Evaluating the Impact of Green Coffee Bean Powder on the Quality of Whole Wheat Bread: A Comprehensive Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Breadmaking Materials
2.1.2. Chemicals and Reagents
2.2. Methods
2.2.1. Formulation of WWBs (Whole Wheat Breads)
2.2.2. Measurement of Physical Dimensions
2.2.3. Moisture of Crumb
2.2.4. Impedance Analysis
2.2.5. Study of Swelling Percentage
2.2.6. Colorimetry and Microcolorimetry
2.2.7. FTIR Study
2.2.8. Texture
2.2.9. Estimation of Total Phenolic Content
Formulation Extract Preparation
Total Phenolic Content
2.2.10. Microbial Analysis of Bread
Assessment of Total Viable Count (TVC)
2.2.11. Sensory Analysis
2.2.12. Statistical Analysis
3. Results
3.1. Physical Evaluation of WWB Preparations
3.2. Moisture Content
3.3. Analysis of Impedance
3.4. Swelling Percentage
3.5. Colorimetry and Microcolorimetry
3.6. FTIR Study
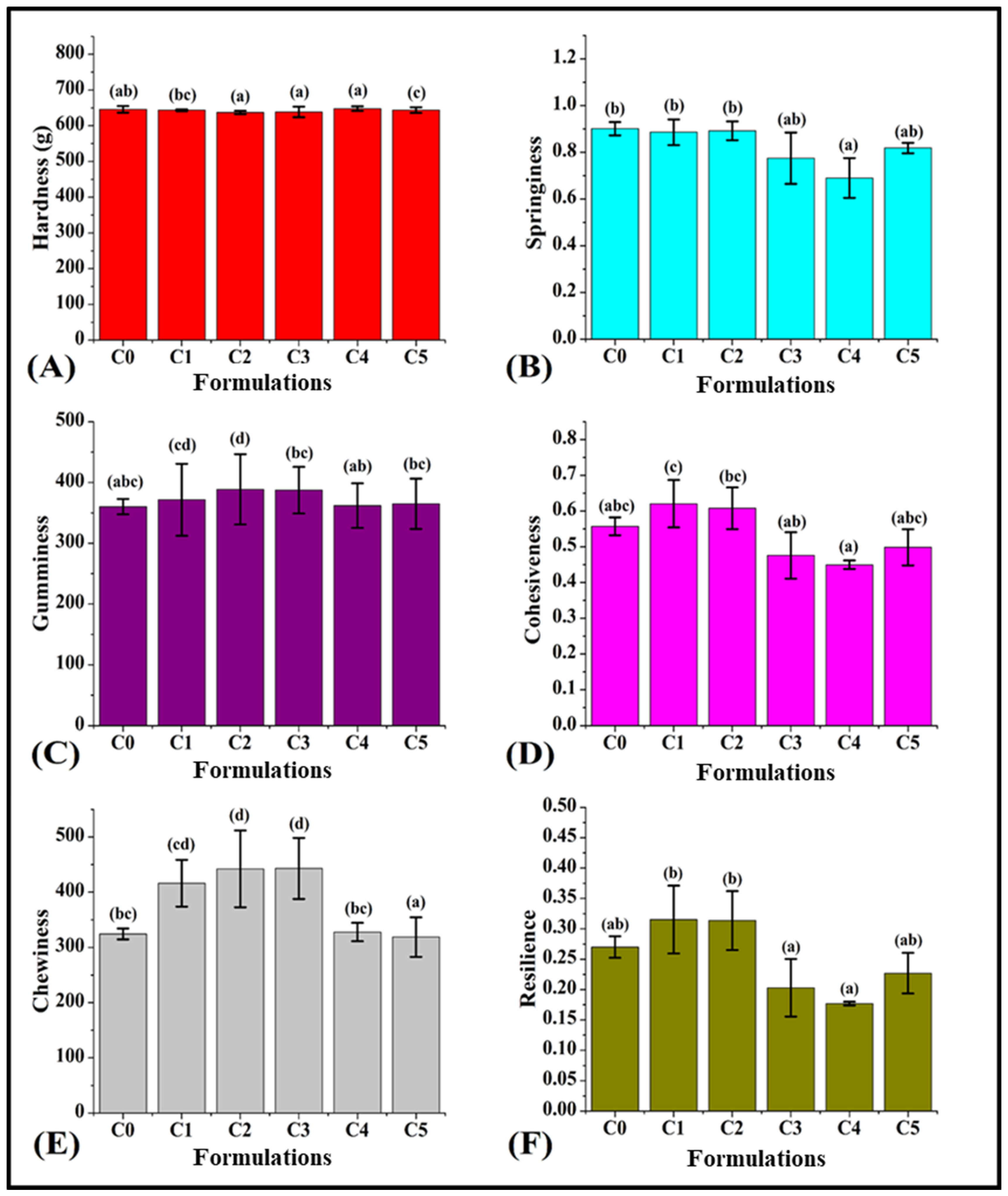
3.7. Texture of Bread
3.8. Total Phenolic Content (TPC)
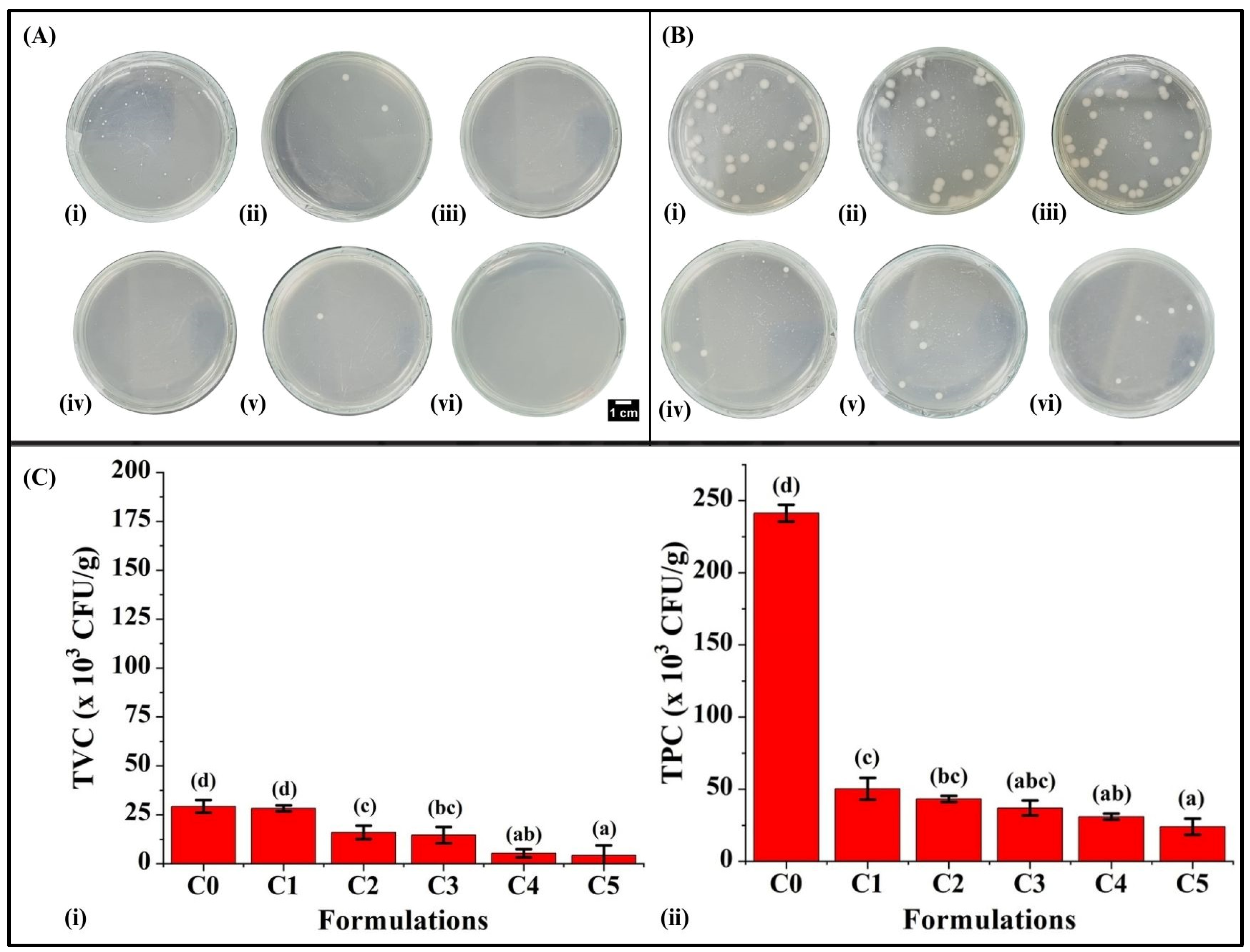
3.9. Microbiological Analysis
3.10. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cauvain, S.P.; Young, L.S. Technology of Breadmaking, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007; XXII+397p. [Google Scholar]
- Savvaidis, I.N.; Al Katheeri, A.; Lim, S.H.E.; Lai, K.S.; Abushelaibi, A. Traditional foods, food safety practices, and food culture in the Middle East. In Food Safety in the Middle East; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–31. [Google Scholar]
