Recent Advances in Light Penetration Depth for Postharvest Quality Evaluation of Fruits and Vegetables
Abstract
:1. Introduction
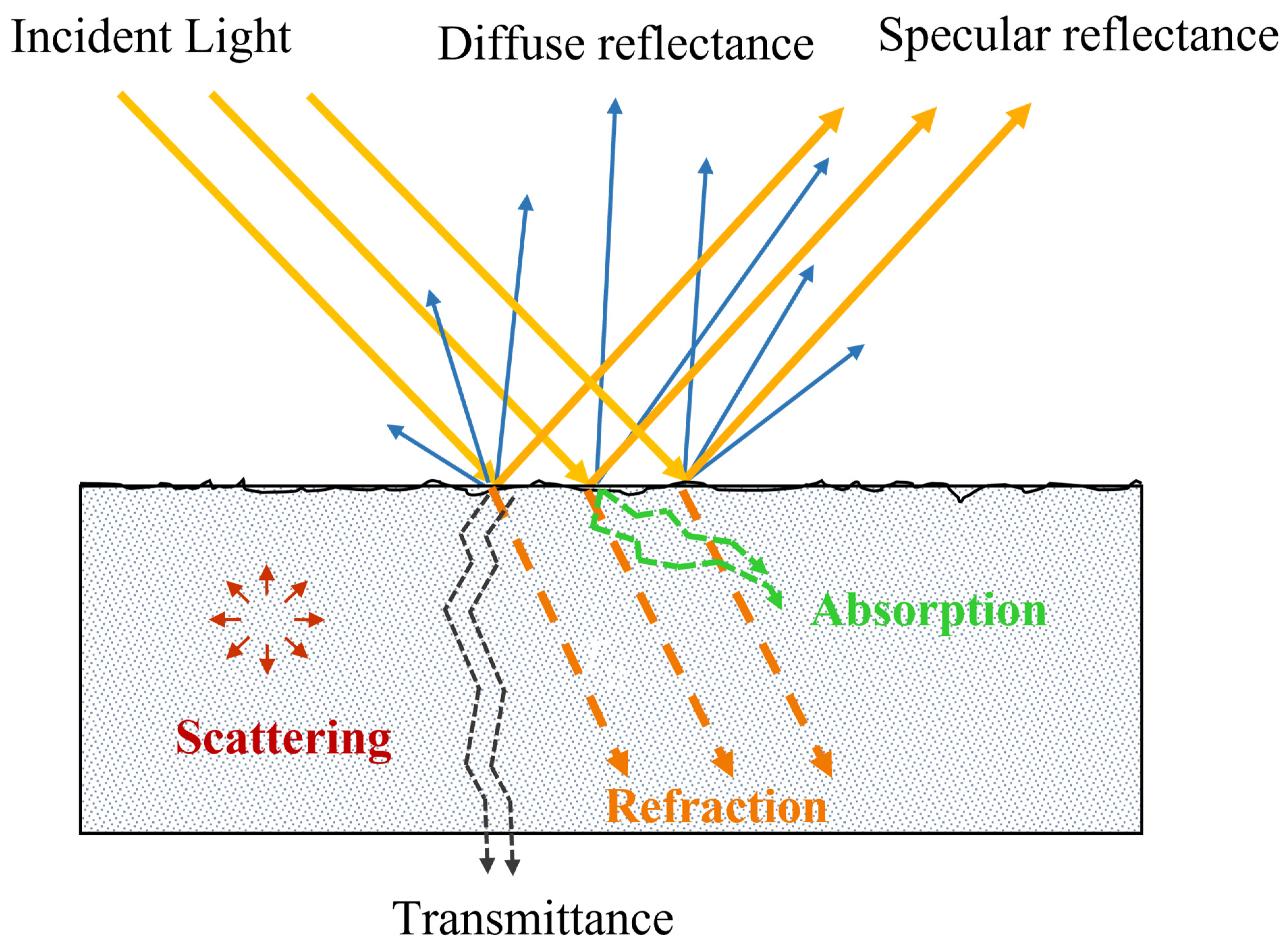
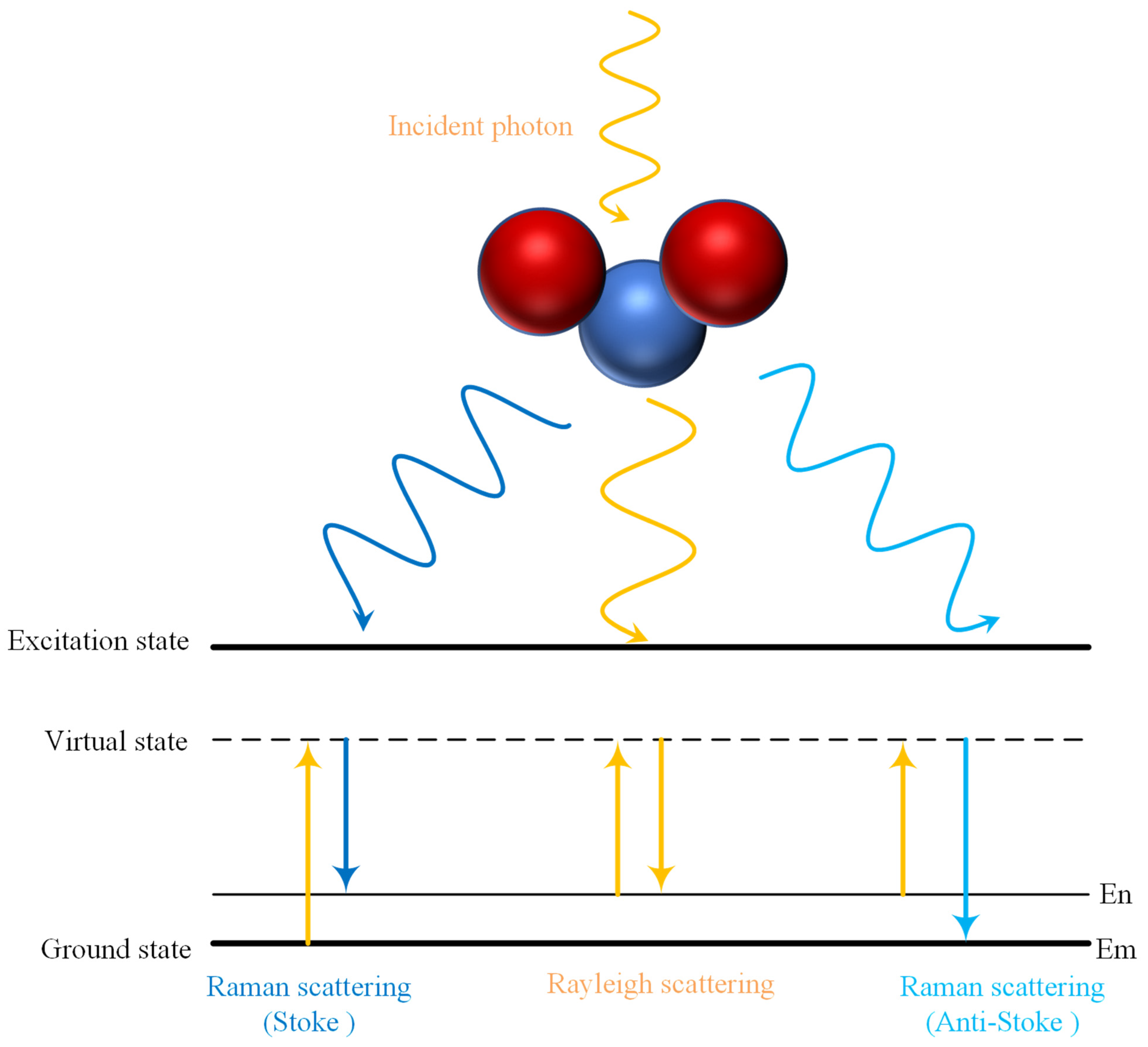
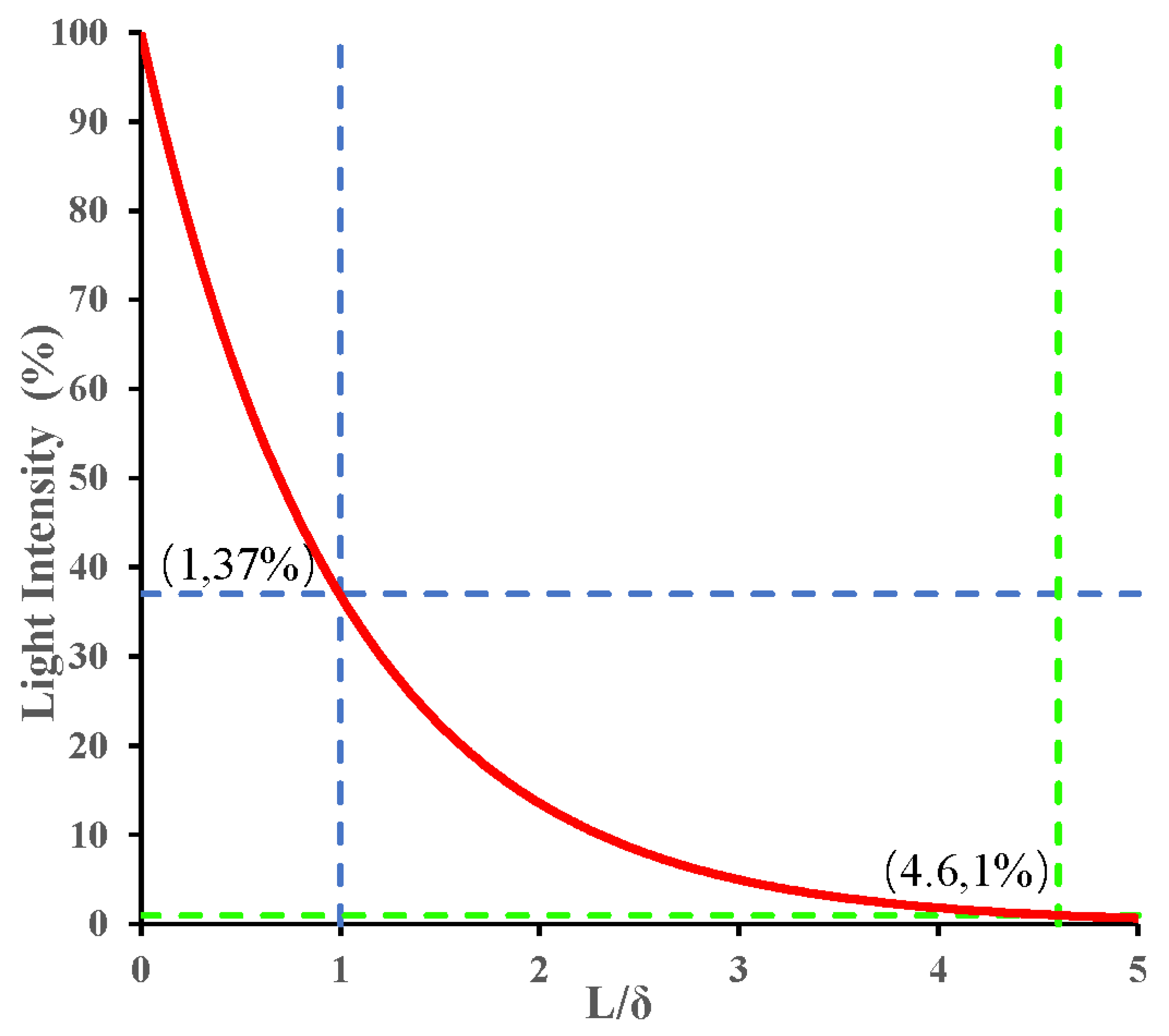
2. The Theory of Absorption, Scattering and Penetration Depth
3. Light Transfer in Turbid Medium
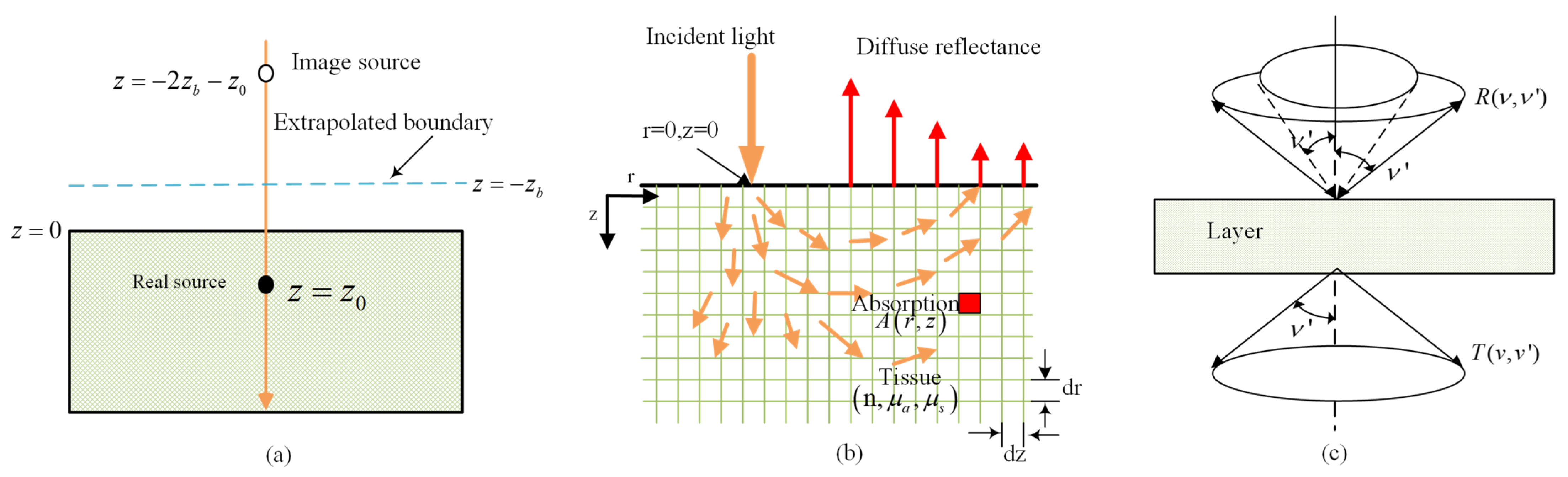
3.1. Diffusion Approximation Theory
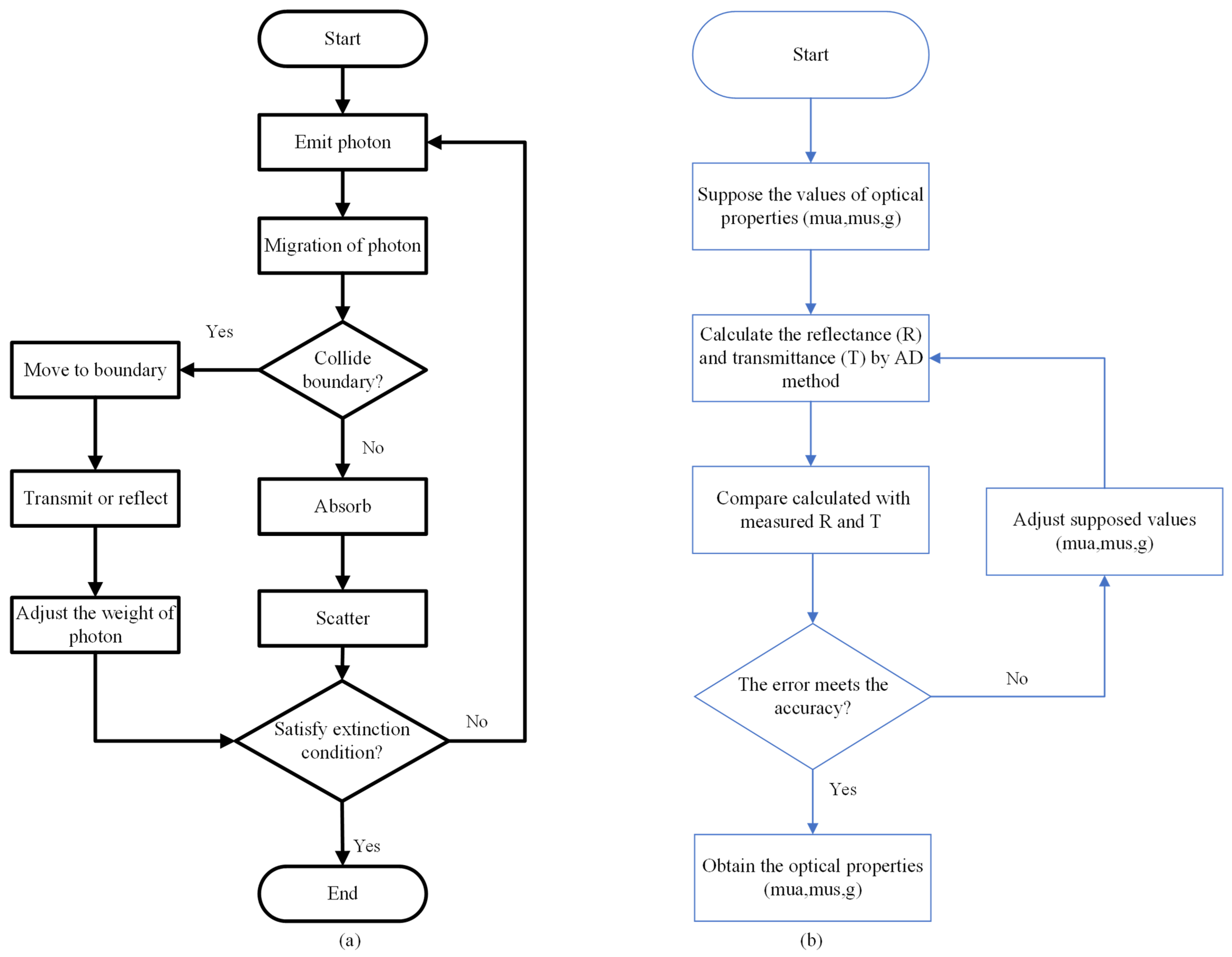
3.2. Monte Carlo Method
3.3. Adding-Doubling Method
4. Optical Detection Techniques
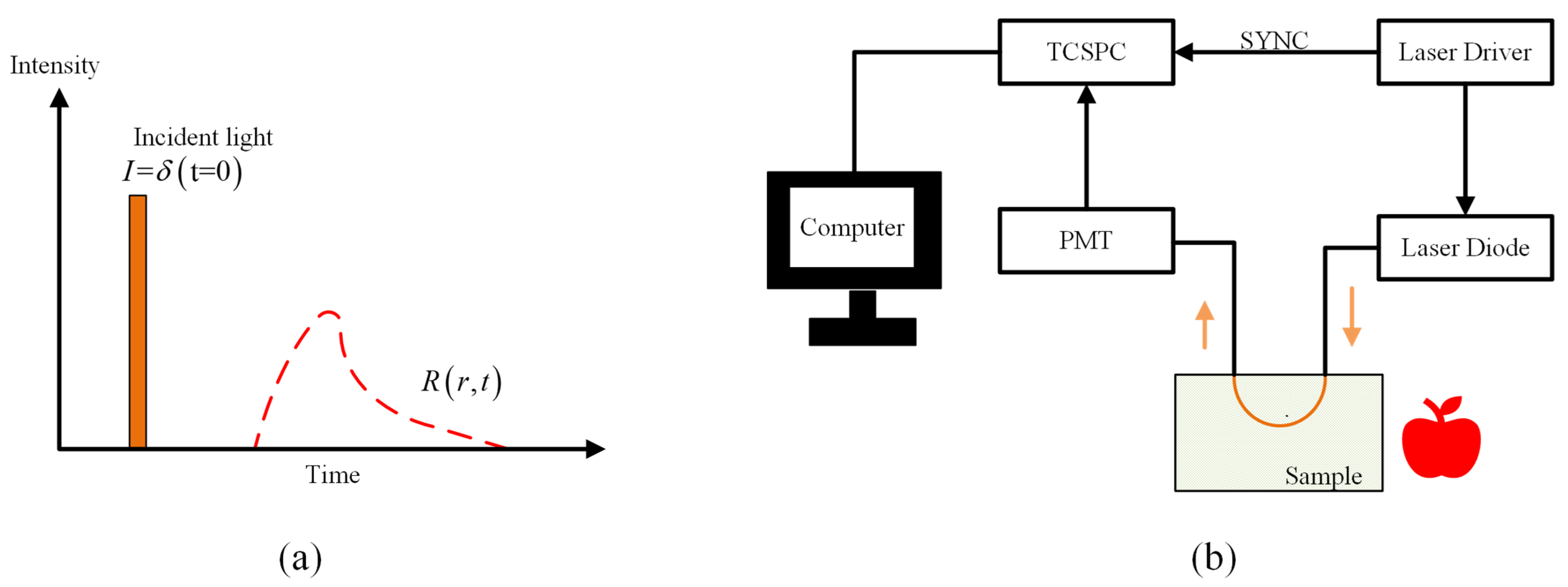
4.1. Time Resolved Spectroscopy
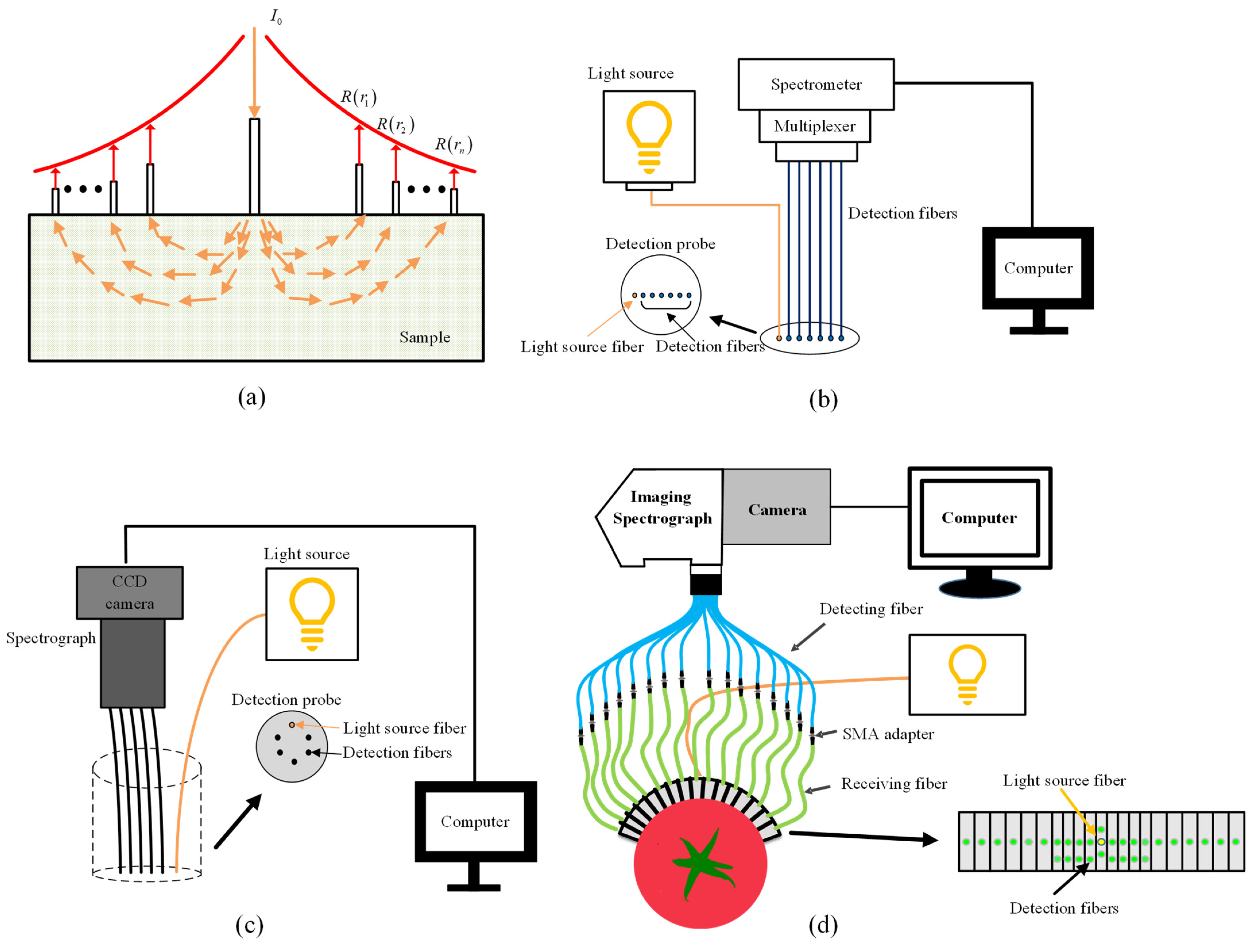
4.2. Spatially Resolved Spectroscopy
4.3. Frequency Domain Technique
4.4. Structured Illumination
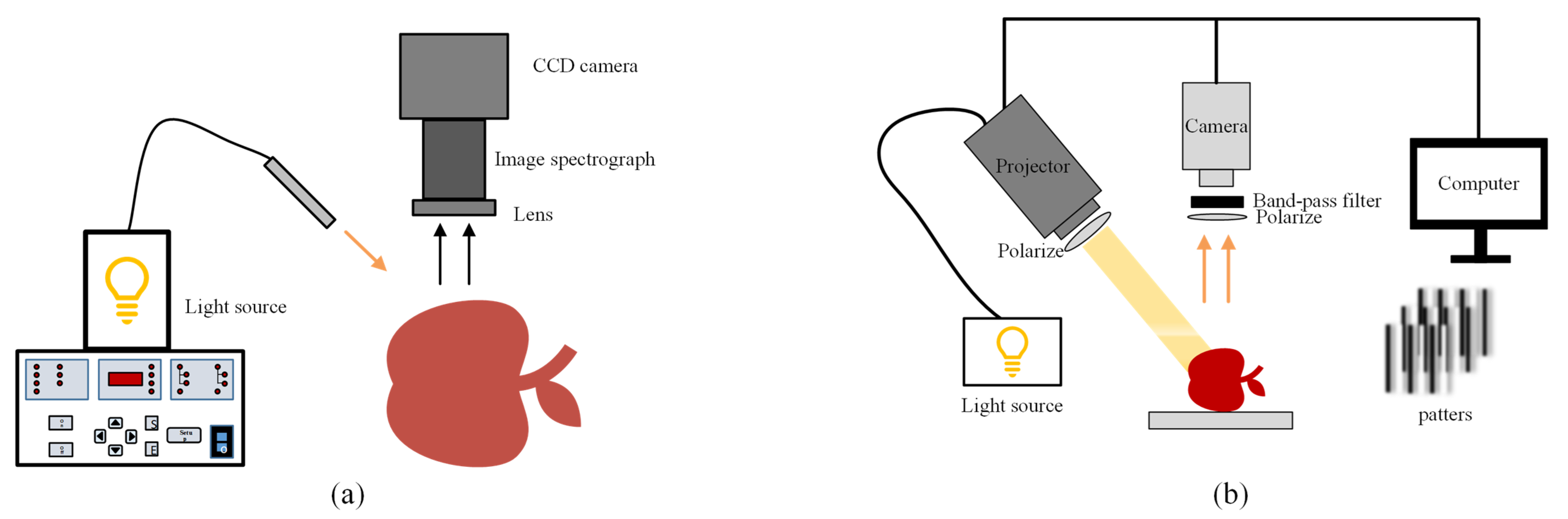
4.4.1. Spatial Frequency Domain Imaging
4.4.2. Structured Illumination Reflectance Imaging
5. Applications
5.1. Analysis of Absorption and Scattering Coefficients
5.2. Maturity and Quality Assessment
| Product | Technique | Year | Parameter | Wavelength (nm) | Model | Result | Reference |
|---|---|---|---|---|---|---|---|
| Apple | TRS | 2020 | Maturity | 540~1064 | Pearson correlation analysis | For chroma: r = −0.604, r = 0.615 | [102] |
| SRS | 2020 | Maturity | 550~1650 | PLSDA | GD: Acc = 100% | [103] | |
| SRS | 2021 | Firmness, SSC | 600~1000 | PLSDA | SSC: R2 = 0.92, Rmse = 0.35% Firmness: R2 = 0.87, Rmse = 0.71 N | [63] | |
| SRS | 2021 | Flesh color | 190~1070 | PLSDA | Skin: R2 = 0.95 Whole flesh, R2 = 0.69 | [104] | |
| SFDI | 2022 | SSC, firmness, color | 450~750 | SVM | Rp = 0.66, 0.73, 0.86 | [105] | |
| Peach | SRS | 2020 | Soluble, TA | 550~1000 | Pearson correlation analysis | r = 0.898 r = −0.776 | [97] |
| SRS | 2021 | Maturity | 550~1000 | SVM | Acc = 94.12%, 95.59% and 94.12% | [99] | |
| SRS | 2022 | Firmness, SSC | 550~1650 | PLSDA | Rp = 0.853, Rmse = 14.76 N Rp = 0.839, Rmse = 0.5 | [90] | |
| Kiwifruit | SRS | 2022 | Firmness, SSC, pH | 650~1000 | PLSDA | R2 = 0.64, 0.67, 0.38 Rmse = 3.63 N/cm2, 0.95%, 0.19% | [100] |
| Mango | TRS | 2015 | Maturity | 540~900 | EP, FD | —— | [106] |
| TRS | 2023 | Maturity, pulp color | 540 | Logistic/exponential model | = 99.8% | [57] | |
| Tomato | SRS | 2018 | SSC, pH | 400~1100 900~1300 | PLSDA | For 400~1100: Rp = 0.729, 0.743 For 900~1300: Rp = 0.815, 0.741 | [101] |
| SRS | 2018 | Firmness | 550~1650 | PLSDA | Rp = 0.859, Rmse = 1.00 | [66] | |
| SRS | 2020 | Maturity | 550~1650 | SVM | Acc = 98.3% | [67] |
5.3. Defect Detection
5.4. Analysis of Light Penetration Depth
6. Challenges and Prospects
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.H.; Sarpong, F.; Zhou, C.S. Use of Ultrasonic Cleaning Technology in the Whole Process of Fruit and Vegetable Processing. Foods 2022, 11, 2874. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.J.; Wei, S.; Yang, D.Y. Morphological measurement for carrot based on three-dimensional reconstruction with a ToF sensor. Postharvest Biol. Technol. 2023, 197, 112216. [Google Scholar] [CrossRef]
- Fadchar, N.A.; Dela Cruz, J.C. A non-destructive approach of young coconut maturity detection using acoustic vibration and neural network. In Proceedings of the 16th IEEE International Colloquium on Signal Processing and its Applications (CSPA), Langkawi, Malaysia, 28–29 February 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 136–140. [Google Scholar]
- Hassoun, A.; Aït-Kaddour, A.; Sahar, A.; Cozzolino, D. Monitoring Thermal Treatments Applied to Meat Using Traditional Methods and Spectroscopic Techniques: A Review of Advances over the Last Decade. Food Bioprocess Technol. 2021, 14, 195–208. [Google Scholar] [CrossRef]
- Suhaime, N.; Sairi, M.; Abbas, Z.; Nafis, N.B.N.; Othman, Z.; Adnan, A.S.M.; Shamsulkamal, A.R.; Paiman, S.; Mohamed, T.N. Microwave technique for moisture content and pH determination during pre-harvest of mango cv. chok anan. Sains Malays. 2018, 47, 1571–1578. [Google Scholar] [CrossRef]
- Chen, Z.; Lian, X.F.; Zhou, M.C.; Zhang, X.L.; Wang, C. Quantitation of L-cystine in Food Supplements and Additives Using 1H qNMR: Method Development and Application. Foods 2023, 12, 2421. [Google Scholar] [CrossRef]
- Jiang, H.Z.; Jiang, X.S.; Ru, Y.; Chen, Q.; Li, X.P.; Xu, L.Y.; Zhou, H.P.; Shi, M.H. Rapid and non-destructive detection of natural mildew degree of postharvest camellia oleifera fruit based on hyperspectral imaging. Infrared Phys. Technol. 2022, 123, 104169. [Google Scholar] [CrossRef]
- Yao, K.S.; Sun, J.; Chen, C.; Xu, M.; Zhou, X.; Cao, Y.; Tian, Y. Non-destructive detection of egg qualities based on hyperspectral imaging. J. Food Eng. 2022, 325, 111024. [Google Scholar] [CrossRef]
- Yan, C.; Jiang, X.S.; Shen, F.; He, X.M.; Fang, Y.; Liu, Q.; Zhou, H.P.; Liu, X.Q. Visible/near-infrared spectroscopy combined with machine vision for dynamic detection of aflatoxin B-1 contamination in peanut. Spectrosc. Spectr. Anal. 2020, 40, 3865–3870. [Google Scholar]
