22 (21.52) | 107.45 (1.63) | 3188.23 (64.31) | 8.22 (0.06) | 26.63 (0.06) | 2.2075 (0.0042) | |
| Blank | 1304.64 (1.80) | 18.67 (0.11) | 2.39 (0.01) | 4.04 (0.01) | 22.97 (0.06) | 26.5800 (N/A) | |
| 3 | Raw material | 6503.01 (35.57) | 5152.03 (11.76) | 603.05 (3.21) | 1556.03 (1.77) | 62.17 (0.76) | 2.0200 (0.0024) |
| Supernatant | 1200.95 (4.08) | 325.18 (0.98) | 10.24 (0.07) | 78.10 (0.13) | 19.05 (0.37) | 32.8539 (0.2814) | |
| Solid residue | 397.38 (2.69) | 117.23 (2.31) | 369.08 (6.79) | 216.90 (6.32) | 23.33 (0.03) | 2.6643 (0.1004) | |
| Blank | 1030.14 (1.18) | 20.21 (0.13) | 3.10 (0.05) | 3.73 (0.01) | 23.34 (0.11) | 26.1800 (N/A) | |
| 4 | Raw material | 6512.51 (37.01) | 1662.35 (6.28) | 4664.07 (21.43) | 1546.07 (19.54) | 60.40 (0.35) | 2.0093 (0.0050) |
| Supernatant | 1171.50 (3.45) | 107.88 (0.14) | 60.14 (0.67) | 82.31 (0.22) | 22.82 (0.60) | 33.5133 (0.1219) | |
| Solid residue | 304.18 (0.69) | 40.02 (0.62) | 3327.88 (111.28) | 172.19 (2.42) | 25.32 (0.12) | 2.1750 (0.1122) | |
| Blank | 1024.19 (0.85) | 13.18 (0.09) | 1.83 (0.01) | 2.80 (0.01) | 21.84 (0.03) | 26.0900 (N/A) | |
| 5 | Raw material | 6561.75 (17.38) | 3555.06 (21.64) | 2744.65 (13.28) | 1637.96 (5.26) | 67.29 (0.21) | 2.200 (0.0039) |
| Supernatant | 1205.01 (1.13) | 227.20 (0.46) | 45.86 (0.74) | 88.14 (0.17) | 27.69 (0.32) | 33.6827 (0.0696) | |
| Solid residue | 323.78 (9.94) | 71.87 (2.04) | 1899.09 (58.47) | 169.77 (5.34) | 26.61 (0.16) | 2.0818 (0.0774) | |
| Blank | 1057.39 (1.55) | 20.38 (0.09) | 1.95 (0.01) | 4.05 (0.02) | 23.18 (0.05) | 26.3700 (N/A) | |
| 6 | Raw material | 6519.45 (21.43) | 5066.09 (9.03) | 2689.18 (26.97) | 777.18 (2.04) | 58.76 (0.34) | 2.0106 (0.0004) |
| Supernatant | 1202.62 (1.70) | 309.97 (1.07) | 33.84 (0.14) | 42.56 (0.08) | 22.47 (0.47) | 33.5629 (0.0913) | |
| Solid residue | 296.74 (6.34) | 107.36 (2.26) | 1957.48 (34.97) | 87.33 (3.60) | 26.22 (0.05) | 2.1235 (0.0664) | |
| Blank | 1058.73 (3.16) | 20.65 (0.09) | 1.43 (0.01) | 3.85 (0.03) | 22.63 (0.01) | 26.2800 (N/A) | |
| 7 | Raw material | 6451.19 (11.12) | 2855.42 (6.25) | 4064.94 (20.22) | 1376.66 (5.95) | 65.38 (0.68) | 2.0080 (0.0017) |
| Supernatant | 1146.92 (1.29) | 176.27 (1.13) | 59.51 (0.36) | 73.47 (0.25) | 28.58 (1.07) | 33.6337 (0.1532) | |
| Solid residue | 293.33 (18.86) | 71.77 (7.11) | 3010.75 (116.49) | 161.11 (10.98) | 26.13 (0.01) | 1.9928 (0.1410) | |