- Wang, Y.; Jian, C. Sustainable plant-based ingredients as wheat flour substitutes in bread making. npj Sci. Food 2022, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.Z.M.; Shori, A.B.; Baba, A.S. Potential Functional Food Ingredients in Bread and their Health Benefits. Biointerface Res. Appl. Chem. 2021, 12, 6533–6542. [Google Scholar]
- Suchintita Das, R.; Tiwari, B.K.; Garcia-Vaquero, M. The Fundamentals of Bread Making: The Science of Bread. In Traditional European Breads: An Illustrative Compendium of Ancestral Knowledge and Cultural Heritage; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–40. [Google Scholar]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.; Courtin, C.; Gebruers, K.; Delcour, J. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Haruna, M.; Udobi, C.; Ndife, J. Effect of added brewers dry grain on the physico-chemical, microbial and sensory quality of wheat bread. Am. J. Food Nutr. 2011, 1, 39–43. [Google Scholar] [CrossRef]
- Schadow, A.M.; Revheim, I.; Spielau, U.; Dierkes, J.; Schwingshackl, L.; Frank, J.; Hodgson, J.M.; Moreira-Rosário, A.; Seal, C.J.; Buyken, A.E.; et al. The Effect of Regular Consumption of Reformulated Breads on Glycemic Control: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv. Nutr. 2023, 14, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.; Adam, A.; Leenhardt, F.; Scalbert, A.; Remesy, C. Control of the nutritional value of bread. Ind. Cereales 2001, 124, 15–20. [Google Scholar]