- Li, Q.L.; Wu, X.H.; Zheng, J.; Wu, B.; Jian, H.; Sun, C.Z.; Tang, Y.B. Determination of Pork Meat Storage Time Using Near-Infrared Spectroscopy Combined with Fuzzy Clustering Algorithms. Foods 2022, 11, 2101. [Google Scholar] [CrossRef]
- Jiang, H.Z.; Jiang, X.S.; Ru, Y.; Chen, Q.; Xu, L.Y.; Zhou, H.P. Sweetness detection and grading of peaches and nectarines by combining short- and long-wave fourier-transform near-infrared spectroscopy. Anal. Lett. 2021, 54, 1125–1144. [Google Scholar] [CrossRef]
- Jiang, H.Z.; Hu, Y.L.; Jiang, X.S.; Zhou, H.P. Maturity stage discrimination of camellia oleifera fruit using visible and near-Infrared hyperspectral imaging. Molecules 2022, 27, 6318. [Google Scholar] [CrossRef] [PubMed]
- Ghooshkhaneh, N.G.; Mollazade, K. Optical Techniques for Fungal Disease Detection in Citrus Fruit: A Review. Food Bioprocess Technol. 2023, 16, 1668–1689. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.H.; Fei, Y.Q.; Liu, Y.; Shen, L.X.; Zhuang, Z.L.; Zhang, X. Research on the prediction of green plum acidity based on improved XGBoost. Sensors 2021, 21, 930. [Google Scholar] [CrossRef]
- Wang, S.M.; Sun, J.; Fu, H.; Xu, M.; Tang, N.Q.; Cao, Y.; Yao, K.S.; Jing, J.P. Identification of red jujube varieties based on hyperspectral imaging technology combined with CARS-IRIV and SSA-SVM. J. Food Process Eng. 2022, 45, e14137. [Google Scholar] [CrossRef]
- Zhou, C.X.; Wang, H.H.; Liu, Y.; Ni, X.Y.; Liu, Y. Green plums surface defect fetection based on deep learning methods. IEEE Access 2022, 10, 100397–100407. [Google Scholar] [CrossRef]
- Rizzolo, A.; Vanoli, M. Time-resolved technique for measuring optical properties and quality of food. In Light Scattering Technology for Food Property, Quality and Safety Assessment; CRC Press: Boca Raton, FL, USA, 2016; pp. 187–224. [Google Scholar]
- Torricelli, A.; Contini, D.; Dalla Mora, A.; Martinenghi, E.; Tamborini, D.; Villa, F.; Tosi, A.; Spinelli, L. Recent advances in time-resolved NIR spectroscopy for nondestructive assessment of fruit quality. In Proceedings of the 9th Nut and Vegetable Production Engineering Symposium, Milano, Italy, 19–22 May 2015; pp. 43–48. [Google Scholar]
- Luo, Y.F.; Jiang, X.; Fu, X.P. Spatial frequency domain imaging system calibration, correction and application for pear surface damage detection. Foods 2021, 10, 2151. [Google Scholar] [CrossRef]
- Xing, S.Q.; Zhang, J.M.; Luo, Y.F.; Yang, Y.; Fu, X.P. Extracting tissue optical properties and detecting bruised tissue in pears quickly and accurately based on spatial frequency domain imaging and machine learning. Foods 2023, 12, 238. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Li, R.C.; Lu, R.F. Structured-illumination reflectance imaging (SIRI) for enhanced detection of fresh bruises in apples. Postharvest Biol. Technol. 2016, 117, 89–93. [Google Scholar] [CrossRef]
- Si, W.; Xiong, J.; Huang, Y.P.; Jiang, X.S.; Hu, D. Quality assessment of fruits and vegetables based on spatially resolved spectroscopy: A review. Foods 2022, 11, 1198. [Google Scholar] [CrossRef]
- Lu, R.F.; Van Beers, R.; Saeys, W.; Li, C.Y.; Cen, H.Y. Measurement of optical properties of fruits and vegetables: A review. Postharvest Biol. Technol. 2020, 159, 111003. [Google Scholar] [CrossRef]
- Hu, D.; Lu, R.F.; Ying, Y.B. Spatial-frequency domain imaging coupled with frequency optimization for estimating optical properties of two-layered food and agricultural products. J. Food Eng. 2020, 277, 109909. [Google Scholar] [CrossRef]
- Wang, A.C.; Lu, R.F.; Xie, L.J. A sequential method for measuring the optical properties of two-layer media with spatially-resolved diffuse reflectance: Simulation study. In Proceedings of the Conference on Sensing for Agriculture and Food Quality and Safety VIII, Baltimore, MD, USA, 20–21 April 2016. [Google Scholar]
- Sujatha, N.; Nivetha, K.B.; Singhal, A. Optimal source to detector separation for extracting sub-dermal chromophores in fiber optic diffuse reflectance spectroscopy: A simulation study. In Proceedings of the Conference on Biophotonics—Photonic Solutions for Better Health Care IV, Brussels, Belgium, 14–17 April 2014. [Google Scholar]
- Wilson, B.C.; Jacques, S.L. Optical reflectance and transmittance of tissues: Principles and applications. IEEE J. Quantum Electron. 1990, 26, 2186–2199. [Google Scholar] [CrossRef]
- Lammertyn, J.; Peirs, A.; Baerdemaeker, J.D.; Nicolaï, B. Light penetration properties of NIR radiation in fruit with respect to non-destructive quality assessment. Postharvest Biol. Technol. 2000, 18, 121–132. [Google Scholar] [CrossRef]
- Fraser, D.G. Near infra-red (NIR) light penetration into an apple. Postharvest Biol. Technol. 2001, 22, 191–194. [Google Scholar] [CrossRef]