| Blank | 996.4 (3.07) | 18.21 (0.04) | 2.06 (0.04) | 3.45 (0.03) | 22.43 (0.02) | 26.0000 (N/A) | |
| 8 | Raw material | 6523.22 (10.66) | 5058.85 (7.89) | 577.51 (2.67) | 1573.89 (5.00) | 58.53 (0.63) | 2.0219 (0.0014) |
| Supernatant | 1203.53 (2.85) | 310.61 (0.81) | 11.24 (0.03) | 79.98 (0.61) | 16.36 (0.10) | 32.9357 (0.1282) | |
| Solid residue | 263.17 (3.05) | 91.07 (1.65) | 313.04 (4.29) | 195.00 (2.67) | 26.60 (0.02) | 2.8663 (0.0731) | |
| Blank | 1028.28 (1.97) | 21.51 (0.15) | 2.75 (0.03) | 3.56 (0.01) | 23.47 (0.03) | 26.3900 (N/A) | |
| 9 | Raw material | 6623.26 (13.64) | 5085.61 (17.09) | 4729.36 (20.19) | 54.89 (0.34) | 60.02 (1.17) | 2.0240 (0.0116) |
| Supernatant | 1271.71 (2.05) | 312.50 (3.15) | 67.49 (1.60) | 5.62 (0.01) | 21.77 (0.61) | 33.3218 (0.0891) | |
| Solid residue | 365.01 (6.17) | 111.28 (3.01) | 2920.10 (64.53) | 10.54 (0.25) | 25.86 (0.02) | 2.4397 (0.1109) | |
| Blank | 1133.50 (1.64) | 19.56 (0.21) | 1.42 (0.04) | 3.76 (0.01) | 22.37 (0.02) | 26.1800 (N/A) | |
| 10 | Raw material | 6627.00 (14.15) | 4404.56 (14.10) | 1973.31 (16.19) | 1353.52 (6.19) | 60.70 (0.05) | 2.0226 (0.0068) |
| Supernatant | 1307.83 (1.58) | 268.08 (0.27) | 33.49 (0.75) | 72.87 (0.09) | 24.86 (0.80) | 33.5741 (0.0680) | |
| Solid residue | 306.94 (12.54) | 81.97 (0.40) | 1393.45 (70.29) | 154.66 (2.87) | 27.11 (0.14) | 2.0773 (0.1266) | |
| Blank | 1176.56 (0.92) | 20.92 (0.04) | 1.80 (0.13) | 4.26 (0.01) | 23.37 (0.02) | 26.3000 (N/A) | |
| 11 | Raw material | 6603.18 (5.35) | 4576.45 (30.24) | 3963.80 (12.62) | 591.53 (1.25) | 64.11 (1.75) | 2.0351 (0.0108) |
| Supernatant | 1322.01 (3.50) | 286.46 (1.69) | 58.48 (0.07) | 35.21 (0.18) | 24.78 (0.55) | 33.9115 (0.0661) | |
| Solid residue | 324.45 (4.62) | 77.77 (6.02) | 2734.00 (44.97) | 59.19 (0.42) | 25.87 (0.05) | 2.1344 (0.0662) | |
| Blank | 1199.41 (3.96) | 17.77 (0.11) | 1.32 (0.07) | 3.83 (0.01) | 22.50 (0.01) | 26.5400 (N/A) | |
| 12 | Raw material | 6590.92 (19.17) | 4040.13 (9.20) | 3337.39 (5.87) | 1107.05 (0.28) | 63.02 (1.58) | 2.0208 (0.0059) |
| Supernatant | 1198.58 (2.47) | 253.07 (1.10) | 39.46 (0.24) | 62.21 (0.18) | 22.21 (1.38) | 33.7144 (0.0372) | |
| Solid residue | 338.31 (13.78) | 83.60 (1.98) | 2373.68 (106.55) | 114.34 (0.87) | 26.41 (0.03) | 2.2251 (0.2039) | |
| Blank | 1066.51 (12.72) | 18.81 (0.02) | 1.06 (0.01) | 3.59 (0.01) | 22.63 (0.02) | 26.0000 (N/A) | |
| 13 | Raw material | 6565.79 (12.91) | 3355.81 (17.26) | 2616.61 (4.02) | 1577.68 (9.46) | 65.10 (1.22) | 2.0210 (0.0020) |