- Parenti, O.; Guerrini, L.; Zanoni, B. Techniques and technologies for the breadmaking process with unrefined wheat flours. Trends Food Sci. Technol. 2020, 99, 152–166. [Google Scholar] [CrossRef]
- Yu, L.; Nanguet, A.-L.; Beta, T. Comparison of Antioxidant Properties of Refined and Whole Wheat Flour and Bread. Antioxidants 2013, 2, 370–383. [Google Scholar] [CrossRef]
- Ngozi, A.A. Effect of whole wheat flour on the quality of wheat- baked bread. Glob. J. Food Sci. Technol. 2014, 2, 127–133. [Google Scholar]
- Dziki, D.; Różyło, R.; Gawlik-Dziki, U.; Świeca, M. Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci. Technol. 2014, 40, 48–61. [Google Scholar] [CrossRef]
- Blandino, M.; Sovrani, V.; Marinaccio, F.; Reyneri, A.; Rolle, L.; Giacosa, S.; Locatelli, M.; Bordiga, M.; Travaglia, F.; Coïsson, J.D.; et al. Nutritional and technological quality of bread enriched with an intermediated pearled wheat fraction. Food Chem. 2013, 141, 2549–2557. [Google Scholar] [CrossRef]
- Ning, J.; Hou, G.G.; Sun, J.; Wan, X.; Dubat, A. Effect of green tea powder on the quality attributes and antioxidant activity of whole-wheat flour pan bread. LWT-Food Sci. Technol. 2017, 79, 342–348. [Google Scholar] [CrossRef]
- Babuchowski, A.; Beekmans, F.; Castenmiller, J.; Fenwick, R.; Haber, J.; Hogg, T.; Tomás-Barberán, F. European Technology Platform on FOOD for LIFE the Vision for 2020 and Beyond. 2005. Available online: https://www.researchgate.net/publication/238068520_European_Technology_Platform_on_FOOD_for_LIFE_The_vision_for_2020_and_beyond (accessed on 11 March 2023).