- Shi, S.N.; Tan, Z.J.; Xie, J.; Lu, J. The effects of skin thickness on optical transmission characteristics in fruits tissues. Spectrosc. Spectr. Anal. 2015, 35, 1817–1823. [Google Scholar] [CrossRef]
- Qin, J.W.; Lu, R.F. Monte Carlo simulation for quantification of light transport features in apples. Comput. Electron. Agric. 2009, 68, 44–51. [Google Scholar] [CrossRef]
- Rodríguez-Ortega, A.; Aleixos, N.; Blasco, J.; Albert, F.; Munera, S. Study of light penetration depth of a Vis-NIR hyperspectral imaging system for the assessment of fruit quality. A case study in persimmon fruit. J. Food Eng. 2023, 358, 111673. [Google Scholar] [CrossRef]
- Tian, H.; Xu, H.R.; Ying, Y.B. Can light penetrate through pomelos and carry information for the non-destructive prediction of soluble solid content using Vis-NIRS? Biosyst. Eng. 2022, 214, 152–164. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, R.F.; Wang, X.C. Evaluation of fungal infection in peaches based on optical and microstructural properties. Postharvest Biol. Technol. 2020, 165, 111181. [Google Scholar] [CrossRef]
- Lu, R. Overview of Light Interaction with Food and Biological Materials; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2016; pp. 100–103. [Google Scholar] [CrossRef]
- Liang, Y.X.; Sun, J.H.; Zhang, J.L.; Wang, Y.Y.; Wan, A.C.; Zhang, S.B.; Ye, Z.Y.; Lin, S.T.; Wang, Z.A. Prediction of fiber Rayleigh scattering responses based on deep learning. Sci. China-Inf. Sci. 2023, 66, 222301. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y. A novel spectroscopic technique for studying metal-organic frameworks based on Mie scattering. Anal. Bioanal. Chem. 2023, 415, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Sun, T.; Yao, L.J.; Yang, Z.D.; Wang, A.C.; Ying, Y.B. Monte Carlo: A flexible and accurate technique for modeling light transport in food and agricultural products. Trends Food Sci. Technol. 2020, 102, 280–290. [Google Scholar] [CrossRef]
- Huang, Y.P.; Lu, R.F.; Chen, K.J. Development of a multichannel hyperspectral imaging probe for property and quality assessment of horticultural products. Postharvest Biol. Technol. 2017, 133, 88–97. [Google Scholar] [CrossRef]
- Hartog, A.H. Raman sensors and their applications. In Proceedings of the 22nd International Conference on Optical Fiber Sensors (OFS), Beijing, China, 15–19 October 2012. [Google Scholar]
- Xiao, Z.W.; Xu, Y.C.; Wang, X.Y.; Wang, Y.; Cheng, M.; Liu, S.W.; Qu, J.Z. Optical properties and Monte Carlo multi-layered simulation of potato skin and flesh tissues. Postharvest Biol. Technol. 2023, 205, 112488. [Google Scholar] [CrossRef]
- Zhou, T.T.; Hu, D.; Qiu, D.K.; Yu, S.Q.; Huang, Y.P.; Sun, Z.Z.; Sun, X.L.; Zhou, G.Q.; Sun, T.; Peng, H.H. Analysis of Light Penetration Depth in Apple Tissues by Depth-Resolved Spatial-Frequency Domain Imaging. Foods 2023, 12, 1783. [Google Scholar] [CrossRef]
- Jiang, H.Z.; Jiang, X.S.; Ru, Y.; Chen, Q.; Wang, J.P.; Xu, L.Y.; Zhou, H.P. Detection and visualization of soybean protein powder in ground beef using visible and near-infrared hyperspectral imaging. Infrared Phys. Technol. 2022, 127, 104401. [Google Scholar] [CrossRef]
- Tian, S.J.; Xu, H.R. Mechanical-based and Optical-based Methods for Nondestructive Evaluation of Fruit Firmness. Food Rev. Int. 2023, 39, 4009–4039. [Google Scholar] [CrossRef]
- Xie, D.D.; Guo, W.C. Measurement and Calculation Methods on Absorption and Scattering Properties of Turbid Food in Vis/NIR Range. Food Bioprocess Technol. 2020, 13, 229–244. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, M.; Li, S.X. A modified condensed Monte Carlo simulation of reflectance with focus light beam from scattering medium. Optik 2015, 126, 4876–4879. [Google Scholar] [CrossRef]
- Zhu, C.G.; Liu, Q. Review of Monte Carlo modeling of light transport in tissues. J. Biomed. Opt. 2013, 18, 050902. [Google Scholar] [CrossRef]
- Leyre, S.; Durinck, G.; Van Giel, B.; Saeys, W.; Hofkens, J.; Deconinck, G.; Hanselaer, P. Extended adding-doubling method for fluorescent applications. Opt. Express 2012, 20, 17856–17872. [Google Scholar] [CrossRef]
- Vincely, V.D.; Vishwanath, K. Accuracy of retrieving optical properties from liquid tissue phantoms using a single integrating sphere. Appl. Opt. 2022, 61, 375–385. [Google Scholar] [CrossRef]
- Hu, D.; Lu, R.F.; Huang, Y.P.; Ying, Y.B.; Fu, X.P. Effects of optical variables in a single integrating sphere system on estimation of scattering properties of turbid media. Biosyst. Eng. 2020, 194, 82–98. [Google Scholar] [CrossRef]
- Lopez-Maestresalas, A.; Aernouts, B.; Van Beers, R.; Arazuri, S.; Jarén, C.; De Baerdemaeker, J.; Saeys, W. Bulk Optical Properties of Potato Flesh in the 500–1900 nm Range. Food Bioprocess Technol. 2016, 9, 463–470. [Google Scholar] [CrossRef]