| Supernatant | 1265.22 (1.90) | 215.17 (1.02) | 38.12 (0.76) | 88.68 (0.45) | 25.77 (1.54) | 33.7465 (0.2227) | |
| Solid residue | 389.06 (8.62) | 65.56 (1.14) | 1858.10 (102.53) | 134.39 (5.93) | 26.30 (0.09) | 2.2600 (0.2403) | |
| Blank | 1144.76 (3.77) | 21.33 (0.07) | 2.77 (0.10) | 3.74 (0.01) | 22.69 (0.09) | 26.2200 (N/A) | |
| 14 | Raw material | 6610.83 (15.63) | 3381.24 (13.83) | 4661.51 (15.06) | 801.43 (3.06) | 64.73 (0.96) | 2.0159 (0.0078) |
| Supernatant | 1298.13 (8.16) | 217.14 (0.59) | 64.76 (0.33) | 45.99 (0.21) | 22.71 (1.10) | 33.2502 (0.1086) | |
| Solid residue | 244.24 (8.16) | 54.51 (1.52) | 3016.58 (84.48) | 64.92 (2.72) | 25.79 (0.06) | 2.4151 (0.1632) | |
| Blank | 1143.04 (1.46) | 21.09 (0.06) | 2.46 (0.03) | 3.66 (0.03) | 22.77 (0.06) | 26.2800 (N/A) | |
| Control | Raw material | 13590.21 (97.57) | 99.49 (1.53) | 608.58 (5.07) | 49.46 (0.10) | 68.35 (0.89) | 2.0080 (0.0037) |
| Supernatant | 1626.03 (2.73) | 21.56 (0.01) | 10.79 (0.13) | 5.20 (0.01) | 25.04 (1.00) | 33.6133 (0.0306) | |
| Solid residue | 453.52 (12.34) | 4.50 (0.13) | 411.69 (15.88) | 9.60 (0.16) | 27.15 (0.02) | 2.2144 (0.1392) | |
| Blank | 1076.39 (5.52) | 20.31 (0.10) | 1.49 (0.01) | 3.61 (0.01) | 23.47 (0.05) | 25.9800 (N/A) |
| Run | Na (S) | Na (SR) | Na (TR) | K (S) | K (SR) | K (TR) | Ca (S) | Ca (SR) | Ca (TR) | Mg (S) | Mg (SR) | Mg (TR) | P (S) | P (SR) | P (TR) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 93.06 (0.49) | 5.09 (0.25) | 98.16 (0.42) | 96.12 (0.77) | 1.96 (0.05) | 98.08 (0.73) | 22.08 (0.08) | 79.70 (0.52) | 101.79 (0.55) | 87.29 (0.57) | 12.34 (0.08) | 99.63 (0.64) | 61.78 (0.60) | 35.65 (0.81) | 97.43 (0.62) |
| 2 | 92.97 (0.59) | 7.01 (0.27) | 99.98 (0.67) | 99.83 (0.36) | 2.34 (0.02) | 102.17 (0.37) | 22.61 (0.03) | 75.80 (0.39) | 98.41 (0.42) | 78.38 (0.65) | 22.93 (0.29) | 101.32 (0.68) | 55.44 (0.33) | 45.93 (0.48) | 101.36 (0.39) |
| 3 | 95.03 (0.41) | 8.05 (0.12) | 103.08 (0.35) | 97.58 (0.70) | 3.01 (0.11) | 100.59 (0.60) | 20.38 (0.23) | 80.51 (0.38) | 100.89 (0.37) | 78.53 (0.48) | 18.42 (0.78) | 96.96 (0.35) | 61.46 (0.77) | 40.36 (0.73) | 101.82 (0.71) |
| 4 | 95.83 (1.06) | 5.06 (0.13) | 100.88 (0.98) | 97.95 (0.29) | 2.61 (0.09) | 100.56 (0.22) | 21.00 (0.29) | 76.71 (0.56) | 97.71 (0.66) | 86.46 (0.44) | 12.03 (0.25) | 98.49 (0.19) | 58.73 (0.86) | 40.72 (0.63) | 99.45 (0.59) |