- Gomes, W.P.C.; Pires, J.A.; Teixeira, N.N.; Bortoleto, G.G.; Gutierrez, E.M.R.; Melchert, W.R. Effects of green coffee bean flour fortification on the chemical and nutritional properties of gluten-free cake. J. Food Meas. Charact. 2022, 16, 3451–3458. [Google Scholar] [CrossRef]
- Stelmach, E.; Pohl, P.; Szymczycha-Madeja, A. The content of Ca, Cu, Fe, Mg and Mn and antioxidant activity of green coffee brews. Food Chem. 2015, 182, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Masek, A.; Latos-Brozio, M.; Kałużna-Czaplińska, J.; Rosiak, A.; Chrzescijanska, E. Antioxidant Properties of Green Coffee Extract. Forests 2020, 11, 557. [Google Scholar] [CrossRef]
- Dziki, D.; Gawlik-Dziki, U.; Pecio, Ł.; Różyło, R.; Świeca, M.; Krzykowski, A.; Rudy, S. Ground green coffee beans as a functional food supplement—Preliminary study. LWT 2015, 63, 691–699. [Google Scholar] [CrossRef]
- Ibrahim, U.; Si-Hien, M.D.L.R.L.; Suzihaque, M.U.H.; Hashib, S.A.; Karim, S.F.A. Antimicrobial properties and sensory evaluation of bread enriched with green coffee beans (GCB). IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 062018. [Google Scholar] [CrossRef]
- Dziki, D.; Gawlik-Dziki, U.; Rozyło, R.; Siastała, M.; Kowalczyk, D. Quality of wholemeal wheat bread enriched with green coffee beans. Croat. J. Food Sci. Technol. 2016, 8, 112–119. [Google Scholar] [CrossRef]
- Gallardo-Ignacio, J.; Santibáñez, A.; Oropeza-Mariano, O.; Salazar, R.; Montiel-Ruiz, R.M.; Cabrera-Hilerio, S.; Gonzáles-Cortazar, M.; Cruz-Sosa, F.; Nicasio-Torres, P. Chemical and Biological Characterization of Green and Processed Coffee Beans from Coffea arabica Varieties. Molecules 2023, 28, 4685. [Google Scholar] [CrossRef]
- Kim, I.; Jung, S.; Kim, E.; Lee, J.-W.; Kim, C.-Y.; Ha, J.-H.; Jeong, Y. Physicochemical characteristics of Ethiopian Coffea arabica cv. Heirloom coffee extracts with various roasting conditions. Food Sci. Biotechnol. 2021, 30, 235–244. [Google Scholar] [CrossRef]
- Jain, A.; Pradhan, B.K.; Mahapatra, P.; Ray, S.S.; Chakravarty, S.; Pal, K. Development of a low-cost food color monitoring system. Color Res. Appl. 2021, 46, 430–445. [Google Scholar] [CrossRef]
- Pathak, D.; Majumdar, J.; Raychaudhuri, U.; Chakraborty, R. Characterization of physicochemical properties in whole wheat bread after incorporation of ripe mango peel. J. Food Meas. Charact. 2016, 10, 554–561. [Google Scholar] [CrossRef]
- Dhal, S.; Anis, A.; Shaikh, H.M.; Alhamidi, A.; Pal, K. Effect of Mixing Time on Properties of Whole Wheat Flour-Based Cookie Doughs and Cookies. Foods 2023, 12, 941. [Google Scholar] [CrossRef]
- Gámbaro, A.; Giménez, A.; Ares, G.; Gilardi, V. Influence of enzymes on the texture of brown pan bread. J. Texture Stud. 2006, 37, 300–314. [Google Scholar] [CrossRef]
- Singleton, V.L. Lamuela-Raventos: Analysis of total phenoles and other oxidation substartes and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152. [Google Scholar]
- Humphries, J.M.; Graham, R.D.; Mares, D.J. Application of reflectance colour measurement to the estimation of carotene and lutein content in wheat and triticale. J. Cereal Sci. 2004, 40, 151–159. [Google Scholar] [CrossRef]
- Capuano, E.; Ferrigno, A.; Acampa, I.; Ait-Ameur, L.; Fogliano, V. Characterization of the Maillard reaction in bread crisps. Eur. Food Res. Technol. 2008, 228, 311–319. [Google Scholar] [CrossRef]
- Tamanna, N.; Mahmood, N. Food processing and maillard reaction products: Effect on human health and nutrition. Int. J. Food Sci. 2015, 2015, 526762. [Google Scholar] [CrossRef]