- Ma, C.; Feng, L.; Pan, L.Q.; Wei, K.L.; Liu, Q.; Tu, K.; Zhao, L.; Peng, J. Relationships between optical properties of peach flesh with firmness and tissue structure during storage. Postharvest Biol. Technol. 2020, 163, 111134. [Google Scholar] [CrossRef]
- Joseph, M.; Postelmans, A.; Saeys, W. Characterization of bulk optical properties of pear tissues in the 500 to 1000 nm range as input for simulation-based optimization of laser spectroscopy in diffuse transmittance mode. J. Food Eng. 2023, 340, 111306. [Google Scholar] [CrossRef]
- Gao, M.J.; Guo, W.C.; Huang, X.L.; Du, R.Y.; Zhu, X.H. Effect of pressing and impacting bruises on optical properties of kiwifruit flesh. Postharvest Biol. Technol. 2021, 172, 111385. [Google Scholar] [CrossRef]
- Yang, S.H.; Guo, W.C.; Wang, J.X.; Li, S.R.; Zeng, S.C. Optical properties of melon tissues and Monte Carlo simulation of light propagation through melon fruit. Postharvest Biol. Technol. 2024, 207, 112616. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, R. Non-destructive defect detection of apples by spectroscopic and imaging technologies: A review. Trans. Asabe 2017, 60, 1765–1790. [Google Scholar] [CrossRef]
- Vanoli, M.; Rizzolo, A.; Grassi, M.; Spinelli, L.; Torricelli, A. Modeling mango ripening during shelf life based on pulp color nondestructively measured by time-resolved reflectance spectroscopy. Sci. Hortic. 2023, 310, 111714. [Google Scholar] [CrossRef]
- Ma, T.; Inagaki, T.; Tsuchikawa, S. Validation study on light scattering changes in kiwifruit during postharvest storage using time-resolved transmittance spectroscopy. Sci. Rep. 2023, 13, 16556. [Google Scholar] [CrossRef]
- Sarkar, M.; Gupta, N.; Assaad, M. Nondestructive Food Quality Monitoring Using Phase Information in Time-Resolved Reflectance Spectroscopy. IEEE Trans. Instrum. Meas. 2020, 69, 7787–7795. [Google Scholar] [CrossRef]
- Sarkar, M.; Assaad, M.; Gupta, N. Phase based time resolved reflectance spectroscopy using time-of-flight camera for fruit quality monitoring. In Proceedings of the 15th IEEE Sensors Applications Symposium (SAS), Electr Network, Kuala Lumpur, Malaysia, 9–11 March 2020; IEEE: Piscataway, NJ, USA, 2020. [Google Scholar]
- Zhou, Y.; Fu, X.P.; Ying, Y.B.; Fang, Z.H. An integrated fiber-optic probe combined with support vector regression for fast estimation of optical properties of turbid media. Anal. Chim. Acta 2015, 880, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Do Trong, N.N.; Erkinbaev, C.; Tsuta, M.; De Baerdemaeker, J.; Nicolai, B.; Saeys, W. Spatially resolved diffuse reflectance in the visible and near-infrared wavelength range for non-destructive quality assessment of ‘Braeburn’ apples. Postharvest Biol. Technol. 2014, 91, 39–48. [Google Scholar] [CrossRef]
- Ma, T.; Xia, Y.; Inagaki, T.; Tsuchikawa, S. Rapid and nondestructive evaluation of soluble solids content (SSC) and firmness in apple using Vis-NIR spatially resolved spectroscopy. Postharvest Biol. Technol. 2021, 173, 111417. [Google Scholar] [CrossRef]
- Huang, Y.P.; Lu, R.F.; Hu, D.; Chen, K.J. Quality assessment of tomato fruit by optical absorption and scattering properties. Postharvest Biol. Technol. 2018, 143, 78–85. [Google Scholar] [CrossRef]
- Cen, H.; Lu, R.; Mendoza, F.A.; Ariana, D.P. Assessing multiple quality attributes of peaches using optical absorption and scattering properties. Trans. Asabe 2012, 55, 647–657. [Google Scholar] [CrossRef]
- Huang, Y.P.; Lu, R.F.; Xu, Y.F.; Chen, K.J. Prediction of tomato firmness using spatially-resolved spectroscopy. Postharvest Biol. Technol. 2018, 140, 18–26. [Google Scholar] [CrossRef]
- Huang, Y.P.; Si, W.; Chen, K.J.; Sun, Y. Assessment of tomato maturity in different layers by spatially resolved spectroscopy. Sensors 2020, 20, 7229. [Google Scholar] [CrossRef] [PubMed]
- Cletus, B.; Kunnemeyer, R.; Martinsen, P.; McGlone, A.; Jordan, R. Characterizing liquid turbid media by frequency-domain photon-migration spectroscopy. J. Biomed. Opt. 2009, 14, 024041. [Google Scholar] [CrossRef]
- Bal, U.; Utzinger, U.; Bal, A.; Moral, O.T. The Determination of Absorption and Reduced Scattering Coefficients of Optical Phantoms Using a Frequency-Domain Multi-Distance Method in a Non-contact Manner. Adv. Electr. Comput. Eng. 2020, 20, 3–10. [Google Scholar] [CrossRef]
- Mei, M.; Li, J. An overview on optical non-destructive detection of bruises in fruit: Technology, method, application, challenge and trend. Comput. Electron. Agric. 2023, 213, 108195. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, R.F.; Lu, Y.Z.; Tu, K.; Pan, L.Q. Detection of early decay in peaches by structured-illumination reflectance imaging. Postharvest Biol. Technol. 2019, 151, 68–78. [Google Scholar] [CrossRef]
- He, X.M.; Yang, X.Y.; Fu, X.P.; Jiang, X.; Rao, X.Q. Assessing soluble solid content and texture of pear during shelf-life period by single snapshot spatial frequency domain imaging. Biosyst. Eng. 2021, 212, 252–263. [Google Scholar] [CrossRef]