| 5 | 95.85 (0.35) | 5.06 (0.08) | 100.91 (0.41) | 99.08 (0.17) | 2.08 (0.03) | 101.16 (0.19) | 26.93 (0.47) | 70.99 (0.82) | 97.92 (0.43) | 86.50 (0.22) | 10.64 (0.17) | 97.14 (0.25) | 57.66 (1.25) | 42.44 (1.11) | 100.09 (0.67) |
| 6 | 95.66 (0.19) | 4.80 (0.09) | 100.46 (0.21) | 96.81 (0.32) | 2.23 (0.02) | 99.04 (0.33) | 20.31 (0.11) | 76.71 (0.26) | 97.02 (0.20) | 84.94 (0.20) | 11.81 (0.32) | 96.75 (0.17) | 59.44 (0.62) | 40.31 (0.53) | 99.76 (0.86) |
| 7 | 97.87 (0.58) | 4.43 (0.12) | 102.30 (0.65) | 95.13 (0.45) | 2.43 (0.15) | 97.56 (0.42) | 23.86 (0.16) | 72.72 (0.46) | 96.58 (0.32) | 86.15 (0.39) | 11.41 (0.40) | 97.56 (0.35) | 57.29 (1.02) | 42.93 (0.36) | 100.22 (1.24) |
| 8 | 94.79 (0.63) | 5.72 (0.07) | 100.51 (0.57) | 94.47 (0.23) | 2.55 (0.05) | 97.02 (0.22) | 25.50 (0.13) | 76.76 (0.56) | 102.25 (0.64) | 79.82 (0.56) | 17.54 (0.10) | 97.36 (0.58) | 59.78 (1.36) | 39.62 (0.46) | 99.40 (0.94) |
| 9 | 94.73 (0.37) | 6.62 (0.04) | 101.36 (0.40) | 96.17 (0.76) | 2.63 (0.05) | 98.80 (0.73) | 23.09 (0.50) | 74.14 (0.50) | 97.23 (0.39) | 80.01 (0.20) | 23.05 (0.18) | 103.06 (0.34) | 56.30 (1.30) | 43.51 (0.33) | 99.81 (1.14) |
| 10 | 96.84 (0.38) | 4.79 (0.32) | 101.62 (0.25) | 94.85 (0.22) | 1.91 (0.07) | 96.76 (0.16) | 26.97 (0.58) | 71.67 (1.49) | 98.65 (0.93) | 85.28 (0.27) | 11.69 (0.21) | 96.97 (0.20) | 58.24 (0.53) | 41.73 (0.23) | 99.97 (0.56) |
| 11 | 96.73 (0.67) | 5.15 (0.03) | 101.87 (0.66) | 99.24 (0.63) | 1.77 (0.13) | 101.02 (0.57) | 24.15 (0.04) | 72.20 (0.19) | 96.35 (0.18) | 90.74 (0.68) | 10.49 (0.12) | 101.22 (0.77) | 58.66 (0.70) | 40.44 (0.35) | 99.10 (0.65) |
| 12 | 95.68 (0.48) | 5.74 (0.43) | 101.41 (0.39) | 99.01 (0.43) | 2.30 (0.14) | 101.31 (0.56) | 19.41 (0.10) | 77.42 (0.26) | 96.83 (0.28) | 90.02 (0.18) | 11.29 (0.14) | 101.31 (0.13) | 60.13 (0.96) | 38.17 (0.71) | 98.30 (0.62) |
| 13 | 95.56 (0.77) | 6.57 (0.22) | 102.13 (0.56) | 98.81 (0.38) | 2.17 (0.08) | 100.98 (0.36) | 22.94 (0.40) | 77.57 (0.81) | 100.50 (0.52) | 90.77 (0.28) | 9.35 (0.05) | 100.11 (0.31) | 57.96 (0.68) | 43.17 (0.71) | 101.13 (0.30) |
| 14 | 98.47 (0.22) | 4.40 (0.12) | 102.87 (0.28) | 97.80 (0.55) | 1.92 (0.07) | 99.73 (0.57) | 22.23 (0.16) | 76.96 (0.85) | 99.19 (0.71) | 88.70 (0.35) | 9.60 (0.11) | 98.29 (0.39) | 60.91 (0.58) | 41.49 (0.63) | 102.40 (0.66) |
| Control | 97.81 (0.44) | 3.70 (0.20) | 101.51 (0.28) | 98.66 (0.24) | 5.02 (0.30) | 103.68 (0.26) | 26.52 (0.39) | 73.89 (0.56) | 100.40 (0.77) | 81.48 (0.29) | 21.37 (0.67) | 102.85 (0.49) | 60.77 (1.21) | 40.01 (0.50) | 100.79 (1.11) |