- Amir, I.Z.; Hanida, H.S.; Syafiq, A. Development and physical analysis of high fiber bread incorporated with cocoa (Theobroma cacao sp.) pod husk powder. Int. Food Res. J. 2013, 20, 1301. [Google Scholar]
- Besbes, E.; Jury, V.; Monteau, J.-Y.; Le Bail, A. Characterizing the cellular structure of bread crumb and crust as affected by heating rate using X-ray microtomography. J. Food Eng. 2013, 115, 415–423. [Google Scholar] [CrossRef]
- Wang, S.; Austin, P.; Bell, S. It’sa maze: The pore structure of bread crumbs. J. Cereal Sci. 2011, 54, 203–210. [Google Scholar] [CrossRef]
- Rathnayake, H.A.; Navaratne, S.B.; Navaratne, C.M. Porous Crumb Structure of Leavened Baked Products. Int. J. Food Sci. 2018, 2018, 8187318. [Google Scholar] [CrossRef]
- Vasudevaiah, A.M.; Chaturvedi, A.; Kulathooran, R.; Dasappa, I. Effect of green coffee extract on rheological, physico-sensory and antioxidant properties of bread. J. Food Sci. Technol. 2017, 54, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Onacik-Gür, S.; Szafrańska, A.; Roszko, M.; Stępniewska, S. Interaction of dough preparation method, green tea extract and baking temperature on the quality of rye bread and acrylamide content. LWT 2022, 154, 112759. [Google Scholar] [CrossRef]
- Singh, P.; Yadav, V.; Sahu, D.; Kumar, K.; Kim, D.; Yang, D.; Jayaraman, S.; Jarzębski, M.; Wieruszewski, M.; Pal, K. Exploring Chitosan Lactate as a Multifunctional Additive: Enhancing Quality and Extending Shelf Life of Whole Wheat Bread. Foods 2024, 13, 1590. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, U.; Rahman, N.A.A.; Suzihaque, M.U.H.; Hashib, S.A.; Aziz, R.A.A. Effect of baking conditions on the physical properties of bread incorporated with green coffee beans (GCB). IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 062019. [Google Scholar] [CrossRef]
- Mollakhalili-Meybodi, N.; Sheidaei, Z.; Khorshidian, N.; Nematollahi, A.; Khanniri, E. Sensory attributes of wheat bread: A review of influential factors. J. Food Meas. Charact. 2022, 17, 2172–2181. [Google Scholar] [CrossRef]
- Alpers, T.; Kerpes, R.; Frioli, M.; Nobis, A.; Hoi, K.I.; Bach, A.; Jekle, M.; Becker, T. Impact of Storing Condition on Staling and Microbial Spoilage Behavior of Bread and Their Contribution to Prevent Food Waste. Foods 2021, 10, 76. [Google Scholar] [CrossRef]
- Littardi, P.; Rinaldi, M.; Grimaldi, M.; Cavazza, A.; Chiavaro, E. Effect of Addition of Green Coffee Parchment on Structural, Qualitative and Chemical Properties of Gluten-Free Bread. Foods 2021, 10, 5. [Google Scholar] [CrossRef]
- Abu-Alruz, K. Effects of using composite flour containing wheat flour with different levels of green banana pulp flour on the quality of saj flatbread. Potravin. Slovak J. Food Sci. 2023, 17, 419–432. [Google Scholar] [CrossRef]
- Abdullahi, X.; Xhabiri, G.; Sulejmani, E.; Selimi, F. The effect of some additives on the rheology of dough and quality of bread. Acta Agric. Slov. 2022, 118, 1–7. [Google Scholar] [CrossRef]
- Jumadi, M.; Jamaludin, D. Non-destructive measurement of rock melon fruit properties using Electrical Impedance Spectroscopy (EIS) technique. ASM Sci. J. 2020, 13, 20–26. [Google Scholar]
- Chen, Y.; Wippermann, K.; Rodenbücher, C.; Suo, Y.; Korte, C. Impedance Analysis of Capacitive and Faradaic Processes in the Pt/[Dema][TfO] Interface. ACS Appl. Mater. Interfaces 2024, 16, 5278–5285. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, G.; Nguyen, D.D.; Mallarino, S.; Touzain, S. Analysis of the non-ideal capacitive behaviour for high impedance organic coatings. Prog. Org. Coat. 2014, 77 Pt A, 2045–2053. [Google Scholar] [CrossRef]