- Hu, D.; Fu, X.P.; He, X.M.; Ying, Y.B. Noncontact and Wide-Field Characterization of the Absorption and Scattering Properties of Apple Fruit Using Spatial-Frequency Domain Imaging. Sci. Rep. 2016, 6, 37920. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Fu, X.P.; Li, T.W.; Rao, X.Q. Spatial frequency domain imaging for detecting bruises of pears. J. Food Meas. Charact. 2018, 12, 1266–1273. [Google Scholar] [CrossRef]
- He, X.M.; Li, T.W.; Fu, X.P.; Jiang, X.; Gao, Y.W.; Rao, X.Q. Fast estimation of optical properties of pear using a single snapshot technique combined with a least-squares support vector regression model based on spatial frequency domain imaging. Appl. Opt. 2019, 58, 4075–4084. [Google Scholar] [CrossRef]
- Zhang, J.M.; Fu, X.P.; Luo, Y.F.; Xing, S.Q.; Yang, Y. A Method for Obtaining Optical Properties of Two-Layer Tissue such as Thin-Skinned Fruits by Using Spatial Frequency Domain Imaging. Photonics 2023, 10, 622. [Google Scholar] [CrossRef]
- Cuccia, D.J.; Bevilacqua, F.; Durkin, A.J.; Ayers, F.R.; Tromberg, B.J. Quantitation and mapping of tissue optical properties using modulated imaging. J. Biomed. Opt. 2009, 14, 024012. [Google Scholar] [CrossRef]
- Vervandier, J.; Gioux, S. Single snapshot imaging of optical properties. Biomed. Opt. Express 2013, 4, 2938–2944. [Google Scholar] [CrossRef]
- Nadeau, K.P.; Durkin, A.J.; Tromberg, B.J. Advanced demodulation technique for the extraction of tissue optical properties and structural orientation contrast in the spatial frequency domain. J. Biomed. Opt. 2014, 19, 056013. [Google Scholar] [CrossRef] [PubMed]
- Aguenounon, E.; Dadouche, F.; Uhring, W.; Gioux, S. Single snapshot of optical properties image quality improvement using anisotropic two-dimensional windows filtering. J. Biomed. Opt. 2019, 24, 071611–071621. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, C.K.; Karrobi, K.; Pera, V.; Roblyer, D.; Venugopaian, V. Optical sampling depth in the spatial frequency domain. J. Biomed. Opt. 2019, 24, 071603. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Lu, R.F. Using composite sinusoidal patterns in structured-illumination reflectance imaging (SIRI) for enhanced detection of apple bruise. J. Food Eng. 2017, 199, 54–64. [Google Scholar] [CrossRef]
- Li, R.; Lu, Y.; Lu, R. Structured illumination reflectance imaging for enhanced detection of subsurface tissue bruising in apples. Trans. Asabe 2018, 61, 809–819. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, R. Development of a multispectral structured illumination reflectance imaging (SIRI) system and its application to bruise detection of apples. Trans. Asabe 2017, 60, 1379–1389. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, R. Detection of surface and subsurface defects of apples using structured-illumination reflectance imaging with machine learning algorithms. Trans. Asabe 2018, 61, 1831–1842. [Google Scholar] [CrossRef]
- Cai, Z.L.; Huang, W.Q.; Wang, Q.Y.; Li, J.B. Detection of early decayed oranges by structured-illumination reflectance imaging coupling with texture feature classification models. Front. Plant Sci. 2022, 13, 952942. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Lu, R.F.; Zhang, Z. Detection of subsurface bruising in fresh pickling cucumbers using structured-illumination reflectance imaging. Postharvest Biol. Technol. 2021, 180, 111624. [Google Scholar] [CrossRef]
- Cen, H. Hyperspectral Imaging-Based Spatially-Resolved Technique for Accurate Measurement of the Optical Properties of Horticultural Products; Michigan State University: East Lansing, MI, USA, 2011. [Google Scholar]
- Huang, Y.P.; Wang, D.Z.; Zhou, H.Y.; Yang, Y.T.; Chen, K.J. Ripeness assessment of tomato fruit by optical absorption and scattering coefficient spectra. Spectrosc. Spectr. Anal. 2020, 40, 3556–3561. [Google Scholar]
- Huang, Y.P.; Xiong, J.; Jiang, X.S.; Chen, K.J.; Hu, D. Assessment of firmness and soluble solids content of peaches by spatially resolved spectroscopy with a spectral difference technique. Comput. Electron. Agric. 2022, 200, 107212. [Google Scholar] [CrossRef]
- Tian, S.J.; Tian, H.; Yang, Q.Y.; Xu, H.R. Internal quality assessment of kiwifruit by bulk optical properties and online transmission spectra. Food Control 2022, 141, 109191. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.C.; Pan, L.Q.; Hu, Y.H. Influence of maturity on bruise detection of peach by structured multispectral imaging. Curr. Res. Food Sci. 2023, 6, 100476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, C.; Fan, S. Optical properties of healthy and bruised blueberry tissues in the near-infrared spectral region. In Proceedings of the 2017 ASABE Annual International Meeting, Spokane, WA, USA, 16–19 July 2017. [Google Scholar]