| Design Run | Comparison between Analytical and Bioaccessible K Content | Comparison between Analytical and Bioaccessible Ca Content | Comparison between Analytical and Bioaccessible Mg Content | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anal. Content | Bioac. Content | Anal. Content | Bioac. Content | Label. Differences | Anal. Content | Bioac. Content | Label. Differences | |||||||||
| Amount | RDI (%) | Amount | RDI (%) | Amount | RDI (%) | Amount | RDI (%) | Amount | Percentage | Amount | RDI (%) | Amount | RDI (%) | Amount | Percentage | |
| 1 | 157.164 (1.002) | 7.85 (0.07) | 151.1 (1.2) | 7.55 (0.06) | 461.170 (0.380) | 57.64 (0.03) | 101.8 (0.4) | 12.73 (0.04) | 359.370 | 77.93 | 157.025 (0.112) | 41.87 (0.01) | 137.1 (0.9) | 36.55 (0.24) | 19.93 | 12.69 |
| 2 | 500.336 (0.447) | 25.02 (0.03) | 499.5 (1.8) | 24.97 (0.09) | 458.318 (0.449) | 57.89 (0.03) | 103.6 (0.2) | 12.95 (0.02) | 354.718 | 77.40 | 3.912 (0.115) | 1.04 (0.01) | 3.1 (<0.1) | 0.82 (<0.01) | 0.81 | 20.76 |
| 3 | 515.203 (1.176) | 25.76 (0.08) | 502.7 (3.6) | 25.14 (0.18) | 60.305 (0.321) | 7.54 (0.02) | 12.3 (0.1) | 1.54 (0.02) | 48.005 | 79.60 | 155.603 (0.177) | 41.49 (0.02) | 122.2 (0.7) | 32.59 (0.20) | 33.43 | 21.48 |
| 4 | 166.235 (0.628) | 8.312 (0.04) | 162.8 (0.5) | 8.14 (0.02) | 466.407 (2.143) | 58.30 (0.14) | 98.0 (1.3) | 12.25 (0.17) | 368.407 | 78.99 | 154.607 (1.954) | 41.23 (0.13) | 133.7 (0.7) | 35.64 (0.18) | 20.91 | 13.52 |
| 5 | 355.506 (2.164) | 17.78 (0.14) | 352.2 (0.6) | 17.61 (0.03) | 274.465 (1.328) | 34.31 (0.09) | 73.9 (1.3) | 9.24 (0.16) | 200.565 | 73.07 | 163.796 (0.526) | 43.68 (0.04) | 141.7 (0.4) | 37.78 (0.10) | 22.07 | 13.47 |
| 6 | 506.609 (0.903) | 25.33 (0.06) | 490.4 (1.6) | 24.52 (0.08) | 268.918 (2.697) | 33.61 (0.18) | 54.6 (0.3) | 6.83 (0.04) | 214.318 | 79.70 | 77.718 (0.204) | 20.72 (0.01) | 66.0 (0.2) | 17.60 (0.04) | 11.72 | 15.08 |
| 7 | 285.542 (0.625) | 14.28 (0.04) | 271.6 (1.3) | 13.58 (0.06) | 406.494 (2.022) | 50.81 (0.13) | 97.0 (0.7) | 12.13 (0.08) | 309.494 | 76.14 | 137.666 (0.595) | 36.71 (0.04) | 118.6 (0.5) | 31.62 (0.14) | 19.07 | 13.85 |
| 8 | 505.885 (0.789) | 25.29 (0.05) | 477.9 (1.1) | 23.89 (0.06) | 57.751 (0.267) | 7.22 (0.02) | 14.7 (0.1) | 1.84 (0.01) | 43.051 | 74.55 | 157.389 (0.500) | 41.97 (0.03) | 125.6 (0.9) | 33.50 (0.23) | 31.79 | 20.20 |