- Shaw, G.S.; Biswal, D.; Banerjee, I.; Pramanik, K.; Anis, A.; Pal, K. Preparation, Characterization and Assessment of the Novel Gelatin–tamarind Gum/Carboxymethyl Tamarind Gum-Based Phase-Separated Films for Skin Tissue Engineering Applications. Polym.-Plast. Technol. Eng. 2017, 56, 141–152. [Google Scholar] [CrossRef]
- Wessels, R.; Wentzel, B.; Labuschagne, M. Solvent retention capacity and swelling index of glutenin in hard red wheat flour as possible indicators of rheological and baking quality characteristics. J. Cereal Sci. 2020, 93, 102983. [Google Scholar] [CrossRef]
- Menon, L.; Majumdar, S.D.; Ravi, U. Development and analysis of composite flour bread. J. Food Sci. Technol. 2015, 52, 4156–4165. [Google Scholar] [CrossRef]
- Tolve, R.; Simonato, B.; Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G. Wheat Bread Fortification by Grape Pomace Powder: Nutritional, Technological, Antioxidant, and Sensory Properties. Foods 2021, 10, 75. [Google Scholar] [CrossRef]
- Sahu, D.; Bharti, D.; Kim, D.; Sarkar, P.; Pal, K. Variations in Microstructural and Physicochemical Properties of Candelilla Wax/Rice Bran Oil–Derived Oleogels Using Sunflower Lecithin and Soya Lecithin. Gels 2021, 7, 226. [Google Scholar] [CrossRef]
- Michalska, A.; Amigo-Benavent, M.; Zielinski, H.; del Castillo, M.D. Effect of bread making on formation of Maillard reaction products contributing to the overall antioxidant activity of rye bread. J. Cereal Sci. 2008, 48, 123–132. [Google Scholar] [CrossRef]
- Ishida, P.M.G.; Steel, C.J. Physicochemical and sensory characteristics of pan bread samples available in the Brazilian market. Food Sci. Technol. 2014, 34, 746–754. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Gawlik-Dziki, U.; Sęczyk, Ł.; Dziki, D.; Sikora, M. Interactions of green coffee bean phenolics with wheat bread matrix in a model of simulated in vitro digestion. Food Chem. 2018, 258, 301–307. [Google Scholar] [CrossRef]
- Ye, L.; Zheng, W.; Li, X.; Han, W.; Shen, J.; Lin, Q.; Hou, L.; Liao, L.; Zeng, X. The Role of Gluten in Food Products and Dietary Restriction: Exploring the Potential for Restoring Immune Tolerance. Foods 2023, 12, 4179. [Google Scholar] [CrossRef]
- Cocchi, M.; Foca, G.; Lucisano, M.; Marchetti, A.; Pagani, M.A.; Tassi, L.; Ulrici, A. Classification of Cereal Flours by Chemometric Analysis of MIR Spectra. J. Agric. Food Chem. 2004, 52, 1062–1067. [Google Scholar] [CrossRef]
- Guo, X.-X.; Hu, W.; Liu, Y.; Gu, D.-C.; Sun, S.-Q.; Xu, C.-H.; Wang, X.-C. Rapid analysis and quantification of fluorescent brighteners in wheat flour by Tri-step infrared spectroscopy and computer vision technology. J. Mol. Struct. 2015, 1099, 393–398. [Google Scholar] [CrossRef]
- Sadat, A.; Joye, I.J. Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- Obeidat, S.M.; Hammoudeh, A.Y.; Alomary, A.A. Application of FTIR Spectroscopy for Assessment of Green Coffee Beans According to Their Origin. J. Appl. Spectrosc. 2018, 84, 1051–1055. [Google Scholar] [CrossRef]
- Su, W.H.; Sun, D.W. Fourier transform infrared and Raman and hyperspectral imaging techniques for quality determinations of powdery foods: A review. Rev. Food Sci. Food Saf. 2018, 17, 104–122. [Google Scholar] [CrossRef]
- Echeverri-Giraldo, L.F.; Pinzón Fandiño, M.I.; González Cadavid, L.M.; Rodriguez Marín, N.D.; Moreno Ríos, D.A.; Osorio Pérez, V. Determination of Lipids and Fatty Acids in Green Coffee Beans (Coffea arabica L.) Harvested in Different Agroclimatic Zones of the Department of Quindío, Colombia. Agronomy 2023, 13, 2560. [Google Scholar] [CrossRef]