- Vanoli, M.; Rizzolo, A.; Grassi, M.; Spinelli, L.; Verlinden, B.E.; Torricelli, A. Studies on classification models to discriminate ‘Braeburn’ apples affected by internal browning using the optical properties measured by time-resolved reflectance spectroscopy. Postharvest Biol. Technol. 2014, 91, 112–121. [Google Scholar] [CrossRef]
- Lohner, S.A.; Biegert, K.; Nothelfer, S.; Hohmann, A.; McCormick, R.; Kienle, A. Determining the optical properties of apple tissue and their dependence on physiological and morphological characteristics during maturation. Part 1: Spatial frequency domain imaging. Postharvest Biol. Technol. 2021, 181, 111647. [Google Scholar] [CrossRef]
- Cen, H.Y.; Lu, R.F.; Mendoza, F.A.; Ariana, D.P. Peach maturity/quality assessment using hyperspectral imaging-based spatially-resolved technique. In Proceedings of the Conference on the Sensing for Agriculture and Food Quality and Safety III, Orlando, FL, USA, 26–27 April 2011. [Google Scholar]
- Sun, Y.; Lu, R.F.; Pan, L.Q.; Wang, X.C.; Tu, K. Assessment of the optical properties of peaches with fungal infection using spatially-resolved diffuse reflectance technique and their relationships with tissue structural and biochemical properties. Food Chem. 2020, 321, 126704. [Google Scholar] [CrossRef]
- Qin, J.W.; Lu, R.F. Measurement of the optical properties of fruits and vegetables using spatially resolved hyperspectral diffuse reflectance imaging technique. Postharvest Biol. Technol. 2008, 49, 355–365. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y.P.; Pan, L.Q.; Wang, X.C. Evaluation of the changes in optical properties of peaches with different maturity levels during bruising. Foods 2021, 10, 388. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, J.; Inagaki, T.; Su, Y.; Tsuchikawa, S. Rapid and nondestructive prediction of firmness, soluble solids content, and pH in kiwifruit using Vis-NIR spatially resolved spectroscopy. Postharvest Biol. Technol. 2022, 186, 111841. [Google Scholar] [CrossRef]
- Huang, Y.P.; Lu, R.F.; Chen, K.J. Assessment of tomato soluble solids content and pH by spatially-resolved and conventional Vis/NIR spectroscopy. J. Food Eng. 2018, 236, 19–28. [Google Scholar] [CrossRef]
- Vanoli, M.; Van Beers, R.; Sadar, N.; Rizzolo, A.; Buccheri, M.; Grassi, M.; Lovati, F.; Nicolai, B.; Aernouts, B.; Watte, R.; et al. Time- and spatially-resolved spectroscopy to determine the bulk optical properties of ‘Braeburn’ apples after ripening in shelf life. Postharvest Biol. Technol. 2020, 168, 111233. [Google Scholar] [CrossRef]
- Huang, Y.P.; Yang, Y.T.; Sun, Y.; Zhou, H.Y.; Chen, K.J. Identification of apple varieties using a multichannel hyperspectral imaging system. Sensors 2020, 20, 5120. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.J.; Doi, T.; Arakawa, O.; Zhang, S.H. A novel spatially resolved interactance spectroscopy system to estimate degree of red coloration in red-fleshed apple. Sci. Rep. 2021, 11, 21982. [Google Scholar] [CrossRef]
- Peng, H.H.; Zhang, C.; Sun, Z.Z.; Sun, T.; Hu, D.; Yang, Z.D.; Wang, J.S. Optical property mapping of apples and the relationship with quality properties. Front. Plant Sci. 2022, 13, 873065. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, P.E.; Vanoli, M.; Rizzolo, A.; Grassi, M.; Pimentel, R.M.D.; Spinelli, L.; Torricelli, A. Optical properties, ethylene production and softening in mango fruit. Postharvest Biol. Technol. 2015, 101, 58–65. [Google Scholar] [CrossRef]
- Benmouna, B.; García-Mateos, G.; Sabzi, S.; Fernandez-Beltran, R.; Parras-Burgos, D.; Molina-Martínez, J.M. Convolutional Neural Networks for Estimating the Ripening State of Fuji Apples Using Visible and Near-Infrared Spectroscopy. Food Bioprocess Technol. 2022, 15, 2226–2236. [Google Scholar] [CrossRef]
- Tang, H.; Fei, L.F.; Zhu, H.Y.; Tao, H.J.; Xie, C. A two-stage network for zero-shot low-illumination image restoration. Sensors 2023, 23, 792. [Google Scholar] [CrossRef]
- Ye, M.; Yan, X.; Chen, N.; Jia, M. Intelligent fault diagnosis of rolling bearing using variational mode extraction and improved one-dimensional convolutional neural network. Appl. Acoust. 2023, 202, 109143. [Google Scholar] [CrossRef]
- Li, J.B.; Luo, W.; Han, L.; Cai, Z.L.; Guo, Z.M. Two-wavelength image detection of early decayed oranges by coupling spectral classification with image processing. J. Food Compos. Anal. 2022, 111, 104642. [Google Scholar] [CrossRef]
- Lu, R.; Cen, H.; Huang, M.; Ariana, D.P. Spectral absorption and scattering properties of normal and bruised apple tissue. Trans. Asabe 2010, 53, 263–269. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Hu, D.; Xie, L.J.; Ying, Y.B. Detection of early stage bruise in apples using optical property mapping. Comput. Electron. Agric. 2022, 194, 106725. [Google Scholar] [CrossRef]