| 9 | 508.561 (1.709) | 25.43 (0.11) | 489.1 (3.8) | 24.45 (0.19) | 472.936 (2.019) | 59.12 (0.13) | 109.2 (2.4) | 13.65 (0.30) | 363.736 | 76.91 | 5.489 (0.034) | 1.46 (<0.01) | 4.4 (<0.1) | 1.17 (<0.01) | 1.09 | 19.84 |
| 10 | 440.456 (1.410) | 22.02 (0.09) | 417.8 (1.0) | 20.89 (0.05) | 197.331 (1.619) | 24.67 (0.11) | 53.2 (1.1) | 6.65 (0.14) | 144.131 | 73.04 | 135.352 (0.619) | 36.09 (0.04) | 115.4 (0.4) | 30.78 (0.10) | 19.95 | 14.74 |
| 11 | 457.645 (3.024) | 22.88 (0.20) | 454.2 (2.9) | 22.71 (0.14) | 396.380 (1.262) | 49.55 (0.08) | 95.7 (0.2) | 11.97 (0.02) | 300.680 | 75.86 | 59.153 (0.125) | 15.77 (<0.01) | 53.7 (0.4) | 14.31 (0.11) | 5.45 | 9.22 |
| 12 | 404.013 (0.920) | 20.20 (0.06) | 400.0 (1.8) | 20.00 (0.09) | 333.739 (0.587) | 41.72 (0.04) | 64.8 (0.3) | 8.10 (0.04) | 268.939 | 80.58 | 110.705 (0.028) | 29.52 (<0.01) | 99.7 (0.2) | 26.58 (0.05) | 11.01 | 9.94 |
| 13 | 335.581 (1.726) | 16.78 (0.12) | 331.6 (1.3) | 16.60 (0.06) | 261.661 (0.402) | 32.71 (0.03) | 60.0 (1.0) | 7.50 (0.13) | 201.661 | 77.37 | 157.768 (0.946) | 42.07 (0.06) | 143.2 (0.4) | 38.19 (0.12) | 14.57 | 9.23 |
| 14 | 338.124 (1.383) | 16.91 (0.09) | 330.7 (1.9) | 16.53 (0.09) | 466.151 (1.506) | 58.27 (0.10) | 103.6 (0.7) | 12.95 (0.10) | 362.551 | 77.78 | 80.143 (0.306) | 21.37 (0.02) | 71.1 (0.3) | 18.96 (0.07) | 9.05 | 11.29 |
| Control | 9.949 (0.153) | 0.50 (0.01) | 9.8 (<0.1) | 0.49 (<0.01) | 60.858 (0.507) | 7.61 (0.03) | 16.1 (0.2) | 2.02 (0.03) | 44.758 | 73.54 | 4.946 (0.010) | 1.32 (<0.01) | 4.0 (<0.1) | 1.07 (<0.01) | 0.95 | 19.13 |
| Design Run | Comparison between Analytical and Bioaccessible Na Content | Comparison between Analytical and Bioaccessible P Content | ||||||
|---|---|---|---|---|---|---|---|---|
| Analytical Content | Bioaccessible Content | Analytical Content | Bioaccessible Content | |||||
| Amount | RDI (%) | Amount | RDI (%) | Amount | RDI (%) | Amount | RDI (%) | |
| 1 | 630.10 (2.16) | 26.25 (0.66) | 586.4 (3.1) | 25.50 (0.13) | 5.925 (0.062) | 0.85 (<0.01) | 3.67 (0.04) | 0.52 (<0.01) |
| 2 | 639.82 (3.27 | 26.66 (0.13) | 594.8 (3.8) | 25.86 (0.16) | 6.512 (0.052) | 0.93 (<0.01) | 3.61 (0.02) | 0.52 (<0.01) |
| 3 | 650.301 (3.557) | 27.10 (0.20) | 618.0 (2.7) | 26.87 (0.12) | 6.217 (0.076) | 0.89 (<0.01) | 3.82 (0.05) | 0.55 (0.01) |
| 4 | 651.251 (3.701) | 27.14 (0.27) | 624.1 (6.9) | 27.13 (0.30) | 6.040 (0.035) | 0.86 (<0.01) | 3.55 (0.05) | 0.51 (0.01) |
| 5 | 656.175 (1.738) | 27.34(0.33) | 628.9 (2.3) | 27.35 (0.10) | 6.729 (0.021) | 0.96 (<0.01) | 3.88 (0.08) | 0.55 (0.01) |