- Linlaud, N.; Ferrer, E.; Puppo, M.C.; Ferrero, C. Hydrocolloid Interaction with Water, Protein, and Starch in Wheat Dough. J. Agric. Food Chem. 2011, 59, 713–719. [Google Scholar] [CrossRef]
- Nawrocka, A.; Krekora, M.; Niewiadomski, Z.; Miś, A. FTIR studies of gluten matrix dehydration after fibre polysaccharide addition. Food Chem. 2018, 252, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, S.; Sanabria, M.; Monteiro, M. Influence of the fermentation methods on the resistant starch formation by X-ray diffraction. SN Appl. Sci. 2021, 3, 191. [Google Scholar] [CrossRef]
- Munyendo, L.; Njoroge, D.; Hitzmann, B. The potential of spectroscopic techniques in coffee analysis—A review. Processes 2021, 10, 71. [Google Scholar] [CrossRef]
- Wang, J.; Somasundaran, P. Study of galactomannose interaction with solids using AFM, IR and allied techniques. J. Colloid Interface Sci. 2007, 309, 373–383. [Google Scholar] [CrossRef]
- Paredes, J.; Cortizo-Lacalle, D.; Imaz, A.M.; Aldazabal, J.; Vila, M. Application of texture analysis methods for the characterization of cultured meat. Sci. Rep. 2022, 12, 3898. [Google Scholar] [CrossRef]
- Tóth, M.; Kaszab, T.; Meretei, A. Texture profile analysis and sensory evaluation of commercially available gluten-free bread samples. Eur. Food Res. Technol. 2022, 248, 1447–1455. [Google Scholar] [CrossRef]
- Ibrahim, U.K.; Wahab, N.S.A.; Suzihaque, M.U.H.; Hashib, S.A.; Aziz, R.A.A. Evaluation of antioxidant activity and total Phenolic content in bread incorporated with Green Coffee Beans (GCB). Int. J. Eng. Technol. 2018, 7, 156–160. [Google Scholar]
- Zain, M.Z.M.; Baba, A.S.; Shori, A.B. Effect of polyphenols enriched from green coffee bean on antioxidant activity and sensory evaluation of bread. J. King Saud Univ.Sci. 2018, 30, 278–282. [Google Scholar] [CrossRef]
- Tarlak, F. The Use of Predictive Microbiology for the Prediction of the Shelf Life of Food Products. Foods 2023, 12, 4461. [Google Scholar] [CrossRef]
- Ali, M.A.; Hashish, M.H.; Fekry, M.M. Microbiological quality of some packed and unpacked bread products in Alexandria, Egypt. J. Egypt. Public Health Assoc. 2023, 98, 16. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Ma, F.; Yang, N.; Virginio Filho, E.D.M.; Fisk, I. Impact of agro-forestry systems on the aroma generation of coffee beans. Front. Nutr. 2022, 9, 968783. [Google Scholar] [CrossRef]









| WWB Formulations | Composition | ||||||
|---|---|---|---|---|---|---|---|
| WWF (g) | GCBp (g)/(%) | Yeast (g) | Sugar (g) | Salt (g) | Oil (mL) | Water (mL) | |
| C0 | 220.00 | 0.00 (0.00%) | 7.00 | 29.00 | 2.00 | 22.00 | 220.00 |
| C1 | 217.80 | 2.20 (1.00%) | 7.00 | 29.00 | 2.00 | 22.00 | 220.00 |
| C2 | 215.60 | 4.40 (2.00%) | 7.00 | 29.00 | 2.00 | 22.00 | 220.00 |
| C3 | 213.40 | 6.60 (3.00%) | 7.00 | 29.00 | 2.00 | 22.00 | 220.00 |
| C4 | 211.20 | 8.80 (4.00%) | 7.00 | 29.00 | 2.00 | 22.00 | 220.00 |
| C5 | 209.00 | 11.00 (5.00%) | 7.00 | 29.00 | 2.00 | 22.00 | 220.00 |
| Model Parameters | Formulations | |||||
|---|---|---|---|---|---|---|
| C0 | C1 | C2 | C3 | C4 | C5 | |
| R (Ω) | 100,000 ± 0 | 100,000 ± 0 | 100,000 ± 0 | 100,000 ± 0 | 100,000 ± 0 | 100,000 ± 0 |
| Q (F) | 6.54 × 10−5 ± 1.49 × 10−6 de | 1.16 × 10−5 ± 3.03 × 10−6 a | 2.10 × 10−5 ± 1.00 × 10−6 b | 3.20 × 10−5 ± 6.55 × 10−6 c | 2.46 × 10−5 ± 4.08 × 10−6 bc | 2.05 × 10−5 ± 6.84 × 10−7 ab |
| Q1 (F) | 1.32 × 10−5 ± 6.88 × 10−7 a | 2.72 × 10−5 ± 2.15 × 10−5 a | 1.17 × 10−5 ± 5.77 × 10−7 a | 1.33 × 10−5 ± 2.34 × 10−6a | 1.46 × 10−5 ± 1.77 × 10−6 a | 1.49 × 10−5 ± 9.52 × 10−7 a |