- Yu, S.Q.; Hu, D.; Qiu, D.K.; Jia, T.Z.; Zhou, T.T.; Sun, X.L.; Sun, Z.Z.; Yan, X.J. Profile-based diffuse reflectance corrections for improved optical property measurement of spherical fruit with spatial frequency domain imaging. Postharvest Biol. Technol. 2023, 204, 112453. [Google Scholar] [CrossRef]
- Mollazade, K.; Arefi, A. Optical analysis using monochromatic imaging-based spatially-resolved technique capable of detecting mealiness in apple fruit. Sci. Hortic. 2017, 225, 589–598. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Xie, L.J.; Hu, D.; Ying, Y.B. An artificial neural network model for accurate and efficient optical property mapping from spatial-frequency domain images. Comput. Electron. Agric. 2021, 188, 106340. [Google Scholar] [CrossRef]
- Liu, N.L.; Chen, X.; Liu, Y.; Ding, C.Z.; Tan, Z.J. Deep learning approach for early detection of sub-surface bruises in fruits using single snapshot spatial frequency domain imaging. J. Food Meas. Charact. 2022, 16, 3888–3896. [Google Scholar] [CrossRef]
- Hu, D.; Qiu, D.K.; Yu, S.Q.; Jia, T.Z.; Zhou, T.T.; Yan, X.J. Integration of Optical Property Mapping and Machine Learning for Real-Time Classification of Early Bruises of Apples. Food Bioprocess Technol. 2023, 17, 2745–2756. [Google Scholar] [CrossRef]
- Ge, Y.H.; Tu, S.Y. Identification of chilling injury in kiwifruit using hyperspectral structured-illumination reflectance imaging system (SIRI) with support vector machine (SVM) modelling. Anal. Lett. 2023, 56, 2040–2052. [Google Scholar] [CrossRef]
- Wilson, B.C.; Jeeves, W.P.; Lowe, D.M. In vivo and post mortem measurements of the attenuation spectra of light in mammalian tissues. Photochem. Photobiol. 1985, 42, 153–162. [Google Scholar] [CrossRef]
- Del Bianco, S.; Martelli, F.; Zaccanti, G. Penetration depth of light re-emitted by a diffusive medium: Theoretical and experimental investigation. Phys. Med. Biol. 2002, 47, 4131–4144. [Google Scholar] [CrossRef]
- Hu, D.; Huang, Y.P.; Zhang, Q.; Yao, L.J.; Yang, Z.D.; Sun, T. Numerical simulation on spatial-frequency domain imaging for estimating optical absorption and scattering properties of two-layered horticultural products. Appl. Sci. 2021, 11, 617. [Google Scholar] [CrossRef]
- Ding, C.Z.; Shi, S.N.; Chen, J.J.; Wei, W.; Tan, Z.J. Analysis of Light Transport Features in Stone Fruits Using Monte Carlo Simulation. PLoS ONE 2015, 10, e0140582. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, J.B.; Zhang, D.Y. Near-infrared light penetration depth analysis inside melon with thick peel by a novel strategy of slicing combining with least square fitting method. J. Food Process Eng. 2018, 41, e12886. [Google Scholar] [CrossRef]
- Gomes, A.J.; Turzhitsky, V.; Ruderman, S.; Backman, V. Monte Carlo model of the penetration depth for polarization gating spectroscopy: Influence of illumination-collection geometry and sample optical properties. Appl. Opt. 2012, 51, 4627–4637. [Google Scholar] [CrossRef] [PubMed]
| Model | Advantage | Limitation |
|---|---|---|
| Diffusion approximation equation | Computationally fast and intuitive, wide applicability and suitable for strong scattering media | Unsuitable for low scattering media and unable to handle anisotropic scattering |
| Monte Carlo method | High accuracy, flexibility and wide applicability | Large calculation, time-consuming and difficult to apply in real time |
| Adding-doubling method | Suitable for anisotropic scattering and multilayer media With high accuracy and short calculation time, suitable for real-time applications | Limited to the assumption of uniform media, only suitable for planar layer media and unsuitable for extremely high scattering |
| Technique | Advantage | Disadvantage |
|---|---|---|
| TRS | Good penetration ability, penetrating tissues as deep as about 1 to 2 cm | Requiring expensive and sophisticated instrumentation, as well as good contact between the sample and the detector |
| SRS | Being cost-effective on instruments, high execution efficiency and support for a wide range of spectral range | Is insufficient for analyzing deep defects inside samples |
| FD | Having high resolution and strong noise suppression ability, and the instrument used is cheaper than TRS | Requiring good contact between the sample and the detector, only one single frequency |
| SFDI | Wide field non-contact, imaging depth discrimination and effective signal enhancement | Requiring selection of the feature band and cannot achieve real-time detection |
| SIRI | Is capable of revealing subsurface tissue properties at specific depths and enhancing the visibility of defects | Being unsuitable for online inspection of food quality and limited to defect detection |
| Product | Technique | Year | Wavelength (nm) | (cm−1) | (cm−1) | Reference |
|---|---|---|---|---|---|---|
| Apple | SRS | 2009 | 500~1000 | 0.04~2.52 | 1.02~12.61 | [31] |
| SRS | 2014 | 500~1000 | 0~2.6 | [62] | ||
| TRS | 2014 | 650~1050 | 0.02~0.45 | —— | [94] | |