| 6 | 651.945 (2.143) | 27.16 (0.40) | 623.7 (1.2) | 27.12 (0.05) | 5.876 (0.034) | 0.84 (<0.01) | 3.49 (0.04) | 0.50 (0.01) |
| 7 | 645.119 (1.112) | 26.89 (0.47) | 631.4 (3.7) | 27.45 (0.16) | 6.538 (0.068) | 0.93 (<0.01) | 3.75 (0.07) | 0.54 (0.01) |
| 8 | 652.322 (1.066) | 27.18 (0.53) | 618.4 (4.1) | 26.88 (0.18) | 5.853 (0.063) | 0.84 (<0.01) | 3.50 (0.08) | 0.50 (0.01) |
| 9 | 662.326 (1.364) | 27.60 (0.60) | 627.4 (2.4) | 27.28 (0.11) | 6.002 (0.117) | 0.86 (<0.01) | 3.38 (0.08) | 0.48 (0.01) |
| 10 | 662.700 (1.415) | 27.61 (0.68) | 641.7 (2.5) | 27.90 (0.11) | 6.070 (0.005) | 0.87 (<0.01) | 3.54 (0.04) | 0.51 (<0.01) |
| 11 | 660.318 (0.535) | 27.51 (0.73) | 638.7 (4.4) | 27.77 (0.19) | 6.411 (0.175) | 0.92 (0.01) | 3.76 (0.04) | 0.54 (0.01) |
| 12 | 659.092 (1.917) | 27.46 (0.80) | 630.6 (3.2) | 27.42 (0.14) | 6.302 (0.158) | 0.90 (0.01) | 3.79 (0.06) | 0.54 (0.01) |
| 13 | 656.579 (1.291) | 27.36 (0.87) | 627.4 (5.0) | 27.28 (0.22) | 6.510 (0.122) | 0.93 (0.01) | 3.77 (0.04) | 0.54 (0.01) |
| 14 | 661.083 (1.563) | 27.55 (0.93) | 651.0 (1.4) | 28.30 (0.06) | 6.473 (0.096) | 0.92 (0.01) | 3.94 (0.04) | 0.56 (0.01) |
| Control | 1359.021 (9.757) | 56.63 (0.98) | 1329.3 (6.0) | 55.38 (0.26) | 6.835 (0.089) | 0.98 (0.01) | 4.16 (0.08) | 0.60 (0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-López, A.; Moreno-Baquero, J.M.; Garrido-Fernández, A. Bioaccessibility of Mineral Nutrients in Plain Green Spanish-Style Manzanilla Table Olives Packaged in Nutrient Salt Mixtures. Foods 2024, 13, 2671. https://doi.org/10.3390/foods13172671
López-López A, Moreno-Baquero JM, Garrido-Fernández A. Bioaccessibility of Mineral Nutrients in Plain Green Spanish-Style Manzanilla Table Olives Packaged in Nutrient Salt Mixtures. Foods. 2024; 13(17):2671. https://doi.org/10.3390/foods13172671
Chicago/Turabian StyleLópez-López, Antonio, José María Moreno-Baquero, and Antonio Garrido-Fernández. 2024. "Bioaccessibility of Mineral Nutrients in Plain Green Spanish-Style Manzanilla Table Olives Packaged in Nutrient Salt Mixtures" Foods 13, no. 17: 2671. https://doi.org/10.3390/foods13172671
APA StyleLópez-López, A., Moreno-Baquero, J. M., & Garrido-Fernández, A. (2024). Bioaccessibility of Mineral Nutrients in Plain Green Spanish-Style Manzanilla Table Olives Packaged in Nutrient Salt Mixtures. Foods, 13(17), 2671. https://doi.org/10.3390/foods13172671