| n | 0.7500 ± 0.0500 bc | 0.7633 ± 0.0764 c | 0.7567 ± 0.0153 c | 0.5867 ± 0.0115 a | 0.6267 ± 0.0569 ab | 0.6533 ± 0.0153 abc |
| n1 | 0.7166 ± 0.0058 a | 0.7860 ± 0.0909 a | 0.8190 ± 0.0115 a | 0.9908 ± 0.0160 b | 1.0000 ± 0.0000 b | 1.0000 ± 0.0000 b |
| R2 | 0.9988 ± 0.0007 a | 0.9994 ± 0.0005 a | 0.9998 ± 0.0000 a | 0.9997 ± 0.0002 a | 0.9996 ± 0.0003 a | 0.9997 ± 0.0002 a |
| WWB Formulations | Color Parameters | |||||
|---|---|---|---|---|---|---|
| L* | a* | b* | WI | YI | BI | |
| C0 | 61.73 ± 1.07 b | 6.55 ± 1.61 a | 44.91 ± 1.14 a | 40.60 ± 1.29 b | 103.98 ± 3.75 b | 124.56 ± 7.10 b |
| C1 | 72.34 ± 1.4 c | 6.39 ± 1.01 a | 41.68 ± 1.18 a | 49.53 ± 0.31 c | 82.31 ± 0.86 a | 88.15 ± 1.18 a |
| C2 | 65.21 ± 0.21 b | 6.57 ± 0.47 a | 44.97 ± 3.40 a | 40.86 ± 3.10 b | 103.37 ± 9.20 b | 123.89 ± 15.88 b |
| C3 | 67.31 ± 0.36 b | 7.90 ± 0.40 ab | 59.66 ± 2.57 b | 28.62 ± 2.38 a | 138.30 ± 6.84 c | 204.98 ± 19.77 c |
| C4 | 59.77±0.83 ab | 10.52 ±1.20 b | 59.91 ± 1.20 b | 27.05 ± 1.22 a | 143.23 ± 4.33 c | 222.38 ± 12.47 c |
| C5 | 58.11 ± 0.83 a | 6.19 ± 1.20 a | 52.98 ± 1.03 b | 26.28 ± 0.41 a | 148.29 ± 1.17 c | 233.70 ± 2.58 c |
| WWB Formulations | Color Parameters | |||||
|---|---|---|---|---|---|---|
| Lm* | am* | bm* | WIm | YIm | BIm | |
| C0 | 73.36 ± 0.70 cd | 8.09 ± 0.94 a | 14.82 ± 2.15 ab | 59.64 ± 1.96 bc | 52.33 ± 6.71 ab | 55.09 ± 7.01 ab |
| C1 | 77.97 ± 2.47 d | 8.60 ± 0.17 a | 11.19 ± 1.72 a | 70.73 ± 1.71 c | 18.73 ± 9.07 a | 22.74 ± 7.06 a |
| C2 | 72.09 ± 2.27 bc | 8.88 ± 1.07 a | 16.85 ± 1.10 bc | 67.32 ± 3.17 b | 26.12 ± 11.46 bc | 29.21 ± 10.56 bc |
| C3 | 67.80 ± 0.49 abc | 9.56 ± 0.82 ab | 15.15 ± 2.71 ab | 71.44 ± 0.87 ab | 16.38 ± 6.86 b | 21.37 ± 5.46 bc |
| C4 | 67.01 ± 0.60 ab | 13.87 ± 3.73 b | 18.73 ± 1.22 bc | 78.11 ± 1.83 a | 13.48 ± 5.61 bc | 15.49 ± 4.25 d |
| C5 | 65.38 ± 3.55 a | 7.93 ± 0.21 b | 20.27 ± 0.90 c | 68.46 ± 3.16 a | 24.43 ± 11.91 c | 26.07 ± 11.50 cd |
| Sensory Attributes | Formulations | |||||
|---|---|---|---|---|---|---|
| C0 | C1 | C2 | C3 | C4 | C5 | |
| Appearance | 4.00 ± 0.63 bc | 4.63 ± 0.67 c | 3.81 ± 0.63 bc | 2.63 ± 0.80 a | 2.90 ± 1.10 ab | 2.36 ± 1.36 a |
| Aroma | 3.72 ± 1.10 a | 3.81 ± 0.87 b | 3.36 ± 1.28 a | 2.63 ± 0.80 a | 3.00 ± 1.18 a | 2.54 ± 0.82 a |
| Color | 4.54 ± 0.68 b | 4.36 ± 0.67 b | 4.00 ± 0.63 b | 2.81 ± 0.80 a | 2.72 ± 1.00 a | 2.09 ± 1.13 a |
| Softness | 4.45 ± 0.68 b | 3.90 ± 0.70 ab | 4.27 ± 1.00 b | 3.50 ± 1.21 ab | 3.36 ± 1.20 ab | 2.72 ± 1.34 a |
| Overall Impression | 4.36 ± 0.80 b | 4.27 ± 0.64 b | 3.45 ± 1.12 ab | 2.81 ± 0.87 a | 2.54 ± 0.03 a | 2.27 ± 1.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, R.; Banerjee, D.; Sahu, D.; Tanveer, J.; Banerjee, S.; Jarzębski, M.; Jayaraman, S.; Deng, Y.; Kim, H.; Pal, K. Evaluating the Impact of Green Coffee Bean Powder on the Quality of Whole Wheat Bread: A Comprehensive Analysis. Foods 2024, 13, 2705. https://doi.org/10.3390/foods13172705
Das R, Banerjee D, Sahu D, Tanveer J, Banerjee S, Jarzębski M, Jayaraman S, Deng Y, Kim H, Pal K. Evaluating the Impact of Green Coffee Bean Powder on the Quality of Whole Wheat Bread: A Comprehensive Analysis. Foods. 2024; 13(17):2705. https://doi.org/10.3390/foods13172705
Chicago/Turabian StyleDas, Raima, Debmalya Banerjee, Deblu Sahu, Juwairiya Tanveer, Soumik Banerjee, Maciej Jarzębski, Sivaraman Jayaraman, Yang Deng, Hayeong Kim, and Kunal Pal. 2024. "Evaluating the Impact of Green Coffee Bean Powder on the Quality of Whole Wheat Bread: A Comprehensive Analysis" Foods 13, no. 17: 2705. https://doi.org/10.3390/foods13172705