| SFDI | 2021 | 400~1000 | 0~1.2 | 0~35 | [95] | |
| Peach | SRS | 2011 | 515~1000 | 0~0.5 | 5~20 | [96] |
| SRS | 2020 | 600~1000 | 0.9~4.1 | 5.59~16.12 | [34] | |
| SRS | 2020 | 550~1000 | 0~0.5 | 2~14 | [97] | |
| IS | 2020 | 400~1050 | 0.3~4 | 2~12 | [52] | |
| SRS | 2022 | 550~1650 | —— | —— | [90] | |
| Pear | IS | 2023 | 500~1000 | 0~3 | 10~18 | [53] |
| Kiwifruit | IS | 2021 | 950~1650 | 0~9 | 3~13 | [54] |
| IS | 2022 | 500~1050 | 0~0.8 | 2~10 | [91] | |
| Tomato | SRS | 2018 | 550~1300 | 0~2.5 | 0.8~6 | [64] |
| Blueberry | IS | 2017 | 925~1400 | 0~10 | 0~8 | [93] |
| Mango | TRS | 2023 | 540 | 0.103~0.403 0.106~0.511 | —— | [57] |
| Melon | IS | 2024 | 450~1650 | 0~7.5 | 5~55 | [55] |
| Product | Technique | Year | Type of Defect | Model | Accuracy | Reference |
|---|---|---|---|---|---|---|
| Apple | TRS | 2014 | Internal browning | LDA | 90%, 71% | [94] |
| SRS | 2017 | Mealiness | ANN | 76%, 82% | [114] | |
| SIRI | 2018 | Fresh bruising | BDA | 70%~100% | [83] | |
| SIRI | 2018 | Surface and Subsurface defects | CNN | 98% | [85] | |
| SFDI | 2021 | Early bruising | ANN | MAE = 0.18%, 0.027% Rmse = 0.01, 0.14 | [115] | |
| SFDI | 2022 | Early bruising | —— | —— | [112] | |
| SFDI | 2022 | Subsurface Bruising | cGANs | PSNR = 34.72 dB SSIM = 0.84 | [116] | |
| SFDI | 2023 | Bruises | —— | —— | [113] | |
| SFDI | 2023 | Early bruises | CNN | two-category: 99.16% three-category: 91.50% | [117] | |
| Pear | SFDI | 2018 | Bruising | Discriminant analysis | 90%, 87.5% | [74] |
| SFDI | 2021 | Surface damage | LDA | class 1: 92.5% class 2: 83.8% | [19] | |
| SFDI | 2023 | Bruising | LSTMR | MAE = 0.32%, 0.21% | [20] | |
| Peach | SIRI | 2019 | Early decay | CNN | 98.6%, 97.6% | [71] |
| SRS | 2020 | Fungal infection | PLSDA | 88% | [34] | |
| S-MSI | 2023 | Bruise detection | WSA | S1: 92% S2: 97.43% S3: 99.86% | [92] | |
| Orange | SIRI | 2022 | Early decay | PLSDA | 96.4% | [86] |
| Kiwifruit | SIRI | 2023 | Chilling injury | SVM | 94.2% | [118] |
| Cucumber | SIRI | 2021 | Subsurface bruising | SVM | 94% | [87] |
| Product | Technique | Year | Research Content | Result | Reference |
|---|---|---|---|---|---|
| Apple | SRS-MC | 2009 | Determine the optimum range of perception | δ is 0.43~8.67 cm over the 500~1000 nm; δ is related to wavelength | [31] |
| MC | 2015 | Effects of skin thickness on light transport features in tissues | Thin skin has little effect on δ | [30] | |
| TRS | 2020 | Nondestructive assessment of the internal quality of fruits | δ is related to SDS | [60] | |
| SFDI-MC | 2020 2021 | Estimate and of two-layered horticultural products | Smaller frequency could generate larger δ | [24,121] | |
| Peach | MC | 2015 | Effects of the core and the skin on light transport features | Pericarp has more influence on δ | [122] |
| Citrus | —— | 2015 | Effects of skin thickness on light transport features in tissues | Thick pericarp has great influence on δ | [30] |
| Melon | NIR | 2018 | Study the δ based on three SDS (10, 20 and 30 mm) | Depth ranges were 11.7~12.4, 14.2~15.8 and 16.8~21.0 mm | [123] |
| VIS/NIR | 2024 | Investigate the light propagation through melons | δ is more than 2.5 mm in exocarp, 3 mm in green mesocarp and 3.5 mm in pulp | [55] | |
| Pomelo | VIS/NIR | 2022 | Prediction of soluble solid content | δ determines the design of the detection system for large fruit | [33] |
| Persimmon | HSI-SR | 2023 | Estimate the δ of a HSI system in a Vis-NIR configuration (in 450~1050 nm) | δ is limited to about 2 mm | [32] |
| Turbid media | MC | 2012 | Influence of the illumination-collection geometry and optical properties on δ | δ is related to illumination-collection area and collection angle | [124] |
| SRS-MC | 2016 | Estimate the optical properties of two-layer media | δ is proportional to SDS | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Xiong, J.; Li, Z.; Hu, D.; Sun, Y.; Jin, H.; Zhang, H.; Fang, H. Recent Advances in Light Penetration Depth for Postharvest Quality Evaluation of Fruits and Vegetables. Foods 2024, 13, 2688. https://doi.org/10.3390/foods13172688
Huang Y, Xiong J, Li Z, Hu D, Sun Y, Jin H, Zhang H, Fang H. Recent Advances in Light Penetration Depth for Postharvest Quality Evaluation of Fruits and Vegetables. Foods. 2024; 13(17):2688. https://doi.org/10.3390/foods13172688
Chicago/Turabian StyleHuang, Yuping, Jie Xiong, Ziang Li, Dong Hu, Ye Sun, Haojun Jin, Huichun Zhang, and Huimin Fang. 2024. "Recent Advances in Light Penetration Depth for Postharvest Quality Evaluation of Fruits and Vegetables" Foods 13, no. 17: 2688. https://doi.org/10.3390/foods13172688
APA StyleHuang, Y., Xiong, J., Li, Z., Hu, D., Sun, Y., Jin, H., Zhang, H., & Fang, H. (2024). Recent Advances in Light Penetration Depth for Postharvest Quality Evaluation of Fruits and Vegetables. Foods, 13(17), 2688. https://doi.org/10.3390/foods13172688








